Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
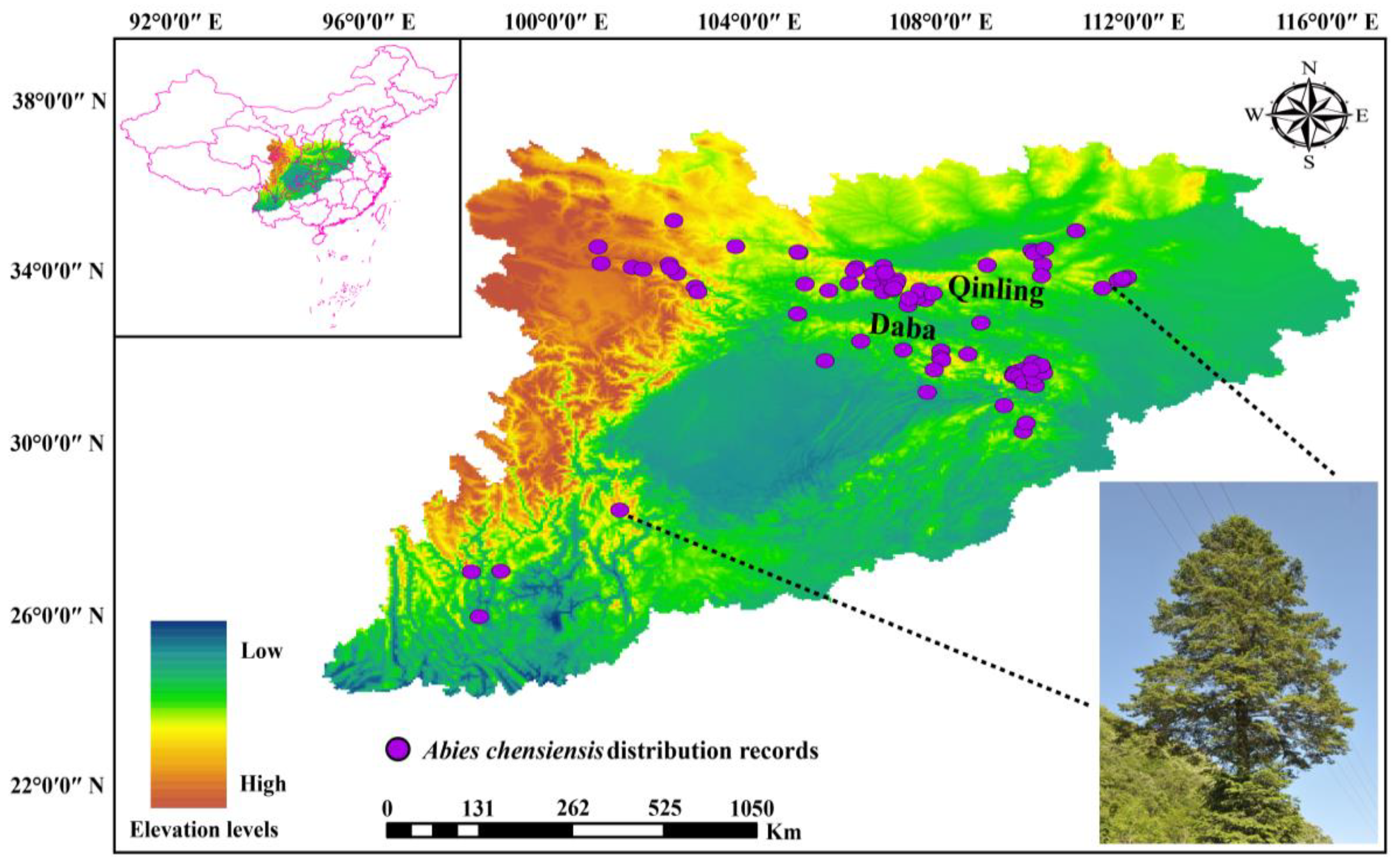
2.1. Species Distribution Data
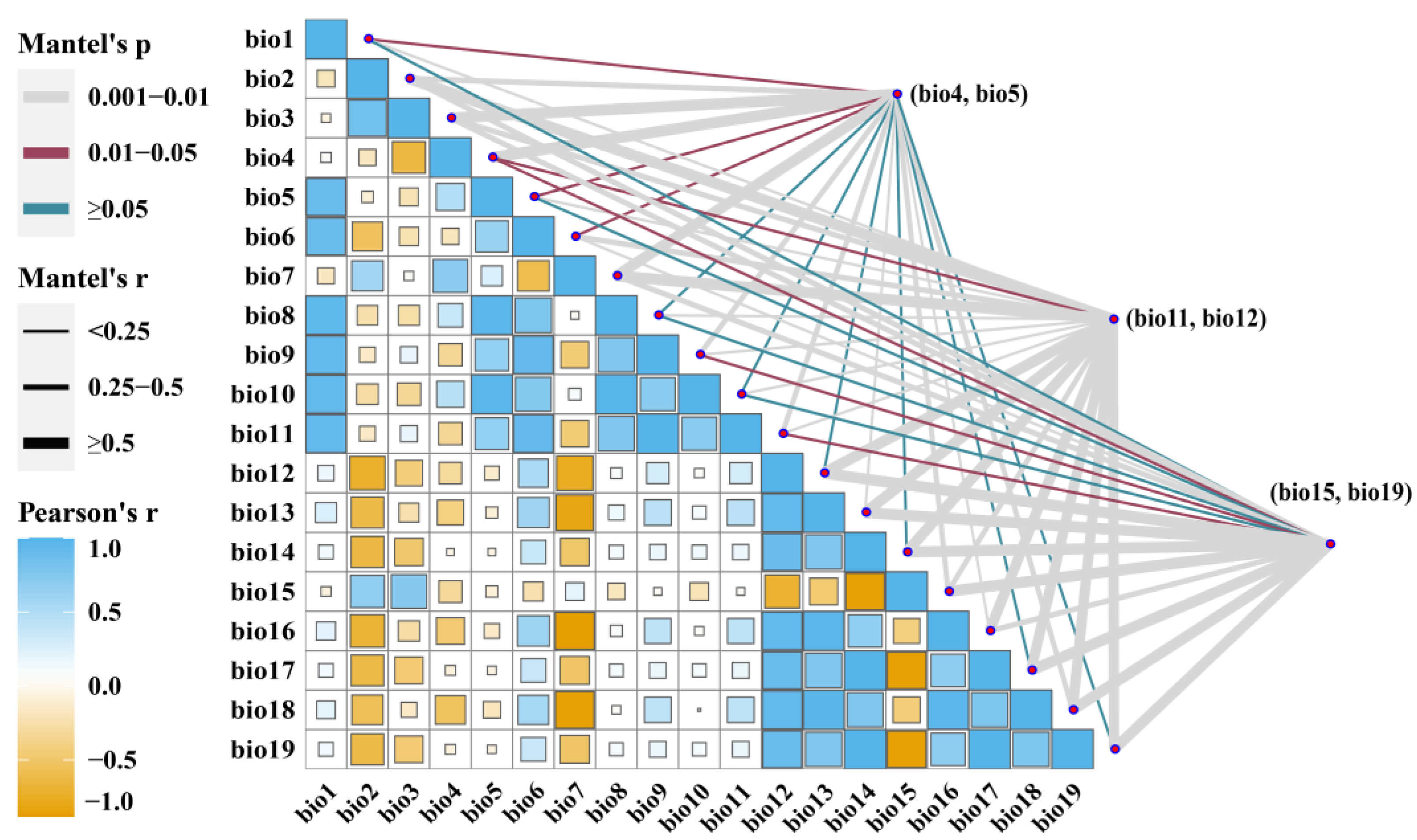
2.2. Bioclimatic Data Acquisition and Screen
2.3. MaxEnt Model Operation and Evaluation
2.4. Classification of Habitat Suitability
2.5. Analysis of Centroid Migration in Suitable Distribution Areas
3. Results
3.1. Accuracy of Model Analysis
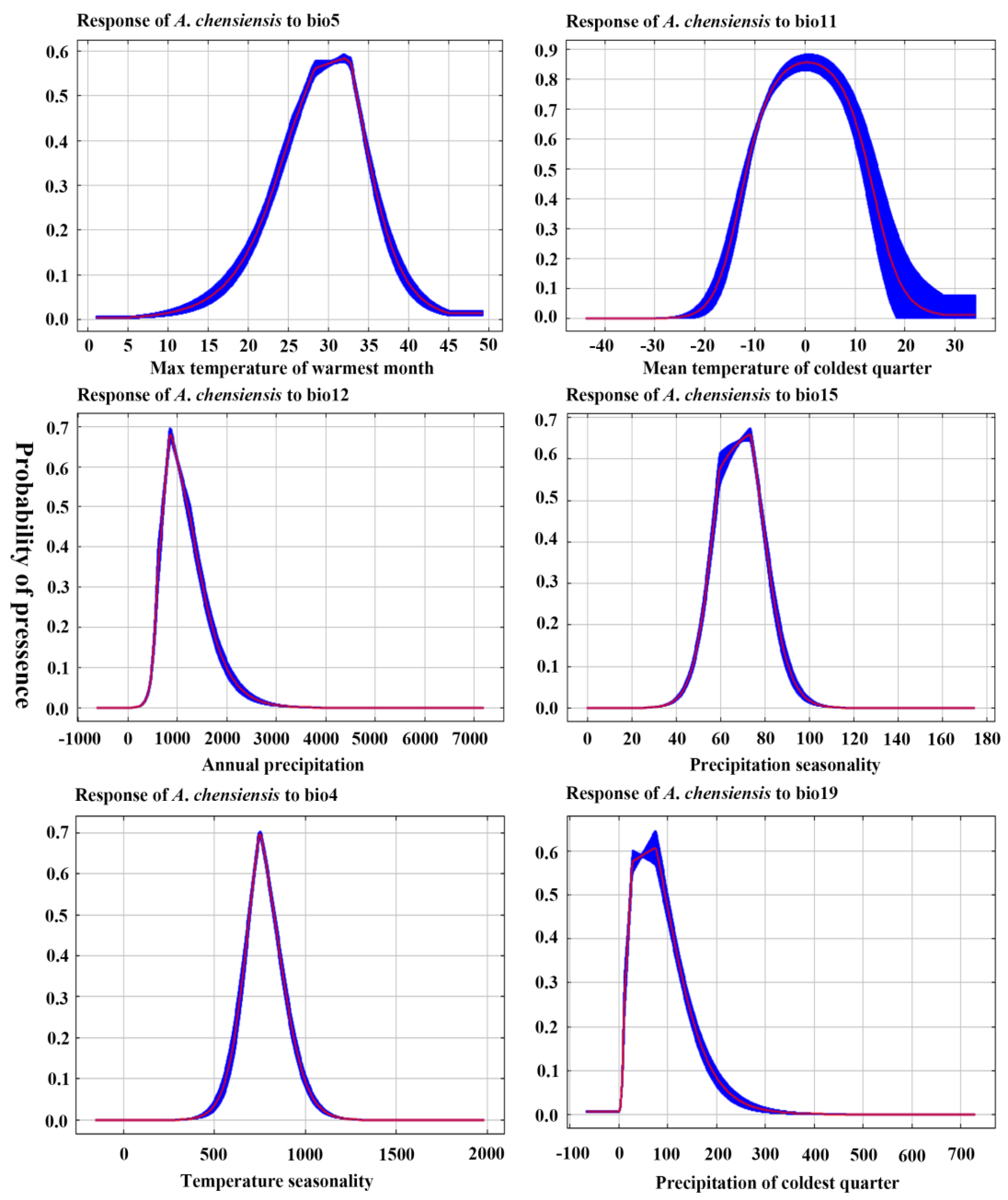
3.2. Dominant Environmental Factors
3.3. Ecological Niche Modeling
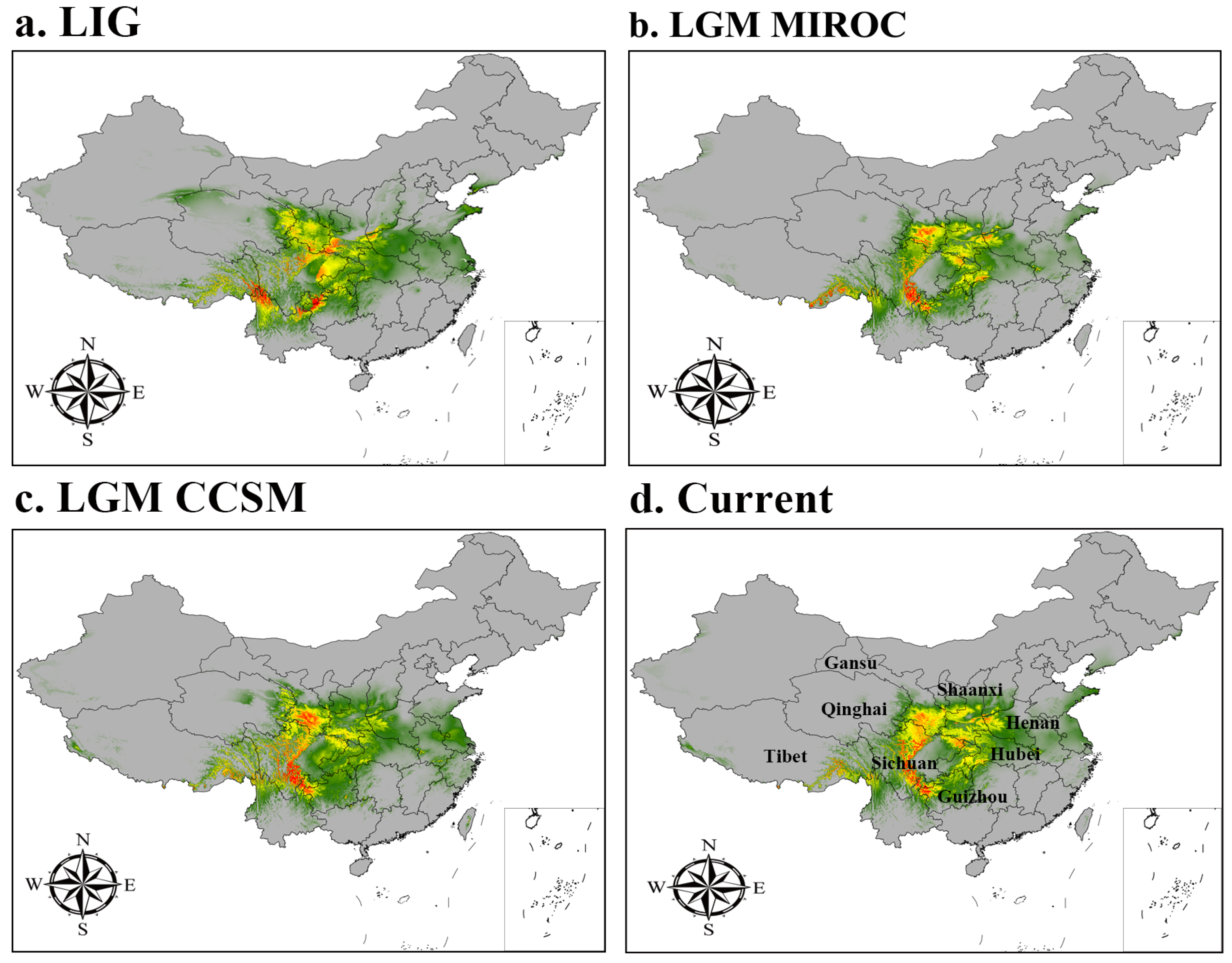
3.3.1. Suitable Areas in the Past
3.3.2. Current Potential Distribution Estimates
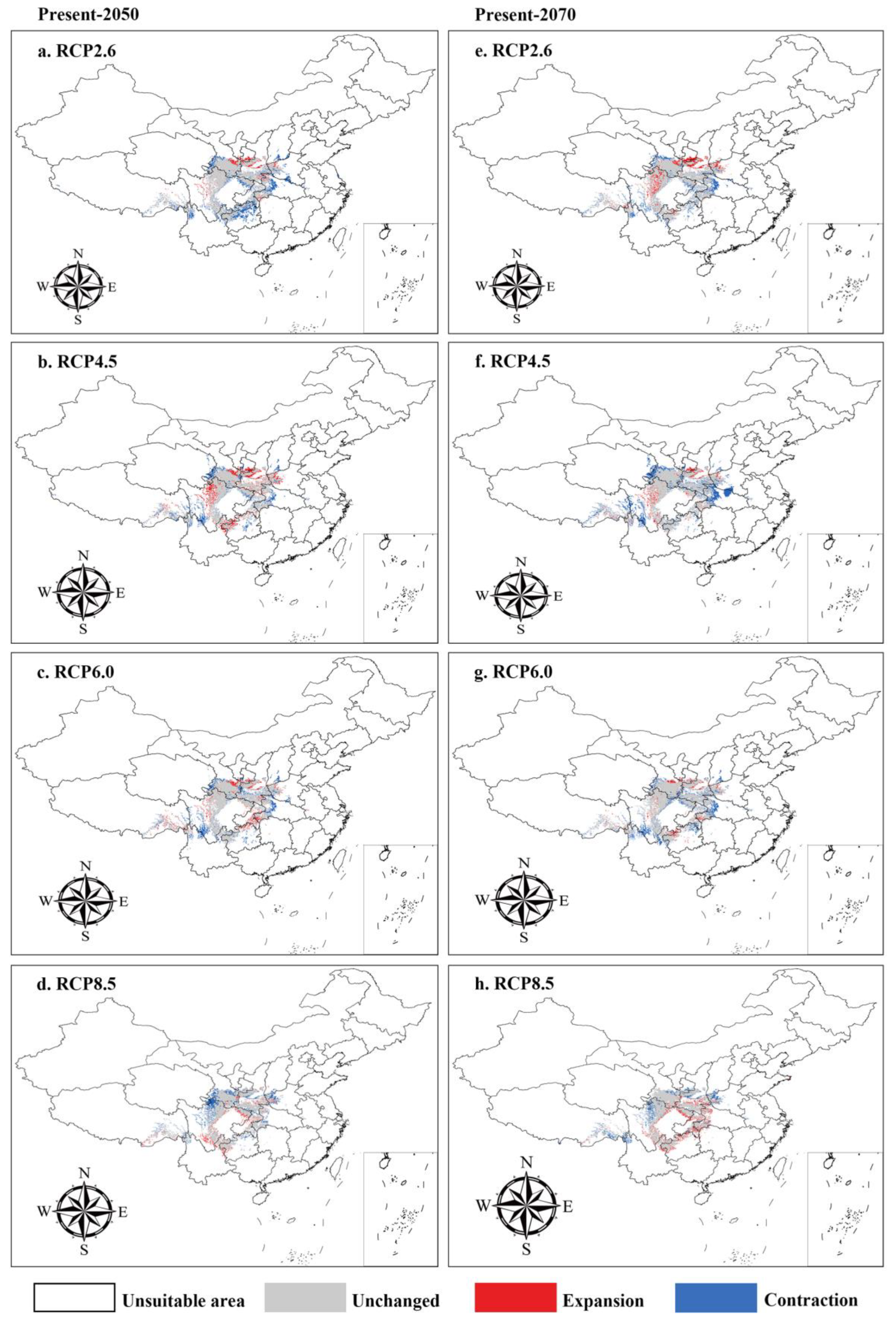
3.4. Suitable Distribution under Future Climate Scenarios
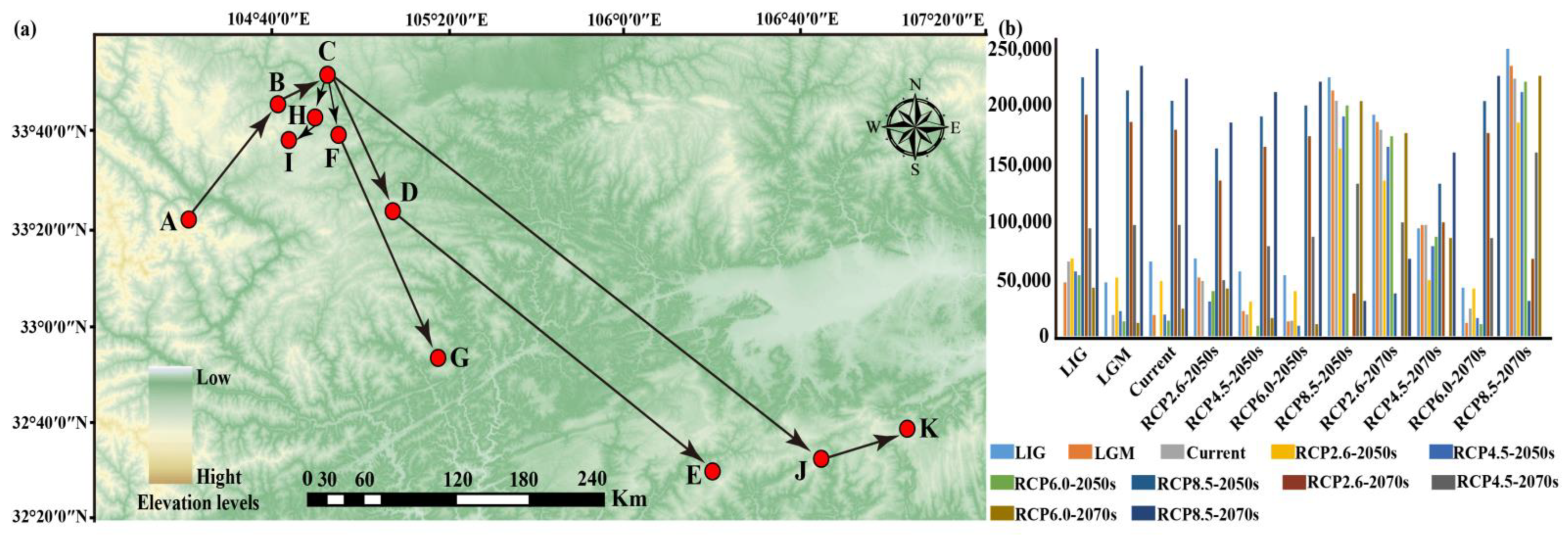
3.5. The Migration Trends of the Geometric Center of Suitable Habitat
4. Discussion
4.1. Response of Species Distribution to Climate Change
4.2. Climate Change Impacts on the Spatial Distribution Patterns of A. chensiensis
4.3. Potential Distribution Area Changes and Conservation of A. chensiensis under Future Climate Scenarios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, J.E.; Brown, C.L.; Conlon, K.; Herring, S.; Kunkel, K.E.; Lawrimore, J.; Luber, G.; Schreck, C.; Smith, A.; Uejio, C. Changes in extreme events and the potential impacts on human health. J. Air Waste Manag. Assoc. 2018, 68, 265–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogorevc, N.; Simčič, M.; Khayatzadeh, N.; Sölkner, J.; Berger, B.; Bojkovski, D.; Horvat, S. Post-genotyping optimization of dataset formation could affect genetic diversity parameters: An example of analyses with alpine goat breeds. BMC Genom. 2021, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Balfagón, D.; Terán, F.; de Oliveira, T.; Santa-Catarina, C.; Gómez-Cadenas, A. Citrus rootstocks modify scion antioxidant system under drought and heat stress combination. Plant Cell Rep. 2022, 41, 593–602. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Qin, X.; Ranjitkar, S.; Lougheed, S.C.; Wang, M.; Zhou, W.; Ouyang, D.; Zhou, Y.; Xu, J.; Zhang, W.; et al. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Sci. Rep. 2018, 8, 5879. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Ueno, K.; Shirako, T.; Nakamura, M.; Minami, M. Identification of Lagopusmuta japonica food plant resources in the Northern Japan Alps using DNA metabarcoding. PLoS ONE 2022, 17, e0252632. [Google Scholar] [CrossRef]
