Functional Adaptation of Vocalization Revealed by Morphological and Histochemical Characteristics of Sonic Muscles in Blackmouth Croaker (Atrobucca nibe)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
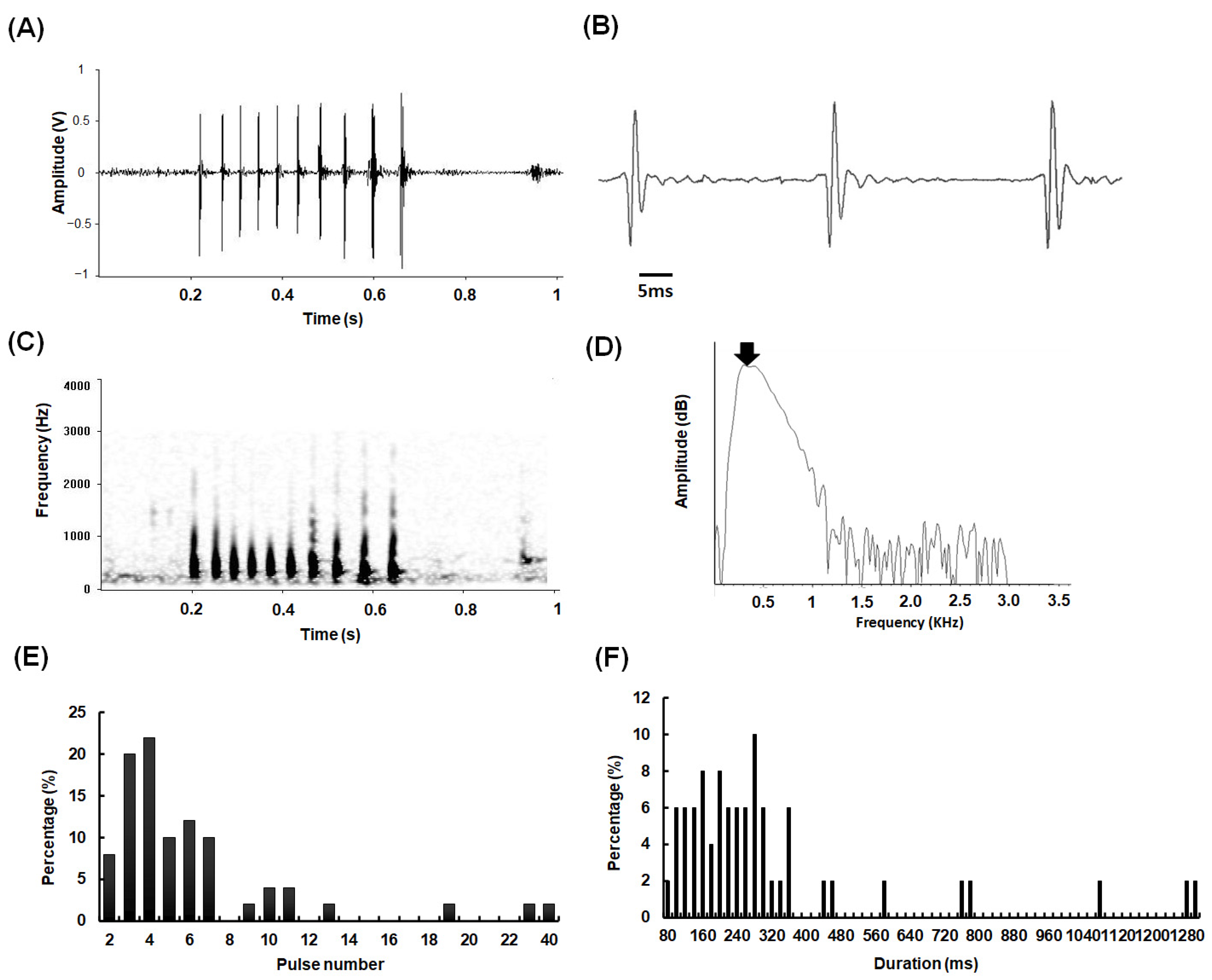
2.2. Sound Recording and Analysis
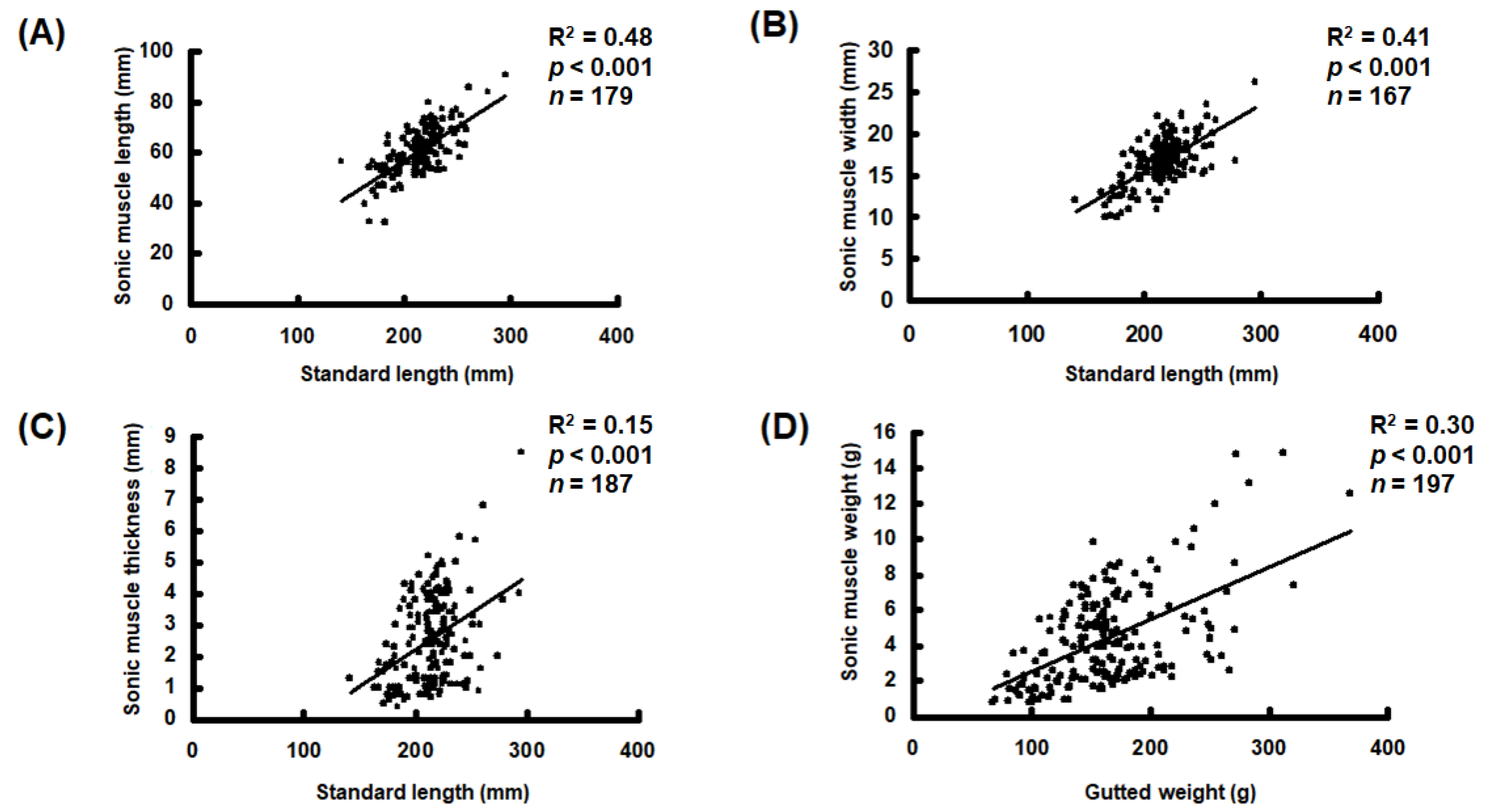
2.3. Morphometry of Sonic Muscles
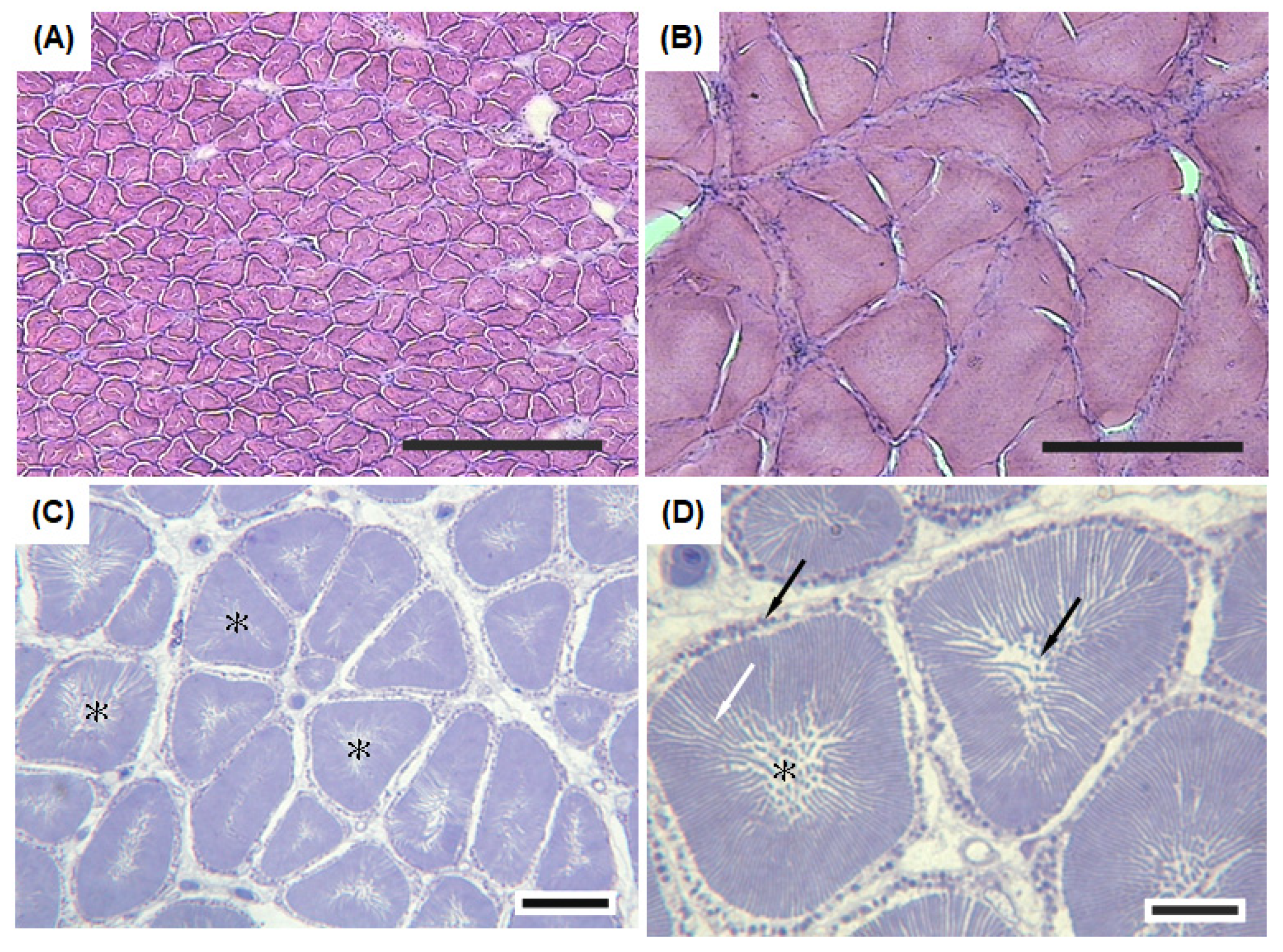
2.4. Histology
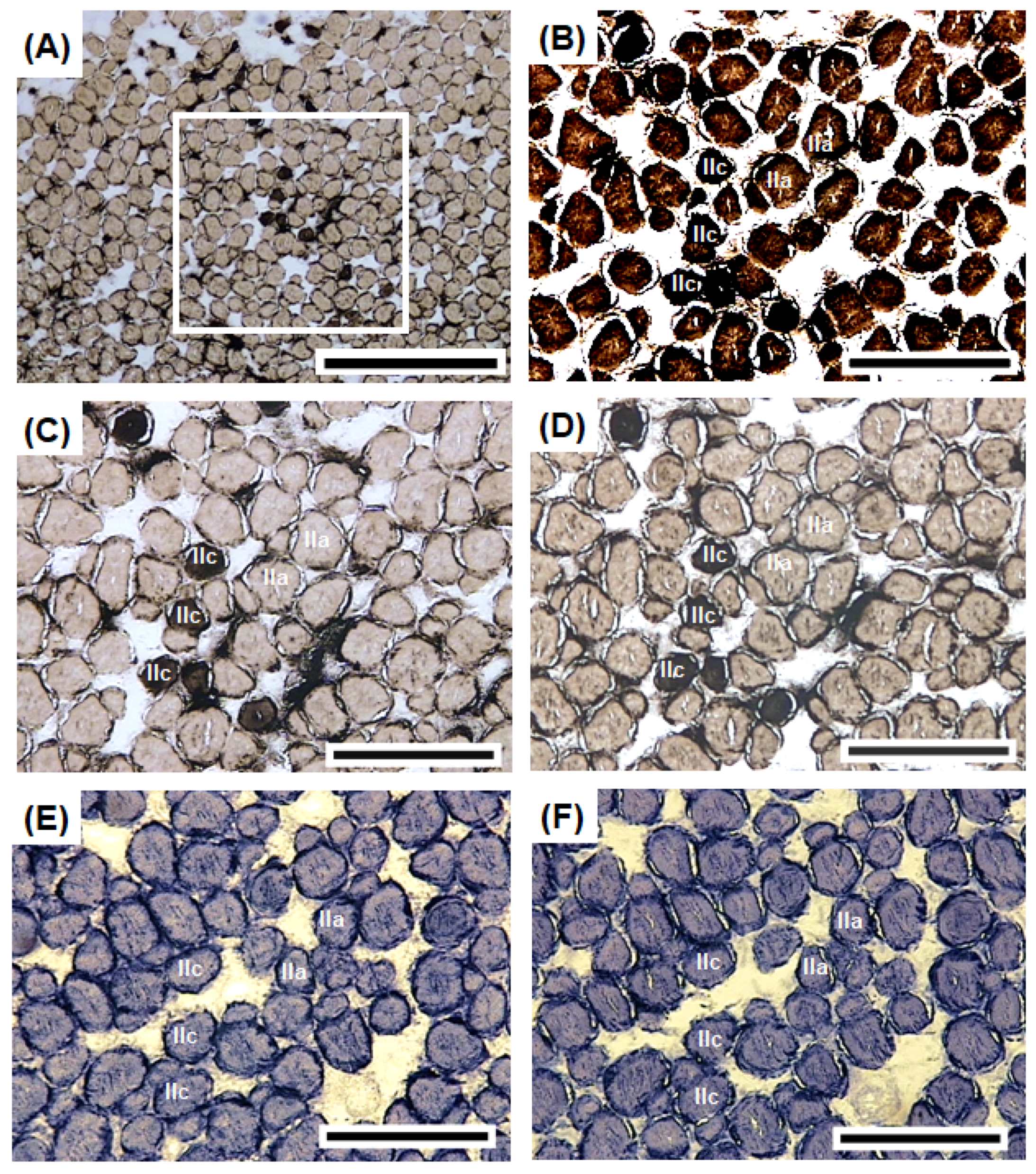
2.5. Histochemistry
2.6. Statistics
3. Results
3.1. Sound Characteristics
3.2. Structure and Organization of Sonic Muscles
3.3. Morphological Characteristics of Sonic Muscles
3.4. Histochemical Characteristics of Sonic Muscles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradbury, J.W.; Vehrencamp, S.L. Principles of Animal Communication, 1st ed.; Sinauer: Sunderland, MA, USA, 1998. [Google Scholar]
- Filiciotto, F.; Buscaino, G. The Role of Sound in the Aquatic Environment. Ecoacoustics Ecol. Role Sounds 2017, 61–79. [Google Scholar] [CrossRef]
- Munk, W.H. Sound Channel in an Exponentially Stratified Ocean, with Application to SOFAR. J. Acoust. Soc. Am. 1974, 55, 220–226. [Google Scholar] [CrossRef]
