Ocruranus–Eohalobia Sclerites from the Cambrian Stage 2 Yanjiahe Formation in South China: Scleritome Reconstruction and Zoological Affinity
Abstract
Simple Summary
Abstract
1. Introduction
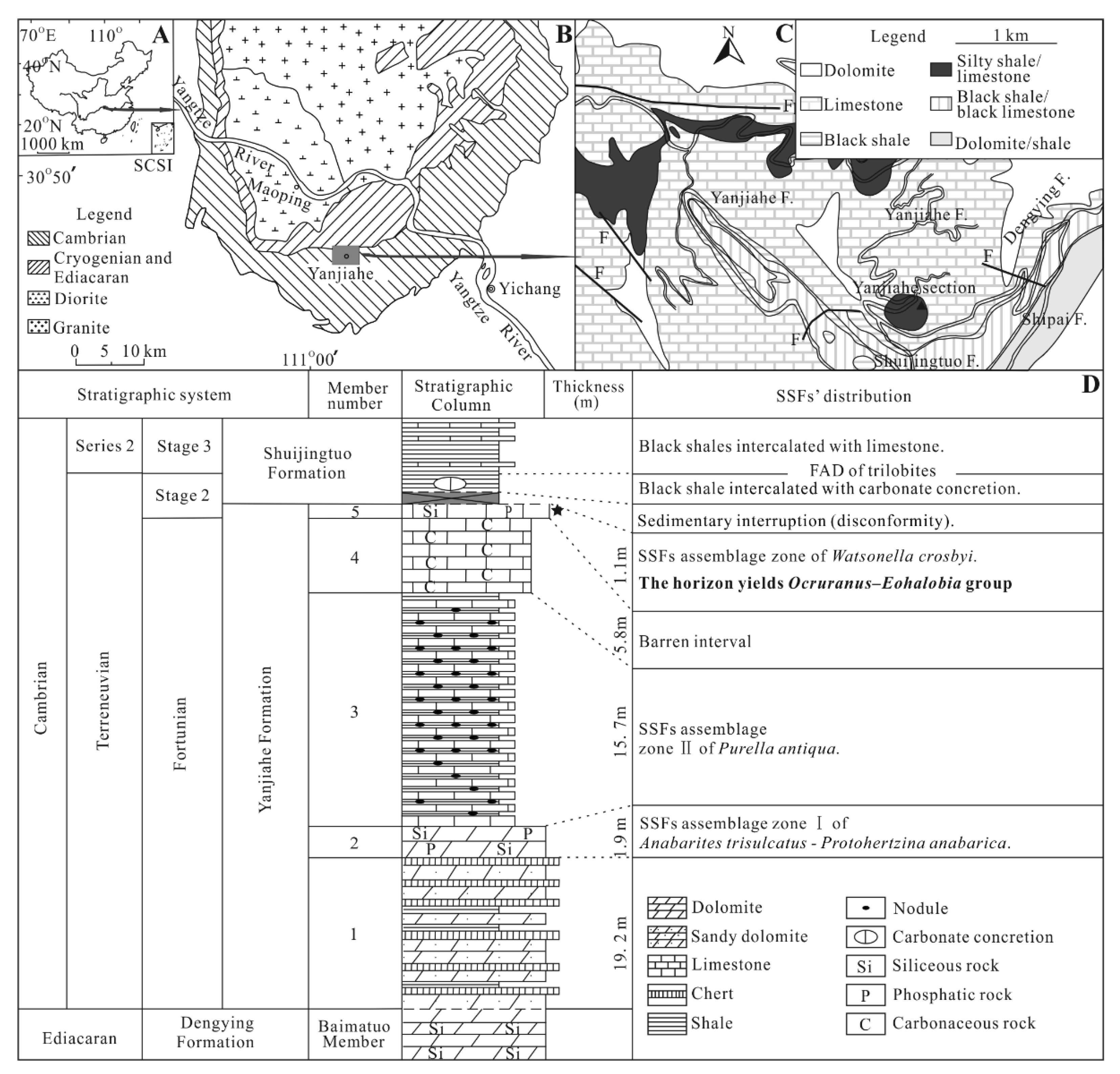
2. Geological Setting, Materials and Methods
3. Results
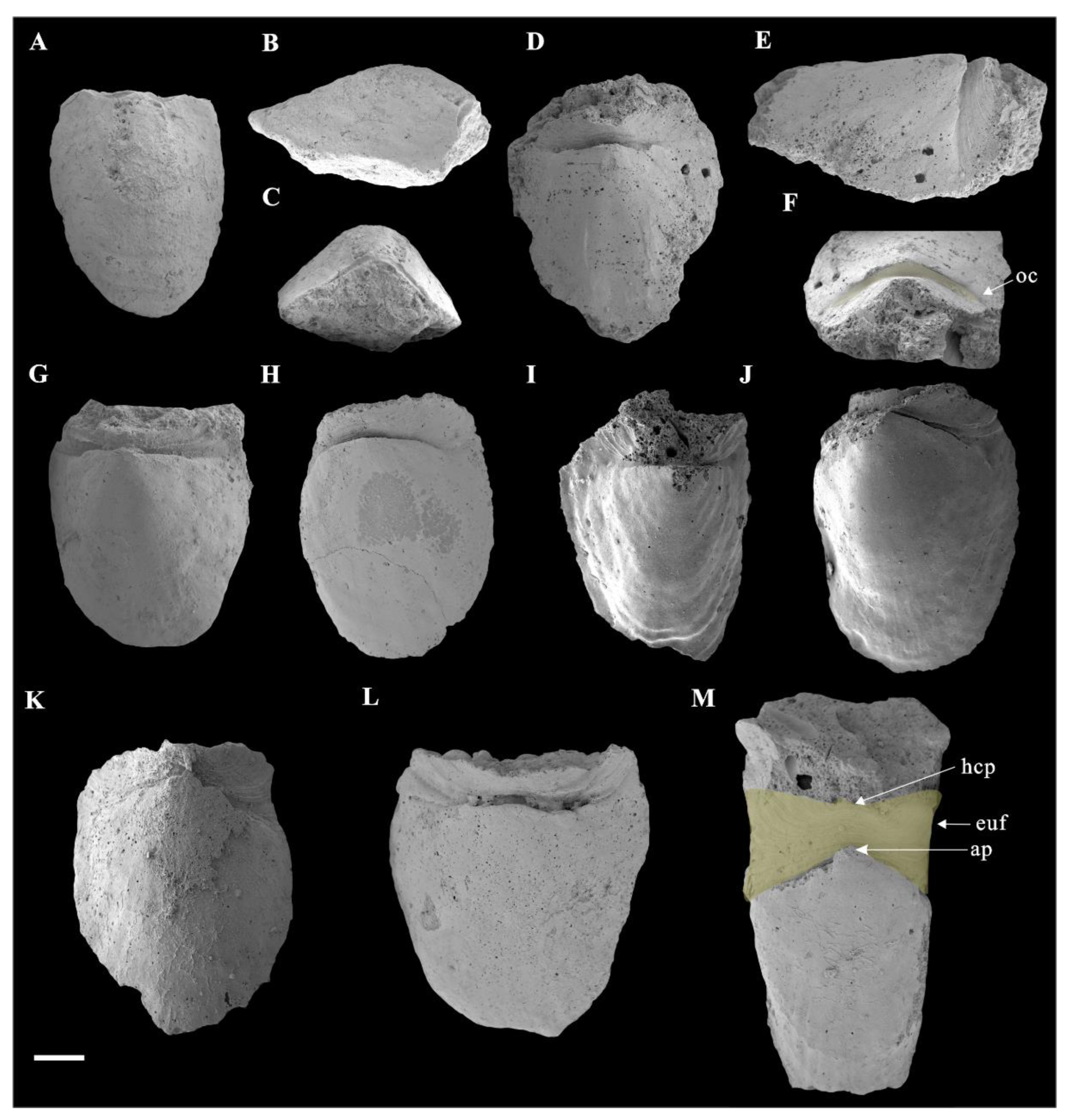
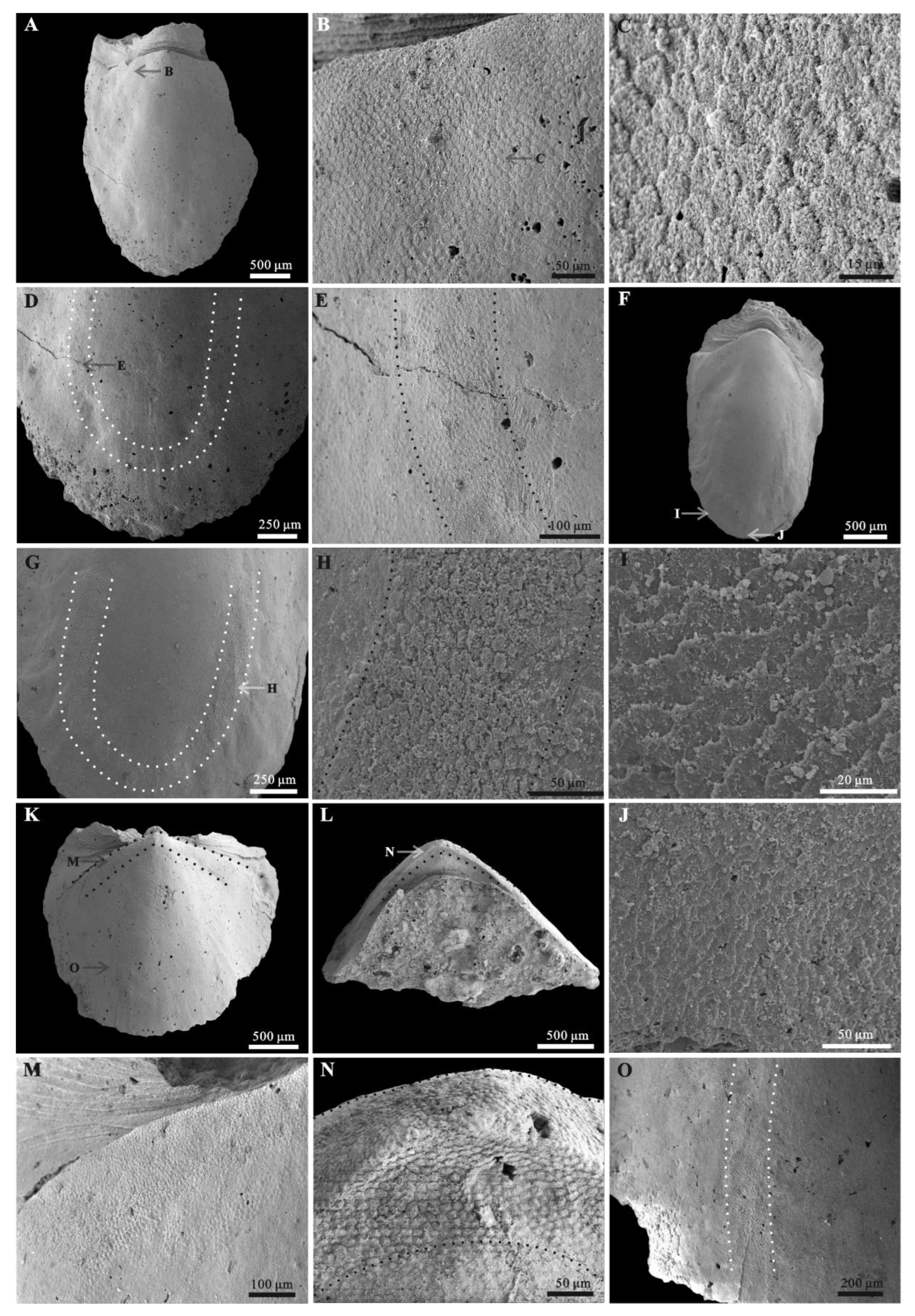
3.1. Morphology of Ocruranus and Eohalobia Sclerites
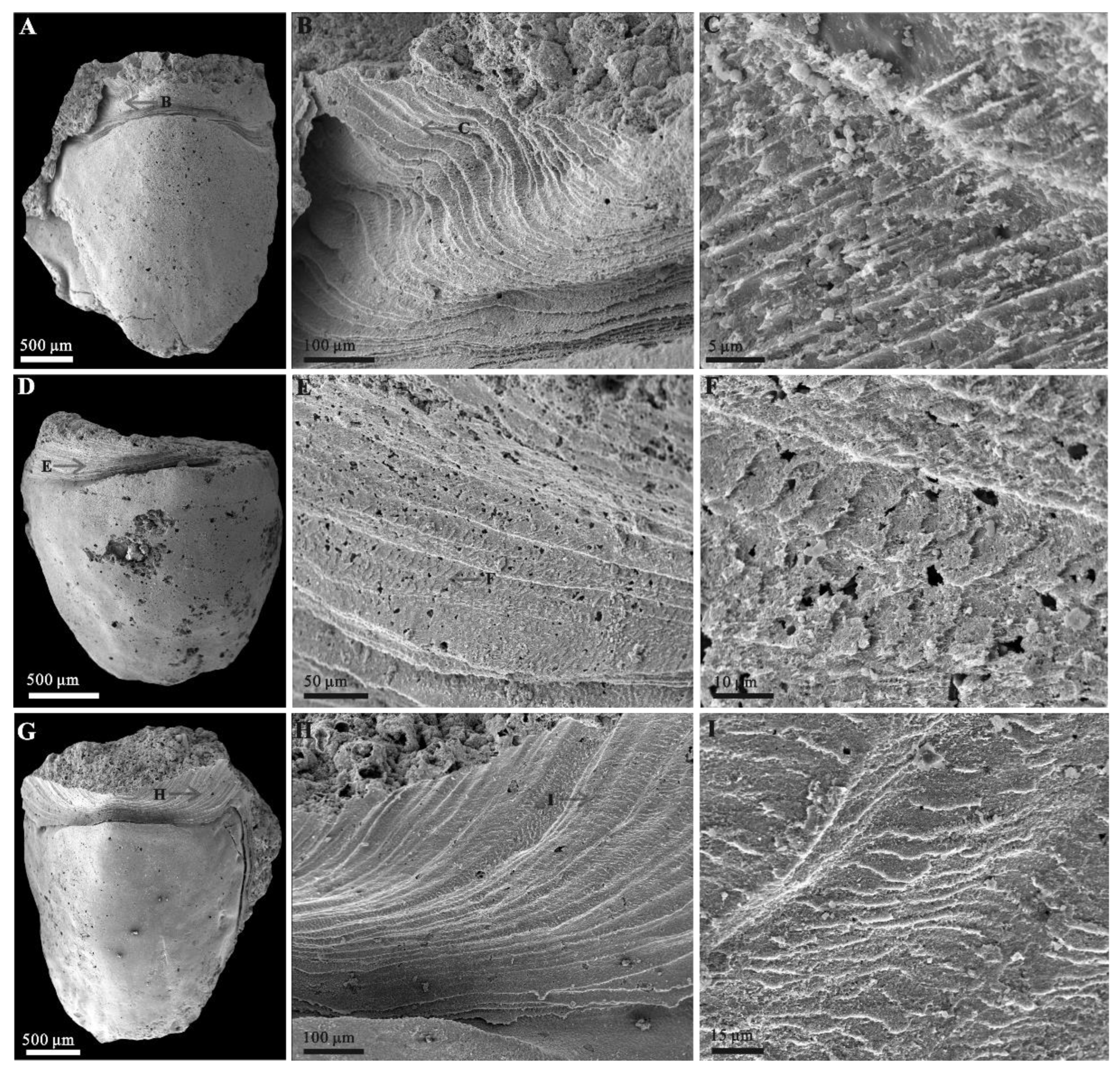
3.2. Microstructures of Eohalobia Sclerites
3.3. Muscle Attachment Zones on Eohalobia Sclerites
4. Discussion
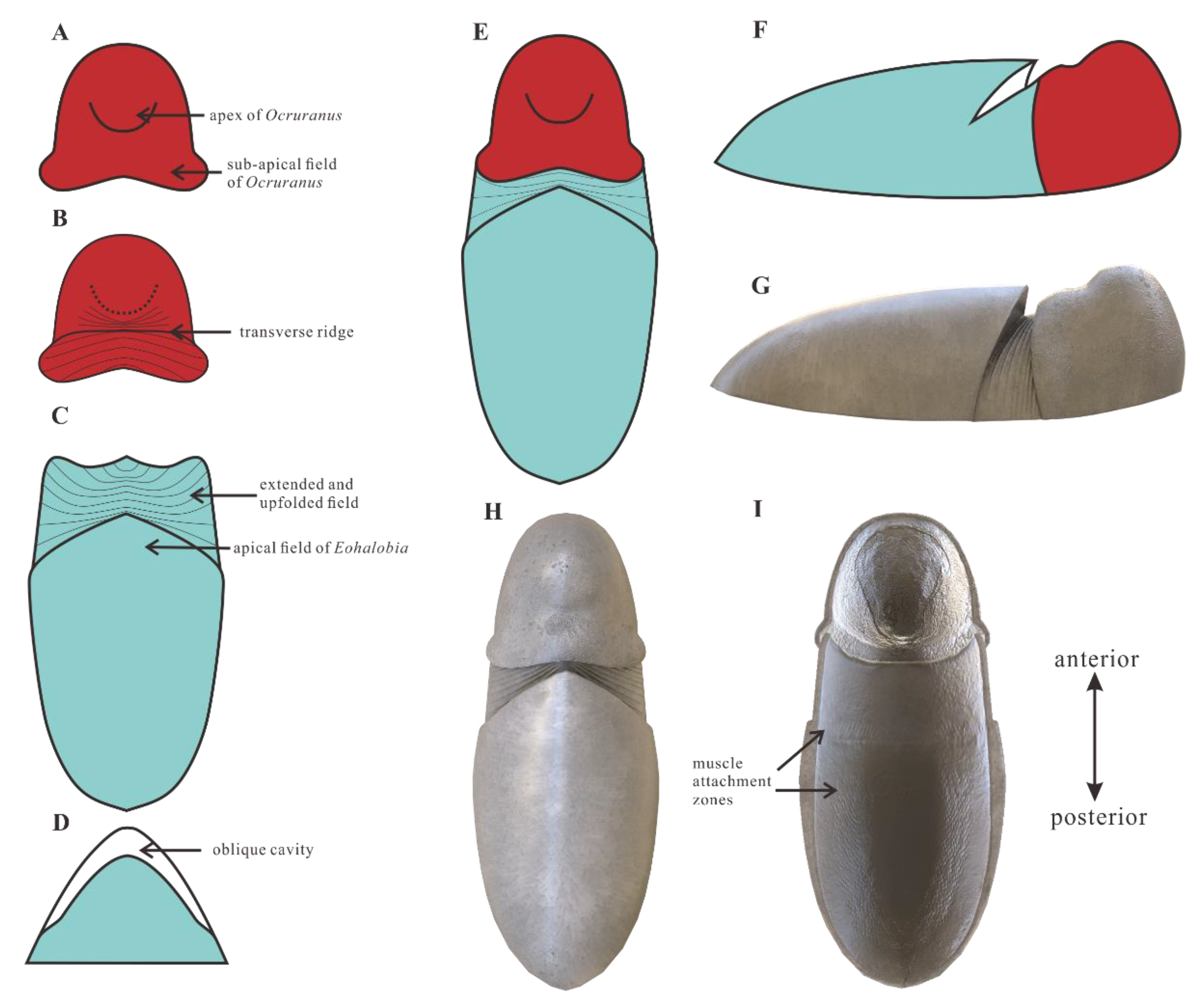
4.1. Ocruranus and Eohalobia Belong to the Same Scleritome
4.2. Zoological Affinity
4.3. Comparison with Early Palaeozoic Polyplacophorans
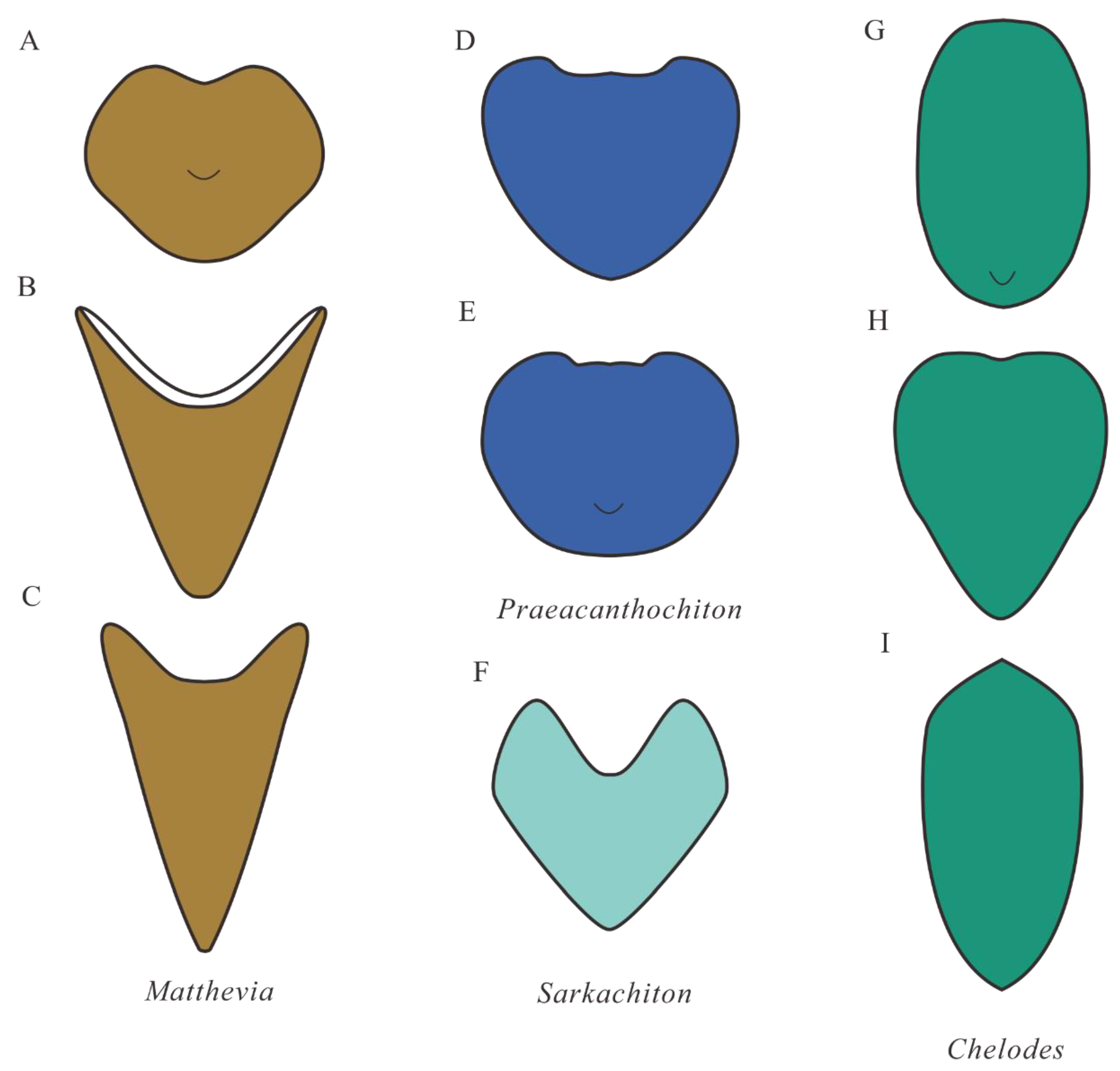
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, Y. Early Cambrian Small Shelly Fossils of China with Special Reference to the Precambrian–Cambrian boundary (2); Nanjing University Publishing House: Nanjing, China, 1989; p. 341. [Google Scholar]
- Bengtson, S.; Conway Morris, S.; Cooper, B.J.; Jell, P.A.; Runnegar, B.N. Early Cambrian Fossils from South Australia. Mem. Ass. Australas. Palaeontols 1990, 9, 364. [Google Scholar]
- Steiner, M.; Li, G.; Qian, Y.; Zhu, M.; Erdtmann, B.D. Neoproterozoic to early Cambrian small shelly fossil assemblages and a revised biostratigraphic correlation of the Yangtze Platform (China). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 254, 67–99. [Google Scholar] [CrossRef]
- Li, G.; Steiner, M.; Zhu, X.; Yang, A.; Wang, H.; Erdtmann, B.D. Early Cambrian metazoan fossil record of South China: Generic diversity and radiation patterns. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 254, 229–249. [Google Scholar] [CrossRef]
- Devaere, L.; Clausen, S.; Steiner, M.; Alvaro, J.J.; Vachard, D. Chronostratigraphic and palaeogeographic significance of an Early Cambrian microfauna from the Heraultia Limestone, northern Montagne Noire, France. Palaeontolo. Electron. 2013, 16, 16.2.17A. [Google Scholar] [CrossRef]
