Flavobacterium psychrophilum: Response of Vaccinated Large Rainbow Trout to Different Strains
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
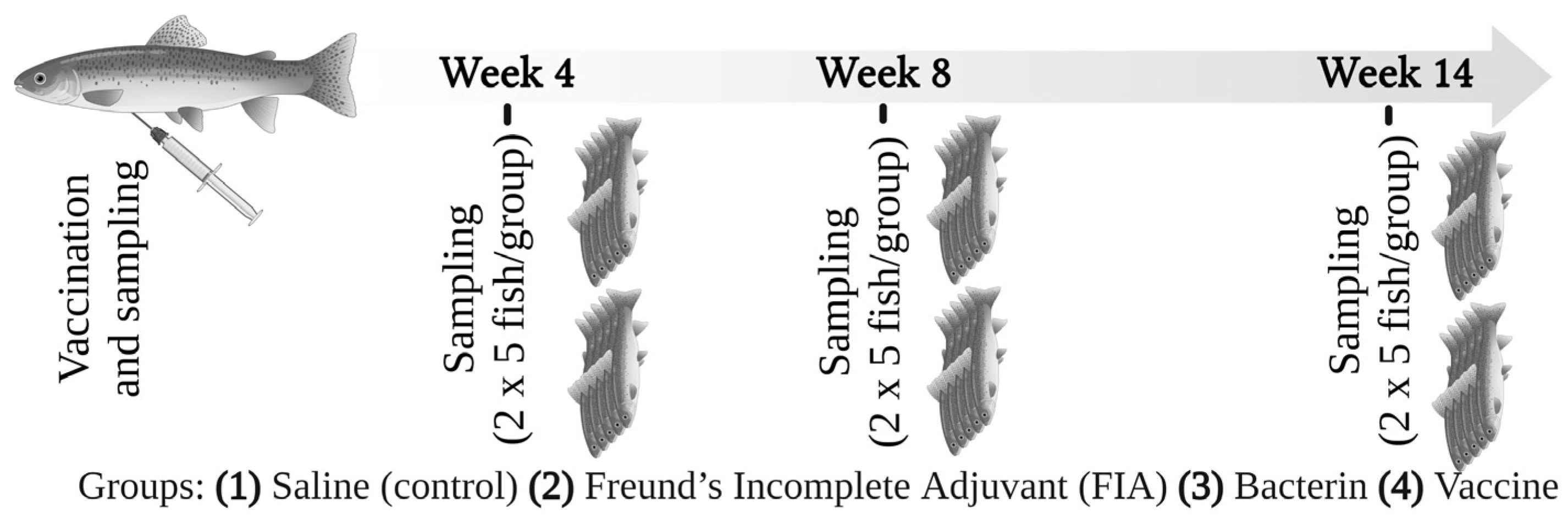
2.1. Fish
2.2. Vaccine Preparation
2.3. Vaccination
2.4. Sampling
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of Ig in Plasma
2.6. Blood Parameters
2.7. Data Analysis
2.8. Ethics
3. Results
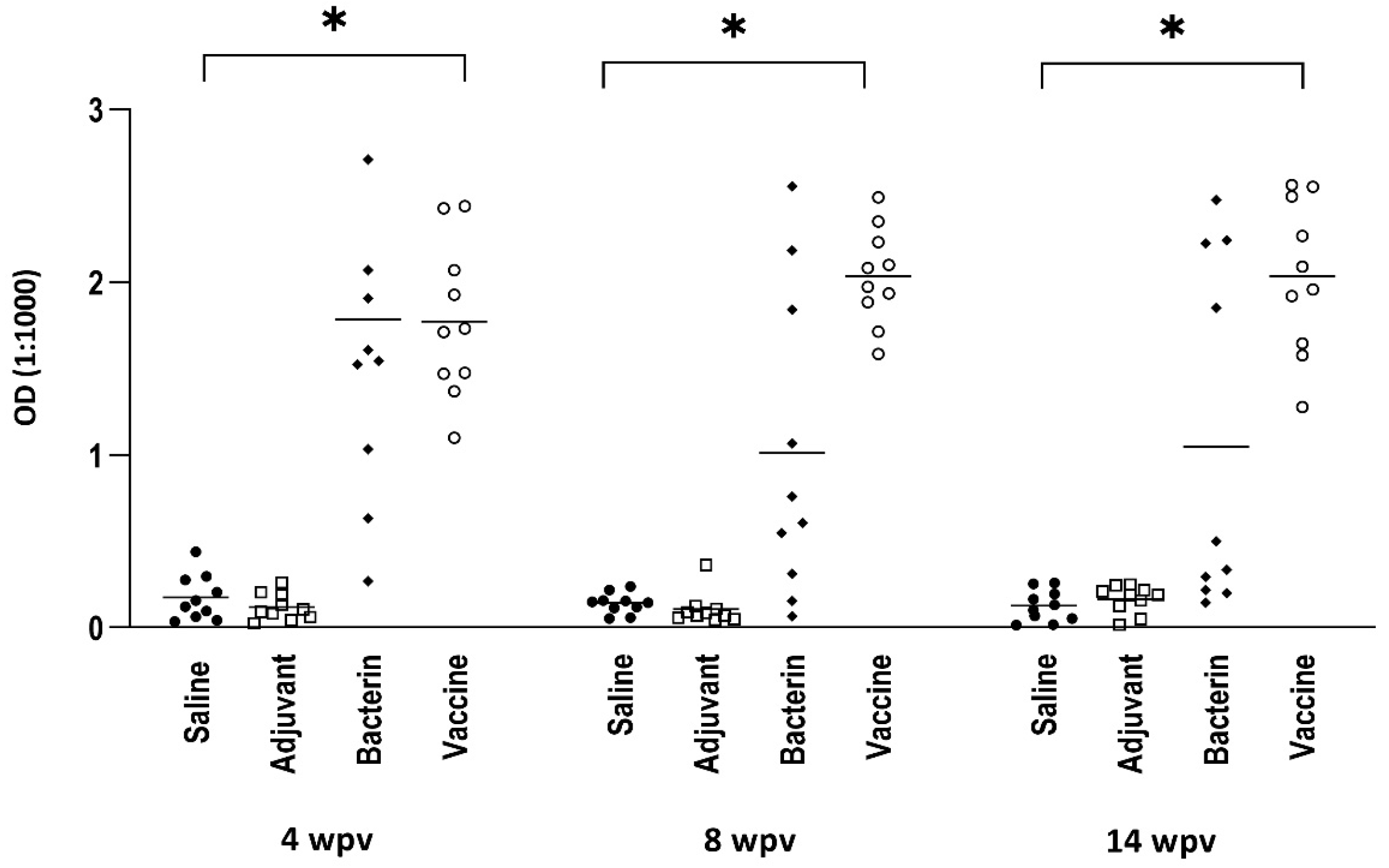
3.1. ELISA
3.1.1. Antibody Reactivity against F. psychrophilum Strain 950106-1/1
3.1.2. Antibody Cross-Reactivity against Heterologous F. psychrophilum Strains
3.2. Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borg, A.F. Studies on Myxobacteria Associated with Diseases in Salmonid Fishes. Ph.D. Thesis, University of Washington, Ann Arbor, MI, USA, 1948. [Google Scholar]
- Lorenzen, E.; Dalsgaard, I.; From, J.; Hansen, E.M.; Hørlyck, V.; Korsholm, H.; Mellergaard, S.; Olesen, N.J. Preliminary investigations of fry mortality syndrome in rainbow trout. Bull. Eur. Assoc. Fish Pathol. 1991, 11, 77–79. [Google Scholar]
- Antaya, C. Current eco-economical impacts of Flavobacterium psychrophilum. MMG 445 Basic Biotechnol. J. 2008, 4, 16–21. [Google Scholar]
- Lorenzen, E.; Dalsgaard, I.; Bernardet, J.-F. Characterization of isolates of Flavobacterium psychrophilum associated with coldwater disease or rainbow trout fry syndrome I: Phenotypic and genomic studies. Dis. Aquat. Org. 1997, 31, 197–208. [Google Scholar] [CrossRef]
- Starliper, C.E. Bacterial coldwater disease of fishes caused by Flavobacterium psychrophilum. J. Adv. Res. 2011, 2, 97–108. [Google Scholar] [CrossRef]
- Kumagai, A.; Yamaoka, S.; Takahashi, K.; Fukuda, H.; Wakabayashi, H. Waterborne Transmission of Flavobacterium psychrophilum in Coho Salmon Egg. Fish Pathol. 2000, 35, 25–28. [Google Scholar] [CrossRef]
