Potential for Omega-3 Fatty Acids to Protect against the Adverse Effect of Phytosterols: Comparing Laboratory Outcomes in Adult Patients on Home Parenteral Nutrition Including Different Lipid Emulsions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patients, and Interventions
2.2. Blood Processing and Overview of Analyses Performed
2.3. Measurement of Fatty Acids in Plasma
2.4. Measurement of Plasma Cytokine Concentrations
2.5. Measurement of Sterol Concentrations
2.6. Statistical Analysis
3. Results
3.1. Sterol and Stanol Concentrations in the Lipid Emulsions and in Plasma
3.2. Plasma Fatty Acids
3.3. Plasma Liver Function Markers and Triglycerides
3.4. Plasma Markers of Inflammation
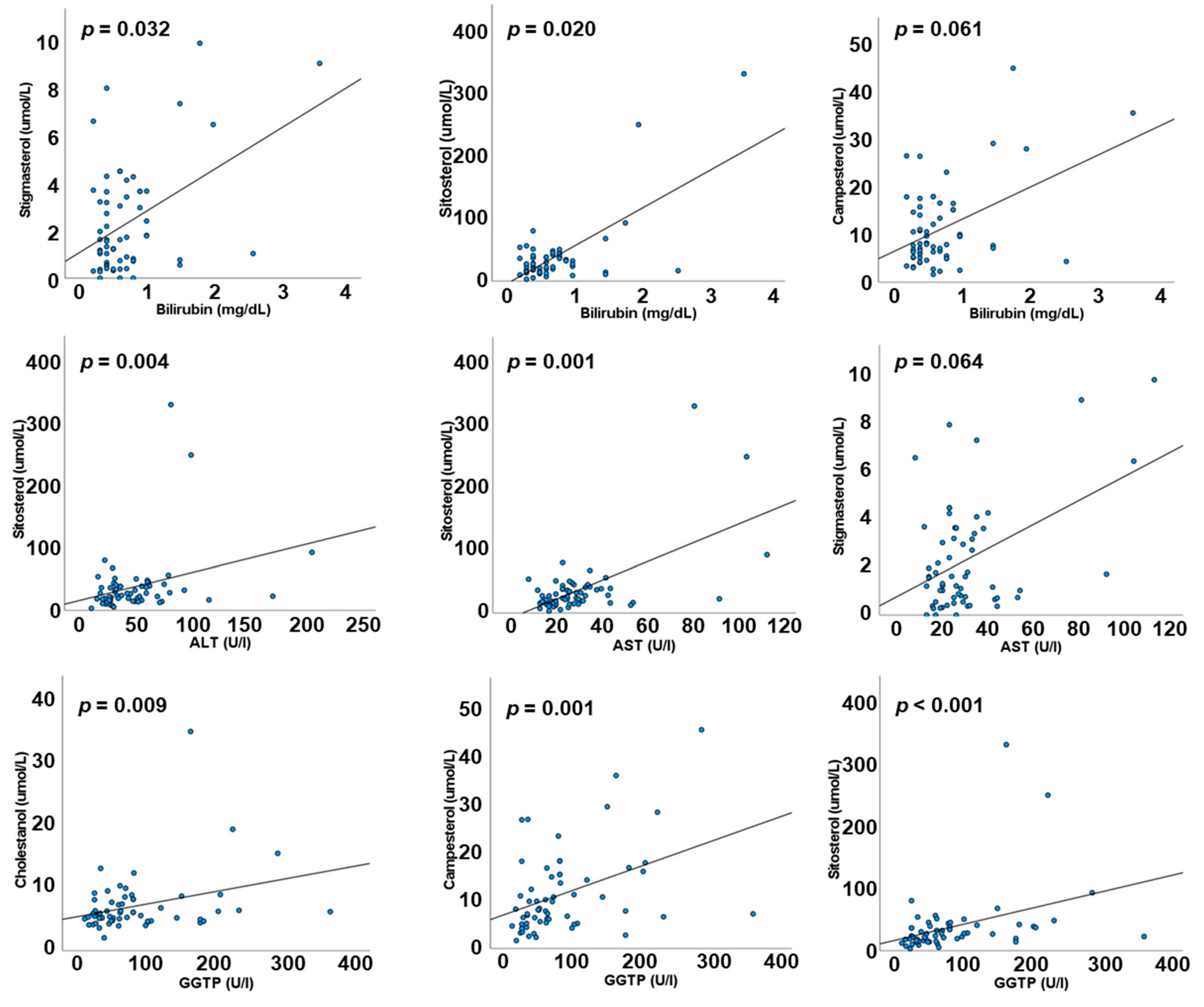
3.5. Correlations between Liver Function Markers and Plasma Sterols and Stanols
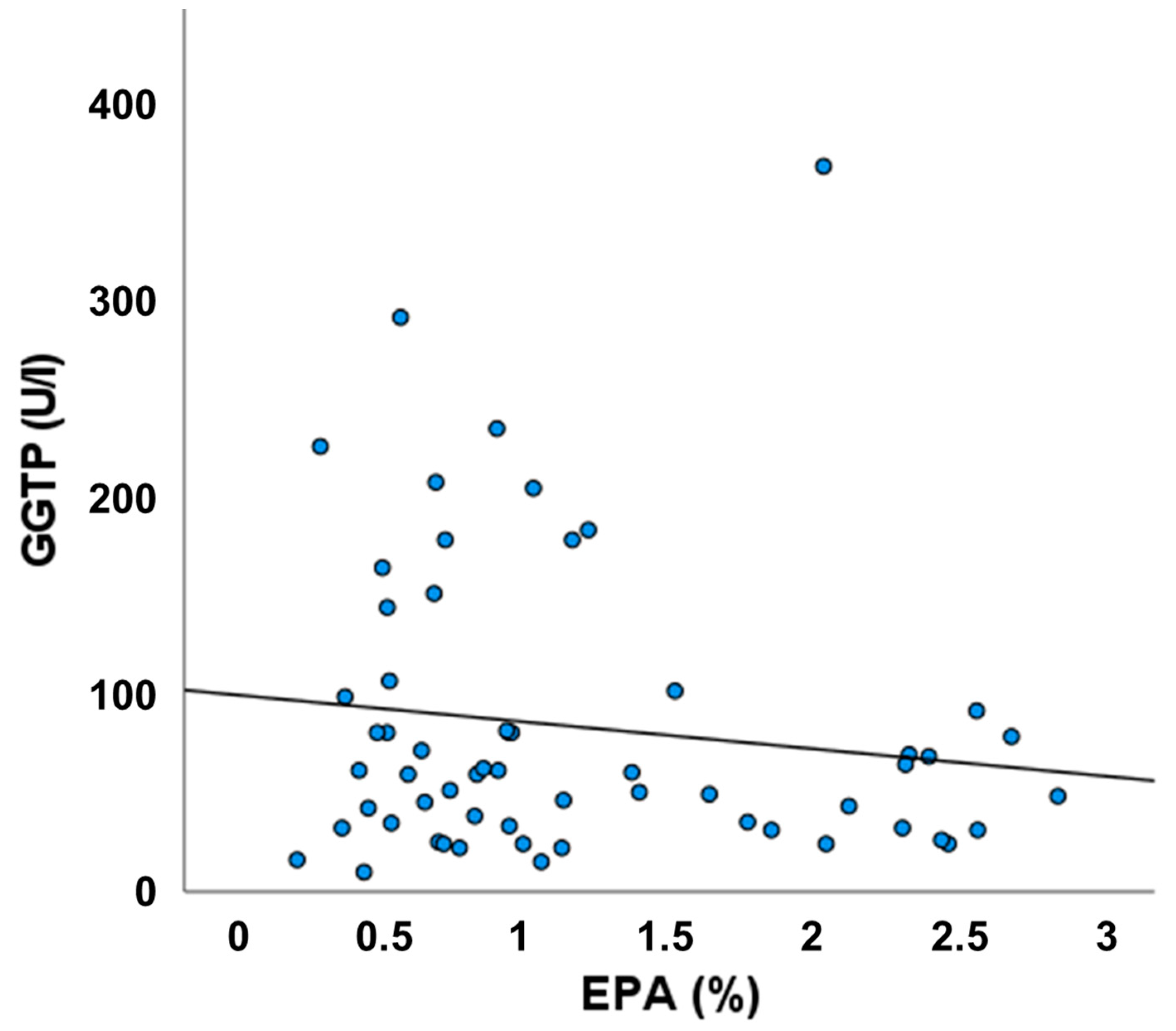
3.6. Relationship between Plasma EPA and GGT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wanten, G.; Calder, P.C.; Forbes, A. Managing adult patients who need home parenteral nutrition. BMJ 2011, 342, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Adolph, M.; Deutz, N.E.; Grau, T.; Innes, J.K.; Klek, S.; Lev, S.; Mayer, K.; Michael-Titus, A.T.; Pradelli, L.; et al. Lipids in the intensive care unit: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2018, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Forchielli, M.L.; Bersani, G.; Tala, S.; Grossi, G.; Puggioli, C.; Masi, M. The spectrum of plant and animal sterols in different oil-derived intravenous emulsions. Lipids 2010, 45, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Llop Talaverón, J.M.; Novak, A.; Suñé Negre, J.M.; Badia Tahull, M.; Leiva Badosa, E.; Ticó Grau, J.R. Phytosterol determination in lipid emulsions for parenteral nutrition. Farm Hospit. 2018, 42, 116–119. [Google Scholar]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef]
- Ahmed, S.; Innes, J.K.; Calder, P.C. Influence of different intravenous lipid emulsions on fatty acid status and laboratory and clinical outcomes in adult patients receiving home parenteral nutrition: A systematic review. Clin. Nutr. 2021, 40, 1115–1122. [Google Scholar] [CrossRef]
- Beath, S.V.; Kelly, D.A. Total parenteral nutrition-induced cholestasis: Prevention and management. Clin. Liver Dis. 2016, 20, 159–176. [Google Scholar] [CrossRef]