- Betts, M.J.; Paterson, J.R.; Jago, J.B.; Jacquet, S.M.; Skovsted, C.B.; Topper, T.P.; Brock, G.A. New lower Cambrian shelly fossil assemblage zones in the lower Hawker Group, Arrowie Basin, South Australia. Gondwana Res. 2016, 36, 163–195. [Google Scholar] [CrossRef]
- Kouchinsky, A.; Bengtson, S.; Landing, E.; Steiner, M.; Vendrasco, M.; Ziegler, K. Terreneuvian stratigraphy and faunas from the Anabar Uplift, Siberia. Acta Palaeontol. Pol. 2017, 62, 311–440. [Google Scholar] [CrossRef]
- Shu, D. Cambrian explosion: Birth of tree of animals. Gondwana Res. 2008, 14, 219–240. [Google Scholar] [CrossRef]
- Shu, D.; Isozaki, Y.; Zhang, X.; Han, J.; Maruyama, S. Birth and early evolution of metazoans. Gondwana Res. 2014, 25, 884–895. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, F.; Yin, Z.; Zeng, H.; Li, G. The Cambrian explosion: Advances and perspectives from China. Sci. China Earth Sci. 2019, 49, 1455–1490. (In Chinese) [Google Scholar]
- Guo, J.; Qiang, Y.; Han, J.; Song, Z.; Wang, W.; Zhang, Z. Recent progresses on Small Shelly Fossils from Cambrian (Terreneuvian) Yanjiahe Formation in Three Gorges. Earth Sci. Front. 2020, 27, 104–115, (In Chinese with English abstract). [Google Scholar]
- Qian, Y.; Bengtson, S. Palaeontology and biostratigraphy of the Early Cambrian Meishucunian Stage in Yunnan Province, South China. Foss. Strat. 1989, 24, 156. [Google Scholar]
- Guo, J.; Li, Y.; Li, G. Small shelly fossils from the Early Cambrian Yanjiahe Formation, Yichang, Hubei, China. Gondwana Res. 2014, 25, 999–1007. [Google Scholar] [CrossRef]
- Guo, J.; Han, J.; Van Iten, H.; Wang, X.; Qiang, Y.; Song, Z.; Wang, W.; Zhang, Z.; Li, G. 2020, A fourteen-faced hexangulaconulariid from the early Cambrian (Stage 2) Yanjiahe Formation, South China. J. Paleontol. 2020, 94, 45–55. [Google Scholar] [CrossRef]