- Madetoja, J.; Nyman, P.; Wiklund, T. Flavobacterium psychrophilum, invasion into and shedding by rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 2000, 43, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Møller, J.D.; Dalsgaard, I. Flavobacterium psychrophilum in rainbow trout, Oncorhynchus mykiss (Walbaum), hatcheries: Studies on broodstock, eggs, fry and environment. J. Fish Dis. 2005, 28, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, I.; Madsen, L. Bacterial pathogens in rainbow trout, Oncorhynchus mykiss (Walbaum), reared at Danish freshwater farms. J. Fish Dis. 2000, 23, 199–209. [Google Scholar] [CrossRef]
- Evensen, Ø.; Lorenzen, E. An immunohistochemical study of Flexibacter psychrophilus infection in experimentally and naturally infected rainbow trout (Oncorhynchus mykiss) fry. Dis. Aquat. Org. 1996, 25, 53–61. [Google Scholar] [CrossRef]
- Madetoja, J.; Nystedt, S.; Wiklund, T. Survival and virulence of Flavobacterium psychrophilum in water microcosms. FEMS Microbiol. Ecol. 2003, 43, 217–223. [Google Scholar] [CrossRef]
- Nematollahi, A.; Decostere, A.; Pasmans, F.; Haesebrouck, F. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 2003, 26, 563–574. [Google Scholar] [CrossRef]
- Bruun, M.S.; Schmidt, A.S.; Madsen, L.; Dalsgaard, I. Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture 2000, 187, 201–212. [Google Scholar] [CrossRef]
- The Council of the European Union. On Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No 2092/91; 834/2007; The Council of the European Union: Bruxelles, Belgium, 2007; Available online: http://data.europa.eu/eli/reg/2007/834/oj (accessed on 10 October 2021).
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.-E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef]
- Buchmann, K. Development of Immunocompetence in Fish. In Principles in Fish Immunology; Buchmann, K., Secombes, C.J., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 495–510. [Google Scholar]
- Hoare, R.; Ngo, T.P.H.; Bartie, K.L.; Adams, A. Efficacy of a polyvalent immersion vaccine against Flavobacterium psychrophilum and evaluation of immune response to vaccination in rainbow trout fry (Onchorynchus mykiss L.). Vet. Res. 2017, 48, 43. [Google Scholar] [CrossRef] [PubMed]
