Responses of Fine Roots at Different Soil Depths to Different Thinning Intensities in a Secondary Forest in the Qinling Mountains, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
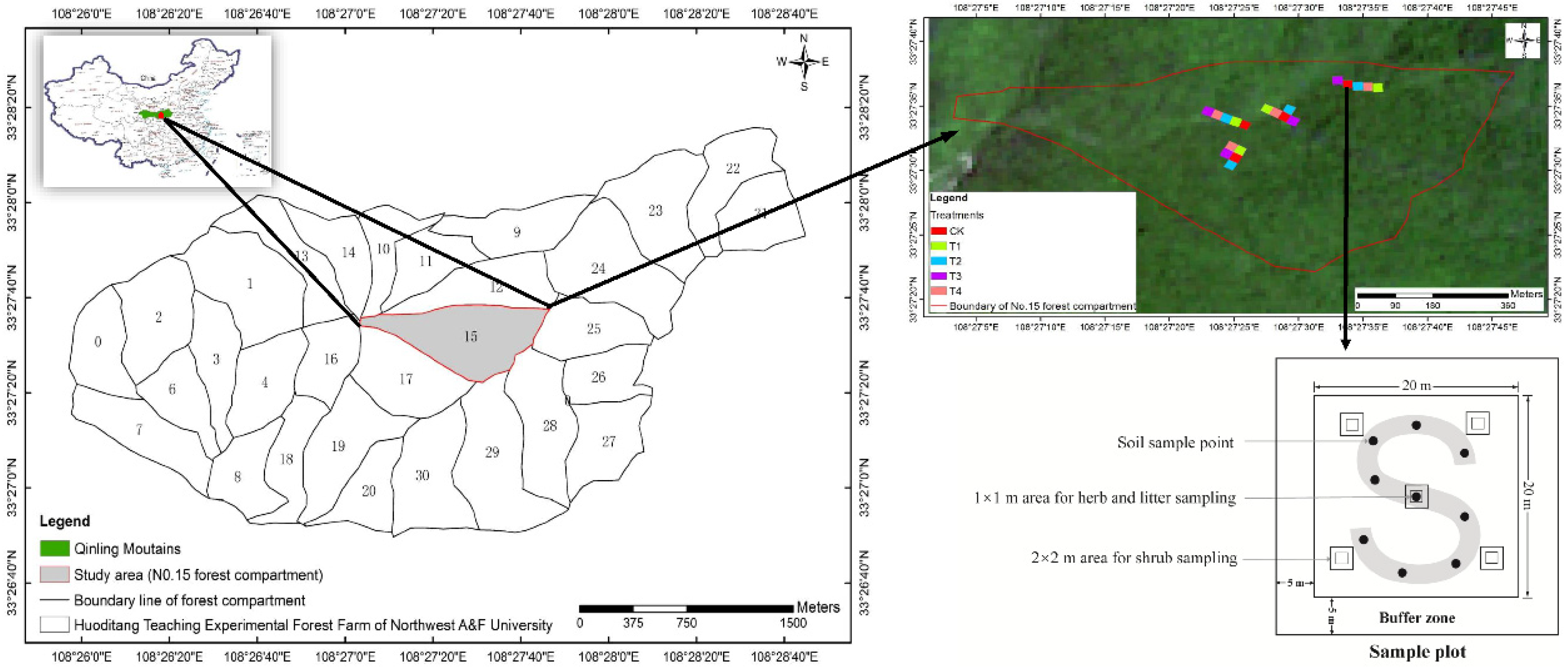
2.1. Study Area
2.2. Experimental Design and Treatments
2.3. Soil, Litter and Vegetation Survey
2.4. Fine-Root Sampling
2.5. Chemical and Biochemical Analyses
2.6. Data Calculation and Analysis
3. Results
3.1. Stand and Soil Properties
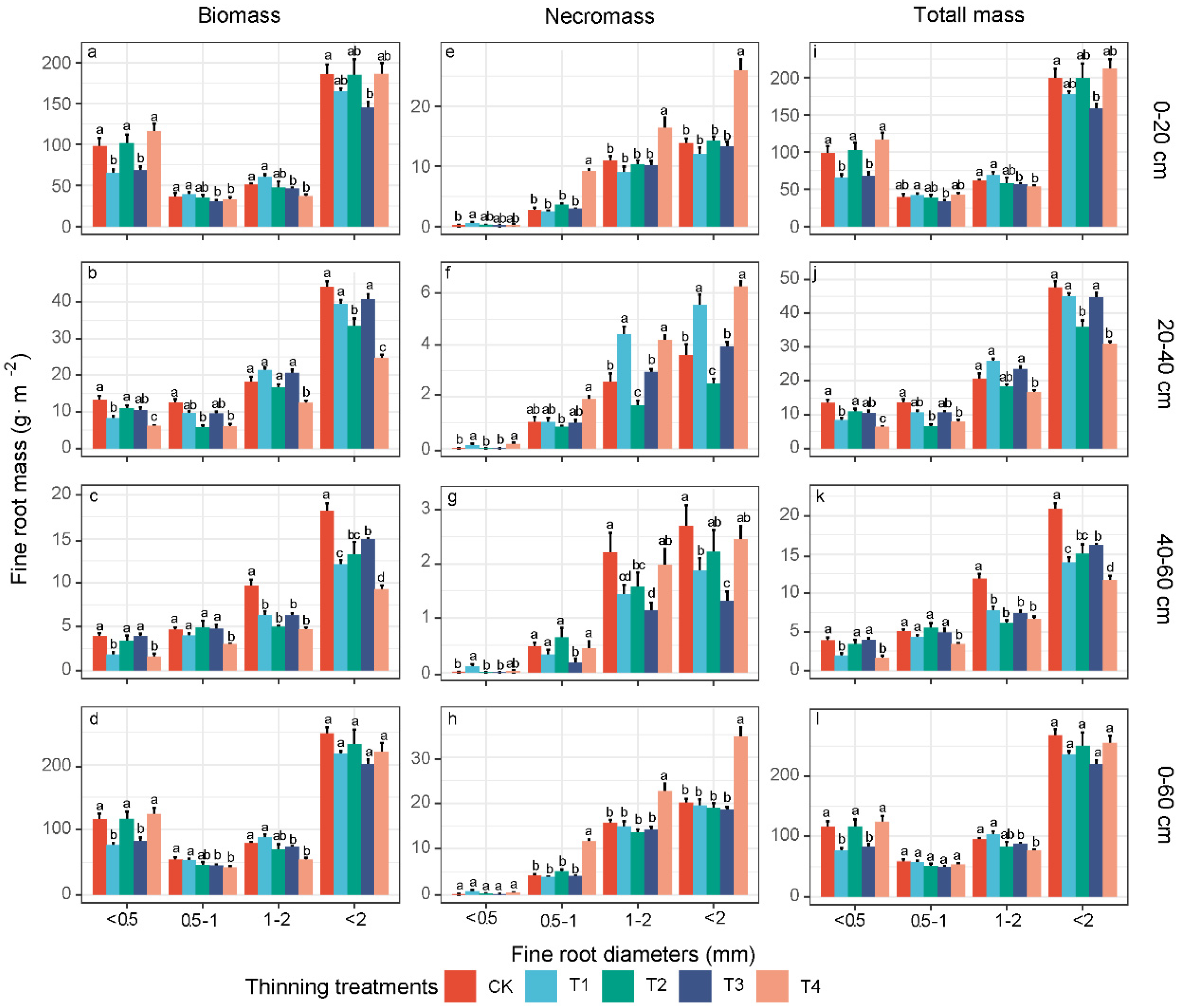
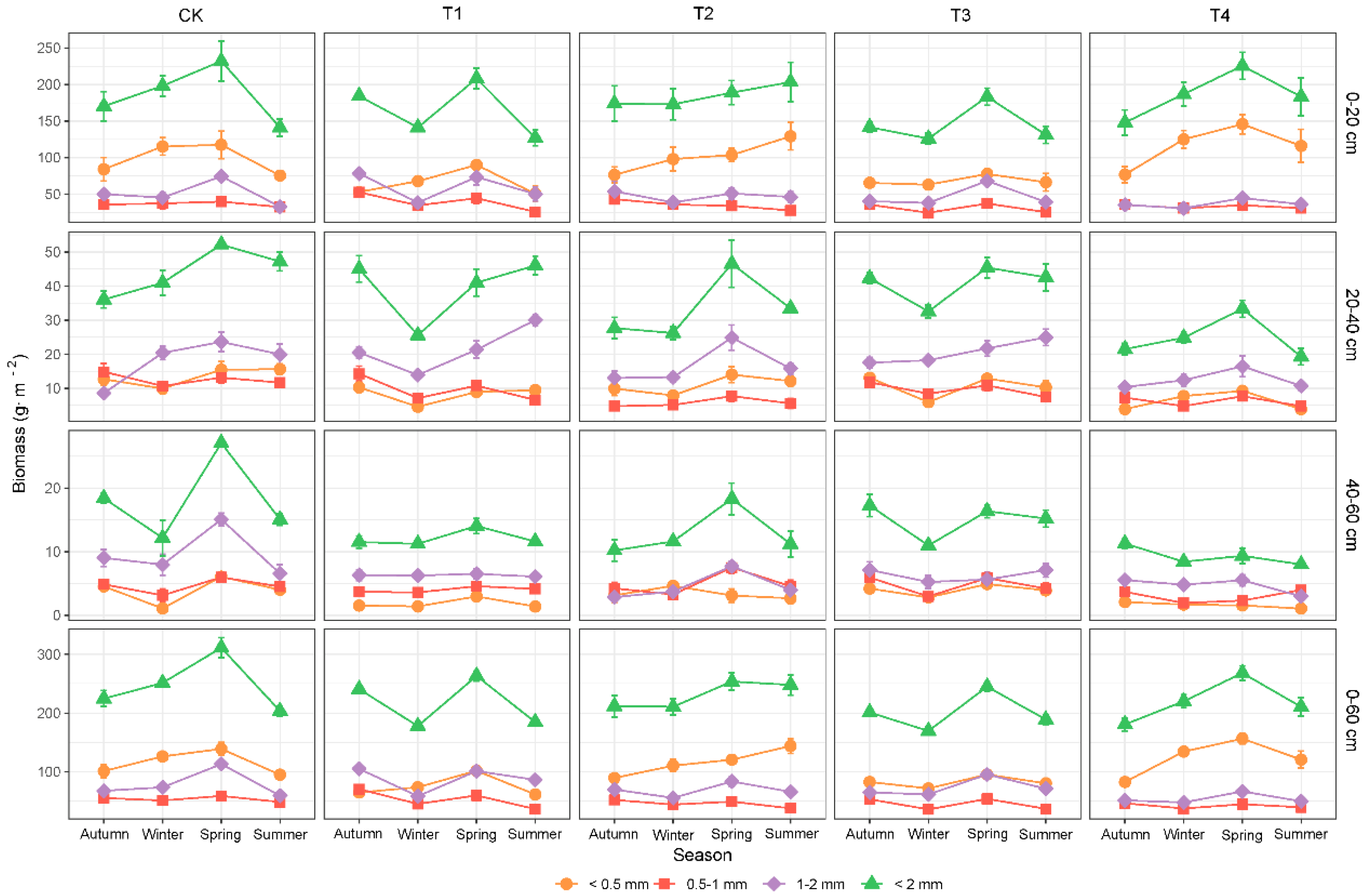
3.2. Fine-Root Biomass, Necromass, and Total Mass of Different Diameter Classes
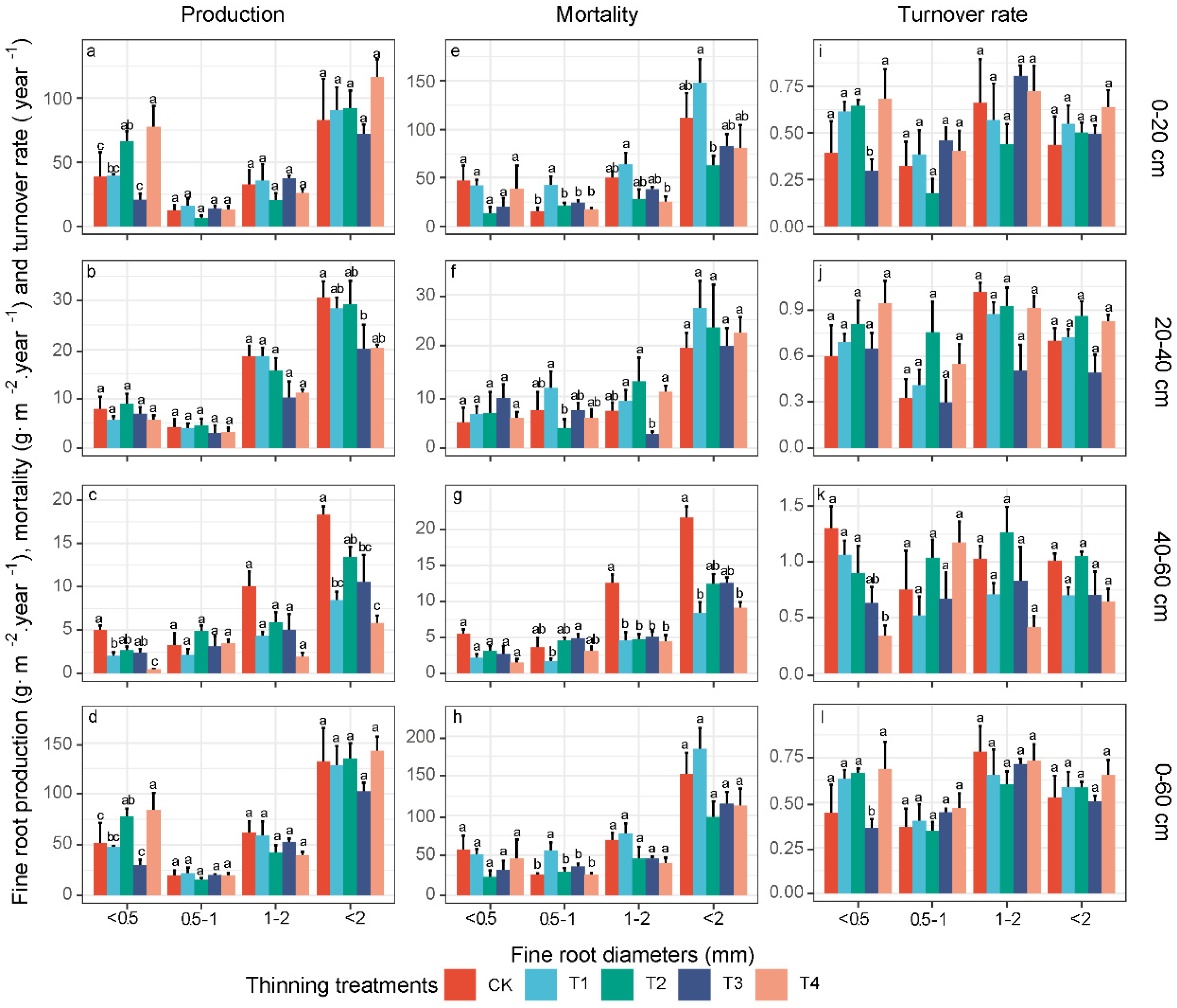
3.3. Fine-Root Production, Mortality and Turnover Rate in Different Diameter Classes
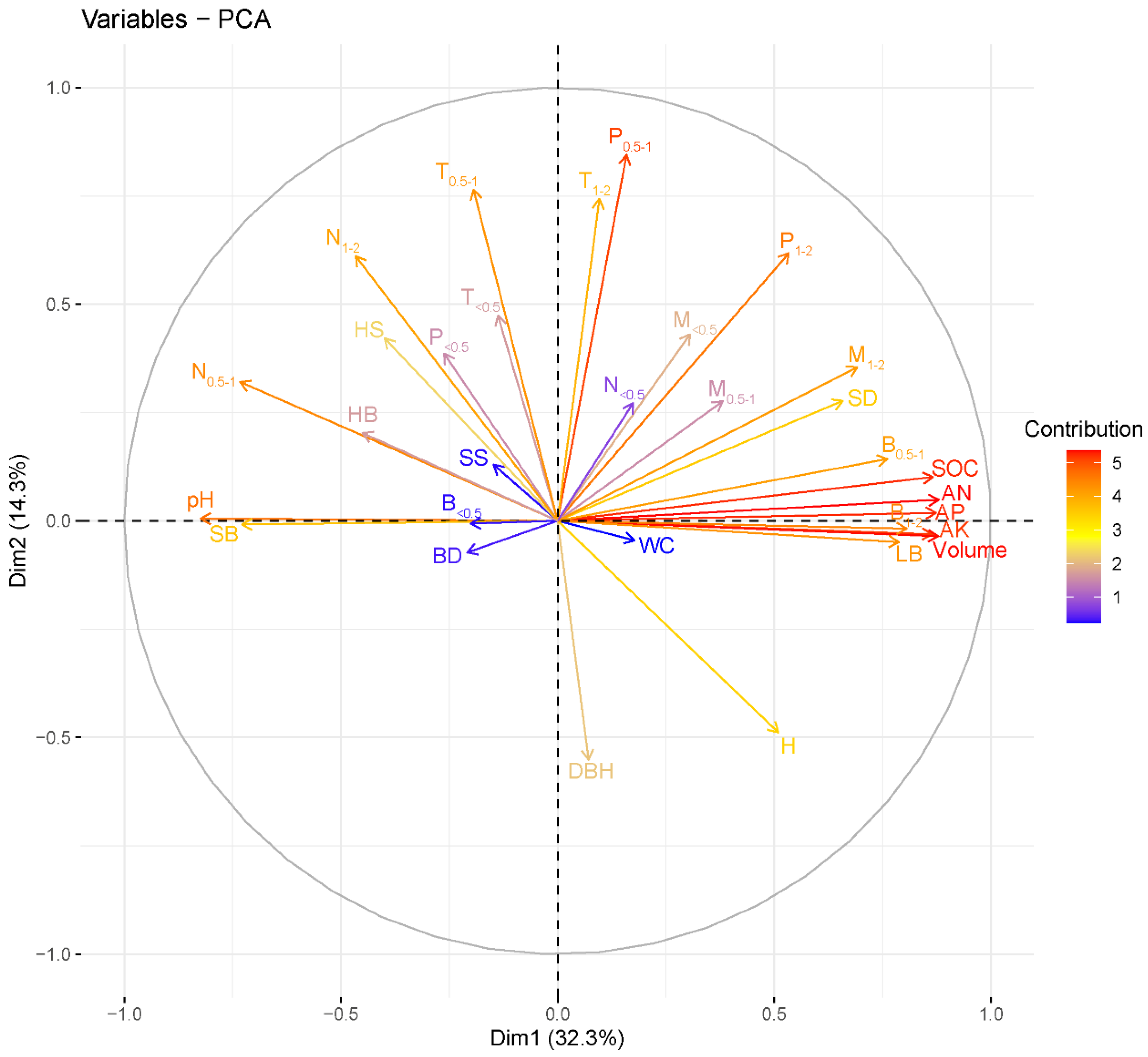
3.4. The Linkages between Fine-Root Characteristics and Stand and Soil Attributes
4. Discussion
4.1. Effects of Thinning on the Fine-Root Biomass, Production and Turnover Rate
4.2. Necromass and Mortality Changes following Thinning
4.3. Response of Deeper-Soil Fine-Root Characteristics to Thinning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Kang, F.; Han, H.; Liu, H.; Zhang, Y. Effect of thinning on partitioned soil respiration in a young Pinus tabulaeformis plantation during growing season. Agric. For. Meteorol. 2015, 214, 473–482. [Google Scholar] [CrossRef]
- Campbell, J.; Alberti, G.; Martin, J.; Law, B. Carbon dynamics of a ponderosa pine plantation following a thinning treatment in the northern Sierra Nevada. For. Ecol. Manag. 2009, 257, 453–463. [Google Scholar] [CrossRef]
- Tian, D.-L.; Peng, Y.-Y.; Yan, W.-D.; Fang, X.; Kang, W.-X.; Wang, G.-J.; Chen, X.-Y. Effects of thinning and litter fall removal on fine root production and soil organic carbon content in Masson pine plantations. Pedosphere 2010, 20, 486–493. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Peng, D.; Liu, X.; Yang, F.; Li, Z.; Cheng, S. Thinning drives C:N:P stoichiometry and nutrient resorption in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Kuehne, C.; Weiskittel, A.R.; Fraver, S.; Puettmann, K.J. Effects of thinning-induced changes in structural heterogeneity on growth, ingrowth, and mortality in secondary coastal Douglas-fir forests. Can. J. For. Res. 2015, 45, 1448–1461. [Google Scholar] [CrossRef]
