Draining the Pleural Space: Lymphatic Vessels Facing the Most Challenging Task
Abstract
Simple Summary
Abstract
1. Lymph Draining and Propulsion
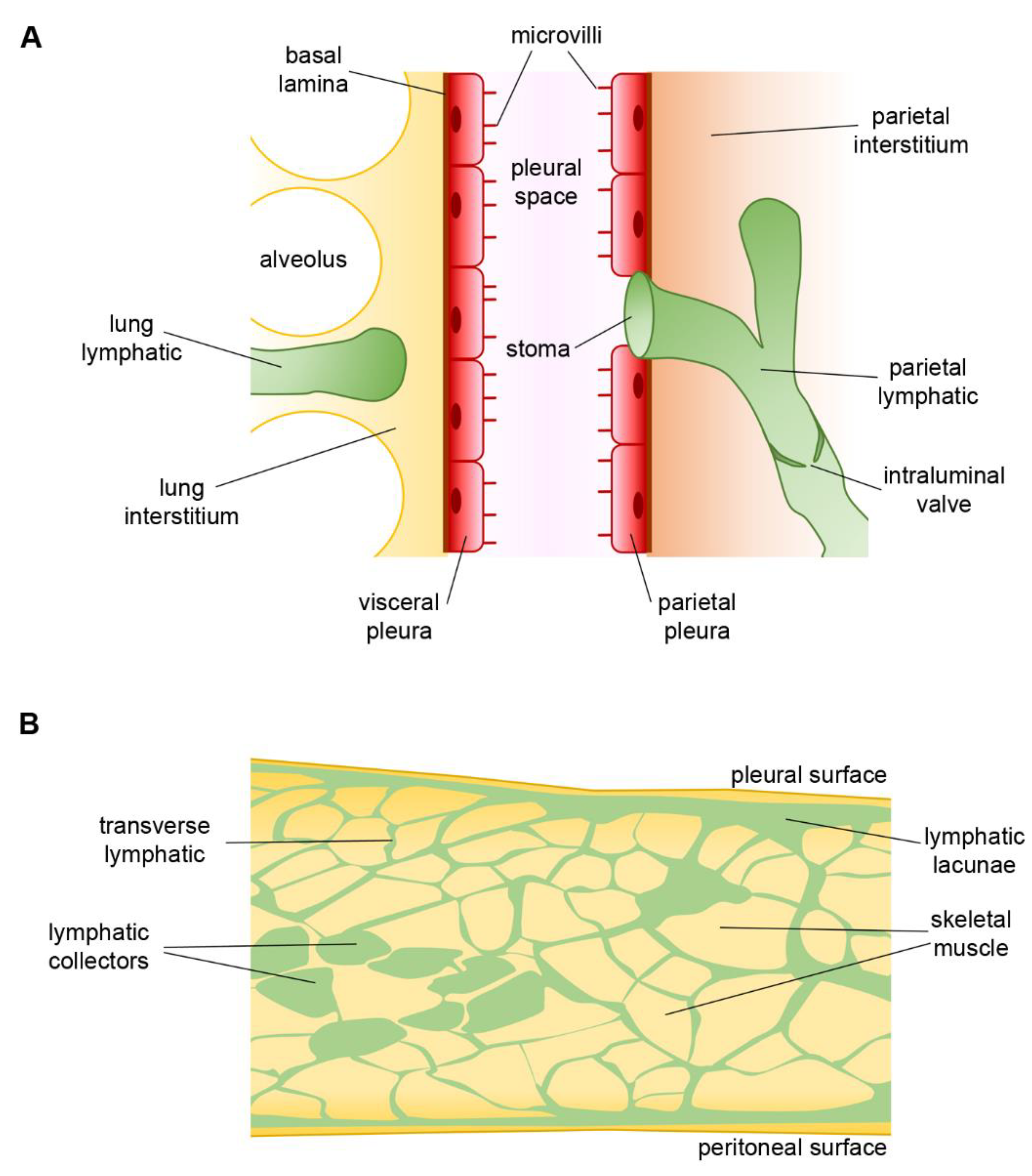
2. The Pleural Space
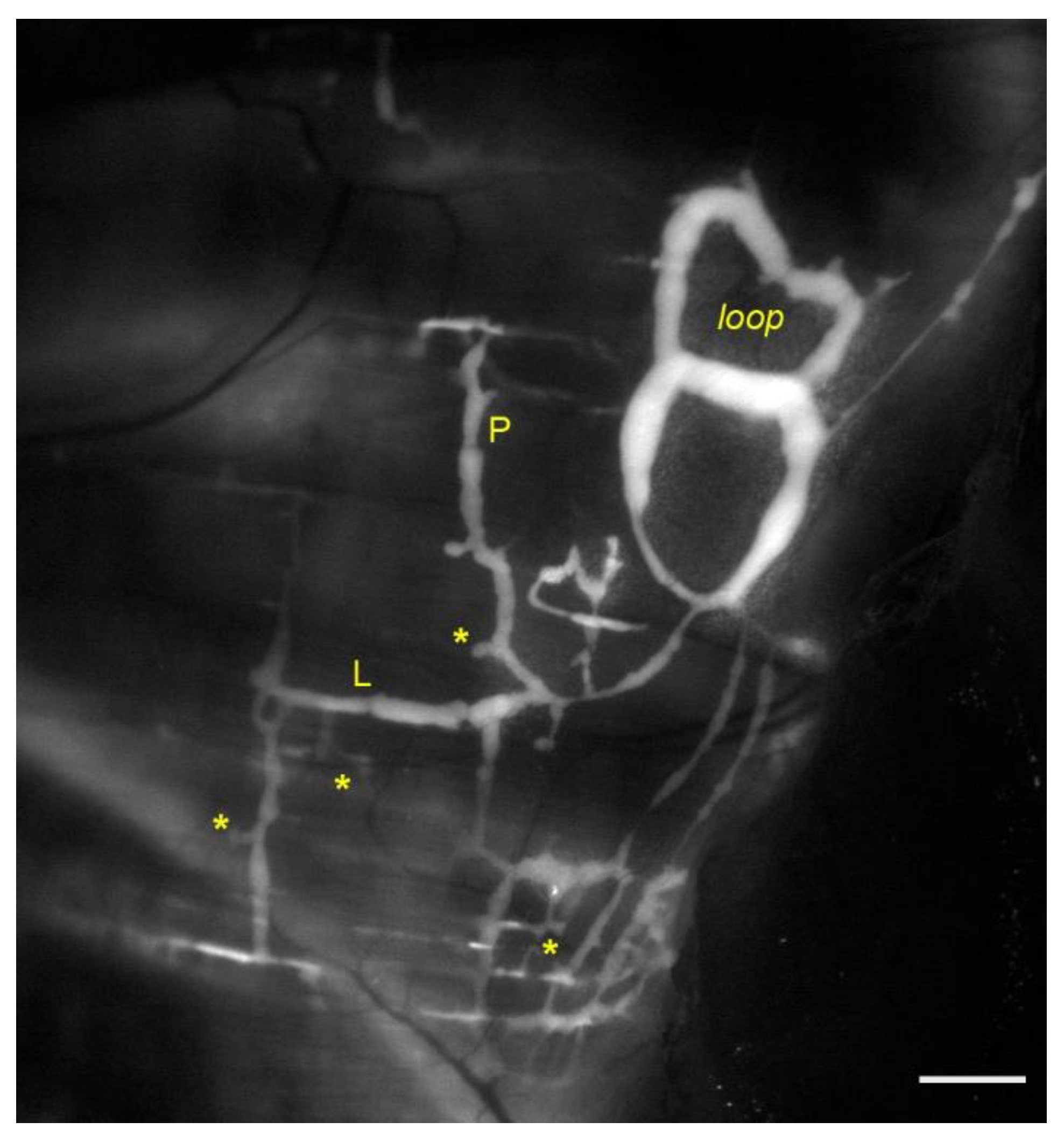
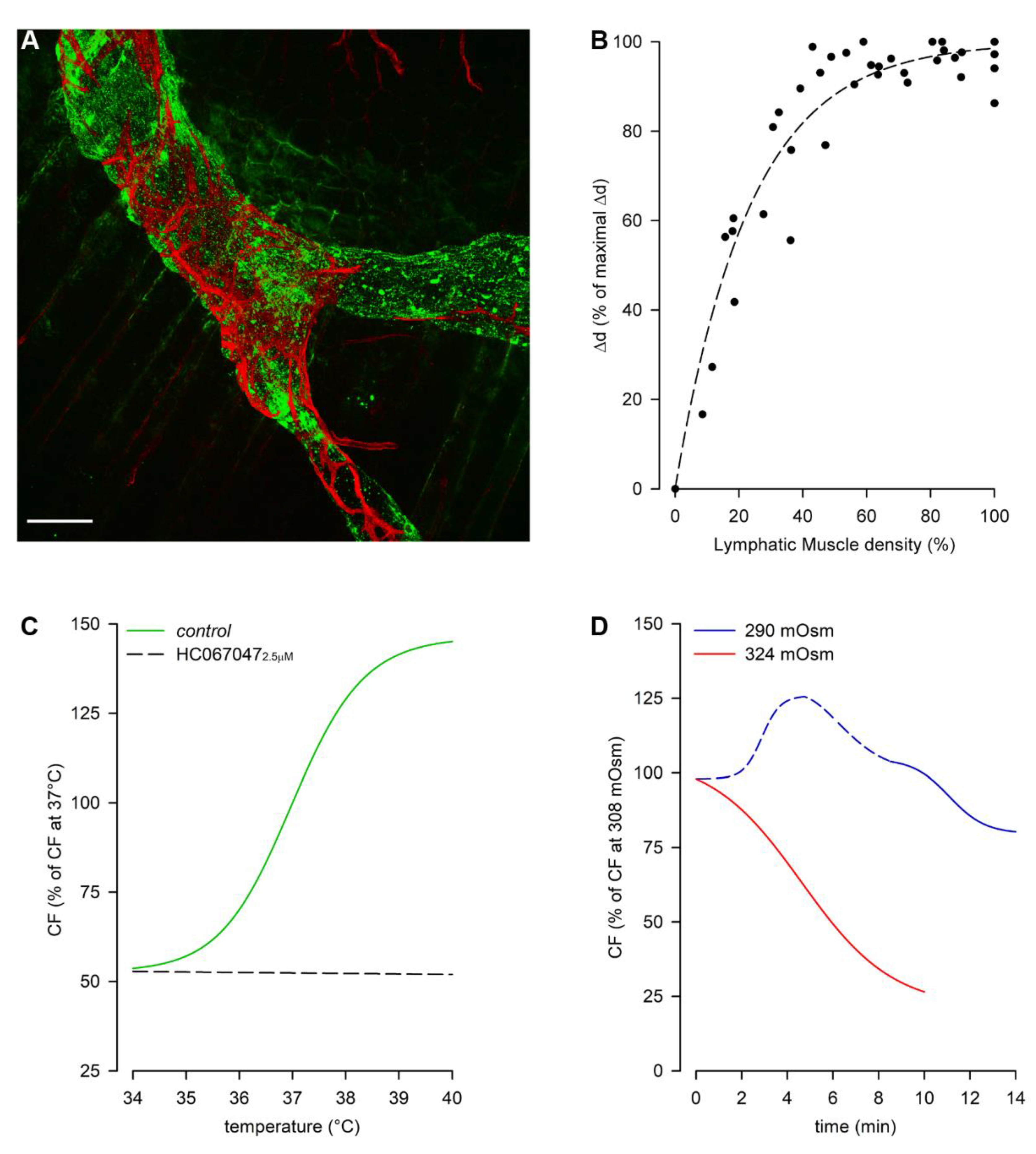
3. Lymphatic Vessels of the Diaphragm
4. Pleural Intercostal Lymphatics
5. Lymphatic Vessels of Airways and Lungs
6. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | intrinsic Contraction Frequency |
| Δd | intrinsic contraction amplitude (Δdiameter) |
| ΔPLymph | Intraluminal Lymphatic hydraulic Pressure gradient |
| ΔPπ | Transmural colloidosmotic Pressure gradient |
| ΔPTM | Transmural hydraulic Pressure gradient |
| ΔPtv | Trans-Valve hydraulic Pressure gradient |
| HA | Hyaluronan |
| HC067047 | selective TRPV4 channels antagonist |
| HCN | Hyperpolarization-activated Cyclic Nucleotide-gated channels |
| If | Sinoatrial node “funny” current of the heart pacemaker |
| Jlymph | Lymph Flow across the lymphatic capillary’s wall |
| LDLs | Low-Density Lipoproteins |
| LECs | Lymphatic Endothelial Cells |
| LMCs | Lymphatic Muscle Cells |
| LYVE1 | Lymphatic Vessels Endothelial Hyaluronan Receptor 1 |
| PEEP | Positive End-Expiratory Pressure |
| Pin | Interstitial hydraulic Pressure |
| PL | Intraluminal Lymphatic hydraulic Pressure |
| Pliq | Pleural hydraulic Pressure |
| Pπ,in | Interstitial colloidosmotic Pressure |
| Pπ,L | Intraluminal Lymphatic colloidosmotic Pressure |
| Prox1 | Prospero homeobox 1 |
| RR | Respiratory Rate |
| STDs | Spontaneous Transient Depolarizations |
| SV | intrinsic Stroke Volume |
| TRPV4 | Transient Receptor Potential channel Vanilloid 4 |
| VEGFC | Vascular Endothelial Growth Factor C |
| VEGFD | Vascular Endothelial Growth Factor D |