- Pepin, N.C.; Seidel, D.J. A global comparison of surface and free-air temperatures at high elevations. J. Geophys. Res. Atmos. 2005, 110, D03104. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araujo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.L. A Study on the Vegetation Ecology of Subalpine Timberline Ecotone. Ph.D. Thesis, Chinese Academy of Sciences, Beijing, China, 1999. [Google Scholar]
- Jump, A.S.; Peuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Grabherr, G. Recent plant diversity changes on Europe’s mountain summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [Green Version]
- Daskalova, G.N.; Myers-Smith, I.H.; Godlee, J.L. Rare and common vertebrates span a wide spectrum of population trends. Nat. Commun. 2020, 11, 4394. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, F.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Renwick, K.M.; Curtis, C.; Kleinhesselink, A.R.; Schlaepfer, D.; Bradley, B.A.; Aldridge, C.; Poulter, B.; Adler, P.B. Multi-model comparison highlights consistency in predicted effect of warming on a semi-arid shrub. GCB 2017, 24, 424–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, O.K.; Ollier, S.; Bertelsmeier, C. Smaller climatic niche shifts in invasive than non-invasive alien ant species. Nat. Commun. 2020, 11, 5213. [Google Scholar] [CrossRef] [PubMed]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. GEB 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Liu, H.; Jacquemyn, H.; He, X.; Chen, W.; Huang, Y.; Yu, S.; Lu, Y.; Zhang, Y. The Impact of Human Pressure and Climate Change on the Habitat Availability and Protection of Cypripedium (Orchidaceae) in Northeast China. Plants 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Din, J.U.; Ali, H.; Kabir, M.; Younas, M.; Ur Rehman, E.; Bari, F.; Hao, W.; Bischof, R.; Nawaz, M.A. Identifying priority landscapes for conservation of snow leopards in Pakistan. PLoS ONE 2020, 15, e0228832. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Elith, J.H.; Graham, C.P.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2010, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.; Tsiftsis, S.; Chapagain, D.J.; Khadka, C.; Bhattarai, P.; Kayastha Shrestha, N.; Alicja Kolanowska, M.; Kindlmann, P. Suitability of Habitats in Nepal for Dactylorhiza hatagirea Now and under Predicted Future Changes in Climate. Plants 2021, 10, 467. [Google Scholar] [CrossRef]