- Yang, B.; Steiner, M.; Li, G.; Keupp, H. Terreneuvian small shelly faunas of East Yunnan (South China) and their biostratigraphic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 398, 28–58. [Google Scholar] [CrossRef]
- Yang, B.; Steiner, M.; Zhu, M.; Li, G.; Liu, J.; Liu, P. Transitional Ediacaran–Cambrian small skeletal fossil assemblages from South China and Kazakhstan: Implications for chronostratigraphy and metazoan evolution. Precambrian Res. 2016, 285, 202–215. [Google Scholar] [CrossRef]
- Jiang, Z. Monoplacophorans and gastropods fauna of the Meishucun Stage from the Meishucun section, Yunnan. Acta Geol. Sin. 1980, 54, 112–123, (In Chinese with English abstract). [Google Scholar]
- Luo, H.; Jiang, Z.; Wu, X.; Song, X.; Ouyang, L. The Sinian–Cambrian boundary in eastern Yunnan, China; The Yunnan People’s Publishing House: Kunming, China, 1982; p. 265, (In Chinese with English abstract). [Google Scholar]
- Liu, D. Earliest Cambrian brachiopods from southwest China. Acta Palaeontol. Sin. 1979, 18, 505–512, (In Chinese with English abstract). [Google Scholar]
- Liu, D. Brachiopods and tommotiids near Precambrian–Cambrian boundary in SW China. In Stratigraphy and Paleontology of systemic boundaries in China: Precambrian–Cambrian Boundary; Zhang, W., Ed.; Nanjing Institute of Geology and Paleontology, Academica Sinica (Compiled), Nanjing University Publishing House: Nanjing, China, 1987; pp. 345–400. [Google Scholar]
- Yu, W. Yangtze micromolluscan fauna in Yangtze region of China with notes on Precambrian–Cambrian boundary. In Stratigraphy and Paleontology of systemic boundaries in China: Precambrian–Cambrian Boundary; Zhang, W., Ed.; Nanjing Institute of Geology and Paleontology, Academica Sinica (Compiled), Nanjing University Publishing House: Nanjing, China, 1987; pp. 19–344. [Google Scholar]
- Steiner, M.; Yang, B.; Hohl, S.; Zhang, L.; Chang, S. Cambrian small skeletal fossil and carbon isotope records of the southern Huangling Anticline, Hubei (China) and implications for chemostratigraphy of the Yangtze Platform. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 554, 109817. [Google Scholar] [CrossRef]