| VEGFR3 | Vascular Endothelial Growth Factor Receptor 3 |
| VT | Tidal Volume |
References
- Wiig, H.; Swartz, M.A. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef]
- Leak, L.V. Studies on the permeability of lymphatic capillaries. J. Cell Biol. 1971, 50, 300–323. [Google Scholar] [CrossRef]
- Trzewik, J.; Mallipattu, S.K.; Artmann, G.M.; Delano, F.A.; Schmid-Schönbein, G.W. Evidence for a second valve system in lymphatics: Endothelial microvalves. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 1711–1717. [Google Scholar] [CrossRef]
- Bazigou, E.; Wilson, J.T.; Moore, J.E.J. Primary and secondary lymphatic valve development: Molecular, functional and mechanical insights. Microvasc. Res. 2014, 96, 38–45. [Google Scholar] [CrossRef]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef]
- Aukland, K.; Reed, R.K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol. Rev. 1993, 73, 1–78. [Google Scholar] [CrossRef]
- Leak, L.V.; Burke, J.F. Ultrastructural studies on the lymphatic anchoring filaments. J. Cell Biol. 1968, 36, 129–149. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W. Microlymphatics and lymph flow. Physiol. Rev. 1990, 70, 987–1028. [Google Scholar] [CrossRef]
- Muthuchamy, M.; Gashev, A.; Boswell, N.; Dawson, N.; Zawieja, D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 920–922. [Google Scholar] [CrossRef]
- Bazigou, E.; Makinen, T. Flow control in our vessels: Vascular valves make sure there is no way back. Cell. Mol. Life Sci. 2013, 70, 1055–1066. [Google Scholar] [CrossRef]
- Zweifach, B.W.; Prather, J.W. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am. J. Physiol. 1975, 228, 1326–1335. [Google Scholar] [CrossRef]
