Plant-Soil Mediated Effects of Long-Term Warming on Soil Nematodes of Alpine Meadows on the Qinghai–Tibetan Plateau
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
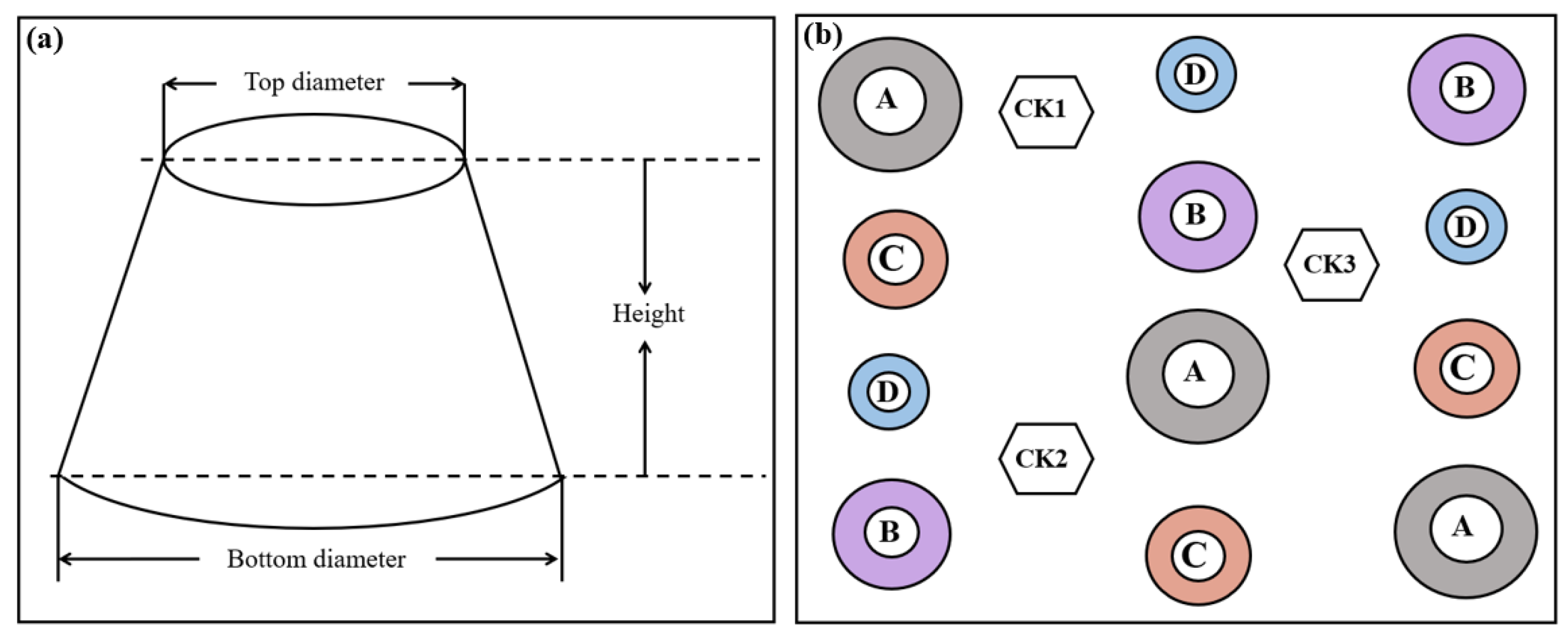
2.2. Experimental Design
2.3. Plant and Soil Sampling
2.4. Determination of Soil Physical and Chemical Indexes
2.5. Soil Nematodes Extraction and Identification
2.6. Ecological Indices of Soil Nematodes
2.7. Statistical Analysis
3. Results
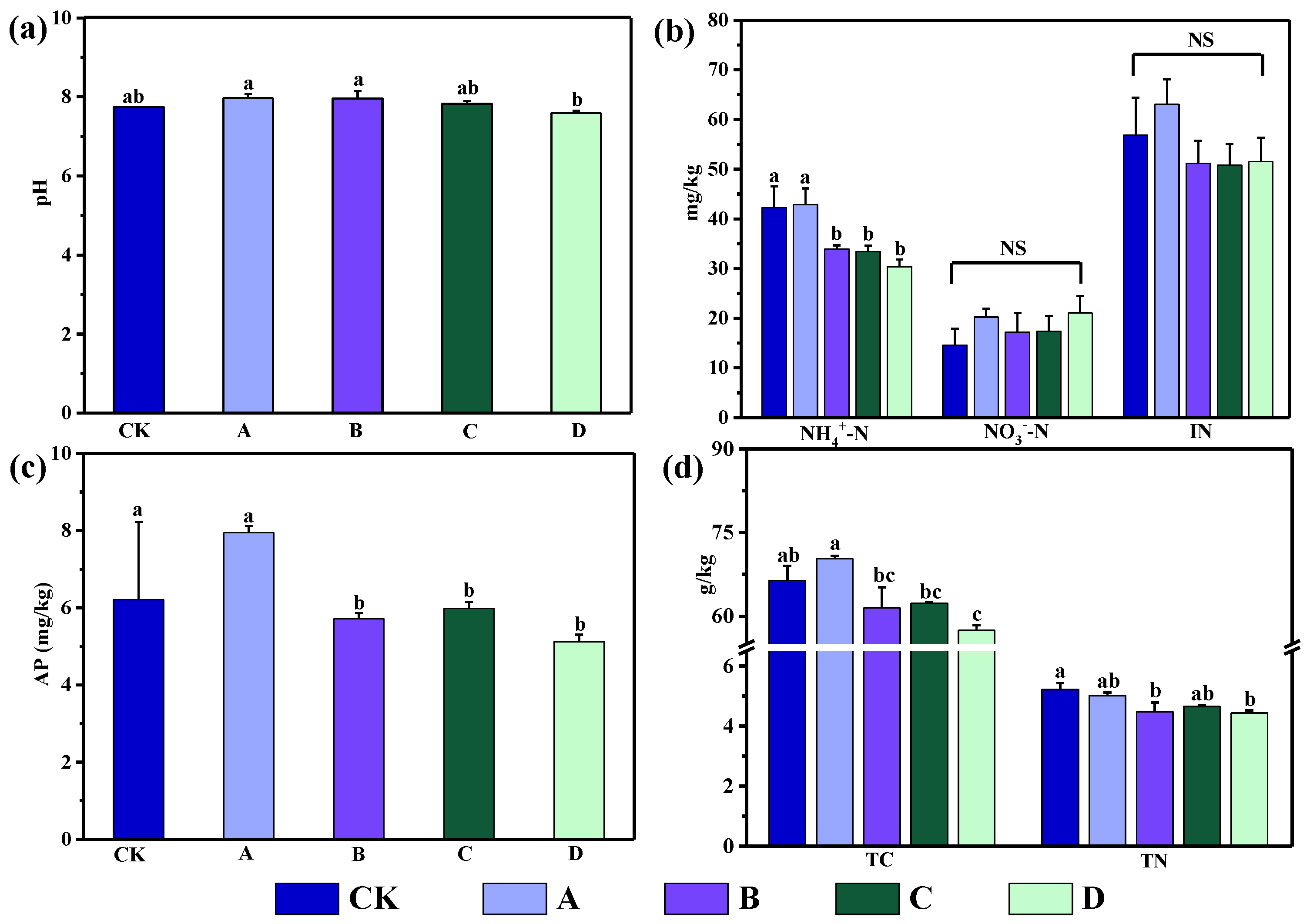
3.1. Soil Physicochemical Properties and Plant Species Diversity
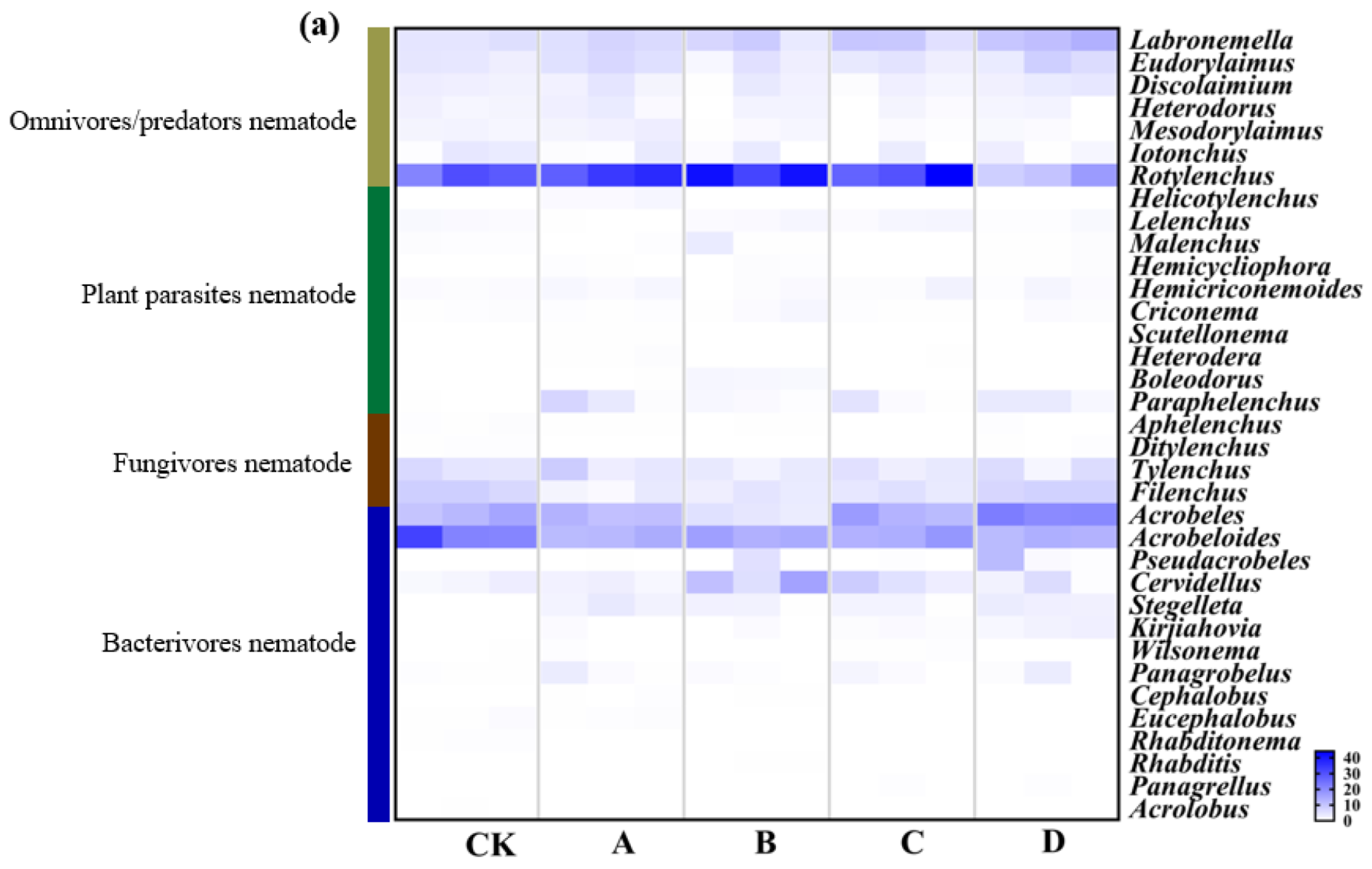
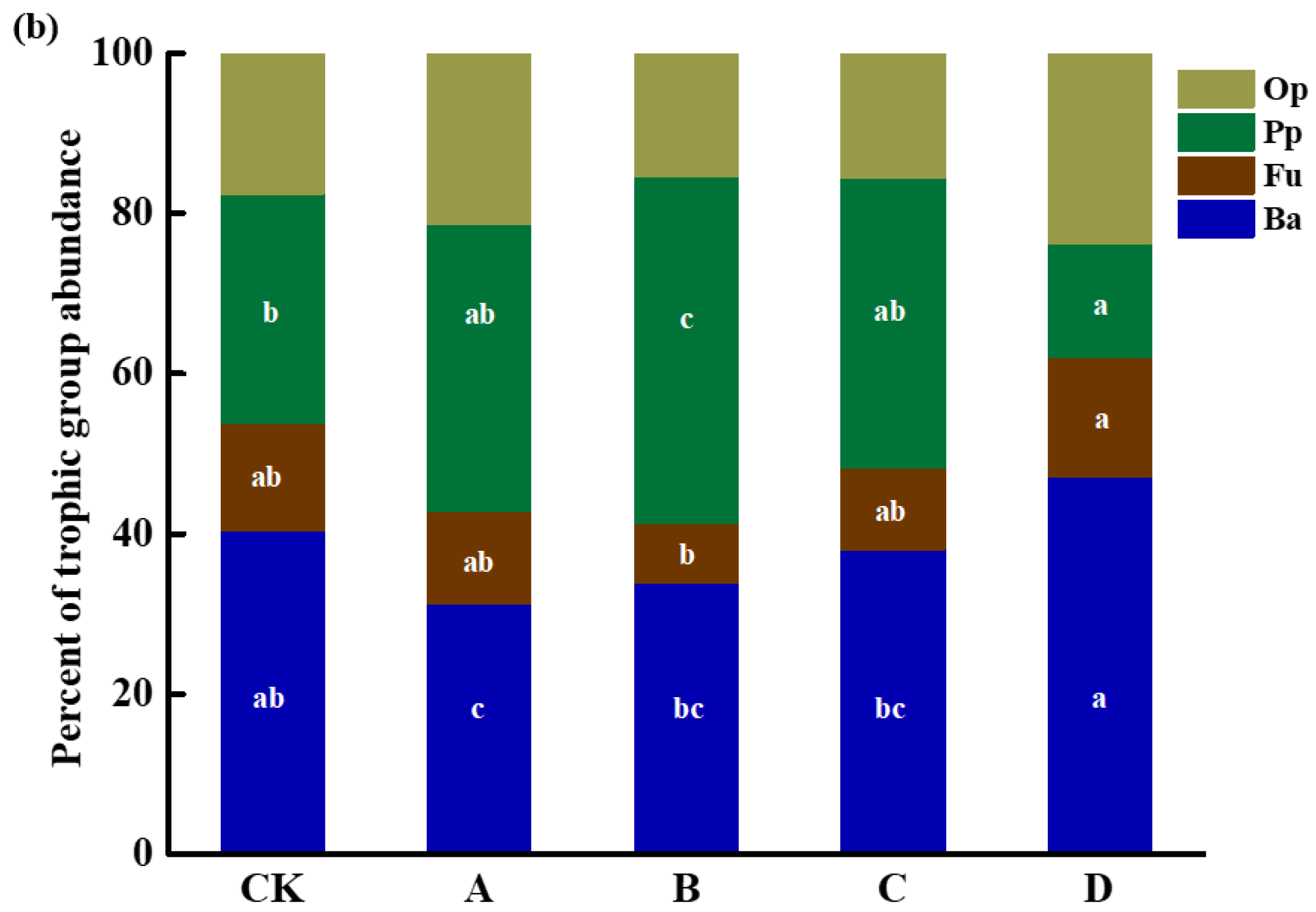
3.2. Soil Nematode Community Composition under Long-Term Gradient Warming
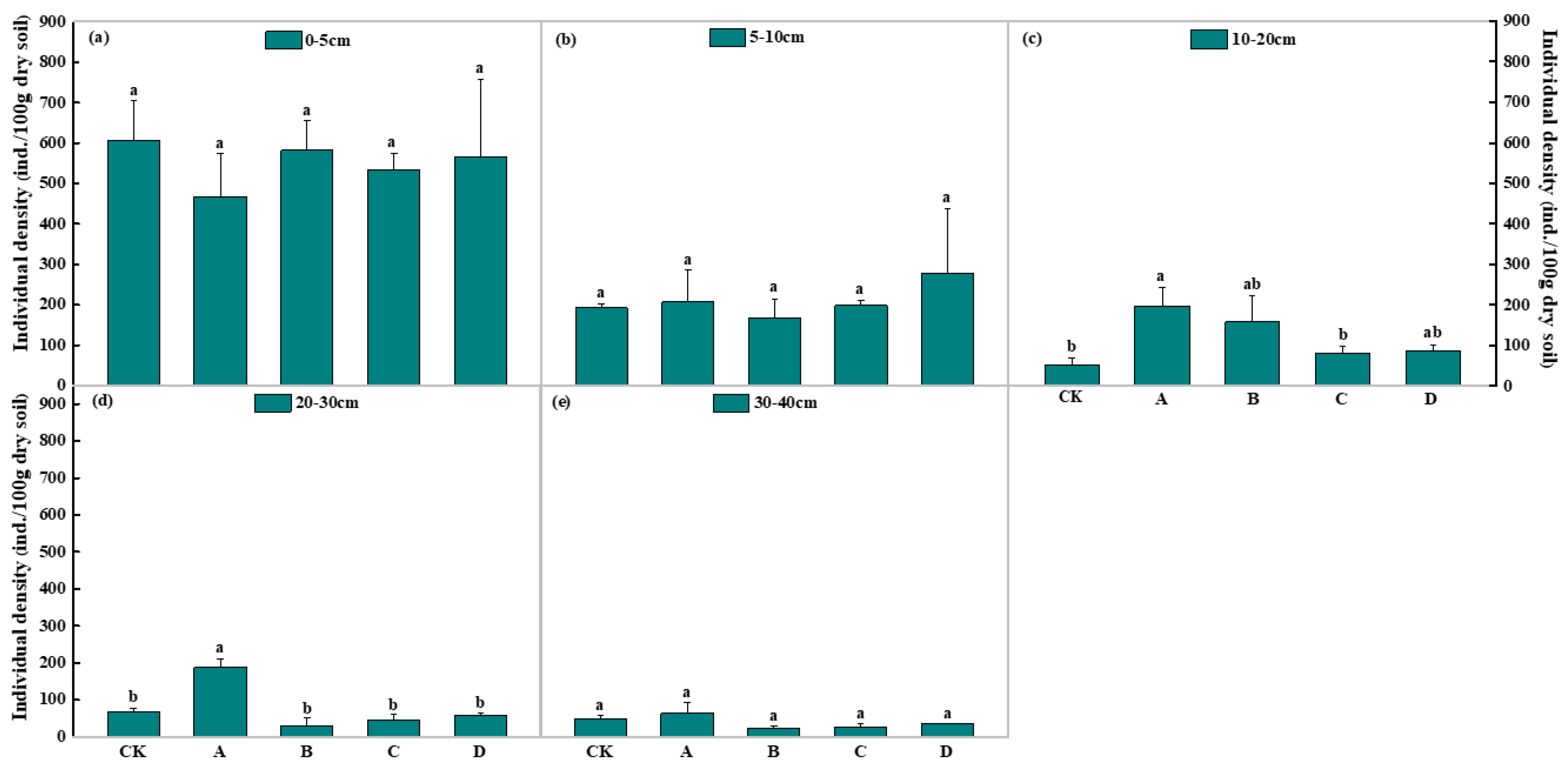
3.3. Soil Nematode Density and Distribution under the Long-Term Gradient Warming
3.4. The Response of Ecological Indices to Long-Term Warming
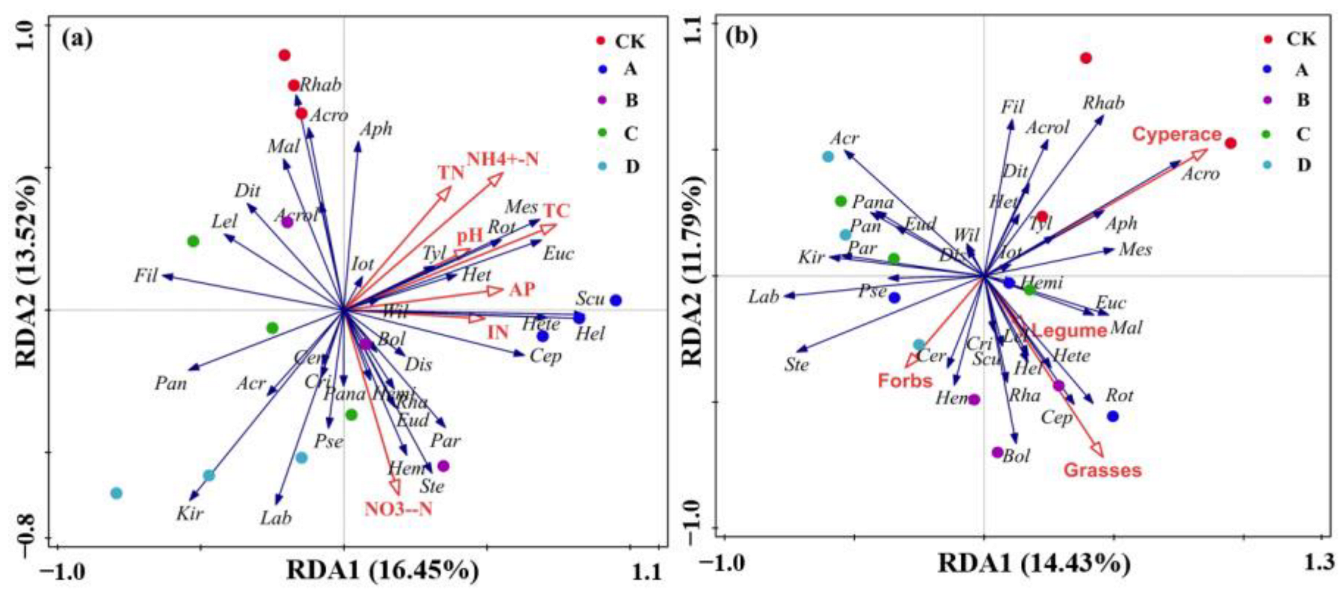
3.5. The Relationship of Soil Properties and Plant Functional Groups with the Soil Nematode Communities
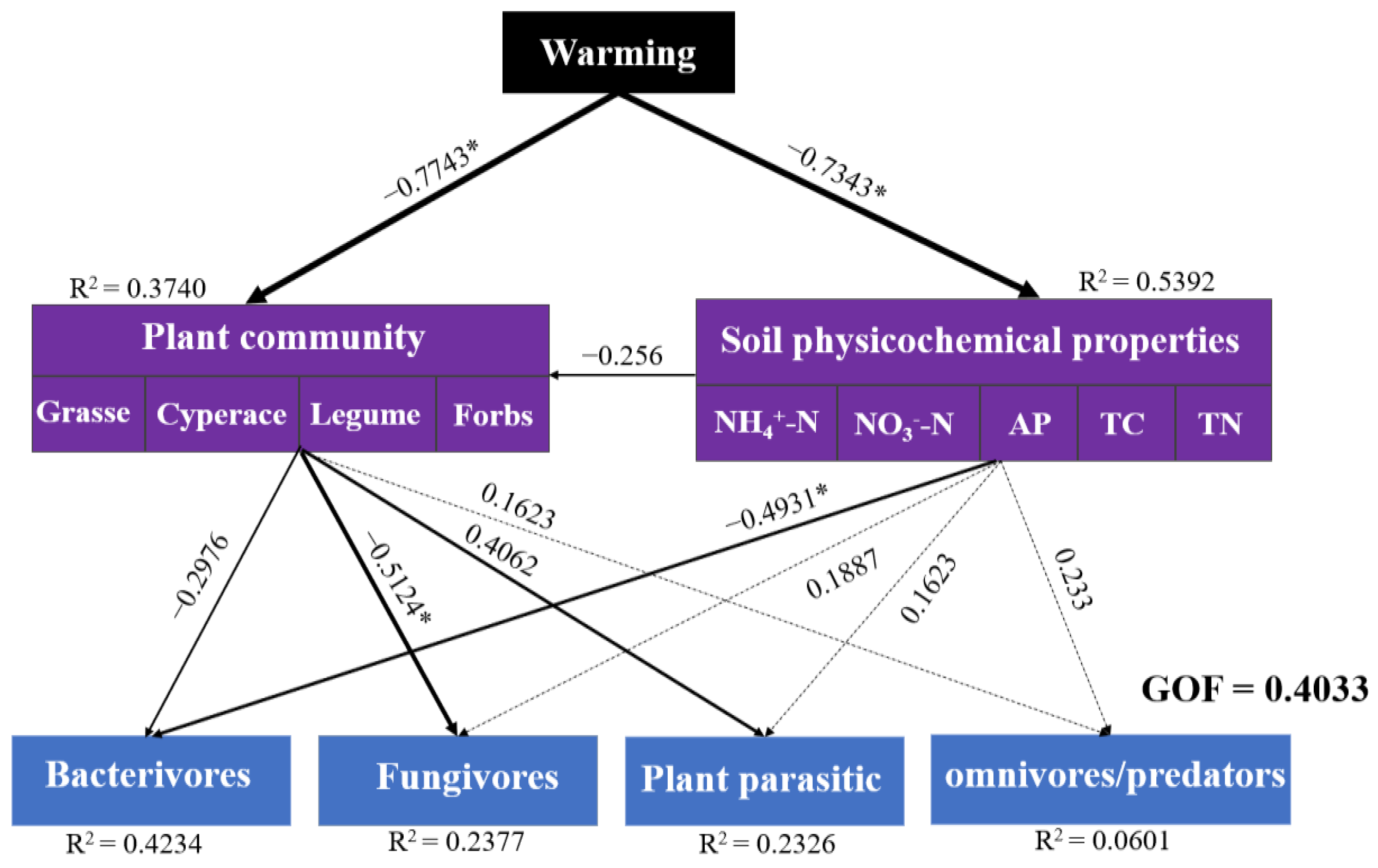
3.6. Relationship between Soil Properties, Plant Diversity and Soil Nematodes Communities
4. Discussion
4.1. The Impact of Long-Term Gradient Warming on Plant Communities