- Liu, T.S. A Monograph of the Genus Abies; National Taiwan University: Taipei, China, 1971. [Google Scholar]
- Li, Y.-L.; Yang, X.-W.; Li, S.-M.; Shen, Y.-H.; Zeng, H.-W.; Liu, X.-H.; Tang, J.; Zhang, W.-D. Terpenoid constituents of Abies chensiensis with potential anti-inflammatory activity. JNP 2009, 72, 1065–1068. [Google Scholar] [CrossRef]
- Compilation Group of Chinese Herbal Medicine in China. Compilation of Chinese Herbal Drugs; People’s Medical Publishing House: Beijing, China, 1975. [Google Scholar]
- Zhu, X.H.; Liu, G.W.; Ru, G.X.; Du, Y.D.; Chen, C. Community quantitative characteristics and dynamics of endangered plant species Abies chensiensis. Chin. J. Ecol. 2007, 26, 1942–1946. [Google Scholar]
- Li, Q.M.; Xie, Z.Q.; Sun, Y.L. Study on seedling adaptability of Abies chensiensis. Forest 2008, 21, 481–485. [Google Scholar]
- Shao, Y.; Xiang, Q. Species delimitation and phylogeography of the Abies chensiensis complex inferred from morphological and molecular data. Bot. J. Linn. Soc. 2015, 177, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.L.; Li, Q.M.; Yang, J.Y.; Xie, Z.Q. Morphological variation in cones and seeds in Abies chensiensis. Acta Ecol. Sin. 2005, 25, 176–181. [Google Scholar]
- Zhang, W.; Xiaobo, X.U.; Zhou, J.; Xie, Z. Population dynamics of endangered plant species Abies chensiensis. Chin. J. Appl. Ecol. 2005, 16, 1799–1804. [Google Scholar]
- Wang, J.; Abbott, R.J.; Peng, Y.L.; Du, F.K.; Liu, J.-Q. Species delimitation and biogeography of two fir species (Abies) in central china: Cytoplasmic dna variation. Heredity 2011, 107, 362–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Abbott, R.J.; Ingvarsson, P.K.; Liu, J. Increased genetic divergence between two closely related fir species in areas of range overlap. Ecol. Evol. 2014, 4, 1019–1029. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.Q.; Wang, S.F.; Li, Y.; Song, Q.Y.; Gao, K. Terpenoids with anti-inflammatory activity from Abies chensiensis. Fitoterapia 2016, 111, 87–94. [Google Scholar] [CrossRef]