- Hukkinen, M.; Mutanen, A.; Nissinen, M.; Merras-Salmio, L.; Gylling, H.; Pakarinen, M.P. Parenteral plant sterols accumulate in the liver reflecting their increased serum levels and portal inflammation in children with intestinal failure. J. Parenter. Ent. Nutr. 2017, 41, 1014–1022. [Google Scholar] [CrossRef]
- Premkumar, M.H.; Carter, B.A.; Hawthorne, K.M.; King, K.; Abrams, S.A. High rates of resolution of cholestasis in parenteral nutrition associated liver disease with fish oil-based lipid emulsion monotherapy. J. Pediatr. 2013, 162, 793–798. [Google Scholar] [CrossRef]
- Muhammed, R.; Bremner, R.; Protheroe, S.; Johnson, T.; Holden, C.; Murphy, M.S. Resolution of parenteral nutrition-associated jaundice on changing from a soybean oil emulsion to a complex mixed-lipid emulsion. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 797–802. [Google Scholar] [CrossRef]
- Prince, E.; Lazare, F.B.; Treem, W.R.; Xu, J.; Iqbal, J.; Pan, X.; Josekutty, J.; Walsh, M.; Anderson, V.; Mahmood Hussain, M.; et al. Omega-3 fatty acids prevent hepatic steatosis independent of PPAR-alpha activity, in a murine model of parenteral nutrition-associated liver disease. J. Parenter. Enter. Nutr. 2014, 38, 608–616. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Kotiya, P.; Zhao, X.; Cheng, P.; Zhu, X.; Xiao, Z.; Wang, J. Fish-oil and soy oil-based lipid emulsions in neonatal parenteral nutrition; a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2016, 70, 1106–1115. [Google Scholar] [CrossRef]
- Nandivada, P.; Fell, G.L.; Gura, K.M.; Puder, M. Lipid emulsions in the treatment and prevention of parenteral nutrition-associated liver disease in infants and children. Am. J. Clin. Nutr. 2016, 103, 629S–634S. [Google Scholar] [CrossRef]
- Edward, R.-R.; Innes, J.K.; Marino, L.V.; Calder, P.C. Influence of different intravenous lipid emulsions on growth, development and laboratory and clinical outcomes in hospitalised paediatric patients: A systematic review. Clin. Nutr. 2018, 37, 765–783. [Google Scholar] [CrossRef]
- Gura, K.M.; Calkins, K.L.; Premkumar, M.H.; Puder, M. Use of intravenous soybean and fish oil emulsions in pediatric intestinal failure-associated liver disease: A multicentre integrated analysis report on extrahepatic adverse events. J. Pediatr. 2022, 241, 173–180. [Google Scholar] [CrossRef]