- Jörgensen, K.; Granath, G.; Lindahl, B.D.; Strengbom, J. Forest management to increase carbon sequestration in boreal Pinus sylvestris forests. Plant Soil 2021, 466, 165–178. [Google Scholar] [CrossRef]
- Ding, Y.; Leppälammi-Kujansuu, J.; Helmisaari, H.-S. Fine root longevity and below-and aboveground litter production in a boreal Betula pendula forest. For. Ecol. Manag. 2019, 431, 17–25. [Google Scholar] [CrossRef]
- Zhou, W.; Guan, K.; Peng, B.; Tang, J.; Jin, Z.; Jiang, C.; Grant, R.; Mezbahuddin, S. Quantifying carbon budget, crop yields and their responses to environmental variability using the ecosys model for US Midwestern agroecosystems. Agric. For. Meteorol. 2021, 307, 108521. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, H.Y. Positive species mixture effects on fine root turnover and mortality in natural boreal forests. Soil Biol. Biochem. 2018, 121, 130–137. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.Y. Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: Literature review and meta-analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Neumann, M.; Godbold, D.L.; Hirano, Y.; Finér, L. Improving models of fine root carbon stocks and fluxes in European forests. J. Ecol. 2020, 108, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Aussenac, G. Interactions between forest stands and microclimate: Ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef]
- Wang, D.; Olatunji, O.A.; Xiao, J. Thinning increased fine root production, biomass, turnover rate and understory vegetation yield in a Chinese fir plantation. For. Ecol. Manag. 2019, 440, 92–100. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a Turkey-oak stand in relation to seasonal changes in soil moisture in the Southern Apennines, Italy. Ecol. Res. 2012, 27, 1015–1025. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, N.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y. Short-term effects of low intensity thinning on the fine root dynamics of Pinus massoniana plantations in the three gorges reservoir area, China. Forests 2017, 8, 428. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Shibata, H.; Takagi, K.; Nomura, M.; Kurima, N.; Fukazawa, T.; Satoh, F.; Sasa, K. Effects of clear-cutting on nitrogen leaching and fine root dynamics in a cool-temperate forested watershed in northern Japan. For. Ecol. Manag. 