- Gang, G.H.; Cho, G.; Kwak, Y.S.; Park, E.H. Distribution of rhizosphere and endosphere fungi on the first-class endangered plant cypripedium japonicum. Mycobiology 2017, 45, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Choi, J.E.; Lee, B.Y.; Yu, J.N.; Lim, C.E. Genetic diversity and structure of an endangered medicinal herb: Implications for conservation. AoB Plants 2018, 10, ply021. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Hajima, T.; Sudo, K.; Nagashima, T.; Takemura, T.; Okajima, H.; Kawamiya, M. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. 2011, 4, 845–872. [Google Scholar] [CrossRef] [Green Version]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R. Community Climate System Model Version 4. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.X.; Kou, Y.X.; Zhang, L.; Zeng, W.D.; Zhang, Z.Y. Suitable distribution of endangered species Pseudotaxus chienii (Cheng) Cheng (Taxaceae) in five periods using niche modeling. Chin. J. Ecol. 2020, 39, 600–613. [Google Scholar]
- Meng, J.; Li, M.; Guo, J.; Zhao, D.; Tao, J. Predicting suitable environments and potential occurrences for Cinnamomum camphora (Linn.) Presl. Forests 2021, 12, 1126. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent; AT and T Research: Atlanta, GA, USA, 2005; Volume 190, pp. 231–259. [Google Scholar]
- Young, N.; Carter, L.; Evangelista, P. A MaxEnt Model v3. 3.3 e Tutorial (ArcGIS v10); Natural Resource Ecology Laboratory, Colorado State University and the National Institute of Invasive Species Science: Fort Collins, CO, USA, 2011. [Google Scholar]
- Moreno, R.; Zamora, R.; Molina, J.R.; Vasquez, A.; Miguel, M.A. Predictive modeling of microhabitats for endemic birds in south chilean temperate forests using maximum entropy (maxent). Ecol. Inform. 2011, 6, 364–370. [Google Scholar] [CrossRef]
- Fielding, A. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [Green Version]
- Laurent, P.; Mouillot, F.; Yue, C.; Ciais, P.; Moreno, M.V.; Nogueira, J.M.P. FRY, a global database of fire patch functional traits derived from space-borne burned area products. Sci. Data 2018, 5, 180132. [Google Scholar] [CrossRef] [Green Version]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Shi, J. The Potential Global Distribution of Sirex juvencus (Hymenoptera: Siricidae) under Near Current and Future Climatic Conditions as