4.2. The Impact of Long-Term Warming on Soil Nematodes Communities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, M.; Ellis, J.E.; Epstein, H.E. Regional analysis of climate, primary production, and livestock density in Inner Mongolia. J. Environ. Qual. 2004, 33, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Chuckran, P.F.; Reibold, R.; Throop, H.L.; Reed, S.C. Multiple mechanisms determine the effect of warming on plant litter decomposition in a dryland. Soil Biol. Biochem. 2020, 145, 107799. [Google Scholar] [CrossRef]
- Ye, R.; Liu, G.F.; Chang, H.; Shan, Y.M.; Mu, L.; Wen, C.; Te, R.; Wu, N.T.; Shi, L.; Liu, Y.H.; et al. Response of plant traits of Stipa breviflora to grazing intensity and fluctuation in annual precipitation in a desert steppe, northern China. Glob. Ecol. Conserv. 2020, 24, e01237. [Google Scholar] [CrossRef]
- Wang, W.Y.; Ma, Y.G.; Xu, J.; Wang, H.C.; Zhu, J.F.; Zhou, H.K. The uptake diversity of soil nitrogen nutrients by main plant species in Kobresia humilis alpine meadow on the Qinghai-Tibet Plateau. Sci. China Earth Sci. 2012, 55, 1688–1695. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.Y.; Wang, Y.F.; Gongbuzeren Zhang, M.H.; Ji, B.M. Ecological consequence of nomad settlement policy in the pasture area of Qinghai-Tibetan Plateau: From plant and soil perspectives. J. Environ. Manag. 