- Davis, M.J.; Rahbar, E.; Gashev, A.A.; Zawieja, D.C.; Moore, J.E.J. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H48–H60. [Google Scholar] [CrossRef]
- Dixon, J.B.; Greiner, S.T.; Gashev, A.A.; Cote, G.L.; Moore, J.E.; Zawieja, D.C. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 2006, 13, 597–610. [Google Scholar] [CrossRef]
- Moriondo, A.; Solari, E.; Marcozzi, C.; Negrini, D. Lymph flow pattern in pleural diaphragmatic lymphatics during intrinsic and extrinsic isotonic contraction. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H60–H70. [Google Scholar] [CrossRef]
- Zawieja, D.C.; Davis, K.L.; Schuster, R.; Hinds, W.M.; Granger, H.J. Distribution, propagation, and coordination of contractile activity in lymphatics. Am. J. Physiol. 1993, 264, H1283–H1291. [Google Scholar] [CrossRef]
- Scallan, J.P.; Zawieja, S.D.; Castorena-Gonzalez, J.A.; Davis, M.J. Lymphatic pumping: Mechanics, mechanisms and malfunction. J. Physiol. 2016, 594, 5749–5768. [Google Scholar] [CrossRef]
- McHale, N.G.; Meharg, M.K. Co-ordination of pumping in isolated bovine lymphatic vessels. J. Physiol. 1992, 450, 503–512. [Google Scholar] [CrossRef]
- von der Weid, P.-Y.; Zawieja, D.C. Lymphatic smooth muscle: The motor unit of lymph drainage. Int. J. Biochem. Cell Biol. 2004, 36, 1147–1153. [Google Scholar] [CrossRef]
- Bridenbaugh, E.A.; Gashev, A.A.; Zawieja, D.C. Lymphatic muscle: A review of contractile function. Lymphat. Res. Biol. 2003, 1, 147–158. [Google Scholar] [CrossRef]
- von der Weid, P.-Y. Lymphatic Vessel Pumping. Adv. Exp. Med. Biol. 2019, 1124, 357–377. [Google Scholar] [CrossRef]
- West, J.B.; Luks, A.M. Respiratory Physiology, 11th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2020; ISBN 9781975139186. [Google Scholar]