- Xueqin, J.; Kania, P.W.; Buchmann, K. Comparative effects of four feed types on white spot disease susceptibility and skin immune parameters in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2012, 35, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Marana, M.H.; Sepúlveda, D.; Chen, D.; Al-Jubury, A.; Jaafar, R.M.; Kania, P.W.; Henriksen, N.H.; Krossøy, B.; Dalsgaard, I.; Lorenzen, N.; et al. A pentavalent vaccine for rainbow trout in Danish aquaculture. Fish Shellfish Immunol. 2019, 88, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Rochat, T.; Fujiwara-Nagata, E.; Calvez, S.; Dalsgaard, I.; Madsen, L.; Calteau, A.; Lunazzi, A.; Nicolas, P.; Wiklund, T.; Bernardet, J.-F.; et al. Genomic Characterization of Flavobacterium psychrophilum Serotypes and Development of a Multiplex PCR-Based Serotyping Scheme. Front. Microbiol. 2017, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U.; Hørder, M.; Rej, R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: Approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 3. IFCC method for alanine aminotransferase (L-alanine: 2-oxoglutarate aminotransferase, EC 2.6.1.2). J. Clin. Chem. Clin. Biochem. 1986, 24, 481–495. [Google Scholar] [PubMed]
- King, E.J.; Armstrong, A.R. A convenient method for determining serum and bile phosphatase activity. Can. Med. Assoc. J. 1934, 31, 376–381. [Google Scholar]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic Determination of Total Serum Cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Kimberly, M.M.; Leary, E.T.; Cole, T.G.; Waymack, P.P. Selection, Validation, Standardization, and Performance of a Designated Comparison Method for HDL-Cholesterol for Use in the Cholesterol Reference Method Laboratory Network. Clin. Chem. 1999, 45, 1803–1812. [Google Scholar]
- Weichselbaum, T.E. An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am. J. Clin. Pathol. 1946, 10, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, G.; David, H. Quantitative Determination of Serum Triglycerides by the Use of Enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, N.; Lorenzen, N.; Dalsgaard, I.; Clausen, T.; Buchmann, K. Autovaccine-Forsøg (YDS) på Dansk Dambrug. 2017. (In Danish). Available online: www.danskakvakultur.dk (accessed on 1 October 2021).