2020, 260, 110114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, D.G.; Jiang, Z.H.; Sun, P.; Xiao, H.L.; Wu, Y.L.; Chen, J.G. Changes in the soil microbial communities of alpine steppe at Qinghai-Tibetan Plateau under different degradation levels. Sci. Total Environ. 2018, 651, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.Z.; Jing, S.S. Response of soil nematodes to climate change: A review. Acta Ecol. Sin. 2015, 35, 6857–6867. [Google Scholar]
- Li, C.; Lang, Q.; Zhang, F.; Zhao, D.; Liu, H.; Zhou, J. Study on the integrated system of prediction of the range of volcanic collapse in Changbai Mountain. IOP Conf. Ser. Earth Environ. Sci. 2020, 558, 032017. [Google Scholar] [CrossRef]
- Renčo, M.; Gömöryová, E.; Čerevková, A. The effect of soil type and ecosystems on the soil nematode and microbial communities. Helminthologia 2020, 57, 129–144. [Google Scholar] [CrossRef]
- Xue, H.Y.; Luo, D.Q.; Hu, F.; Li, H.X.; Wang, J.S.; Qu, X.L.; Wang, H.Y.; Yu, B.Z.; Sun, Q. Effect of short-term enclosure on soil nematode communities in an alpine meadow in Northern Tibet. Acta Ecol. Sin. 2016, 36, 6139–6148. [Google Scholar]
- Li, S.; Song, M.; Jing, S. Effects of different carbon inputs on soil nematode abundance and community composition. Appl. Soil Ecol. 2021, 163, 103915. [Google Scholar] [CrossRef]
