A Comparative Study of Mesenchymal Stem Cell-Derived Extracellular Vesicles’ Local and Systemic Dose-Dependent Administration in Rat Spinal Cord Injury
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Mesenchymal Stem Cells
2.2. Isolation of Extracellular Vesicles
2.3. Ultrastructural Assessment of Extracellular Vesicles
2.4. Flow Cytometry
2.5. Spinal Cord Injury and MSC-EV Therapy
2.6. BBB Locomotor Rating Scale
2.7. Recording of Electrophysiological Parameters
2.8. Morphological Analysis
2.9. Immunofluorescence Analysis
2.10. RNA Isolation, cDNA Synthesis and Real-Time PCR
2.11. Statistical Analysis
3. Results
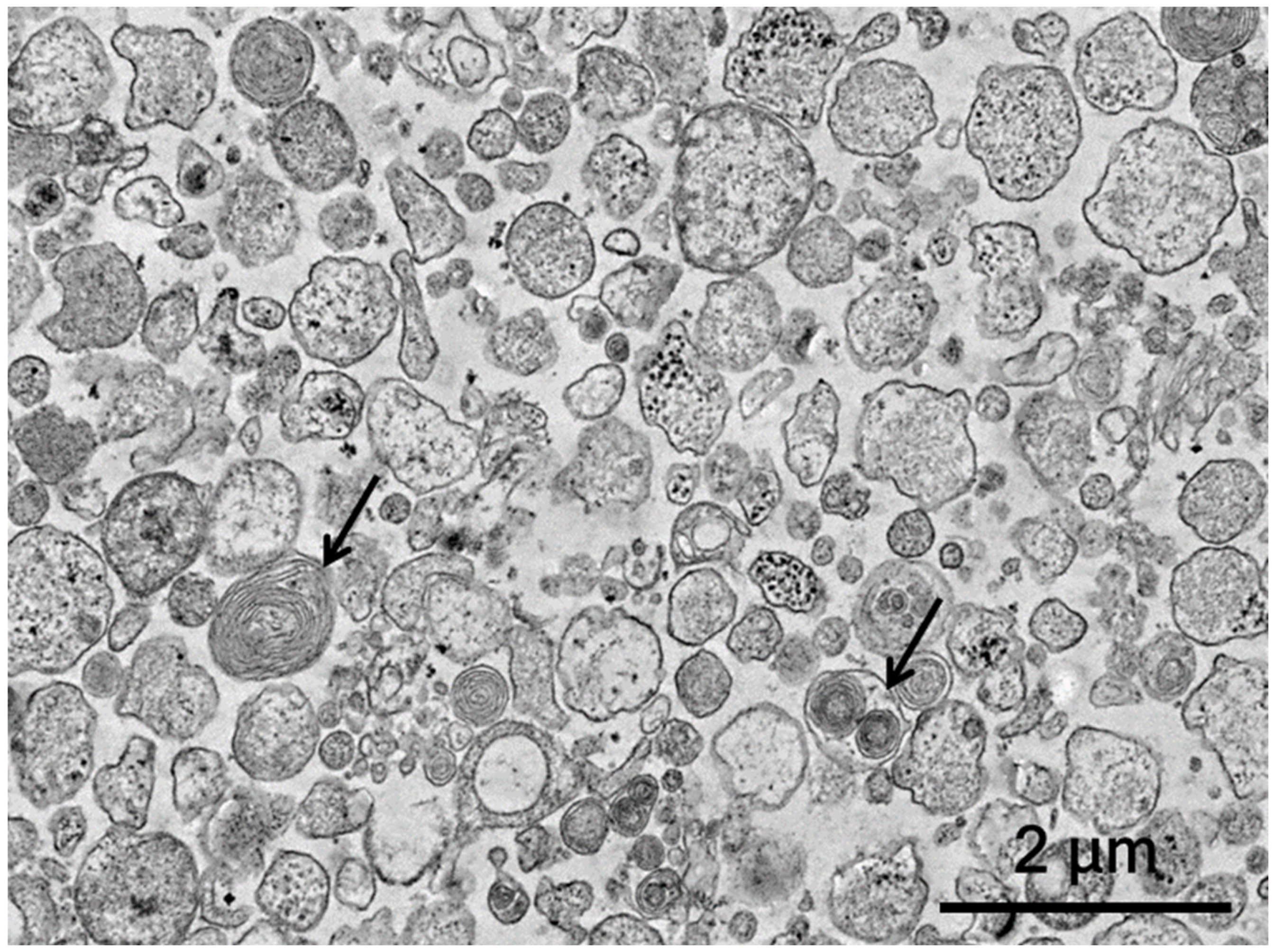
3.1. Assessment of Extracellular Vesicles
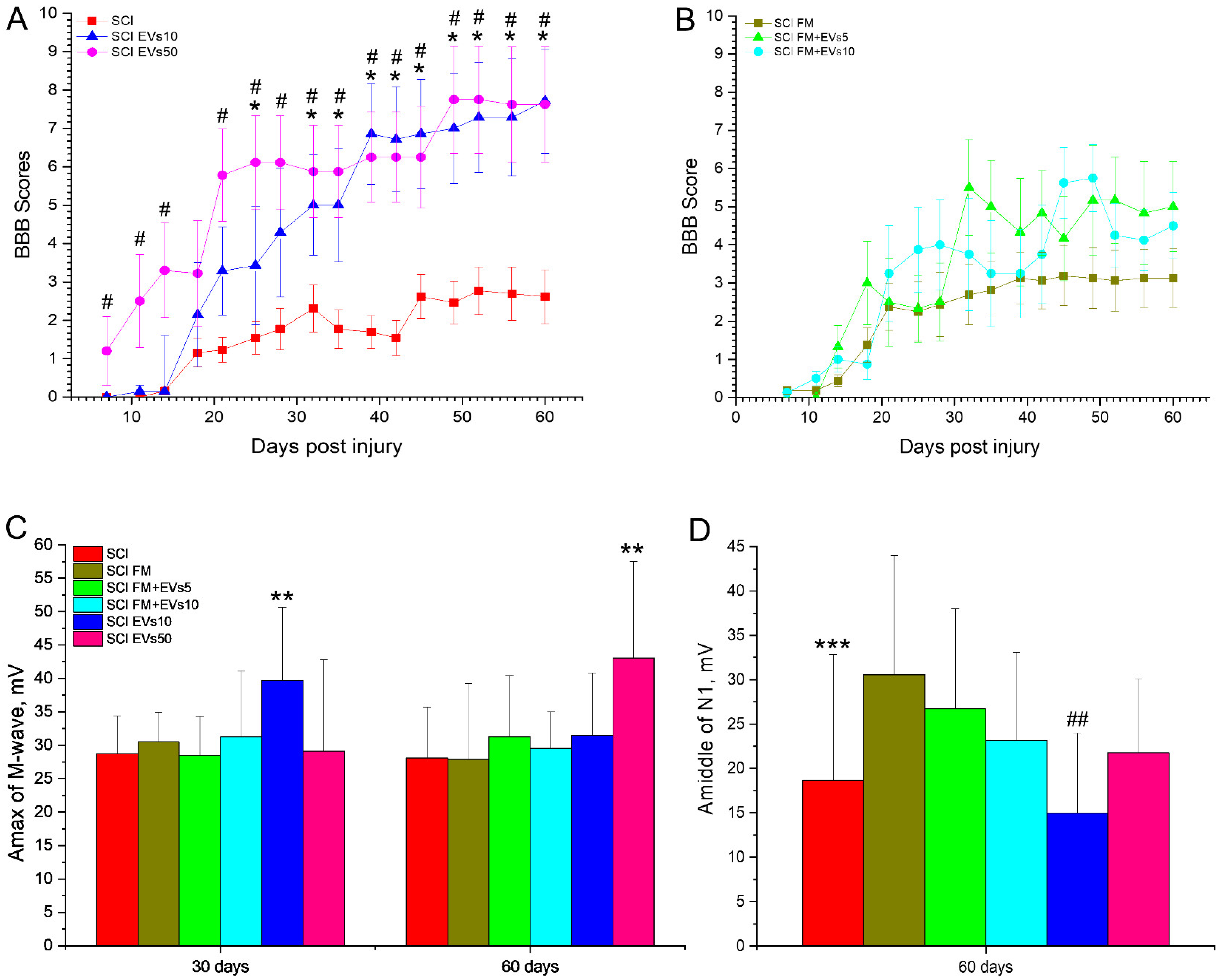
3.2. Assessment of Locomotor Activity
3.3. Electrophysiology Results
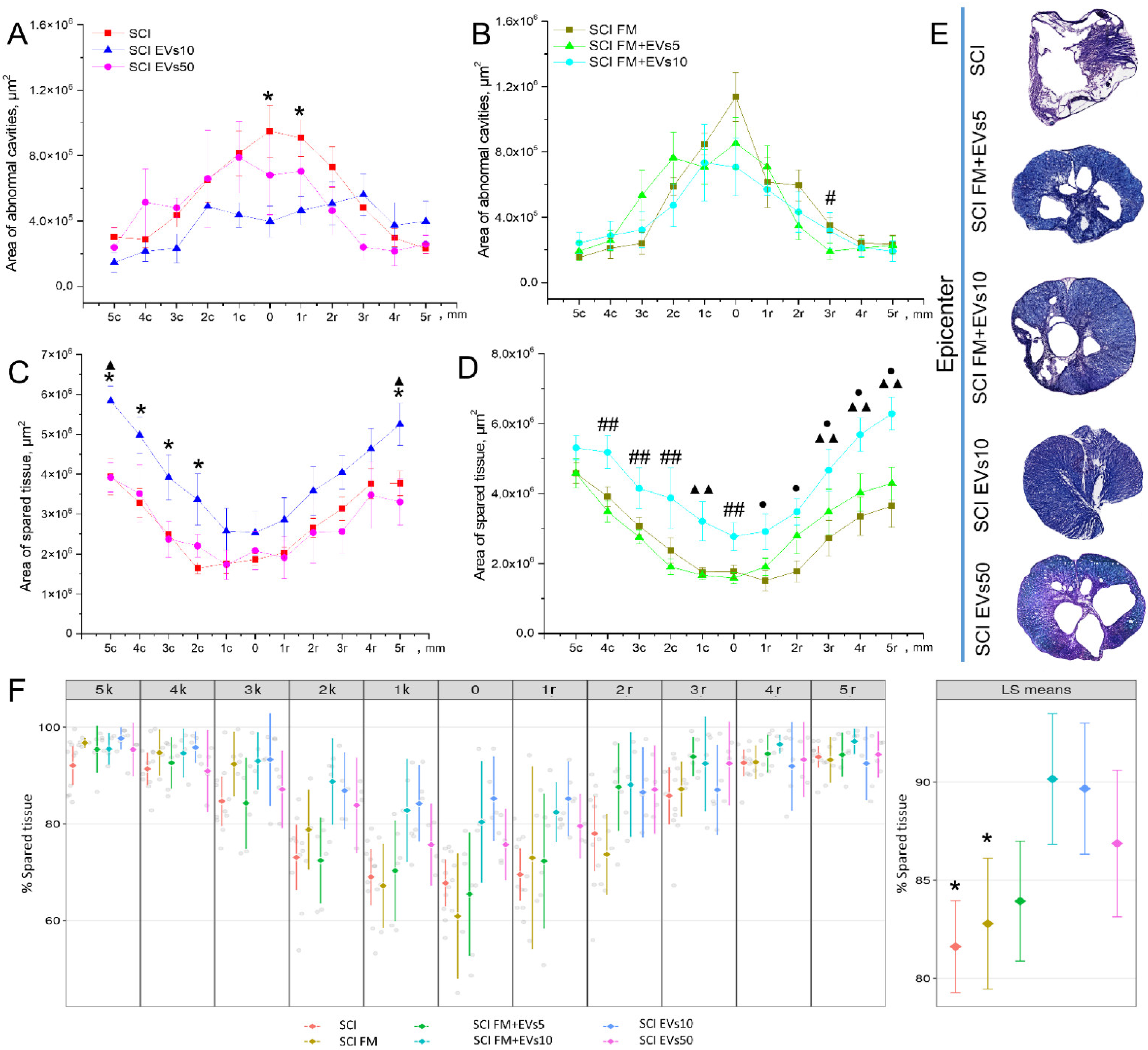
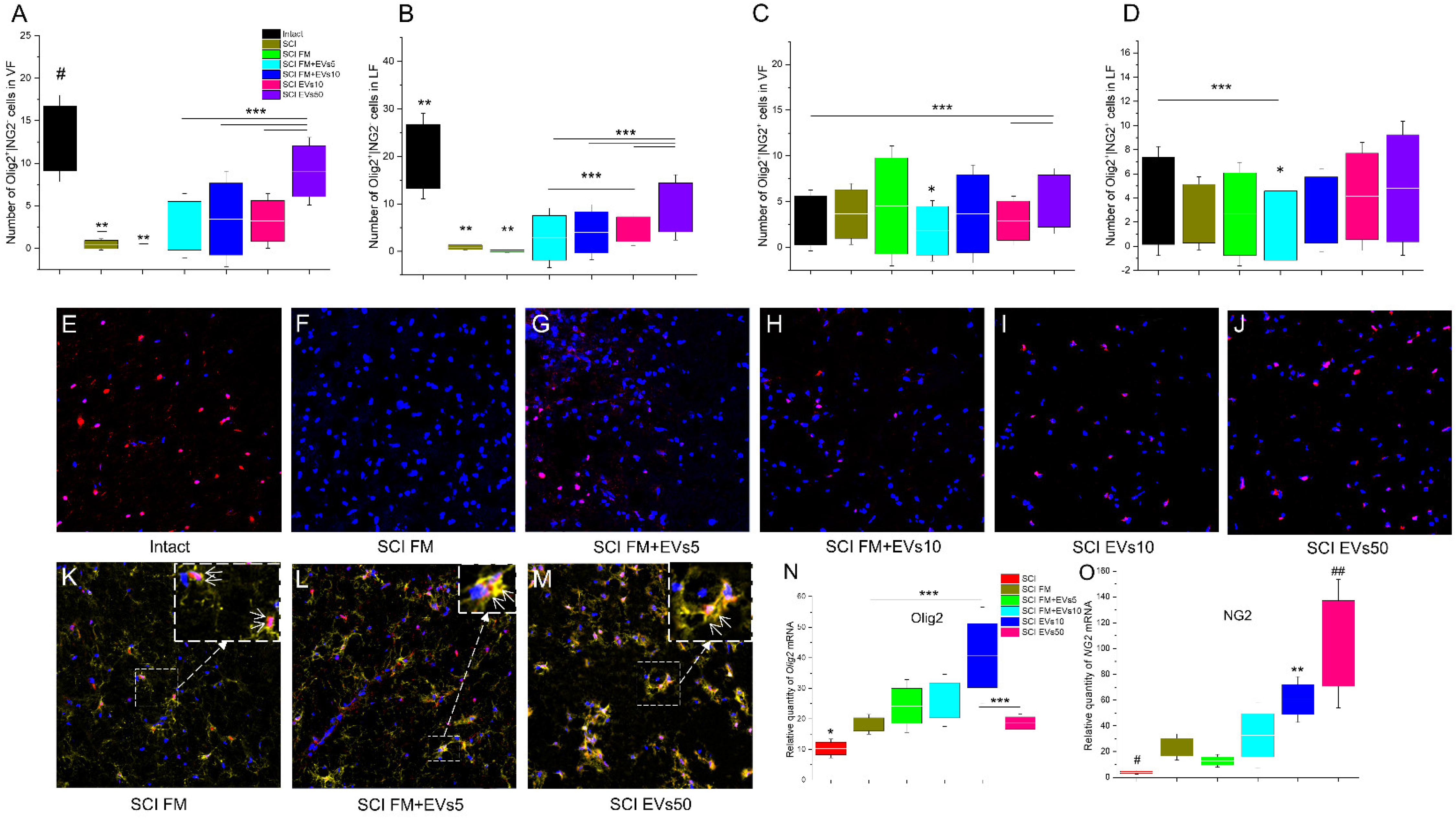
3.4. Morphometrical Assessment
3.5. Immunofluorescence Results
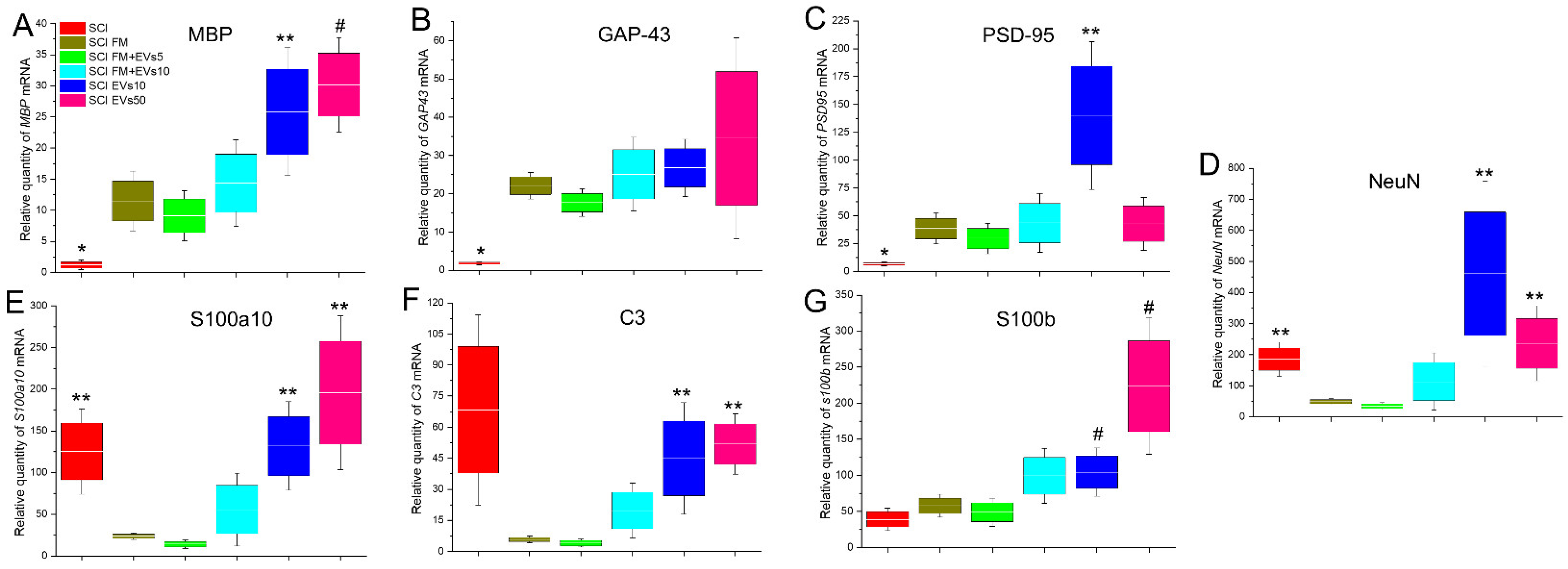
3.6. Analysis of mRNA Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackery, A.; Tator, C.; Krassioukov, A. A Global Perspective on Spinal Cord Injury Epidemiology. J. Neurotrauma 2004, 21, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- DeVivo, M.J. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord 2012, 50, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Ding, H.; Zhou, H.; Wei, Z.; Liu, L.; Pan, D.; Feng, S. Epidemiology of worldwide spinal cord injury: A literature review. J. Neurorestoratol. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Eckert, M.J.; Martin, M.J. Trauma: Spinal Cord Injury. Surg. Clin. N. Am. 2017, 97, 1031–1045. [Google Scholar] [CrossRef]