- Moriondo, A.; Bianchin, F.; Marcozzi, C.; Negrini, D. Kinetics of fluid flux in the rat diaphragmatic submesothelial lymphatic lacunae. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1182–H1190. [Google Scholar] [CrossRef]
- Moriondo, A.; Solari, E.; Marcozzi, C.; Negrini, D. Spontaneous activity in peripheral diaphragmatic lymphatic loops. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H987–H995. [Google Scholar] [CrossRef]
- Negrini, D.; Ballard, S.T.; Benoit, J.N. Contribution of lymphatic myogenic activity and respiratory movements to pleural lymph flow. J. Appl. Physiol. 1994, 76, 2267–2274. [Google Scholar] [CrossRef]
- Shinohara, H. Lymphatic system of the mouse diaphragm: Morphology and function of the lymphatic sieve. Anat. Rec. 1997, 249, 6–15. [Google Scholar] [CrossRef]
- Negrini, D.; Mukenge, S.; Del Fabbro, M.; Gonano, C.; Miserocchi, G. Distribution of diaphragmatic lymphatic stomata. J. Appl. Physiol. 1991, 70, 1544–1549. [Google Scholar] [CrossRef]
- Negrini, D.; Del Fabbro, M.; Gonano, C.; Mukenge, S.; Miserocchi, G. Distribution of diaphragmatic lymphatic lacunae. J. Appl. Physiol. 1992, 72, 1166–1172. [Google Scholar] [CrossRef]
- Grimaldi, A.; Moriondo, A.; Sciacca, L.; Guidali, M.L.; Tettamanti, G.; Negrini, D. Functional arrangement of rat diaphragmatic initial lymphatic network. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H876–H885. [Google Scholar] [CrossRef]
- Barrett, K.E.; Barman, S.M.; Boitano, S.; Brux, H. Ganong’s Review of Medical Physiology; McGraw-Hill: New York, NY, USA, 2012; ISBN 9780071780032. [Google Scholar]
- Species-Specific Information: Mouse. Available online: https://web.jhu.edu/animalcare/procedures/mouse.html (accessed on 28 December 2021).
- Species-Specific Information: Rat. Available online: https://web.jhu.edu/animalcare/procedures/rat.html (accessed on 28 December 2021).