2006, 225, 257–261. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Wu, M.; Xue, Y.; Ma, L.; Zhou, J. Effect of aboveground intervention on fine root mass, production, and turnover rate in a Chinese cork oak (Quercus variabilis Blume) forest. Plant Soil 2013, 368, 201–214. [Google Scholar] [CrossRef]
- Liu, C.; Xiang, W.; Zou, L.; Lei, P.; Zeng, Y.; Ouyang, S.; Deng, X.; Fang, X.; Liu, Z.; Peng, C. Variation in the functional traits of fine roots is linked to phylogenetics in the common tree species of Chinese subtropical forests. Plant Soil 2019, 436, 347–364. [Google Scholar] [CrossRef]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef]
- Hertel, D.; Strecker, T.; Müller-Haubold, H.; Leuschner, C. Fine root biomass and dynamics in beech forests across a precipitation gradient–is optimal resource partitioning theory applicable to water-limited mature trees? J. Ecol. 2013, 101, 1183–1200. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, N.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y.; Lei, L.; Zeng, L.; Wang, X. Characteristics of fine roots of Pinus massoniana in the Three Gorges Reservoir Area, China. Forests 2017, 8, 183. [Google Scholar] [CrossRef]
- Xiao, C.W.; Sang, W.G.; Wang, R.-Z. Fine root dynamics and turnover rate in an Asia white birch forest of Donglingshan Mountain, China. For. Ecol. Manag. 2008, 255, 765–773. [Google Scholar] [CrossRef]
- Zobel, R.W.; Kinraide, T.B.; Baligar, V.C. Fine root diameters can change in response to changes in nutrient concentrations. Plant Soil 2007, 297, 243–254. [Google Scholar] [CrossRef]
- Montagnoli, A.; Di Iorio, A.; Terzaghi, M.; Trupiano, D.; Scippa, G.; Chiatante, D. Influence of soil temperature and water content on fine-root seasonal growth of European beech natural forest in Southern Alps, Italy. Eur. J. For. Res. 2014, 133, 957–968. [Google Scholar] [CrossRef]
- Peng, S.; Chen, H.Y. Global responses of fine root biomass and traits to plant species mixtures in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2021, 30, 289–304. [Google Scholar] [CrossRef]
- Lopez, B.C.; Sabate, S.; Gracia, C. Thinning effects on carbon allocation to fine roots in a Quercus ilex forest. Tree Physiol. 2003, 23, 1217–1224. [Google Scholar] [CrossRef]