- Sandrow-Feinberg, H.R.; Houlé, J. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015, 1619, 12–21. [Google Scholar] [CrossRef]
- Xu, P.; Yang, X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transpl. 2019, 28, 36–46. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.O.; Gracheva, O.A.; Mukhutdinova, D.M.; Chelyshev, Y.A.; Rizvanov, A.A. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen. Res. 2019, 14, 227. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control. Release 2017, 264, 112–126. [Google Scholar] [CrossRef]
- Mo, M.; Zhou, Y.; Li, S.; Wu, Y. Three-Dimensional Culture Reduces Cell Size By Increasing Vesicle Excretion. Stem Cells 2018, 36, 286–292. [Google Scholar] [CrossRef]
- Galieva, L.R.; James, V.; Mukhamedshina, Y.O.; Rizvanov, A.A. Therapeutic Potential of Extracellular Vesicles for the Treatment of Nerve Disorders. Front. Neurosci. 2019, 13, 163. [Google Scholar] [CrossRef]
- Katsman, D.; Stackpole, E.J.; Domin, D.R.; Farber, D.B. Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS ONE 2012, 7, 50417. [Google Scholar] [CrossRef]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Beer Stolz, D.; Sullivan, M.L.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood J. Am. Soc. Hematol. 2012, 119, 756–766. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Benito-Martin, A.; Di Giannatale, A.; Ceder, S.; Peinado, H. The new deal: A potential role for secreted vesicles in innate immunity and tumor progression. Front. Immunol. 2015, 6, 66. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Chen, Z.; Xu, L.; Chang, M.; Wang, K.; Deng, C.; Gu, Y.; Zhou, S.; Shen, Y.; et al. Biogenesis and function of extracellular vesicles in pathophysiological processes of skeletal muscle atrophy. Biochem. Pharmacol. 2022, 198, 114954. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 07, 18–30. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, J.; Wang, T.; Hei, Y.; Li, H.; Wang, X.; Wang, L.; Zhao, R.; Liu, W.; et al. Tail-vein injection of MSC-derived small extracellular vesicles facilitates the restoration of hippocampal neuronal morphology and function in APP/PS1 mice. Cell Death Discov. 2021, 7, 230. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Laso-García, F.; Frutos, M.D.C.G.-D.; Rodríguez-Frutos, B.; Pascual-Guerra, J.; Fuentes, B.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. White Matter Repair After Extracellular Vesicles Administration in an Experimental Animal Model of Subcortical Stroke. Sci. Rep. 2017, 7, 44433. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef]
- Jaimes, Y.; Naaldijk, Y.; Wenk, K.; Leovsky, C.; Emmrich, F. Mesenchymal stem cell-derived microvesicles modulate lipopolysaccharides-induced inflammatory responses to microglia cells. Stem Cells 2017, 35, 812–823. [Google Scholar] [CrossRef]