- Moriondo, A.; Boschetti, F.; Bianchin, F.; Lattanzio, S.; Marcozzi, C.; Negrini, D. Tissue contribution to the mechanical features of diaphragmatic initial lymphatics. J. Physiol. 2010, 588, 3957–3969. [Google Scholar] [CrossRef]
- Mazzoni, M.C.; Skalak, T.C.; Schmid-Schönbein, G.W. Effects of skeletal muscle fiber deformation on lymphatic volumes. Am. J. Physiol. 1990, 259, H1860–H1868. [Google Scholar] [CrossRef]
- Moriondo, A.; Solari, E.; Marcozzi, C.; Negrini, D. Diaphragmatic lymphatic vessel behavior during local skeletal muscle contraction. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H193–H205. [Google Scholar] [CrossRef]
- Negrini, D.; Moriondo, A.; Mukenge, S. Transmural pressure during cardiogenic oscillations in rodent diaphragmatic lymphatic vessels. Lymphat. Res. Biol. 2004, 2, 69–81. [Google Scholar] [CrossRef]
- Ohtani, O.; Ohtani, Y. Organization and developmental aspects of lymphatic vessels. Arch. Histol. Cytol. 2008, 71, 1–22. [Google Scholar] [CrossRef]
- Negrini, D.; Marcozzi, C.; Solari, E.; Bossi, E.; Cinquetti, R.; Reguzzoni, M.; Moriondo, A. Hyperpolarization-activated cyclic nucleotide-gated channels in peripheral diaphragmatic lymphatics. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H892–H903. [Google Scholar] [CrossRef]
- McCloskey, K.D.; Toland, H.M.; Hollywood, M.A.; Thornbury, K.D.; McHale, N.G. Hyperpolarisation-activated inward current in isolated sheep mesenteric lymphatic smooth muscle. J. Physiol. 1999, 521 Pt 1, 201–211. [Google Scholar] [CrossRef]
- von der Weid, P.-Y.; Rahman, M.; Imtiaz, M.S.; van Helden, D.F. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: Pharmacology and implication for spontaneous contractility. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1989–H2000. [Google Scholar] [CrossRef]
- Biel, M.; Wahl-Schott, C.; Michalakis, S.; Zong, X. Hyperpolarization-activated cation channels: From genes to function. Physiol. Rev. 2009, 89, 847–885. [Google Scholar] [CrossRef]
- Baruscotti, M.; Bucchi, A.; Difrancesco, D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol. Ther. 2005, 107, 59–79. [Google Scholar] [CrossRef]
- BoSmith, R.E.; Briggs, I.; Sturgess, N.C. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br. J. Pharmacol. 1993, 110, 343–349. [Google Scholar] [CrossRef]
- Bucchi, A.; Tognati, A.; Milanesi, R.; Baruscotti, M.; DiFrancesco, D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J. Physiol. 2006, 572, 335–346. [Google Scholar] [CrossRef]
- Zawieja, D.C. Contractile physiology of lymphatics. Lymphat. Res. Biol. 2009, 7, 87–96. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Bartolini, B.; Viola, M.; Negrini, D.; Moriondo, A. Acute Exposure of Collecting Lymphatic Vessels to Low-Density Lipoproteins Increases Both Contraction Frequency and Lymph Flow: An In Vivo Mechanical Insight. Lymphat. Res. Biol. 2020, 18, 146–155. [Google Scholar] [CrossRef]
- Negrini, D.; Del Fabbro, M. Subatmospheric pressure in the rabbit pleural lymphatic network. J. Physiol. 1999, 520 Pt 3, 761–769. [Google Scholar] [CrossRef]
- Boriek, A.M.; Rodarte, J.R.; Reid, M.B. Shape and tension distribution of the passive rat diaphragm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R33–R41. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Lymphatic Vessels and Their Surroundings: How Local Physical Factors Affect Lymph Flow. Biology 2020, 9, 463. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Bistoletti, M.; Baj, A.; Giaroni, C.; Negrini, D.; Moriondo, A. TRPV4 channels’ dominant role in the temperature modulation of intrinsic contractility and lymph flow of rat diaphragmatic lymphatics. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H507–H518. [Google Scholar] [CrossRef]
- Everaerts, W.; Zhen, X.; Ghosh, D.; Vriens, J.; Gevaert, T.; Gilbert, J.P.; Hayward, N.J.; McNamara, C.R.; Xue, F.; Moran, M.M.; et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl. Acad. Sci. USA 2010, 107, 19084–19089. [Google Scholar] [CrossRef]
- Thorneloe, K.S.; Sulpizio, A.C.; Lin, Z.; Figueroa, D.J.; Clouse, A.K.; McCafferty, G.P.; Chendrimada, T.P.; Lashinger, E.S.R.; Gordon, E.; Evans, L.; et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urina. J. Pharmacol. Exp. Ther. 2008, 326, 432–442. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Temperature-dependent modulation of regional lymphatic contraction frequency and flow. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H879–H889. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Fluid Osmolarity Acutely and Differentially Modulates Lymphatic Vessels Intrinsic Contractions and Lymph Flow. Front. Physiol. 2018, 9, 871. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Interplay between Gut Lymphatic Vessels and Microbiota. Cells 2021, 10, 2584. [Google Scholar] [CrossRef]