Predicted by the Maximum Entropy Model. Insects 2021, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Matías, L.; Linares, J.C.; Sánchez-Miranda, Á.; Jump, A.S. Contrasting growth forecasts across the geographical range of Scots pine due to altitudinal and latitudinal differences in climatic sensitivity. Glob. Change Biol. 2017, 23, 4106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.L.; He, J.; Xie, L.; Cui, G.F. Prediction of the suitable area of the chinese white Pines (Pinus subsect. Strobus) under climate changes and implications for their conservation. Forests 2020, 11, 996. [Google Scholar]
- Pandey, B.; Khatiwada, J.R.; Zhang, L.; Pan, K.; Dakhil, M.A.; Xiong, Q.; Yadav, R.; Siwakoti, M.; Tariq, A.; Olatunji, O.A.; et al. Energy-water and seasonal variations in climate underlie the spatial distribution patterns of gymnosperm species richness in China. Ecol. Evol. 2020, 10, 9474–9485. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, A.M.; Ng, J.; Aeschbach, W.; Kipfer, R.; Kulongoski, J.T.; Severinghaus, J.P.; Stute, M. Widespread six degrees Celsius cooling on land during the Last Glacial Maximum. Nature 2021, 593, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.P.; Wang, Y.; Xiong, Q.L.; Wu, X.G.; Sun, X.M.; Huang, Y.M.; Zhang, L.; Pan, K.W. Distribution changes and refugia of three spruce taxa since the last interglacial. J. Appl. Ecol. 2018, 29, 2411–2421. [Google Scholar]
- Liu, J.; Möller, M.; Provan, J.; Gao, L.M.; Poudel, R.C.; Li, D.Z. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytol. 2013, 199, 1093–1108. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Liu, L.; Qi, W.; Li, S.; Hu, Z. Combining the least cost path method with population genetic data and species distribution models to identify landscape connectivity during the late Quaternary in Himalayan hemlock. Ecol. Evol. 2015, 5, 5781–5791. [Google Scholar] [CrossRef] [Green Version]
- Olvmo, M.; Holmer, B.; Thorsson, S.; Reese, H.; Lindberg, F. Sub-arctic palsa degradation and the role of climatic drivers in the largest coherent palsa mire complex in Sweden (Vissátvuopmi), 1955–2016. Sci. Rep. 2020, 10, 8937. [Google Scholar] [CrossRef]
- Li, Q.M. Characteristics of Cones and Seeds of Two Firs in the Qinling-Daba Mountains and Endangering Causes for Abies chensiensis. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2008. [Google Scholar]