- Chettri, J.K.; Deshmukh, S.; Holten-Andersen, L.; Jafaar, R.M.; Dalsgaard, I.; Buchmann, K. Comparative evaluation of administration methods for a vaccine protecting rainbow trout against Yersinia ruckeri O1 biotype 2 infections. Vet. Immunol. Immunopathol. 2013, 154, 42–47. [Google Scholar] [CrossRef]
- Madsen, L.; Dalsgaard, I. Comparative studies of Danish Flavobacterium psychrophilum isolates: Ribotypes, plasmid profiles, serotypes and virulence. J. Fish Dis. 2000, 23, 211–218. [Google Scholar] [CrossRef]
- LaFrentz, B.R.; LaPatra, S.E.; Jones, G.R.; Cain, K.D. Passive immunization of rainbow trout, Oncorhynchus mykiss (Walbaum), against Flavobacterium psychrophilum, the causative agent of bacterial coldwater disease and rainbow trout fry syndrome. J. Fish Dis. 2003, 26, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, B.N.; Olsen, R.H.; Furevik, A.; Souhoka, R.A.; Gauthier, D.; Brudeseth, B. Efficacy of a divalent and a multivalent water-in-oil formulated vaccine against a highly virulent strain of Flavobacterium psychrophilum after intramuscular challenge of rainbow trout (Oncorhynchus mykiss). Vaccine 2013, 31, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Madetoja, J.; Lönnström, L.G.; Björkblom, C.; Uluköy, G.; Bylund, G.; Syvertsen, C.; Gravningen, K.; Norderhus, E.A.; Wiklund, T. Efficacy of injection vaccines against Flavobacterium psychrophilum in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2006, 29, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, T.; Dalsgaard, I. Survival of Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) serum in vitro. Fish Shellfish Immunol. 2002, 12, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.A. Cytophaga psychrophila, the Causative Agent of Bacterial Cold-Water Disease in Salmonid Fish. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1987. [Google Scholar]
- Lorenzen, E. Studies on Flexibacter psychrophilus in Relation to Rainbow Trout Fry Syndrome (RTFS). Ph.D. Thesis, National Veterinary Laboratory, Århus and Royal Veterinary and Agricultural University, Copenhagen, Denmark, 1994. [Google Scholar]
- Hoare, R.; Jung, S.J.; Ngo, T.P.H.; Bartie, K.; Bailey, J.; Thompson, K.D.; Adams, A. Efficacy and safety of a non-mineral oil adjuvanted injectable vaccine for the protection of Atlantic salmon (Salmo salar L.) against Flavobacterium psychrophilum. Fish Shellfish Immunol. 2019, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bruce, T.J.; Sudheesh, P.S.; Knupp, C.; Loch, T.P.; Faisal, M.; Cain, K.D. Assessment of cross-protection to heterologous strains of Flavobacterium psychrophilum following vaccination with a live-attenuated coldwater disease immersion vaccine. J. Fish Dis. 2019, 42, 75–84. [Google Scholar] [CrossRef]
- Coskun, O.F.; Aydin, D.; Duman, F. Comparison of some blood parameters of rainbow trout (Oncorhynchus mykiss) living in running and still water. Iran J. Fish Sci. 2016, 15, 497–507. [Google Scholar]
- Chen, Y.E.; Jin, S.; Wang, G.L. Study on blood physiological and biochemical indices of Vibrio alginolyticus disease of Lateolabrax japonicus. J. Oceanogr. Taiwan Strait. 2005, 241, 104–108. [Google Scholar]
- Cnaani, A.; Tinman, S.; Avidar, Y.; Ron, M.; Hulata, G. Comparative study of biochemical parameters in response to stress in Oreochromis aureus, O. mossambicus and two strains of O. niloticus. Aquac. Res. 2004, 35, 1434–1440. [Google Scholar] [CrossRef]