- Sasdelli, A.S.; Agostini, F.; Pazzeschi, C.; Guidetti, M.; Lal, S.; Pironi, L. Assessment of Intestinal Failure Associated Liver Disease according to different diagnostic criteria. Clin. Nutr. 2019, 38, 1198–1205. [Google Scholar] [CrossRef]
- Cuerda, C.; Pironi, L.; Arends, J.; Bozzetti, F.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. Home Artificial Nutrition & Chronic Intestinal Failure Special Interest Group of ESPEN. ESPEN practical guideline: Clinical nutrition in chronic intestinal failure. Clin. Nutr. 2021, 40, 5196–5220. [Google Scholar]
- Fisk, H.L.; West, A.L.; Childs, C.E.; Burdge, G.C.; Calder, P.C. The use of gas chromatography to analyze compositional changes of fatty acids in rat liver tissue during pregnancy. J. Vis. Exp. 2014, 13, 51445. [Google Scholar] [CrossRef]
- Mackay, D.S.; Jones, P.J.; Myrie, S.B.; Plat, J.; Lütjohann, D. Methodological considerations for the harmonization of non-cholesterol sterol bio-analysis. J. Chromatog. B 2014, 957, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.T.; Bowron, A.; Mills, K.A.; Massoud, A.; Casteels, M.; Milla, P. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. J. Gastroenterol. 1993, 105, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.E.; Braun, L.P.; Mercer, L.D.; Sherrill, M.; Stevens, J.; Javid, P.J. The effect of lipid restriction on the prevention of parenteral nutrition-associated cholestasis in surgical infants. J. Peditr. Surg. 2013, 48, 573–578. [Google Scholar] [CrossRef]
- Nandivada, P.; Cowan, E.; Carlson, S.J.; Chang, M.; Gura, K.M.; Puder, M. Mechanisms for the effects of fish oil lipid emulsions in the management of parenteral nutrition-associated liver disease. Prostaglandins. Leukot. Essent. Fat. Acids 2013, 89, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A.; Fidler Mis, N.; Goulet, O.; van den Akker, C.H.P.; Wu, J.; Koletzko, B.; ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin. Nutr. 2018, 37, 2324–2336. [Google Scholar] [CrossRef]
- Carter, B.A.; Taylor, O.A.; Prendergast, D.R.; Zimmerman, T.L.; Von Furstenberg, R.; Moore, D.D.; Karpen, S.J. Stigmasterol, a soy lipid–derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Ped. Res. 2007, 62, 301–306. [Google Scholar] [CrossRef]