- Gayer, C.P.; Basson, M.D. The effects of mechanical forces on intestinal physiology and pathology. Cell. Signal. 2009, 21, 1237–1244. [Google Scholar] [CrossRef]
- Benoit, J.N.; Zawieja, D.C.; Goodman, A.H.; Granger, H.J. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am. J. Physiol. 1989, 257, H2059–H2069. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W. Mechanisms causing initial lymphatics to expand and compress to promote lymph flow. Arch. Histol. Cytol. 1990, 53, 107–114. [Google Scholar] [CrossRef]
- McGeown, J.G.; McHale, N.G.; Thornbury, K.D. The role of external compression and movement in lymph propulsion in the sheep hind limb. J. Physiol. 1987, 387, 83–93. [Google Scholar] [CrossRef]
- Mizuno, R.; Koller, A.; Kaley, G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am. J. Physiol. 1998, 274, R790–R796. [Google Scholar] [CrossRef]
- Fox, J.L.R.; von der Weid, P.-Y. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br. J. Pharmacol. 2002, 136, 1210–1218. [Google Scholar] [CrossRef]
- von der Weid, P.-Y.; Muthuchamy, M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2010, 17, 263–276. [Google Scholar] [CrossRef]
- Von Der Weid, P.-Y.; Rehal, S. Lymphatic pump function in the inflamed gut. Ann. N. Y. Acad. Sci. 2010, 1207 Suppl, E69–E74. [Google Scholar] [CrossRef]
- Chen, Y.; Rehal, S.; Roizes, S.; Zhu, H.-L.; Cole, W.C.; von der Weid, P.-Y. The pro-inflammatory cytokine TNF-α inhibits lymphatic pumping via activation of the NF-κB-iNOS signaling pathway. Microcirculation 2017, 24, e12364. [Google Scholar] [CrossRef]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef]
- Telinius, N.; Baandrup, U.; Rumessen, J.; Pilegaard, H.; Hjortdal, V.; Aalkjaer, C.; Boedtkjer, D.B. The human thoracic duct is functionally innervated by adrenergic nerves. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H206–H213. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.B.; Gsponer, D.; Montoya-Zegarra, J.A.; Schneider, M.; Scholkmann, F.; Tacconi, C.; Noerrelykke, S.F.; Proulx, S.T.; Detmar, M. A Distinct Role of the Autonomic Nervous System in Modulating the Function of Lymphatic Vessels under Physiological and Tumor-Draining Conditions. Cell Rep. 2019, 27, 3305–3314.e13. [Google Scholar] [CrossRef] [PubMed]
- Mariassy, A.T.; Wheeldon, E.B. The pleura: A combined light microscopic, scanning, and transmission electron microscopic study in the sheep. I. Normal pleura. Exp. Lung Res. 1983, 4, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Moriondo, A.; Mukenge, S.; Negrini, D. Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H263–H269. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, D.H. The anatomy of the lymphatics of the lungs and chest wall. Thorax 1970, 25, 255–256. [Google Scholar] [CrossRef][Green Version]
- Leak, L.V.; Jamuar, M.P. Ultrastructure of pulmonary lymphatic vessels. Am. Rev. Respir. Dis. 1983, 128, S59–S65. [Google Scholar] [CrossRef] [PubMed]
- Sozio, F.; Rossi, A.; Weber, E.; Abraham, D.J.; Nicholson, A.G.; Wells, A.U.; Renzoni, E.A.; Sestini, P. Morphometric analysis of intralobular, interlobular and pleural lymphatics in normal human lung. J. Anat. 2012, 220, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Sozio, F.; Borghini, A.; Sestini, P.; Renzoni, E. Pulmonary lymphatic vessel morphology: A review. Ann. Anat.-Anat. Anz. 2018, 218, 110–117. [Google Scholar] [CrossRef]
- Egashira, R.; Tanaka, T.; Imaizumi, T.; Senda, K.; Doki, Y.; Kudo, S.; Fukuoka, J. Differential distribution of lymphatic clearance between upper and lower regions of the lung. Respirology 2013, 18, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E. Lung lymphatic anatomy and correlates. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2010, 17, 337–343. [Google Scholar] [CrossRef]
- Wigle, J.T.; Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996, 15, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Bálint, L.; Jakus, Z. Mechanosensation and Mechanotransduction by Lymphatic Endothelial Cells Act as Important Regulators of Lymphatic Development and Function. Int. J. Mol. Sci. 2021, 22, 3955. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.V.; Mäkinen, T.; Mäkelä, T.P.; Saarela, J.; Virtanen, I.; Ferrell, R.E.; Finegold, D.N.; Kerjaschki, D.; Ylä-Herttuala, S.; Alitalo, K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002, 21, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Detmar, M. The rediscovery of the lymphatic system: Old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002, 16, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; Veikkola, T.; Mustjoki, S.; Karpanen, T.; Catimel, B.; Nice, E.C.; Wise, L.; Mercer, A.; Kowalski, H.; Kerjaschki, D.; et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001, 20, 4762–4773. [Google Scholar] [CrossRef] [PubMed]