- Turovsky, E.; Golovicheva, V.; Varlamova, E.; Danilina, T.; Goryunov, K.; Shevtsova, Y.; Pevzner, I.B.; Zorova, L.D.; Babenko, V.A.; Evtushenko, E.A.; et al. Mesenchymal stromal cell-derived extracellular vesicles afford neuroprotection by modulating PI3K/AKT pathway and calcium oscillations. Int. J. Biol. Sci. 2022, 18, 5345–5368. [Google Scholar] [CrossRef]
- Goncalves, M.B.; Malmqvist, T.; Clarke, E.; Hubens, C.J.; Grist, J.; Hobbs, C.; Trigo, D.; Risling, M.; Angeria, M.; Damberg, P.; et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J. Neurosci. 2015, 35, 15731–15745. [Google Scholar] [CrossRef]
- Han, T.; Song, P.; Wu, Z.; Xiang, X.; Liu, Y.; Wang, Y.; Fang, H.; Niu, Y.; Shen, C. MSC secreted extracellular vesicles carrying TGF-beta upregulate Smad 6 expression and promote the regrowth of neurons in spinal cord injured rats. Stem Cell Rev. Rep. 2021, 18, 1078–1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Wang, J.; Yi, H.; Song, Y. Extracellular Vesicles in the Pathogenesis, Treatment, and Diagnosis of Spinal Cord Injury: A Mini-Review. Curr. Stem Cell Res. Ther. 2022, 17, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.; Shulman, I.; Ogurcov, S.; Kostennikov, A.; Zakirova, E.; Akhmetzyanova, E.; Rogozhin, A.; Masgutova, G.; James, V.; Masgutov, R.; et al. Mesenchymal stem cell therapy for spinal cord contusion: A comparative study on small and large animal models. Biomolecules 2019, 9, 811. [Google