- Mutanen, A.; Nissinen, M.J.; Lohi, J.; Heikkilä, P.; Gylling, H.; Pakarinen, M.P. Serum plant sterols, cholestanol and cholesterol precursors associate with histological liver injury in pediatric onset intestinal failure. Am. J. Clin. Nutr. 2014, 100, 1085–1094. [Google Scholar] [CrossRef]
- Bhogal, H.K.; Sanyal, A.J. The molecular pathogenesis of cholestasis in sepsis. Front. Biosci. 2013, 5, 87–96. [Google Scholar] [CrossRef]
- Kosters, A.; Karpen, S.J. The role of inflammation in cholestasis: Clinical and basic aspects. Semin. Liver Dis. 2010, 30, 186–194. [Google Scholar] [CrossRef]
- Geier, A.; Fickert, P.; Trauner, M. Mechanisms of disease: Mechanisms and clinical implications of cholestasis in sepsis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 574–585. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A.; Walsh, S.W.; Willey, A.; Yager, D.R.; Fowler, A.A.; Graham, M.F. Linoleic acid induces interleukin-8 production by Crohn's human intestinal smooth muscle cells via arachidonic acid metabolites. Am. J. Physiol. 2004, 286, G528–G537. [Google Scholar] [CrossRef]
- Leik, C.E.; Walsh, S.W. Linoleic acid but not oleic acid upregulates production of interleukin-8 by human vascular smooth muscle cells via arachidonic acid metabolites under conditions of oxidative stress. J. Soc. Gynecol. Investig. 2005, 12, 593–598. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 PUFA and inflammation: From membrane to nucleus and from bench to bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Keller, H.; Dreyer, C.; Medin, J.; Mahfoudi, A.; Ozato, K.; Wahli, W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc. Natl. Acad. Sci. USA 1993, 90, 2160–2164. [Google Scholar] [CrossRef]
- Botta, M.; Audano, M.; Sahebkar, A.; Sirtori, C.R.; Mitro, N.; Ruscica, M. PPAR agonists and metabolic syndrome: An established role? Int. J. Mol. Sci. 2018, 19, 1197. [Google Scholar] [CrossRef]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and metabolic partitioning of fatty acids within the liver in the context of non-alcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 248–255. [Google Scholar] [CrossRef]