- Kerber, M. Mikrofossilien aus unterkambrischen gesteinen der Montagne Noire, Frankreich. Palaeontogr. Abt. A 1988, 202, 127–203. [Google Scholar]
- Peel, J.S.; Skovsted, C.B. Problematic cap-shaped fossils from the Lower Cambrian of North-East Greenland. Paläontol. Z. 2005, 79, 461–470. [Google Scholar] [CrossRef]
- Esakova, N.V.; Zhegallo, E.A. Biostratigraphy and fauna of Lower Cambrian of Mongolia. Trudy Sovmestnoj Rossijsko-Mongol’skoj Paleontologičeskoj Ekspeditsii 1996, 46, 216. (In Russsian) [Google Scholar]
- Vendrasco, M.J.; Li, G.; Porter, S.M.; Fernandez, C.Z. New data on the enigmatic Ocruranus–Eohalobia group of Early Cambrian small skeletal fossils. Palaeontology 2009, 52, 1373–1396. [Google Scholar] [CrossRef]
- Skovsted, C.B.; Brock, G.A.; Topper, T.P. First occurrence of a new Ocruranus-like helcionelloid mollusc from the lower Cambrian of East Gondwana. Gondwana Res. 2012, 22, 256–261. [Google Scholar] [CrossRef]
- Conway Morris, S.; Caron, J.B. Halwaxiids and the early evolution of the lophotrochozoans. Science 2007, 315, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, S. The cap-shaped Cambrian fossil Maikhanella and the relationship between coeloscleritophorans and molluscs. Lethaia 1992, 25, 401–420. [Google Scholar] [CrossRef]
- Siegmund, H. The Ocruranus–Eohalobia group of small shelly fossils from the Lower Cambrian of Yunnan. Lethaia 1997, 30, 285–291. [Google Scholar] [CrossRef]
- Vinther, J. The origins of molluscs. Palaeontology 2015, 58, 19–34. [Google Scholar] [CrossRef]
- Chen, P. Discovery of lower Cambrian small shelly fossils from Jijiapo, Yichang, West Hubei and its significance. Prof. Pap. Stratigr. Palaeontol. 1984, 13, 49–64, (In Chinese with English abstract). [Google Scholar]