Scholar] [CrossRef]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. nparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; Aimaletdinov, A.M.; Bondar, O.V.; Starostina, I.G.; Gorshkova, N.V.; Neustroeva, O.A.; Kletukhina, S.K.; Kurbangaleeva, S.V.; Vorobev, V.V.; Garanina, E.E.; et al. Immunosuppressive properties of cytochalasin B-induced membrane vesicles of mesenchymal stem cells: Comparing with extracellular vesicles derived from mesenchymal stem cells. Sci. Rep. 2020, 10, 10740. [Google Scholar] [CrossRef]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.U.; Sung, S.E.; Kang, K.K.; Choi, J.H.; Lee, S.; Sung, M.; Yang, S.Y.; Kim, S.-K.; Kim, Y.I.; Lim, J.-H.; et al. Therapeutic potential of mesenchymal stem cells (MSCs) and MSC-derived extracellular vesicles for the treatment of spinal cord injury. Int. J. Mol. Sci. 2021, 22, 13672. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, K.A.; Nguyen, T.T.; Prabhakara, K.S.; Toledano Furman, N.E.; Srivastava, A.K.; Harting, M.T.; Cox, C.S., Jr.; Olson, S.D. Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Modify Microglial Response and Improve Clinical Outcomes in Experimental Spinal Cord Injury. Sci. Rep. 2018, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.M.A.; Lozano, V.; Oyagüez, I.; Casado, M.A. Surgical treatment of pressure ulcers with a fibrin sealant in patients with spinal cord injury: A cost-consequence analysis. Adv. Ski. Wound Care 2015, 28, 503–507. [Google Scholar] [CrossRef]
- Hyatt, A.J.; Wang, D.; Kwok, J.C.; Fawcett, J.W.; Martin, K.R. Controlled release of chondroitinase ABC from fibrin gel reduces the level of inhibitory glycosaminoglycan chains in lesioned spinal cord. J. Control. Release 2010, 147, 24–29. [Google Scholar] [CrossRef]