- Jakus, Z.; Gleghorn, J.P.; Enis, D.R.; Sen, A.; Chia, S.; Liu, X.; Rawnsley, D.R.; Yang, Y.; Hess, P.R.; Zou, Z.; et al. Lymphatic function is required prenatally for lung inflation at birth. J. Exp. Med. 2014, 211, 815–826. [Google Scholar] [CrossRef]
- Huang, X.Z.; Wu, J.F.; Ferrando, R.; Lee, J.H.; Wang, Y.L.; Farese, R.V.J.; Sheppard, D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol. Cell. Biol. 2000, 20, 5208–5215. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder-Geleff, S.; Soleiman, A.; Kowalski, H.; Horvat, R.; Amann, G.; Kriehuber, E.; Diem, K.; Weninger, W.; Tschachler, E.; Alitalo, K.; et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: Podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999, 154, 385–394. [Google Scholar] [CrossRef]
- Schacht, V.; Ramirez, M.I.; Hong, Y.-K.; Hirakawa, S.; Feng, D.; Harvey, N.; Williams, M.; Dvorak, A.M.; Dvorak, H.F.; Oliver, G.; et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003, 22, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Uhrin, P.; Zaujec, J.; Breuss, J.M.; Olcaydu, D.; Chrenek, P.; Stockinger, H.; Fuertbauer, E.; Moser, M.; Haiko, P.; Fässler, R.; et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 2010, 115, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.I.; Millien, G.; Hinds, A.; Cao, Y.; Seldin, D.C.; Williams, M.C. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev. Biol. 2003, 256, 61–72. [Google Scholar] [CrossRef]
- Banerji, S.; Ni, J.; Wang, S.X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Prevo, R.; Banerji, S.; Ferguson, D.J.; Clasper, S.; Jackson, D.G. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 2001, 276, 19420–19430. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, G.; Negrini, D.; Gonano, C. Direct measurement of interstitial pulmonary pressure in in situ lung with intact pleural space. J. Appl. Physiol. 1990, 69, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, G.; Negrini, D.; Gonano, C. Parenchymal stress affects interstitial and pleural pressures in in situ lung. J. Appl. Physiol. 1991, 71, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Parker, J.C.; Kvietys, P.R.; Perry, M.A. The pulmonary interstitium in capillary exchange. Ann. N. Y. Acad. Sci. 1982, 384, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Beretta, E.; Romanò, F.; Sancini, G.; Grotberg, J.B.; Nieman, G.F.; Miserocchi, G. Pulmonary Interstitial Matrix and Lung Fluid Balance From Normal to the Acutely Injured Lung. Front. Physiol. 2021, 12, 781874. [Google Scholar] [CrossRef] [PubMed]
- Moriondo, A.; Marcozzi, C.; Bianchin, F.; Reguzzoni, M.; Severgnini, P.; Protasoni, M.; Raspanti, M.; Passi, A.; Pelosi, P.; Negrini, D. Impact of mechanical ventilation and fluid load on pulmonary glycosaminoglycans. Respir. Physiol. Neurobiol. 2012, 181, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Chua, F.; Gauldie, J.; Laurent, G.J. Pulmonary fibrosis: Searching for model answers. Am. J. Respir. Cell Mol. Biol. 2005, 33, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M. Lymphangiogenesis and Lesion Heterogeneity in Interstitial Lung Diseases. Clin. Med. Insights. Circ. Respir. Pulm. Med. 2015, 9, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lara, A.R.; Cosgrove, G.P.; Janssen, W.J.; Huie, T.J.; Burnham, E.L.; Heinz, D.E.; Curran-Everett, D.; Sahin, H.; Schwarz, M.I.; Cool, C.D.; et al. Increased lymphatic vessel length is associated with the fibroblast reticulum and disease severity in usual interstitial pneumonia and nonspecific interstitial pneumonia. Chest 2012, 142, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Ebina, M.; Shibata, N.; Ohta, H.; Hisata, S.; Tamada, T.; Ono, M.; Okaya, K.; Kondo, T.; Nukiwa, T. The disappearance of subpleural and interlobular lymphatics in idiopathic pulmonary fibrosis. Lymphat. Res. Biol. 2010, 8, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Salloum, J.; O’Donovan, P.B.; Mascha, E.J.; Mehta, A.C.; Matthay, M.A.; Arroliga, A.C. Acute pulmonary edema after lung transplantation: The pulmonary reimplantation response. Chest 1999, 116, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, R.; Muz, J.; Fietsam, R.J.; Thomas, G.A.; Welsh, R.J.; Miller, J.E.; Stephenson, L.W.; Baciewicz, F.A.J. Reestablishment of lymphatic drainage after canine lung transplantation. J. Thorac. Cardiovasc. Surg. 1993, 106, 167–171. [Google Scholar] [CrossRef]
- Lee, J.C.; Christie, J.D. Primary graft dysfunction. Clin. Chest Med. 2011, 32, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Dashkevich, A.; Heilmann, C.; Kayser, G.; Germann, M.; Beyersdorf, F.; Passlick, B.; Geissler, H.J. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann. Thorac. Surg. 2010, 90, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, K.; Monzon-Medina, M.E.; Padera, R.F.; Wang, H.; George, G.; Toprak, D.; Abdelnour, E.; D’Agostino, E.; Goldberg, H.J.; et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J. Clin. Investig. 2015, 125, 4255–4268. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, R.H.; Achen, M.G.; Stacker, S.A. The evolving role of lymphatics in cancer metastasis. Curr. Opin. Immunol. 2018, 53, 64–73. [Google Scholar] [CrossRef]