- Guo, J.; Li, G.; Qiang, Y.; Song, Z.; Zhang, Z.; Han, J.; Wang, W. Watsonella crosbyi from the lower Cambrian (Terreneuvian, Stage 2) Yanjiahe Formation in Three Gorges Area, South China. Palaeoworld 2021, 31, 1–19. [Google Scholar] [CrossRef]
- Runnegar, B. Shell microstructures of Cambrian molluscs replicated by phosphate. Alcheringa 1985, 9, 245–257. [Google Scholar] [CrossRef]
- Kouchinsky, A. Shell microstructures of the Early Cambrian Anabarella and Watsonella as new evidence on the origin of the Rostroconchia. Lethaia 1999, 32, 173–180. [Google Scholar] [CrossRef]
- Kouchinsky, A. Shell microstructures in Early Cambrian molluscs. Acta Palaeontol. Pol. 2000, 45, 119–150. [Google Scholar]
- Feng, W.; Sun, W. Phosphate replicated and replaced microstructure of molluscan shells from the earliest Cambrian of China. Acta Palaeontol. Pol. 2003, 48, 21–30. [Google Scholar]
- Ushatinskaya, G.; Parkhaev, P.Y. Preservation of imprints and casts of cells of the outer mantle epithelium in the shells of Cambrian brachiopods, mollusks, and problematics. Paleontol. J. 2005, 39, 251–263. [Google Scholar]
- Vendrasco, M.J.; Porter, S.M.; Kouchinsky, A.; Li, G.; Fernandez, C.Z. Shell microstructures in early Mollusks. The Festivus 2010, 42, 43–54. [Google Scholar]
- Vendrasco, M.J.; Checa, A.G.; Kouchinsky, A. Shell microstructure of the early bivalve Pojetaia and the independent origin of nacre within the mollusca. Palaeontology 2011, 54, 825–850. [Google Scholar] [CrossRef]
- Vendrasco, M.J.; Rodríguez-Navarro, A.B.; Checa, A.G.; Devaere, L.; Porter, S.M. To infer the early evolution of Mollusc shell microstructures. Key Eng. Mater. 2016, 672, 113–133. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Yun, H.; Li, G. Complex hierarchical microstructures of Cambrian mollusk Pelagiella: Insight into early biomineralization and evolution. Sci. Rep. 2017, 7, 1935. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Skovsted, C.B.; Yun, H.; Li, G.; Pan, B. Shell microstructures of the helcionelloid mollusc Anabarella australis from the lower Cambrian (Series 2) Xinji Formation of North China. J. Syst. Palaeontol. 2019, 17, 1479–1489. [Google Scholar] [CrossRef]