- Coll, L.; Balandier, P.; Picon-Cochard, C.; Prévosto, B.; Curt, T. Competition for water between beech seedlings and surrounding vegetation in different light and vegetation composition conditions. Ann. For. Sci. 2003, 60, 593–600. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Zhu, Q.; Fu, S.; Chen, H.Y. Rapid increases in fine root biomass and production following cessation of anthropogenic disturbances in degraded forests. Land Degrad. Dev. 2018, 29, 461–470. [Google Scholar] [CrossRef]
- Olesinski, J.; Lavigne, M.B.; Kershaw, J.A., Jr.; Krasowski, M.J. Fine-root dynamics change during stand development and in response to thinning in balsam fir (Abies balsamea L. Mill.) forests. For. Ecol. Manag. 2012, 286, 48–58. [Google Scholar] [CrossRef]
- Asaye, Z.; Zewdie, S. Fine root dynamics and soil carbon accretion under thinned and un-thinned Cupressus lusitanica stands in, Southern Ethiopia. Plant Soil 2013, 366, 261–271. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Wang, D. Response of multi-ecological component stoichiometry and tree nutrient resorption to medium-term whole-tree harvesting in secondary forests in the Qinling Mountains, China. For. Ecol. Manag. 2021, 498, 119573. [Google Scholar] [CrossRef]
- Delian, W. Studies on Runoff and Its Water Quality in the Forestry Watershed of Huoditang in Qingling Mountain. Master’s Thesis, Northwest Agriculture and Forestry University of Science and Technology, Xianyang, China, 2004. [Google Scholar]
- Yuan, J.; Cheng, F.; Zhu, X.; Li, J.; Zhang, S. Respiration of downed logs in pine and oak forests in the Qinling Mountains, China. Soil Biol. Biochem. 2018, 127, 1–9. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Zhao, X.; Chao, Z.; Wang, Y.; Zhang, X.; Wang, D. The linkages of plant, litter and soil C:N:P stoichiometry and nutrient stock in different secondary mixed forest types in the Qinling Mountains, China. PeerJ 2020, 8, e9274. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Liu, L.; Han, L.; Wang, D. Coupling of different plant functional group, soil, and litter nutrients in a natural secondary mixed forest in the Qinling Mountains, China. Environ. Sci. Pollut. R. 2021, 28, 66272–66286. [Google Scholar] [CrossRef]