- Hughes, G.M.; Nemcsók, J. Effects of low pH alone and combined with copper sulphate on blood parameters of rainbow trout. Environ. Poll. 1988, 55, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Peres, H.; Santos, S.; Oliva-Teles, A. Blood chemistry profile as indicator of nutritional status in European seabass (Dicentrarchus labrax). Fish Physiol. Biochem. 2014, 40, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, N.; Guijarro, A.I.; López-Patiño, M.A.; Martínez-Álvarez, R.; Delgado, M.J. Daily and seasonal variations in haematological and blood biochemical parameters in the tench, Tinca tinca Linnaeus, 1758. Aquac. Res. 2005, 36, 1185–1196. [Google Scholar] [CrossRef]
- Midtlyng, P.J.; Reitan, L.J.; Speilberg, L. Experimental studies on the efficacy and side-effects of intraperitoneal vaccination of Atlantic salmon (Salmo salar L.) against furunculosis. Fish Shellfish Immunol. 1996, 6, 335–350. [Google Scholar] [CrossRef]
- Manera, M.; Britti, D. Assessment of blood chemistry normal ranges in rainbow trout. J. Fish Biol. 2006, 69, 1427–1434. [Google Scholar] [CrossRef]

| ALAT U/L | ALP U/L | CHOL mmol/L | HDL mmol | TP g/L | TRIG mmol | |
|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| 4 wpv | ||||||
| Saline | 1.18 ± 0.3 | 74.01 ± 6.74 | 6.76 ± 0.35 | 1.68 ± 0.08 | 27.28 ± 0.95 | 2.26 ± 0.35 |
| Adjuvant | 2.36 ± 0.38 * | 53.37 ± 6.62 | 5.78 ± 0.41 | 1.28 ± 0.10 * | 24.48 ± 1.25 | 2.60 ± 0.32 |
| Bacterin | 2.46 ± 2.68 * | 81.97 ± 21.07 | 5.64 ± 0.64 | 1.31 ± 0.1 * | 24.66 ± 1.35 | 2.38 ± 0.24 |
| Vaccine | 1.46 ± 0.32 | 64.66 ± 9.16 | 5.29 ± 0.25 * | 1.25 ± 0.09 * | 27.36 ± 1.49 | 2.52 ± 0.28 |
| 8 wpv | ||||||
| Saline | 4.69 ± 0.83 | 101.84 ± 10.58 | 8.33 ± 0.49 | 1.57 ± 0.06 | 34.52 ± 1.6 | 3.51 ± 0.33 |
| Adjuvant | 4.02 ± 0.67 | 90.4 ± 10.37 | 7.62 ± 0.44 | 1.54 ± 0.04 | 34.54 ± 1.17 | 3.18 ± 0.37 |
| Bacterin | 3.49 ± 0.52 | 81.76 ± 9.05 | 7.14 ± 0.47 | 1.52 ± 0.06 | 32.5 ± 1.53 | 2.73 ± 0.25 |
| Vaccine | 4.4 ± 0.43 | 81.91 ± 7.57 | 3.39 ± 0.28 | 1.52 ± 0.03 | 35.18 ± 1 | 3.39 ± 0.35 |
| 14 wpv | ||||||
| Saline | 5.34 ± 0.85 | 77.38 ± 11.25 | 8.55 ± 0.72 | 1.64 ± 0.08 | 30.78 ± 1.9 | 3.15 ± 0.53 |
| Adjuvant | 3.51 ± 0.40 | 66.05 ± 9.41 | 7.42 ± 0.71 | 1.66 ± 0.01 | 28.42 ± 2.22 | 2.54 ± 0.26 |
| Bacterin | 8.37 ± 1.42 | 89.95 ± 11.71 | 8.25 ± 0.29 | 1.79 ± 0.05 | 31.99 ± 0.82 | 2.73 ± 0.1 |
| Vaccine | 10.76 ± 1.93 * | 108.92 ± 16.74 | 9.11 ± 0.51 | 1.69 ± 0.05 | 35.02 ± 1.33 | 3.88 ± 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marana, M.H.; Dalsgaard, I.; Kania, P.W.; Mohamed, A.; Hannibal, J.; Buchmann, K. Flavobacterium psychrophilum: Response of Vaccinated Large Rainbow Trout to Different Strains. Biology 2022, 11, 1701. https://doi.org/10.3390/biology11121701
Marana MH, Dalsgaard I, Kania PW, Mohamed A, Hannibal J, Buchmann K. Flavobacterium psychrophilum: Response of Vaccinated Large Rainbow Trout to Different Strains. Biology. 2022; 11(12):1701. https://doi.org/10.3390/biology11121701
Chicago/Turabian StyleMarana, Moonika H., Inger Dalsgaard, Per Walter Kania, Abdu Mohamed, Jens Hannibal, and Kurt Buchmann. 2022. "Flavobacterium psychrophilum: Response of Vaccinated Large Rainbow Trout to Different Strains" Biology 11, no. 12: 1701. https://doi.org/10.3390/biology11121701
APA StyleMarana, M. H., Dalsgaard, I., Kania, P. W., Mohamed, A., Hannibal, J., & Buchmann, K. (2022). Flavobacterium psychrophilum: Response of Vaccinated Large Rainbow Trout to Different Strains. Biology, 11(12), 1701. https://doi.org/10.3390/biology11121701