- Carter, J.G.; Lawrence, D.R.; Sanders, H. Shell microstructural data for the Bivalvia. Part II. Orders Nuculoida and Solemyoida. In Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends; Carter, J.G., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1990; Volume 1, pp. 303–319. [Google Scholar]
- Vendrasco, M.J.; Porter, S.M.; Kouchinsky, A.; Li, G.; Fernandez, C.Z. New data on molluscs and their shell microstructures from the Middle Cambrian Gowers Formation, Australia. Palaeontology 2010, 53, 97–135. [Google Scholar] [CrossRef]
- Parkhaev, P.Y. Muscle scars of the Cambrian univalved mollusks and their significance for systematics. Paleontol. J. 2002, 36, 453–459. [Google Scholar]
- Parkhaev, P.Y. New data on the morphology of shell muscles in Cambrian helcionelloid mollusks. Paleontol. J. 2004, 38, 254–256. [Google Scholar]
- Parkhaev, P.Y. New data on the morphology of ancient gastropods of the genus Aldanella Vostokova, 1962 (Archaeobranchia, Pelagielliformes). Paleontol. J. 2006, 40, 244–252. [Google Scholar] [CrossRef]
- Allyn, G.S. Amphineura. In Treatise on Invertebrate Paleontology, Part I, Mollusca 1; Moore, R.C., Ed.; Geological Society of America and University of Kansas Press: Lawrence, KS, USA, 1960; pp. 41–76. [Google Scholar]
- Runnegar, B.; Pojeta, J., Jr.; Taylor, M.E.; Collins, D. New species of the Cambrian and Ordovician chitons Matthevia and Chelodes from Wisconsin and Queensland: Evidence for the early history of Polyplacophoran mollusks. J. Paleontol. 1979, 53, 1374–1394. [Google Scholar]
- Cherns, L. Early Palaeozoic diversification of chitons (Polyplacophora, Mollusca) based on new data from the Silurian of Gotland, Sweden. Lethaia 2004, 37, 445–456. [Google Scholar] [CrossRef]