- Kota, B.P.; Huang, T.H.; Roufogalis, B.D. An overview on biological mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef]
- Gonyon, T.; Tomaso, A.E., Jr.; Kotha, P.; Owen, H.; Patel, D.; Carter, P.W.; Cronin, J.; Green, J.B.D. Interactions between parenteral lipid emulsions and container surfaces. PDA J. Pharm. Sci. Technol. 2013, 67, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Dumas, G.J.; Shoaie, C.; Zheng, Z.; Mackinnon, B.; Al-Aweel, I.; Bristrian, B.R.; Pursley, M.; Driscoll, D.F. Incidence of hypertriglyceridemia in critically ill neonates receiving lipid injectable emulsions in glass versus plastic containers: A retrospective analysis. J. Pediatr. 2008, 152, 232–236. [Google Scholar] [CrossRef] [PubMed]
| ClinOleic | SMOFLipid | Intralipid | |
|---|---|---|---|
| Number of patients | 21 | 17 | 20 |
| Age range, years (mean) | 19–91 (60.3) | 27–84 (54.5) | 25–89 (59.0) |
| TPN duration, months (mean) | 26–72 (46.5) | 24–40 (33.4) | 32–78 (48.2) |
| Male (n) | 8 | 7 | 10 |
| Female (n) | 13 | 10 | 10 |
| Etiology of intestinal failure (n): | |||
| Bowel obstruction | 2 | 2 | 2 |
| Mesenteric ischemia | 5 | 3 | 3 |
| Surgical complications | 3 | 4 | 5 |
| Crohn’s disease | 3 | 3 | 4 |
| Adhesion ileus | 3 | 1 | 2 |
| Radiation enteropathy | 4 | 2 | 2 |
| Malabsorption | 1 | 2 | 2 |
| Sterol or Stanol | ClinOleic | Intralipid | SMOFLipid |
|---|---|---|---|
| Cholesterol | 5.37 ± 0.67 | 27.65 ± 1.14 * | 42.00 ± 1.88 ‡,¶ |
| Cholestanol | 0.06 ± 0.02 | 0.22 ± 0.01 * | 0.35 ± 0.02 ‡,¶ |
| Lathosterol | 0.08 ± 0.02 | 0.25 ± 0.01 * | 0.23 ± 0.01 ¶ |
| Campesterol | 1.88 ± 0.22 | 7.05 ± 0.33 * | 2.89 ± 0.10 ‡,¶ |
| Sitosterol | 18.31 ± 2.16 | 24.08 ± 0.76 * | 12.46 ± 0.25 ‡,¶ |
| Campestanol | 0.06 ± 0.01 | 0.16 ± 0.02 * | 0.07 ± 0.01 ‡ |
| Stigmasterol | 1.12 ± 0.15 | 7.44 ± 0.30 * | 2.67 ± 0.07 ‡,¶ |
| Sitostanol | 0.77 ± 0.09 | 1.49 ± 0.03 * | 0.54 ± 0.04 ‡,¶ |
| Sterol or Stanol | ClinOleic | Intralipid | SMOFLipid |
|---|---|---|---|
| Cholesterol (mmol/L) | 3.40 (2.65, 3.95) | 2.94 (2.59, 3.33) | 2.89 (2.36, 3.88) |
| Cholestanol (μmol/L) | 5.45 (4.63, 6.51) | 6.14 (4.87, 8.80) | 6.44 (5.5, 8.22) |
| Lathosterol (μmol/L) | 10.85 (7.51, 16.28) | 11.64 (3.69, 14.81) | 12.39 (6.59, 19.94) |
| Campesterol (μmol/L) | 4.95 (3.19, 6.80) | 15.17 * (9.99, 17.94) | 7.13 ‡‡ (6.33, 9.68) |