- Benetková, P.; Háněl, L.; Frouz, J. Nematode Assemblages Development Twenty-One Years after the Introduction of Meadow Soil into Bare Post Mining Spoil Heap. Diversity 2022, 14, 567. [Google Scholar] [CrossRef]
- Wang, M.; De Deyn, G.B.; Bezemer, T.M. Separating effects of soil microorganisms and nematodes on plant community dynamics. Plant Soil 2019, 441, 455–467. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.K.; Liang, W.J.; Li, Q. Recent progress and future directions of soil nematode ecology in China. Biodivers. Sci. 2018, 26, 1060–1073. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.W.; Kou, X.C.; Li, Y.B.; Lü, X.T.; Wang, J.K.; Li, Q. Soil nematode community composition and stability under different nitrogen additions in a semiarid grassland. Glob. Ecol. Conserv. 2020, 22, e00965. [Google Scholar] [CrossRef]
- Guan, P.; Zhang, X.; Yu, J.; Cheng, Y.; Li, Q.; Andriuzzi, W.S.; Liang, W. Soil microbial food web channels associated with biological soil crusts in desertification restoration: The carbon flow from microbes to nematodes. Soil Biol. Biochem. 2018, 116, 82–90. [Google Scholar] [CrossRef]
- Pan, F.J.; Yan, R.R.; Zhao, J.L.; Li, L.H.; Hu, Y.F.; Jiang, Y.; Shen, J.; McLaughlin, N.B.; Zhao, D.; Xin, X.P. Effects of grazing intensity on soil nematode community structure and function in different soil layers in a meadow steppe. Plant Soil 2021, 471, 33–46. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Sánchez-Moreno, S. Biochar addition rate determines contrasting shifts in soil nematode trophic groups in outdoor mesocosms: An appraisal of underlying mechanisms. Appl. Soil. Ecol. 2021, 158, 103788. [Google Scholar] [CrossRef]
- Zhang, A.; Olatunji, O.A.; Tariq, A.; Li, T.; Wang, R.; Jiang, Y. Sulfur deposition changed the community structure of soil nematodes by affecting omnivores-predators. Sci. Total Environ. 2021, 771, 144912. [Google Scholar] [CrossRef]
- Landesman, W.J.; Treonis, A.M.; Dighton, J. Effects of a one-year rainfall manipulation on soil nematode abundances and community composition. Pedobiologia 2011, 54, 87–91. [Google Scholar] [CrossRef]
- Song, M.; Li, X.; Jing, S.; Lei, L.; Wang, J.; Wan, S. Responses of soil nematodes to water and nitrogen additions in an old-field grassland. Appl. Soil Ecol. 2016, 102, 53–60. [Google Scholar] [CrossRef]
- Thakur, M.P.; Reich, P.B.; Fisichelli, N.A.; Stefanski, A.; Cesarz, S.; Dobies, T.; Rich, R.L.; Hobbie, S.E.; Eisenhauer, N. Nematode community shifts in response to experimental warming and canopy conditions are associated with plant community changes in the