- Wanninger, A.; Haszprunar, G. Chiton myogenesis: Perspectives for the development and evolution of larval and adult muscle systems in molluscs. J. Morphol. 2002, 251, 103–113. [Google Scholar] [CrossRef]
- Brusca, R.C.; Brusca, G.L. Invertebrates, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2003; p. 936. [Google Scholar]
- Sampson, L.V. The musculature of chiton. J. Morphol. 1985, 11, 595–628. [Google Scholar] [CrossRef][Green Version]
- Vendrasco, M.J.; Runnegar, B. The Cambrian and early Ordovician stem group chitons (Mollusca: Polyplacophora) from Utah and Missouri. J. Paleontol. 2004, 78, 675–689. [Google Scholar] [CrossRef]
- Dzik, J. Machaeridians, chitons, and conchiferan molluscs of Mójcza Limestone. Palaeontol. Pol. 1994, 53, 213–252. [Google Scholar]
- Dzik, J. Turrilepadida and other Machaeridia. In Problematic Fossil Taxa; Hoffman, A., Nitechi, M.N., Eds.; Oxford University Press: New York, NY, USA, 1986; pp. 116–134. [Google Scholar]
- Cherns, L. Chelodes and closely related Polyplacophora (Mollusca) from the Silurian of Gotland, Sweden. Palaeontology 1998, 41, 545–573. [Google Scholar]
- Walcott, C.D. Note on some Paleozoic pteropods. Am. J. Sci. 1885, 30, 17–21. [Google Scholar] [CrossRef]
- Bergenhayn, J.R.M. Cambrian and Ordovician loricates from North America. J. Paleontol. 1960, 34, 168–178. [Google Scholar]
- Davidson, T.; King, W. On the Trimerellidae, a Palaeozoic family of the Palliobranchs or Brachiopoda. Q. J. Geol. Soc. Lond. 1874, 30, 124–173. [Google Scholar] [CrossRef]
- Runnegar, B.; Pojeta, J., Jr. Origin and diversification of the Mollusca. In The Mollusca Evolution; Trueman, E.R., Clarke, M.R., Eds.; Academic Press: Orlando, FL, USA, 1985; Volume 10, pp. 1–57. [Google Scholar]
- Yochelson, E.L. Mattheva, a proposed new class of mollusks. U.S. Geol. Surv. Prof. Pap. 1966, 523–B, B1–B11. [Google Scholar]
- Dzik, J. Evolution of ‘small shelly fossils’ assemblages. Acta Palaeontol. Pol. 1994, 39, 247–313. [Google Scholar]