- Kaarakka, L.; Tamminen, P.; Saarsalmi, A.; Kukkola, M.; Helmisaari, H.-S.; Burton, A.J. Effects of repeated whole-tree harvesting on soil properties and tree growth in a Norway spruce (Picea abies (L.) Karst.) stand. For. Ecol. Manag. 2014, 313, 180–187. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.; Yang, K.; Yu, L.; Zhang, J. Nutrient removal under different harvesting scenarios for larch plantations in northeast China: Implications for nutrient conservation and management. For. Ecol. Manag. 2017, 400, 150–158. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Hart, S.A.; Chen, H.Y. Fire, logging, and overstory affect understory abundance, diversity, and composition in boreal forest. Ecol. Monogr. 2008, 78, 123–140. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Simplifying the decision matrix for estimating fine root production by the sequential soil coring approach. Acta Oecologica 2013, 48, 54–61. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, X.; Guan, W.; Liao, B.; Chen, Y.; Li, M.; Zhong, C. Fine root functional group based estimates of fine root production and turnover rate in natural mangrove forests. Plant Soil 2017, 413, 83–95. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.; Cavard, X.; Laganière, J.; Reich, P.B.; Bergeron, Y.; Pare, D.; Yuan, Z. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2013, 101, 210–219. [Google Scholar] [CrossRef]
- De Vos, B.; Van Meirvenne, M.; Quataert, P.; Deckers, J.; Muys, B. Predictive quality of pedotransfer functions for estimating bulk density of forest soils. Soil Sci. Soc. Am. J. 2005, 69, 500–510. [Google Scholar] [CrossRef]
- Brunner, I.; Bakker, M.R.; Björk, R.G.; Hirano, Y.; Lukac, M.; Aranda, X.; Børja, I.; Eldhuset, T.D.; Helmisaari, H.S.; Jourdan, C.; et al. Fine-root turnover rates of European forests revisited: An analysis of data from sequential coring and ingrowth cores. Plant Soil 2012, 362, 357–372. [Google Scholar] [CrossRef]
- De Boeck, P.; Bakker, M.; Zwitser, R.; Nivard, M.; Hofman, A.; Tuerlinckx, F.; Partchev, I. The estimation of item response models with the lmer function from the lme4 package in R. J. Stat. Softw. 2011, 39, 1–28. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statstical Computing. Version 3.3.2. R. Foundation for Statistical Computing. 2017. Available online: http://www.R-project.org/ (accessed on 9 January 2022).