- Liu, X.; Zhang, Y.; Li, P. Spatial Variation Characteristics of Soil Erodibility in the Yingwugou Watershed of the Middle Dan River. China Int. J. Environ. Res. Public Health 2020, 17, 3568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, X.; Zhou, J. Study on reproduction ecology of endangered species Abies chensiensis. Acta Ecol. Sin. 2006, 26, 2417–2424. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: Retrospect and prospect. J. Biogeogr. 2009, 36, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Dimitrov, N.B.; Meyers, L.A. Uncertainty analysis of species distribution models. PLoS ONE 2019, 14, e0214190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.J.; Shu, Q.; Zhou, S.Z.; Zhao, Z.J.; Zhang, J.M. Review and prospects of Quaternary glaciation research in China. J. Glaciol. Geocryol. 2004, 26, 235–243. [Google Scholar]
- Willis, K.J.; Niklas, K.J. The role of Quaternary environmental change in plant macroevolution: The exception or the rule? Philosophical transactions of the Royal Society of London. Ser. B Biol. Sci. 2004, 359, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Abbott, R.J.; Yu, Q.S.; Lin, K.; Liu, J.Q. Pleistocene climate change and the origin of two desert plant species, Pugionium cornutum and Pugionium dolabratum (Brassicaceae), in northwest China. New Phytol. 2013, 199, 277–287. [Google Scholar] [CrossRef]
- Kusky, T.; Liang, G.; Xiang, S.; Guo, X.; Xu, X. A critical examination of evidence for a quaternary glaciation in mt. laoshan, eastern China. China J. Asian Earth Sci. 2011, 40, 403–416. [Google Scholar] [CrossRef]
- Qiu, Y.X.; Fu, C.X.; Comes, H.P. Plant molecular phylogeography in china and adjacent regions: Tracing the genetic imprints of quaternary climate and environmental change in the world’s most diverse temperate flora. Mol. Phylogenet. Evol. 2011, 59, 225–244. [Google Scholar] [CrossRef]
- Zeng, M. Study on the distribution and potential suitable area of rare and endangered plant Cercidiphyllum japonicum. Master’s Thesis, China West Normal University, Nanchong, China, 2021. [Google Scholar]
- Zhao, H.X. Response of Potential Geographical Distribution of Eight China’s First-Class Rare and Endangered Plants to Climate Change and Analysis of GAP. Master’s Thesis, Northwest Normal University, Lanzhou, China, 2021. [Google Scholar]