- Fine, M.L.; Parmentier, E. Mechanisms of Fish Sound Production. In Sound Communication in Fishes; Animal Signals and Communication; Ladich, F., Ed.; Springer: Vienna, Austria, 2015; pp. 77–126. ISBN 978-3-7091-1846-7. [Google Scholar]
- Lobel, P.S.; Kaatz, I.M.; Rice, A.N. Acoustical Behavior of Coral Reef Fishes. In Reproduction and Sexuality in Marine Fishes: Patterns and Processes; University of California Press: Berkley, CA, USA, 2010; p. 307. [Google Scholar]
- Amorim, M.C.P. Diversity of Sound Production in Fish. Commun. Fishes 2006, 1, 71–104. [Google Scholar]
- Ladich, F. Ecology of Sound Communication in Fishes. Fish Fish. 2019, 20, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, E.; Diogo, R.; Fine, M.L. Multiple Exaptations Leading to Fish Sound Production. Fish Fish. 2017, 18, 958–966. [Google Scholar] [CrossRef]
- Mok, H.-K.; Parmentier, E.; Chiu, K.-H.; Tsai, K.-E.; Chiu, P.-H.; Fine, M.L. An Intermediate in the Evolution of Superfast Sonic Muscles. Front. Zool. 2011, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.L.; Waybright, T.D. Grunt Variation in the Oyster Toadfish Opsanus Tau: Effect of Size and Sex. PeerJ 2015, 3, e1330. [Google Scholar] [CrossRef] [PubMed]