| Sitosterol (μmol/L) | 23.18 (13.5, 48.6) | 34.2 (19.0, 42.2) | 21.8 (15.0, 27.6) |
| Stigmasterol (μmol/L) | 0.52 (0.31, 0.87) | 3.55 * (2.13, 4.40) | 1.58 ‡ (1.09, 1.76) |
| Fatty Acid | ClinOleic | Intralipid | SMOFLipid |
|---|---|---|---|
| Myristic (14:0) | 1.04 (0.84, 1.32) | 1.12 (0.92, 1.42) | 1.13 (1.02, 1.40) |
| Palmitic (16:0) | 25.97 (24.46, 27.35) | 24.66 (23.85, 25.63) | 25.31 (24.38, 28.73) |
| Palmitoleic (16:1n-7) | 4.27 (2.27, 5.11) | 3.79 (2.90, 4.23) | 3.74 (3.03, 4.61) |
| Stearic (18:0) | 7.34 (6.84, 8.08) | 7.96 (6.88, 9.24) | 7.67 (6.91, 8.80) |
| Oleic (18:1n-9) | 31.34 (27.64, 33.38) | 21.78 * (20.8, 23.51) | 25.27 ‡‡ (23.84, 29.7) |
| Vaccenic (18:1n-7) | 2.55 (2.13, 2.80) | 2.15 * (1.96, 2.28) | 2.46 (1.90, 2.67) |
| Linoleic (18:2n-6) | 14.25 (12.01, 19.00) | 22.67 ** (21.21, 26.08) | 16.06 ‡‡‡ (12.99, 19.87) |
| α-Linolenic (18:3n-3) | 0.42 (0.35, 0.50) | 0.91 ** (0.73, 1.10) | 0.60 ‡‡ (0.48, 0.71) |
| Dihomo-γ-linolenic (20:3n-6) | 1.69 (1.37, 2.03) | 1.86 (1.51, 2.16) | 1.51 (1.17, 1.86) |
| Arachidonic (20:4n-6) | 6.86 (6.07, 8.33) | 7.03 * (5.85, 7.68) | 5.77 ‡,¶ (5.13, 6.18) |
| Eicosapentaenoic (20:5n-3) | 0.65 (0.45, 0.75) | 0.95 * (0.69, 1.22) | 2.21 ‡‡,¶¶ (1.62, 2.41) |
| Docosapentaenoic (22:5n-3) | 0.54 (0.45, 0.64) | 0.55 (0.45, 0.64) | 0.88 ‡‡‡,¶¶ (0.69, 1.21) |
| Docosahexaenoic (22:6n-3) | 1.61 (1.20, 2.11) | 1.78 (1.41, 2.42) | 3.52 ‡‡‡,¶¶ (3.04, 4.18) |
| Marker | ClinOleic | Intralipid | SMOFLipid |
|---|---|---|---|
| Total bilirubin (mg/dL) | 0.6 (0.4, 0.8) | 0.6 (0.4, 0.9) | 0.4 (0.3, 0.7) |
| ALT (U/L) | 46 (27, 61) | 36 (29, 59) | 34 (26, 60) |
| AST (U/L) | 28 (21, 43) | 26 (21, 36) | 25 (18, 32) |
| GGT (U/L) | 50 (25, 101) | 80 (35, 150) | 61 (38, 75) |
| Triglycerides (mg/dL) | 178 (114, 236) | 94 * (83, 146) | 111 ‡ (70, 148) |
| Marker | ClinOleic | Intralipid | SMOFLipid |
|---|---|---|---|
| Total bilirubin (mg/dL) | 9.5 | 10.0 | 5.8 |
| ALT (U/L) | 28.6 | 15.0 | 29.4 |
| AST (U/L) | 23.8 | 25.0 | 11.8 |
| GGT (U/L) | 29.0 | 55.0 | 23.5 |
| Triglycerides (mg/dL) | 52.4 | 15.0 * | 11.8 ¶ |
| Marker | ClinOleic | Intralipid | SMOFLipid |
|---|---|---|---|
| CRP (mg/L) | 4.10 (0.60, 5.95) | 6.36 * (5.57, 10.00) | 5.49 ¶ (5.01, 10.09) |