- Liao, Y.; Fan, H.; Wei, X.; Wu, J.; Duan, H.; Fu, X.; Liu, W.; Wang, H.; Zhan, X.; Tang, P. Competition increased fine root biomass in Chinese fir (Cunninghamia lanceolata) plantations in Subtropical China. For. Ecol. Manag. 2019, 435, 151–157. [Google Scholar] [CrossRef]
- Luiro, J.; Kukkola, M.; Saarsalmi, A.; Tamminen, P.; Helmisaari, H.-S. Logging residue removal after thinning in boreal forests: Long-term impact on the nutrient status of Norway spruce and Scots pine needles. Tree Physiol. 2010, 30, 78–88. [Google Scholar] [CrossRef][Green Version]
- Lechuga, V.; Carraro, V.; Viñegla, B.; Carreira, J.A.; Linares, J.C. Managing drought-sensitive forests under global change. Low competition enhances long-term growth and water uptake in Abies pinsapo. For. Ecol. Manag. 2017, 406, 72–82. [Google Scholar] [CrossRef]
- Noguchi, K.; Han, Q.; Araki, M.G.; Kawasaki, T.; Kaneko, S.; Takahashi, M.; Chiba, Y. Fine-root dynamics in a young hinoki cypress (Chamaecyparis obtusa) stand for 3 years following thinning. J. For. Res.-Jpn. 2011, 16, 284–291. [Google Scholar] [CrossRef]
- Eissenstat, D.; Wells, C.; Yanai, R.; Whitbeck, J. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Eshel, A.; Beeckman, T. Plant Roots: The Hidden Half; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Schroeer, A.E.; Hendrick, R.L.; Harrington, T.B. Root, ground cover, and litterfall dynamics within canopy gaps in a slash pine (Pinus elliottii Engelm.) dominated forest. Ecoscience 1999, 6, 548–555. [Google Scholar] [CrossRef]
- McGuire, J.P.; Mitchell, R.J.; Moser, E.B.; Pecot, S.D.; Gjerstad, D.H.; Hedman, C.W. Gaps in a gappy forest: Plant resources, longleaf pine regeneration, and understory response to tree removal in longleaf pine savannas. Can. J. For. Res. 2001, 31, 765–778. [Google Scholar] [CrossRef]
- Juodvalkis, A.; Kairiukstis, L.; Vasiliauskas, R. Effects of thinning on growth of six tree species in north-temperate forests of Lithuania. Eur. J. For. Res. 2005, 124, 187–192. [Google Scholar] [CrossRef]
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ritter, L.; Ares, A.; Goldstein, G. Thinning of loblolly pine plantations in subtropical Argentina: Impact on microclimate and understory vegetation. For. Ecol. Manag. 2017, 384, 236–247. [Google Scholar] [CrossRef]
- Boot, R.G. The significance of size and morphology of root systems for nutrient acquisition and competition. In Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants; Lambers, H., Cambridge, M.L., Konings, H., Pons, T.L., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Joslin, J.; Gaudinski, J.B.; Torn, M.S.; Riley, W.; Hanson, P.J. Fine-root turnover patterns and their relationship to root diameter and soil depth in a 14C-labeled hardwood forest. New Phytol. 2006, 172, 523–535. [Google Scholar] [CrossRef]
- Di Iorio, A.; Montagnoli, A.; Terzaghi, M.; Scippa, G.S.; Chiatante, D. Effect of tree density on root distribution in Fagus sylvatica stands: A semi-automatic digitising device approach to trench wall method. Trees 2013, 27, 1503–1513. [Google Scholar] [CrossRef]
- Sun, T.; Dong, L.; Mao, Z.; Li, Y. Fine root dynamics of trees and understorey vegetation in a chronosequence of Betula platyphylla stands. For. Ecol. Manag. 2015, 346, 1–9. [Google Scholar] [CrossRef]
- Jones, R.H.; Mitchell, R.J.; Stevens, G.N.; Pecot, S.D. Controls of fine root dynamics across a gradient of gap sizes in a pine woodland. Oecologia 2003, 134, 132–143. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.C.; Macdonald, S.E.; Quideau, S.A. Understory plant community composition is associated with fine-scale above-and below-ground resource heterogeneity in mature lodgepole pine (Pinus contorta) forests. PLoS ONE 2016, 11, e0151436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of forest growth to C: N: P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Hertel, D.; Harteveld, M.A.; Leuschner, C. Conversion of a tropical forest into agroforest alters the fine root-related carbon flux to the soil. Soil Biol. Biochem. 2009, 41, 481–490. [Google Scholar] [CrossRef]