temperate-boreal forest ecotone. Oecologia 2014, 175, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.G.; Sui, X.; Li, Y.; Jia, M.Q.; Wang, Z.W.; Han, G.D.; Wang, L.C. The response of soil nematode fauna to climate drying and warming in Stipa breviflora desert steppe in Inner Mongolia, China. J. Soil Sediments 2020, 20, 2166–2180. [Google Scholar] [CrossRef]
- Simmons, B.L.; Wall, D.H.; Adams, B.J.; Ayres, E.; Barrett, J.E.; Virginia, R.A. Long-term experimental warming reduces soil nematode populations in the McMurdo Dry Valleys, Antarctica. Soil Biol. Biochem. 2009, 41, 2052–2060. [Google Scholar] [CrossRef]
- Matute, M.M. Soil nematodes of Brassica rapa: Influence of temperature and pH. Adv. Nat. Sci. 2013, 6, 20–26. [Google Scholar] [CrossRef]
- Dong, Z.K.; Hou, R.X.; Chen, Q.Y.; Ouyang, Z.; Ge, F. Response of soil nematodes to elevated temperature in conventional and no-tillage cropland systems. Plant Soil 2013, 373, 907–918. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.C.; Dong, S.K. Adaptive management of alpine grassland ecosystems over Tibetan Plateau. Pratacult. Sci. 2019, 36, 933–938. [Google Scholar]
- Zhao, X.Q.; Zhou, X.M. Ecological basis of Alpine meadow ecosystem management in Tibet: Haibei Alpine Meadow Ecosystem Research Station. Ambio 1999, 28, 642–647. [Google Scholar] [CrossRef]
- Zhang, C.; Willis, C.G.; Klein, J.A.; Ma, Z.; Li, J.; Zhou, H.; Zhao, X. Recovery of plant species diversity during long-term experimental warming of a species-rich alpine meadow community on the Qinghai-Tibet plateau. Biol. Conserv. 2017, 213, 218–224. [Google Scholar] [CrossRef]
- Hooper, D.J.; Hallmann, J.; Subbotin, S.A. Method for extraction, proceeding and detection of plant and soil nematodes. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; CABI Publishing: Egham, UK, 2005; pp. 53–86. [Google Scholar] [CrossRef]
- Xie, H. Taxonomy of Plant Nematodes; Higher Education Press: Beijing, China, 2005. [Google Scholar]
- Yeates, G.W.; Bonges, T.; de Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-Anoutline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [CrossRef]
- Mulder, C.; Schouten, A.J.; Hund-Rinke, K.; Breure, A.M. The use of nematodes in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fert. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T.; De Goede, R.G. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Sanchez, G.; Trinchera, L.; Russolillo, G. plspm: Tools for Partial Least Squares Path Modeling (PLS-PM). R Package Version 0.4.9. 2015. Available online: https://github.com/gastonstat/plspm (accessed on 10 November 2021).