- Onuki, A.; Somiya, H. Two Types of Sounds and Additional Spinal Nerve Innervation to the Sonic Muscle in John Dory, Zeus Faber (Zeiformes: Teleostei). J. Mar. Biol. Assoc. UK 2004, 84, 843–850. [Google Scholar] [CrossRef]
- Connaughton, M.A. Sound Generation in the Searobin (Prionotus Carolinus), a Fish with Alternate Sonic Muscle Contraction. J. Exp. Biol. 2004, 207, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Huang, B.-Q.; Chien, Y.-Y. Histochemical Characteristics of Sonic Muscle Fibers in Tigerperch, Terapon Jarbua. Zool. Stud. 1998, 37, 56–62. [Google Scholar]
- Connaughton, M.A.; Fine, M.L.; Taylor, M.H. Weakfish Sonic Muscle: Influence of Size, Temperature and Season. J. Exp. Biol. 2002, 205, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.S.; Dewan, A.K.; Tricas, T.C. Fast Drum Strokes: Novel and Convergent Features of Sonic Muscle Ultrastructure, Innervation, and Motor Neuron Organization in the Pyramid Butterflyfish (Hemitaurichthys Polylepis). J. Morphol. 2013, 274, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, E.; Solagna, L.; Bertucci, F.; Fine, M.L.; Nakae, M.; Compère, P.; Smeets, S.; Raick, X.; Lecchini, D. Simultaneous Production of Two Kinds of Sounds in Relation with Sonic Mechanism in the Boxfish Ostracion Meleagris and O. Cubicus. Sci. Rep. 2019, 9, 4962. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, E.; Marucco Fuentes, E.; Millot, M.; Raick, X.; Thiry, M. Sound Production, Hearing Sensitivity, and in-Depth Study of the Sound-Producing Muscles in the Cowfish (Lactoria Cornuta). J. Anat. 2021, 238, 956–969. [Google Scholar] [CrossRef]
- Tavolga, W.N. Sonic Characteristics and Mechanisms in Marine Fishes. In Marine Bio-Acoust.; Tavolga, W.N., Ed.; Pergamon: New York, NY, USA, 1964; Volume 1, pp. 195–211. [Google Scholar]
- Rome, L.C.; Syme, D.A.; Hollingworth, S.; Lindstedt, S.L.; Baylor, S.M. The Whistle and the Rattle: The Design of Sound Producing Muscles. Proc. Natl. Acad. Sci. USA 1996, 93, 8095–8100. [Google Scholar] [CrossRef]
- Fine, M.L.; Malloy, K.L.; King, C.; Mitchell, S.L.; Cameron, T.M. Movement and Sound Generation by the Toadfish Swimbladder. J. Comp. Physiol. A 2001, 187, 371–379. [Google Scholar] [CrossRef]
- Parmentier, E.; Lagardère, J.-P.; Braquegnier, J.-B.; Vandewalle, P.; Fine, M.L. Sound Production Mechanism in Carapid Fish: First Example with a Slow Sonic Muscle. J. Exp. Biol. 2006, 209, 2952–2960. [Google Scholar] [CrossRef][Green Version]