- Ford, J.D.; Vanderbilt, W.; Berrang-Ford, L. Authorship in IPCC AR5 and its implications for content: Climate change and Indigenous populations in WGII. Clim. Change 2012, 113, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Nzei, J.M.; Mwanzia, V.M.; Ngarega, B.K.; Musili, P.M.; Wang, Q.F.; Chen, J.M.; Li, Z.Z. Ecological Niche Modeling of Water Lily (Nymphaea L.) Species in Australia under Climate Change to Ascertain Habitat Suitability for Conservation Measures. Plants 2022, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. The response of the Qinling tree line trees’radial growth to climate based on the Vaganov-Shashkin model. Master’s Thesis, Northwestern University, Xi’an, China, 2018. [Google Scholar]
- Dong, Y.P.; Shi, X.H.; Sun, S.S.; Sun, J.P.; Hui, B.; He, D.F.; Chong, F.B.; Yang, Z. Co-evolution of the Cenozoic tectonics, geomorphology, environment and ecosystem in the Qinling Mountains and adjacent areas, Central China. Geosystems Geoenvironment 2022, 1, 100032. [Google Scholar] [CrossRef]
- Rana, H.K.; Luo, D.; Rana, S.K.; Sun, H. Geological and climatic factors affect the population genetic connectivity in Mirabilis himalaica (Nyctaginaceae): Insight from phylogeography and dispersal corridors in the Himalaya-Hengduan Biodiversity Hotspot. Front. Plant Sci. 2020, 10, 1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.K.; Luo, D.; Rana, H.K.; O’Neill, A.R.; Sun, H. Geoclimatic factors influence the population genetic connectivity of Incarvillea arguta (Bignoniaceae) in the Himalaya-Hengduan Mountains biodiversity hotspot. J. Syst. Evol. 2021, 59, 151–168. [Google Scholar] [CrossRef]
| Type | Variable | Code Unit | Code Unit |
|---|---|---|---|
| Temperature seasonality (standard deviation) | Bio4 | °C | |
| Max temperature of warmest month | Bio5 | °C | |
| Climate | Mean temperature of coldest quarter | Bio11 | °C |
| Annual precipitation | Bio12 | mm | |
| Precipitation seasonality (coefficient of variation) | Bio15 | mm | |
| Precipitation of the coldest quarter | Bio19 | mm |
| Period | Area of Each Suitable Region (×104 km2) | |||
|---|---|---|---|---|
| Marginally Suitable Region | Moderately Suitable Region | Highly Suitable Region | Total Suitable Region | |