- McKay Fletcher, D.M.; Ruiz, S.; Dias, T.; Petroselli, C.; Roose, T. Linking root structure to functionality: The impact of root system architecture on citrate-enhanced phosphate uptake. New Phytol. 2020, 227, 376–391. [Google Scholar] [CrossRef]
| If | Fine-Root Production | Fine-Root Mortality |
|---|---|---|
| ΔL + ΔD ≥ 0 and ΔD ≥ 0 | ΔL + ΔD | ΔD |
| ΔL ≥ 0 and ΔD ≤ 0 | ΔL | 0 |
| ΔL ≤ 0 and ΔD ≤ 0 | 0 | |ΔL| |
| Characteristic | Depth (cm) | CK | T1 | T2 | T3 | T4 | p |
|---|---|---|---|---|---|---|---|
| Tree | 0% | 15% | 30% | 40% | 60% | ||
| Stem density (trees ha−1) | 1306 ± 158 a | 1006 ± 128 ab | 845 ± 148 b | 614 ± 83 b | 595 ± 161 b | <0.001 | |
| DBH (cm) | 16.77 ± 1.07 | 18.41 ± 1.33 | 17.74 ± 1.13 | 17.06 ± 1.16 | 17.31 ± 0.74 | 0.35 | |
| Tree height (m) | 14.03 ± 0.67 | 14.72 ± 0.97 | 13.8 ± 0.61 | 13.15 ± 0.63 | 12.59 ± 0.35 | 0.16 | |
| Volume (m3 ha−1) | 250.5 ± 21.56 a | 240.08 ± 10.68 a | 167.33 ± 17.14 b | 117.42 ± 4.08 bc | 105.72 ± 16.87 c | <0.001 | |
| Understory plants | |||||||
| Shrub biomass (t ha−1) | 3.41 ± 0.06 c | 3.92 ± 0.23 bc | 4.45 ± 0.16 ab | 4.94 ± 0.21 ab | 4.97 ± 0.58 a | <0.01 | |
| Herb biomass (t ha−1) | 0.62 ± 0.04 b | 0.79 ± 0.12 ab | 0.87 ± 0.13 ab | 1.02 ± 0.12 a | 1.02 ± 0.18 a | <0.001 | |
| Litter biomass (t ha−1) | 3.6 ± 0.18 a | 2.93 ± 0.28 ab | 2.78 ± 0.31 b | 2.29 ± 0.27 bc | 1.9 ± 0.17 c | <0.001 | |
| Shannon-Wiener–herb | 1.96 ± 0.05 b | 2.13 ± 0.08 ab | 2.28 ± 0.13 ab | 2.22 ± 0.13 ab | 2.39 ± 0.15 a | <0.05 | |
| Shannon-Wiener–shrub | 1.84 ± 0.09 b | 1.91 ± 0.17 b | 2.25 ± 0.03 a | 2.01 ± 0.05 b | 2.19 ± 0.13 ab | <0.05 | |
| Soil | |||||||
| Water content (%) | 0–20 | 44.25 ± 3.08 | 36.01 ± 2.6 | 33.13 ± 3.61 | 44.25 ± 3.74 | 34.44 ± 1.5 | 0.051 |
| 20–40 | 33.22 ± 2.79 | 29.17 ± 1.42 | 30.58 ± 2.32 | 30.46 ± 1.65 | 27.48 ± 2.2 | 0.44 | |
| 40–60 | 29.04 ± 1.1 | 23.3 ± 1.13 | 25.9 ± 1.59 | 26.86 ± 1.56 | 28.41 ± 2.61 | 0.18 | |
| Bulk density (g cm−3) | 0–20 | 1.1 ± 0.05 | 1.26 ± 0.05 | 1.29 ± 0.11 | 1.1 ± 0.05 | 1.31 ± 0.04 | 0.076 |
| 20–40 | 1.33 ± 0.05 | 1.41 ± 0.05 | 1.41 ± 0.05 | 1.4 ± 0.05 | 1.44 ± 0.06 | 0.63 | |
| 40–60 | 1.41 ± 0.05 | 1.56 ± 0.03 | 1.49 ± 0.05 | 1.48 ± 0.04 | 1.47 ± 0.07 | 0.36 | |
| SOC (g kg−1) | 0–20 | 26.64 ± 5.08 a | 23.83 ± 3.18 a | 9.22 ± 0.47 b | 9.54 ± 1.59 b | 7.37 ± 0.55 b | <0.001 |
| 20–40 | 14.47 ± 1.05 a | 15.82 ± 1.05 a | 7.65 ± 0.47 b | 5.53 ± 0.67 b | 5.76 ± 0.45 b | <0.001 | |
| 40–60 | 13.34 ± 0.78 a | 8.75 ± 1.15 b | 5.08 ± 0.52 c | 4.48 ± 0.37 c | 3.77 ± 0.2 c | <0.001 | |
| AN (mg kg−1) | 0–20 | 35.56 ± 2.65 a | 29.37 ± 3.93 a | 10.07 ± 0.98 b | 9.14 ± 0.42 b | 7.96 ± 0.4 b | <0.001 |
| 20–40 | 16.64 ± 1.58 a | 16.01 ± 1.38 a | 7.97 ± 0.14 b | 6.95 ± 0.6 b | 6.37 ± 0.18 b | <0.001 | |
| 40–60 | 9.37 ± 1.33 a | 7.59 ± 0.94 ab | 7.19 ± 0.32 ab | 5.52 ± 0.36 bc | 4.95 ± 0.12 c | <0.01 | |
| AP (mg kg−1) | 0–20 | 4.38 ± 0.74 a | 3.58 ± 0.39 ab | 3.15 ± 0.41 ab | 1.84 ± 0.28 b | 2.16 ± 0.3 b | <0.01 |
| 20–40 | 2.46 ± 0.29 ab | 3.15 ± 0.54 a | 1.95 ± 0.33 ab | 1.36 ± 0.16 b | 1.18 ± 0.18 b | <0.01 | |
| 40–60 | 1.86 ± 0.24 a | 1.78 ± 0.08 a | 1.12 ± 0.06 b | 0.73 ± 0.04 c | 0.71 ± 0.1 c | <0.001 | |
| AK (mg kg−1) | 0–20 | 156.19 ± 11.78 ab | 185.2 ± 7.26 a | 134.78 ± 12.64 b | 146.29 ± 5.87 ab | 110.81 ± 12.08 b | <0.01 |
| 20–40 | 110.49 ± 6.98 a | 97.33 ± 9.32 ab | 101.85 ± 6.63 ab | 80.37 ± 8.67 ab | 77.15 ± 7.71 b | <0.05 | |
| 40–60 | 91.69 ± 12.45 a | 96.3 ± 11.96 a | 45.27 ± 5.39 b | 42.49 ± 6.26 b | 43.88 ± 4.38 b | <0.001 | |
| pH | 0–20 | 6.29 ± 0.14 | 6.14 ± 0.11 | 6.38 ± 0.08 | 6.42 ± 0.14 | 6.6 ± 0.07 | 0.11 |
| 20–40 | 5.99 ± 0.18 | 6.02 ± 0.09 | 6.07 ± 0.17 | 6.28 ± 0.08 | 6.37 ± 0.04 | 0.18 | |
| 40–60 | 5.75 ± 0.09 b | 6.11 ± 0.05 a | 6.1 ± 0.09 a | 6.21 ± 0.07 a | 6.37 ± 0.08 a | <0.05 |