- Cherns, L. Silurian chitons as indicators of rocky shores and lowstand on Gotland, Sweden. Palaios 1999, 14, 172–179. [Google Scholar] [CrossRef]


| Specimens Number | Length of Extended and Upfolded Field (mm) | Width of Extended and Upfolded Field (=Width of Eohalobia Sclerites) (mm) | Length of Eohalobia Sclerites (mm) |
|---|---|---|---|
| CUBar70–25 | 0.300 | 2.083 | 2.951 |
| CUBar121–7 | 0.319 | 1.605 | - |
| CUBar110–7 | 0.318 | 2.053 | 2.220 |
| CUBar228–5 | 0.430 | 2.046 | 2.339 |
| CUBar212–1 | 0.554 | 1.900 | 2.579 |
| CUBar227–3 | 0.531 | 1.349 | 1.840 |
| CUBar47–28 | 0.536 | 2.422 | 3.075 |
| CUBar132–3 | 0.657 | 1.588 | 2.655 |
| CUBar126–12 | 0.589 | 2.062 | 2.622 |
| CUBar121–11 | 0.650 | 2.697 | 3.643 |
| CUBar40–1 | 0.841 | 2.153 | 2.824 |
| CUBar212–2 | 1.027 | 1.817 | 3.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Guo, J.; Pan, B.; Qiang, Y.; Li, G.; Peng, J.; Sun, J.; Han, J. Ocruranus–Eohalobia Sclerites from the Cambrian Stage 2 Yanjiahe Formation in South China: Scleritome Reconstruction and Zoological Affinity. Biology 2022, 11, 1648. https://doi.org/10.3390/biology11111648
Song Z, Guo J, Pan B, Qiang Y, Li G, Peng J, Sun J, Han J. Ocruranus–Eohalobia Sclerites from the Cambrian Stage 2 Yanjiahe Formation in South China: Scleritome Reconstruction and Zoological Affinity. Biology. 2022; 11(11):1648. https://doi.org/10.3390/biology11111648
Chicago/Turabian StyleSong, Zuchen, Junfeng Guo, Bing Pan, Yaqin Qiang, Guoxiang Li, Jiaxin Peng, Jie Sun, and Jian Han. 2022. "Ocruranus–Eohalobia Sclerites from the Cambrian Stage 2 Yanjiahe Formation in South China: Scleritome Reconstruction and Zoological Affinity" Biology 11, no. 11: 1648. https://doi.org/10.3390/biology11111648
APA StyleSong, Z., Guo, J., Pan, B., Qiang, Y., Li, G., Peng, J., Sun, J., & Han, J. (2022). Ocruranus–Eohalobia Sclerites from the Cambrian Stage 2 Yanjiahe Formation in South China: Scleritome Reconstruction and Zoological Affinity. Biology, 11(11), 1648. https://doi.org/10.3390/biology11111648