| LIG | 109.77 | 65.13 | 28.74 | 203.64 |
| LGM (CCSM) | 102.26 | 74.82 | 30.59 | 207.67 |
| LGM (MIROC) | 87.11 | 49.04 | 21.41 | 157.55 |
| Current | 96.04 | 48.48 | 35.38 | 179.91 |
| 2050RCP2.6 | 89.23 | 54.73 | 20.40 | 164.35 |
| 2050RCP4.5 | 109.55 | 56.82 | 19.06 | 185.43 |
| 2050RCP6.0 | 78.55 | 46.56 | 16.95 | 142.05 |
| 2050RCP8.5 | 94.42 | 45.59 | 23.03 | 163.04 |
| 2070RCP2.6 | 95.33 | 50.18 | 19.20 | 164.70 |
| 2070RCP4.5 | 89.30 | 49.51 | 9.63 | 144.44 |
| 2070RCP6.0 | 99.34 | 49.17 | 22.96 | 171.47 |
| 2070RCP8.5 | 95.14 | 46.82 | 31.20 | 173.16 |
| Species | Period | Area of Each Suitable Region (×104 Km2) | |||
|---|---|---|---|---|---|
| Unsuitable Region | Unchanged Region | Expansion Region | Contraction Region | ||
| Abies chensiensis | Current vs. RCP2.6-2050s | 889.29 | 42.34 | 3.25 | 14.00 |
| Current vs. RCP4.5-2050s | 894.40 | 37.14 | 8.45 | 8.90 | |
| Current vs. RCP6.0-2050s | 893.47 | 40.52 | 5.07 | 9.82 | |
| Current vs. RCP8.5-2050s | 897.51 | 36.61 | 3.81 | 8.86 | |
| Current vs. RCP2.6-2070s | 894.11 | 39.40 | 6.18 | 9.19 | |
| Current vs. RCP4.5-2070s | 887.94 | 41.85 | 3.74 | 15.36 | |
| Current vs. RCP6.0-2070s | 892.25 | 42.52 | 3.66 | 11.04 | |
| Current vs. RCP8.5-2070s | 891.08 | 39.23 | 7.24 | 9.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, P.-B.; Wang, L.-Y.; Wang, L.-J.; Jia, Y.; Li, Z.-H.; Bai, G.; Zhao, R.-M.; Liang, W.; Wang, H.-Y.; Guo, F.-X.; et al. Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia. Biology 2022, 11, 1659. https://doi.org/10.3390/biology11111659
Dong P-B, Wang L-Y, Wang L-J, Jia Y, Li Z-H, Bai G, Zhao R-M, Liang W, Wang H-Y, Guo F-X, et al. Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia. Biology. 2022; 11(11):1659. https://doi.org/10.3390/biology11111659
Chicago/Turabian StyleDong, Peng-Bin, Li-Yang Wang, Ling-Juan Wang, Yun Jia, Zhong-Hu Li, Gang Bai, Rui-Ming Zhao, Wei Liang, Hong-Yan Wang, Feng-Xia Guo, and et al. 2022. "Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia" Biology 11, no. 11: 1659. https://doi.org/10.3390/biology11111659
APA StyleDong, P.-B., Wang, L.-Y., Wang, L.-J., Jia, Y., Li, Z.-H., Bai, G., Zhao, R.-M., Liang, W., Wang, H.-Y., Guo, F.-X., & Chen, Y. (2022). Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia. Biology, 11(11), 1659. https://doi.org/10.3390/biology11111659