| IL-1β (pg/mL) | 1.00 (0.54, 1.39) | 0.96 (0.63, 1.39) | 0.80 (0.43, 1.51) |
| IL-6 (pg/mL) | 5.07 (1.94, 5.80) | 2.99 (2.36, 4.93) | 3.09 (2.16, 5.12) |
| IL-8 (pg/mL) | 36.4 (10.2, 34.8) | 9.6 ** (4.6, 12.3) | 10.6 (5.3, 26.8) |
| IL-10 (pg/mL) | 1.92 (0.88, 1.86) | 1.90 (1.02, 2.70) | 1.85 (1.32, 2.36) |
| IFN-γ (pg/mL) | 2.59 (0.13, 3.88) | 1.26 (0.66, 6.00) | 1.12 (0.32, 2.23) |
| TNF-α (pg/mL) | 19.6 (16.3, 21.9) | 14.6 (12.5, 20.3) | 16.0 (13.7, 18.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osowska, S.; Kunecki, M.; Sobocki, J.; Tokarczyk, J.; Majewska, K.; Burkacka, M.; Radkowski, M.; Makarewicz-Wujec, M.; Fisk, H.L.; Mashnafi, S.; et al. Potential for Omega-3 Fatty Acids to Protect against the Adverse Effect of Phytosterols: Comparing Laboratory Outcomes in Adult Patients on Home Parenteral Nutrition Including Different Lipid Emulsions. Biology 2022, 11, 1699. https://doi.org/10.3390/biology11121699
Osowska S, Kunecki M, Sobocki J, Tokarczyk J, Majewska K, Burkacka M, Radkowski M, Makarewicz-Wujec M, Fisk HL, Mashnafi S, et al. Potential for Omega-3 Fatty Acids to Protect against the Adverse Effect of Phytosterols: Comparing Laboratory Outcomes in Adult Patients on Home Parenteral Nutrition Including Different Lipid Emulsions. Biology. 2022; 11(12):1699. https://doi.org/10.3390/biology11121699
Chicago/Turabian StyleOsowska, Sylwia, Marek Kunecki, Jacek Sobocki, Joanna Tokarczyk, Krystyna Majewska, Magdalena Burkacka, Marek Radkowski, Magdalena Makarewicz-Wujec, Helena L. Fisk, Sultan Mashnafi, and et al. 2022. "Potential for Omega-3 Fatty Acids to Protect against the Adverse Effect of Phytosterols: Comparing Laboratory Outcomes in Adult Patients on Home Parenteral Nutrition Including Different Lipid Emulsions" Biology 11, no. 12: 1699. https://doi.org/10.3390/biology11121699
APA StyleOsowska, S., Kunecki, M., Sobocki, J., Tokarczyk, J., Majewska, K., Burkacka, M., Radkowski, M., Makarewicz-Wujec, M., Fisk, H. L., Mashnafi, S., Baumgartner, S., Plat, J., & Calder, P. C. (2022). Potential for Omega-3 Fatty Acids to Protect against the Adverse Effect of Phytosterols: Comparing Laboratory Outcomes in Adult Patients on Home Parenteral Nutrition Including Different Lipid Emulsions. Biology, 11(12), 1699. https://doi.org/10.3390/biology11121699