- Romanelli, P.; Bieler, L.; Scharler, C.; Pachler, K.; Kreutzer, C.; Zaunmair, P.; Jakubecova, D.; Mrowetz, H.; Benedetti, B.; Rivera, F.J.; et al. Extracellular Vesicles Can Deliver Anti-inflammatory and Anti-Scarring Activities of Mesenchymal Stromal Cells After Spinal Cord Injury. Front. Neurol. 2019, 10, 1225. [Google Scholar] [CrossRef]
- Go, V.; Sarikaya, D.; Zhou, Y.; Bowley, B.G.E.; Pessina, M.A.; Rosene, D.L.; Zhang, Z.G.; Chopp, M.; Finklestein, S.P.; Medalla, M.; et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells enhance myelin maintenance after cortical injury in aged rhesus monkeys. Exp. Neurol. 2021, 337, 113540. [Google Scholar] [CrossRef]
- Noori, L.; Arabzadeh, S.; Mohamadi, Y.; Mojaverrostami, S.; Mokhtari, T.; Akbari, M.; Hassanzadeh, G. Intrathecal administration of the extracellular vesicles derived from human Wharton’s jelly stem cells inhibit inflammation and attenuate the activity of inflammasome complexes after spinal cord injury in rats. Neurosci. Res. 2021, 170, 87–98. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xia, K.; Wang, J.; Cheng, F.; Shi, K.; Ying, L.; Yu, C.; Xu, H.; Xiao, S.; et al. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact. Mater. 2021, 6, 2523–2534. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Gong, F.; Rong, Y.; Luo, Y.; Tang, P.; Zhou, Z.; Zhou, Z.; Xu, T.; Jiang, T.; et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of a1 neurotoxic reactive astrocytes. J. Neurotrauma 2019, 36, 469–484. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostennikov, A.; Kabdesh, I.; Sabirov, D.; Timofeeva, A.; Rogozhin, A.; Shulman, I.; Rizvanov, A.; Mukhamedshina, Y. A Comparative Study of Mesenchymal Stem Cell-Derived Extracellular Vesicles’ Local and Systemic Dose-Dependent Administration in Rat Spinal Cord Injury. Biology 2022, 11, 1853. https://doi.org/10.3390/biology11121853
Kostennikov A, Kabdesh I, Sabirov D, Timofeeva A, Rogozhin A, Shulman I, Rizvanov A, Mukhamedshina Y. A Comparative Study of Mesenchymal Stem Cell-Derived Extracellular Vesicles’ Local and Systemic Dose-Dependent Administration in Rat Spinal Cord Injury. Biology. 2022; 11(12):1853. https://doi.org/10.3390/biology11121853
Chicago/Turabian StyleKostennikov, Alexander, Ilyas Kabdesh, Davran Sabirov, Anna Timofeeva, Alexander Rogozhin, Ilya Shulman, Albert Rizvanov, and Yana Mukhamedshina. 2022. "A Comparative Study of Mesenchymal Stem Cell-Derived Extracellular Vesicles’ Local and Systemic Dose-Dependent Administration in Rat Spinal Cord Injury" Biology 11, no. 12: 1853. https://doi.org/10.3390/biology11121853
APA StyleKostennikov, A., Kabdesh, I., Sabirov, D., Timofeeva, A., Rogozhin, A., Shulman, I., Rizvanov, A., & Mukhamedshina, Y. (2022). A Comparative Study of Mesenchymal Stem Cell-Derived Extracellular Vesicles’ Local and Systemic Dose-Dependent Administration in Rat Spinal Cord Injury. Biology, 11(12), 1853. https://doi.org/10.3390/biology11121853