- Barany, M. ATPase Activity of Myosin Correlated with Speed of Muscle Shortening. J. Gen. Physiol. 1967, 50, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Guth, L.; Samaha, F.J. Qualitative Differences between Actomyosin ATPase of Slow and Fast Mammalian Muscle. Exp. Neurol. 1969, 25, 138–152. [Google Scholar] [CrossRef]
- Dubowitz, V.; Sewry, C.A.; Oldfors, A. Muscle Biopsy E-Book: A Practical Approach; Elsevier Health Sciences: London, UK, 2020; ISBN 0-7020-7858-1. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 1-119-22082-3. [Google Scholar]
- Connaughton, M.A.; Taylor, M.H. Seasonal and Daily Cycles in Sound Production Associated with Spawning in the Weakfish, Cynoscion Regalis. Environ. Biol. Fishes 1995, 42, 233–240. [Google Scholar] [CrossRef]
- Ueng, J.-P.; Huang, B.-Q.; Mok, H.-K. Sexual Differences in the Spawning Sounds of the Japanese Croaker, Argyrosomus Japonicus (Sciaenidae). Zool. Stud. 2007, 46, 103–110. [Google Scholar]
- Bolgan, M.; Crucianelli, A.; Mylonas, C.C.; Henry, S.; Falguière, J.C.; Parmentier, E. Calling Activity and Calls’ Temporal Features Inform about Fish Reproductive Condition and Spawning in Three Cultured Sciaenidae Species. Aquaculture 2020, 524, 735243. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. Version (06/2021). 2021. Available online: www.fishbase.org (accessed on 11 March 2022).