| Characteristic | df | Source | <0.5 mm | 0.5–1 mm | 1–2 mm | <2 mm |
|---|---|---|---|---|---|---|
| Entire soil profile | ||||||
| Biomass (g m−2) | 4 | T | 10.91 ** | 4.42 * | 9.01 ** | 2.11 |
| Necromass (g m−2) | 4 | T | 2.34 | 58 ** | 11.11 ** | 25.31 ** |
| Total mass (g m−2) | 4 | T | 10.62 ** | 2.33 | 4.15 * | 2.33 |
| Depth-specific response | ||||||
| Biomass (g m−2) | 4 | T | 19.05 ** | 11.75 ** | 16.57 ** | 12.15 ** |
| 2 | D | 1620 ** | 894 ** | 1621 ** | 3488 ** | |
| 8 | T × D | 9.12 ** | 6.44 ** | 6.91 ** | 12.58 ** | |
| Necromass (g m−2) | 4 | T | 6.58 ** | 4.35 ** | 10.82 ** | 13.57 ** |
| 2 | D | 17.74 ** | 57 ** | 431 ** | 580 ** | |
| 8 | T × D | 1.46 | 2.17 * | 6.64 ** | 8.03 ** | |
| Total mass (g m−2) | 4 | T | 17 ** | 6.15 ** | 9.93 ** | 8.85 ** |
| 2 | D | 1558 ** | 1074 ** | 2201 ** | 4109 ** | |
| 8 | T × D | 8.49 ** | 7.9 ** | 9.30 ** | 13.56 ** |
| Characteristic | Diameter(mm) | 0–20 cm | 20–40 cm | 40–60 cm |
|---|---|---|---|---|
| Biomass (%) | <0.05 | 21.44 ± 6.75 | 32.92 ± 8.26 | 42.23 ± 10.18 |
| 0.5–1 | 9.05 ± 2.54 | 38.02 ± 8.54 | 14.39 ± 7.43 | |
| 1–2 | 15.14 ± 4.88 | 17.68 ± 5.01 | 42.51 ± 4.39 | |
| Necromass (%) | <0.05 | 36.17 ± 13.27 | NA | 76.42 ± 25.86 |
| 0.5–1 | 16.51 ± 5.91 | 27.23 ± 19.99 | 33.95 ± 11.6 | |
| 1–2 | 20 ± 10.36 | 46.4 ± 12.96 | 30.63 ± 7.89 | |
| Total mass (%) | <0.05 | 21.35 ± 6.66 | 32.38 ± 7.95 | 41.23 ± 9.84 |
| 0.5–1 | 7.45 ± 2.72 | 33.96 ± 7.4 | 15.06 ± 6.39 | |
| 1–2 | 10.01 ± 1.74 | 17.34 ± 3.07 | 41.08 ± 3.01 | |
| Production (%) | <0.05 | 40.04 ± 14.25 | 19.74 ± 4.09 | 62.29 ± 9.94 |
| 0.5–1 | 19.22 ± 5.27 | 16.52 ± 4.95 | 23.83 ± 10.96 | |
| 1–2 | 20.62 ± 6.28 | 25.19 ± 10.41 | 57.37 ± 8.42 | |
| Mortality (%) | <0.05 | 39.83 ± 14.89 | 46.03 ± 17.65 | 57.21 ± 6.48 |
| 0.5–1 | 36.03 ± 9.42 | 42.48 ± 7.92 | 37.38 ± 5.89 | |
| 1–2 | 36.03 ± 6.28 | 55.04 ± 11.02 | 62.4 ± 1 | |
| Turnover rate (%) | <0.05 | 48.21 ± 8.83 | 29.93 ± 11.18 | 52.15 ± 8.83 |
| 0.5–1 | 32.98 ± 6.62 | 59.15 ± 27.89 | 33.92 ± 9.27 | |
| 1–2 | 19.82 ± 5.21 | 21.07 ± 9.96 | 33.33 ± 9.13 |
| Characteristic | df | Source | <0.5 mm | 0.5–1 mm | 1–2 mm | <2 mm |
|---|---|---|---|---|---|---|
| Entire soil profile | ||||||
| Production (g m−2) | 4 | T | 5.7 ** | 0.39 | 1.5 | 0.75 |
| Mortality (g m−2) | 4 | T | 1.32 | 5.27 ** | 2.2 | 2.23 |
| Turnover (year−1) | 4 | T | 3.27 * | 0.77 | 0.63 | 0.63 |
| Depth-specific response | ||||||
| Production (g m−2) | 4 | T | 2.11 | 0.52 | 2.12 | 2.52 |
| 2 | D | 126.65 ** | 0.53 | 40.43 ** | 153.64 ** | |
| 8 | T × D | 5.49 ** | 1.05 | 1.94 | 2.96 * | |
| Mortality (g m−2) | 4 | T | 1.25 | 1.52 | 1.62 | 1.23 |
| 2 | D | 0.7 | 47.3 ** | 125.66 ** | 123.36 ** | |
| 8 | T × D | 0.82 | 2.23 * | 6.11 ** | 2.36 * | |
| Turnover (year−1) | 4 | T | 1.5 | 1 | 0.66 | 1.93 |
| 2 | D | 2.97 | 4.94 * | 1.54 | 8.34 ** | |
| 8 | T × D | 2.63 * | 1.26 | 2 | 1.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Tian, J.; Yang, H.; Zhang, K.; Wang, D. Responses of Fine Roots at Different Soil Depths to Different Thinning Intensities in a Secondary Forest in the Qinling Mountains, China. Biology 2022, 11, 351. https://doi.org/10.3390/biology11030351
Pang Y, Tian J, Yang H, Zhang K, Wang D. Responses of Fine Roots at Different Soil Depths to Different Thinning Intensities in a Secondary Forest in the Qinling Mountains, China. Biology. 2022; 11(3):351. https://doi.org/10.3390/biology11030351
Chicago/Turabian StylePang, Yue, Jing Tian, Hang Yang, Kai Zhang, and Dexiang Wang. 2022. "Responses of Fine Roots at Different Soil Depths to Different Thinning Intensities in a Secondary Forest in the Qinling Mountains, China" Biology 11, no. 3: 351. https://doi.org/10.3390/biology11030351
APA StylePang, Y., Tian, J., Yang, H., Zhang, K., & Wang, D. (2022). Responses of Fine Roots at Different Soil Depths to Different Thinning Intensities in a Secondary Forest in the Qinling Mountains, China. Biology, 11(3), 351. https://doi.org/10.3390/biology11030351