- Stachura, J.; Wachowska, M.; Kilarski, W.W.; Güç, E.; Golab, J.; Muchowicz, A. The dual role of tumor lymphatic vessels in dissemination of metastases and immune response development. Oncoimmunology 2016, 5, e1182278. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Yazawa, T.; Sato, H.; Suzuki, T.; Ikeda, M.; Hayashi, Y.; Takanashi, Y.; Kitamura, H. Enhancement of pleural dissemination and lymph node metastasis of intrathoracic lung cancer cells by vascular endothelial growth factors (VEGFs). Lung Cancer 2004, 45, 325–337. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Karpanen, T.; Alitalo, K. Role of lymphangiogenic factors in tumor metastasis. Biochim. Biophys. Acta 2004, 1654, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Detmar, M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv. Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kozaki, K.-I.; Karpanen, T.; Koshikawa, K.; Yla-Herttuala, S.; Takahashi, T.; Alitalo, K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 2002, 94, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Reed, H.O.; Wang, L.; Sonett, J.; Chen, M.; Yang, J.; Li, L.; Aradi, P.; Jakus, Z.; D’Armiento, J.; Hancock, W.W.; et al. Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Investig. 2019, 129, 2514–2526. [Google Scholar] [CrossRef] [PubMed]
- Truman, L.A.; Bentley, K.L.; Smith, E.C.; Massaro, S.A.; Gonzalez, D.G.; Haberman, A.M.; Hill, M.; Jones, D.; Min, W.; Krause, D.S.; et al. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am. J. Pathol. 2012, 180, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Doh, S.J.; Yamakawa, M.; Santosa, S.M.; Montana, M.; Guo, K.; Sauer, J.R.; Curran, N.; Han, K.-Y.; Yu, C.; Ema, M.; et al. Fluorescent reporter transgenic mice for in vivo live imaging of angiogenesis and lymphangiogenesis. Angiogenesis 2018, 21, 677–698. [Google Scholar] [CrossRef]
- Redder, E.; Kirschnick, N.; Bobe, S.; Hägerling, R.; Hansmeier, N.R.; Kiefer, F. Vegfr3-tdTomato, a reporter mouse for microscopic visualization of lymphatic vessel by multiple modalities. PLoS ONE 2021, 16, e0249256. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Tan, I.-C.; Marshall, M.V.; Fife, C.E.; Sevick-Muraca, E.M. Lymphatic imaging in humans with near-infrared fluorescence. Curr. Opin. Biotechnol. 2009, 20, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Soltesz, E.G.; Kim, S.; Laurence, R.G.; DeGrand, A.M.; Parungo, C.P.; Dor, D.M.; Cohn, L.H.; Bawendi, M.G.; Frangioni, J.V.; Mihaljevic, T. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann. Thorac. Surg. 2005, 79, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Itkin, M.; Fan, Y. Quantification of Thoracic Lymphatic Flow Patterns Using Dynamic Contrast-enhanced MR Lymphangiography. Radiology 2020, 296, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y.; Zviman, M.M.; Itkin, M. Dynamic contrast-enhanced MR lymphangiography: Feasibility study in swine. Radiology 2014, 273, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Landh, E.; M Moir, L.; Bradbury, P.; Traini, D.; M Young, P.; Ong, H.X. Properties of rapamycin solid lipid nanoparticles for lymphatic access through the lungs & part I: The effect of size. Nanomedicine 2020, 15, 1927–1945. [Google Scholar] [CrossRef] [PubMed]
- Landh, E.; Moir, L.M.; Traini, D.; Young, P.M.; Ong, H.X. Properties of rapamycin solid lipid nanoparticles for lymphatic access through the lungs & part II: The effect of nanoparticle charge. Nanomedicine 2020, 15, 1947–1963. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solari, E.; Marcozzi, C.; Ottaviani, C.; Negrini, D.; Moriondo, A. Draining the Pleural Space: Lymphatic Vessels Facing the Most Challenging Task. Biology 2022, 11, 419. https://doi.org/10.3390/biology11030419
Solari E, Marcozzi C, Ottaviani C, Negrini D, Moriondo A. Draining the Pleural Space: Lymphatic Vessels Facing the Most Challenging Task. Biology. 2022; 11(3):419. https://doi.org/10.3390/biology11030419
Chicago/Turabian StyleSolari, Eleonora, Cristiana Marcozzi, Chiara Ottaviani, Daniela Negrini, and Andrea Moriondo. 2022. "Draining the Pleural Space: Lymphatic Vessels Facing the Most Challenging Task" Biology 11, no. 3: 419. https://doi.org/10.3390/biology11030419
APA StyleSolari, E., Marcozzi, C., Ottaviani, C., Negrini, D., & Moriondo, A. (2022). Draining the Pleural Space: Lymphatic Vessels Facing the Most Challenging Task. Biology, 11(3), 419. https://doi.org/10.3390/biology11030419