- Specht, R. Avisoft-Saslab Pro: Sound Analysis and Synthesis Laboratory; Avisoft Bioacoustics: Berlin, Germany, 2002; pp. 1–723. [Google Scholar]
- Vieira, M.; Pereira, B.P.; Pousão-Ferreira, P.; Fonseca, P.J.; Amorim, M. Seasonal Variation of Captive Meagre Acoustic Signalling: A Manual and Automatic Recognition Approach. Fishes 2019, 4, 28. [Google Scholar] [CrossRef]
- Picciulin, M.; Fiorin, R.; Facca, C.; Malavasi, S. Sound Features and Vocal Rhythms as a Proxy for Locating the Spawning Ground of Sciaena Umbra in the Wild. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1299–1312. [Google Scholar] [CrossRef]
- Ladich, F. Acoustic Communication in Fishes: Temperature Plays a Role. Fish Fish. 2018, 19, 598–612. [Google Scholar] [CrossRef]
- Caiger, P.E.; Dean, M.J.; DeAngelis, A.I.; Hatch, L.T.; Rice, A.N.; Stanley, J.A.; Tholke, C.; Zemeckis, D.R.; Van Parijs, S.M. A Decade of Monitoring Atlantic Cod Gadus Morhua Spawning Aggregations in Massachusetts Bay Using Passive Acoustics. Mar. Ecol. Prog. Ser. 2020, 635, 89–103. [Google Scholar] [CrossRef]
- Carriço, R.; Silva, M.A.; Menezes, G.M.; Fonseca, P.J.; Amorim, M.C.P. Characterization of the Acoustic Community of Vocal Fishes in the Azores. PeerJ 2019, 7, e7772. [Google Scholar] [CrossRef]
- Hill, G.L.; Fine, M.L.; Musick, J.A. Ontogeny of the Sexually Dimorphic Sonic Muscle in Three Sciaenid Species. Copeia 1987, 1987, 708–713. [Google Scholar] [CrossRef]
- Ramcharitar, J.; Gannon, D.P.; Popper, A.N. Bioacoustics of Fishes of the Family Sciaenidae (Croakers and Drums). Trans. Am. Fish. Soc. 2006, 135, 1409–1431. [Google Scholar] [CrossRef]
- Borie, A.; Mok, H.-K.; Chao, N.L.; Fine, M.L. Spatiotemporal Variability and Sound Characterization in Silver Croaker Plagioscion Squamosissimus (Sciaenidae) in the Central Amazon. PLoS ONE 2014, 9, e99326. [Google Scholar] [CrossRef]
- Lin, Y.C.; Mok, H.K.; Huang, B.Q. Sound Characteristics of Big-Snout Croaker, Johnius Macrorhynus (Sciaenidae). J. Acoust. Soc. Am. 2007, 121, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, J.S.; Norbis, W.; Olsson, D.; Fine, M.L. Calls of the Black Drum (Pogonias Cromis: Sciaenidae): Geographical Differences in Sound Production between Northern and Southern Hemisphere Populations. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2011, 315, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chiu, K.-H.; Shiea, J.; Huang, H.-W.; Mok, H.-K. Seasonal Changes in Atrophy-Associated Proteins of the Sonic Muscle in the Big-Snout Croaker, Johnius Macrorhynus (Pisces, Sciaenidae), Identified by Using a Proteomic Approach. Fish Physiol. Biochem. 2011, 37, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Connaughton, M.A.; Fine, M.L.; Taylor, M.H. The Effects of Seasonal Hypertrophy and Atrophy on Fiber Morphology, Metabolic Substrate Concentration and Sound Characteristics of the Weakfish Sonic Muscle. J. Exp. Biol. 1997, 200, 2449–2457. [Google Scholar] [CrossRef]
- Parmentier, E.; Tock, J.; Falguière, J.-C.; Beauchaud, M. Sound Production in Sciaenops Ocellatus: Preliminary Study for the Development of Acoustic Cues in Aquaculture. Aquaculture 2014, 432, 204–211. [Google Scholar] [CrossRef]
- Ono, R.D.; Poss, S.G. Structure and Innervation of the Swim Bladder Musculature in the Weakfish, Cynoscion Regalis (Teleostei: Sciaenidae). Can. J. Zool. 1982, 60, 1955–1967. [Google Scholar] [CrossRef]
- Fine, M.L.; Burns, N.M.; Harris, T.M. Ontogeny and Sexual Dimorphism of Sonic Muscle in the Oyster Toadfish. Can. J. Zool. 1990, 68, 1374–1381. [Google Scholar] [CrossRef]
- Parmentier, E.; Gennotte, V.; Focant, B.; Goffinet, G.; Vandewalle, P. Characterization of the Primary Sonic Muscles in Carapus Acus (Carapidae): A Multidisciplinary Approach. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 2301–2308. [Google Scholar] [CrossRef]
- Loesser, K.E.; Rafi, J.; Fine, M.L. Embryonic, Juvenile, and Adult Development of the Toadfish Sonic Muscle. Anat. Rec. 1997, 249, 469–477. [Google Scholar] [CrossRef]
- Fine, M.L.; Bernard, B.; Harris, T.M. Functional Morphology of Toadfish Sonic Muscle Fibers: Relationship to Possible Fiber Division. Can. J. Zool. 1993, 71, 2262–2274. [Google Scholar] [CrossRef]
- Fine, M.L.; Pennypacker, K.R. Histochemical Typing of Sonic Muscle from the Oyster Toadfish. Copeia 1988, 1988, 130–134. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Mackenzie, M.G.; Elgar, G.; Suzuki, Y.; Watabe, S.; Kinghorn, J.R.; Johnston, I.A. A Genomic Approach to Reveal Novel Genes Associated with Myotube Formation in the Model Teleost, Takifugu Rubripes. Physiol. Genom. 2005, 22, 327–338. [Google Scholar] [CrossRef] [PubMed]

| Type IIa | Type IIc | |
|---|---|---|
| ATPase (pH 9.7) | +++ | ++ |
| ATPase (pH 5.1) | − | +++ |
| ATPase (pH 4.9) | − | +++ |
| NADH-TR | +++ | +++ |
| LDH | +++ | +++ |
| Composition (%) | >95 | |
| Diameter (μm) * | 43 ± 4 (n = 200) | 29 ± 4 (n = 80) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-T.; Huang, B.-Q.; Liao, C.-H. Functional Adaptation of Vocalization Revealed by Morphological and Histochemical Characteristics of Sonic Muscles in Blackmouth Croaker (Atrobucca nibe). Biology 2022, 11, 438. https://doi.org/10.3390/biology11030438
Lee H-T, Huang B-Q, Liao C-H. Functional Adaptation of Vocalization Revealed by Morphological and Histochemical Characteristics of Sonic Muscles in Blackmouth Croaker (Atrobucca nibe). Biology. 2022; 11(3):438. https://doi.org/10.3390/biology11030438
Chicago/Turabian StyleLee, Hung-Tai, Bao-Quey Huang, and Cheng-Hsin Liao. 2022. "Functional Adaptation of Vocalization Revealed by Morphological and Histochemical Characteristics of Sonic Muscles in Blackmouth Croaker (Atrobucca nibe)" Biology 11, no. 3: 438. https://doi.org/10.3390/biology11030438
APA StyleLee, H.-T., Huang, B.-Q., & Liao, C.-H. (2022). Functional Adaptation of Vocalization Revealed by Morphological and Histochemical Characteristics of Sonic Muscles in Blackmouth Croaker (Atrobucca nibe). Biology, 11(3), 438. https://doi.org/10.3390/biology11030438