- Nicholls, E.M.; Carey, S.K. Evapotranspiration and energy partitioning across a forest-shrub vegetation gradient in a subarctic, alpine catchment. J. Hydrol. 2021, 602, 126790. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, H.; Wu, Y.; Li, Y.; Qiao, L.; Wang, J.; Zhai, J.Y.; Song, Y.H.; Zhao, Z.W.; Zhang, Z.H.; et al. Plant-mediated effects of long-term warming on soil microorganisms on the Qinghai-Tibet Plateau. CATENA 2021, 204, 105391. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Guo, R.; Li, H.; Shang, Z. Fencing enclosure alters nitrogen distribution patterns and tradeoff strategies in an alpine meadow on the Qinghai-Tibetan Plateau. Catena 2021, 197, 104948. [Google Scholar] [CrossRef]
- Li, N.; Wang, G.X.; Yang, Y.; Gao, Y.H.; Liu, Y.A.; Liu, G.S. Short-term effects of temperature enhancement on community structure and biomass of alpine meadow in the Qinghai-Tibet Plateau. Acta Ecol. Sin. 2011, 31, 0895–0905. [Google Scholar]
- Xue, H.Y.; Luo, D.Q.; Wang, H.Y.; Qu, X.L. Effects of Free Grazing or Enclosure on Soil Nematodes in Alpine Meadows in North Tibet, China. Acta Pedol. Sin. 2017, 54, 480–492. [Google Scholar]
- Liu, T.; Mao, P.; Shi, L.; Wang, Z.; Fu, S. Contrasting effects of nitrogen deposition and increased precipitation on soil nematode communities in a temperate forest. Soil Biol. Biochem. 2020, 148, 107869. [Google Scholar] [CrossRef]
- Cesarz, S.; Ciobanu, M.; Wright, A.J.; Ebeling, A.; Vogel, A.; Weisser, W.W.; Eisenhauer, N. Plant species richness sustains higher trophic levels of soil nematode communities after consecutive environmental perturbations. Oecologia 2017, 184, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Gao, Q.Z.; ZhaBu, G.Z.; Hu, G.Z.; Li, W.H. Soil nematode community response to warming in alpine meadows of northern Tibet. Pratacult. Sci. 2018, 35, 1528–1538. [Google Scholar]
- Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Crowther, T.W. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, D.M.; Yan, D.H.; Song, X.S.; Yu, Z.L.; Peng, D.; Ting, X.; Weng, B.S. Community structure of soil nematodes under different drought conditions. Geoderma 2018, 325, 110–116. [Google Scholar] [CrossRef]
- Hu, J.; Wu, J.; Ma, M.; Nielsen, U.N.; Wang, J.; Du, G. Nematode communities response to long-term grazing disturbance on Tibetan plateau. Eur. J. Soil Biol. 2015, 69, 24–32. [Google Scholar] [CrossRef]
- Xue, H.Y.; Hu, F.; Luo, D.Q. Effects of alpine meadow plant communities on soil nematode functional structure in Northern Tibet, China. Acta Ecol. Sin. 2013, 33, 1482–1494. [Google Scholar]
- Liu, Y.R.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Yang, Z.M.; He, J.Z. New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol. Biochem. 2018, 118, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Briones, M.J.I.; Ostle, N.J.; McNamara, N.P.; Poskitt, J. Functional shifts of grassland soil communities in response to soil warming. Soil Biol. Biochem. 2009, 41, 315–322. [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, M.; Zhang, X.H.; Liu, X.Y.; Li, L.Q.; Shi, X.Z.; Hu, H.W.; Pan, G.X. Changes in soil nematode abundance and composition under elevated [CO2] and canopy warming in a rice paddy field. Plant Soil 2019, 445, 425–437. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S. Nematodes as drivers of plant performance in natural systems. Trends Plant Sci. 2020, 26, 237247. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Wall, D.H.; Adams, B.J.; Virginia, R.A. Antarctic nematode communities: Observed and predicted responses to climate change. Polar Biol. 2011, 34, 1701–1711. [Google Scholar] [CrossRef]
- Zong, N.; Chai, X.; Shi, P.L.; Ang, J.; Niu, B.; Zhang, X.Z.; He, Y.T. Responses of plant community structure and species composition to warming and N addition in an alpine meadow, northern Tibetan Plateau, China. Chin. J. Appl. Ecol. 2016, 27, 3739–3748. [Google Scholar]
- Ma, Q.H.; Yu, H.Y.; Liu, X.D.; Xu, Z.Z.; Zhou, G.S.; Shi, Y.H. Climatic warming shifts the soil nematode community in a desert steppe. Clim. Change 2018, 150, 243–258. [Google Scholar] [CrossRef]
- Mueller, K.E.; Blumenthal, D.M.; Carrillo, Y.; Cesarz, S.; Ciobanu, M.; Hines, J.; Pabst, S.; Pendall, E.; de Tomasel, C.M.; Wall, D.H.; et al. Elevated CO2 and warming shift the functional composition of soil nematode communities in a semiarid grassland. Soil Biol. Biochem. 2016, 103, 46–51. [Google Scholar] [CrossRef]


| Source | df | Mean Square | F | Sig. |
|---|---|---|---|---|
| Correction model | 24 | 109,718.114 | 9.217 | 0.000 |
| Intercept | 1 | 2,846,987.035 | 239.159 | 0.000 |
| Warming | 4 | 6117.611 | 0.514 | 0.726 |
| Soil layer | 4 | 625,437.533 | 52.540 | 0.000 ** |
| Warming * Soil layer | 16 | 6688.384 | 0.562 | 0.897 |
| Error | 50 | 11,904.136 | ||
| Total | 75 | |||
| Total corrected | 74 |
| CK | A | B | C | D | |
|---|---|---|---|---|---|
| H′ | 2.15 ± 0.02 a | 2.42 ± 0.10 a | 2.1 ± 0.17 a | 2.12 ± 0.14 a | 2.31 ± 0.06 a |
| J′ | 0.70 ± 0.01 ab | 0.78 ± 0.02 a | 0.68 ± 0.03 b | 0.72 ± 0.04 ab | 0.72 ± 0.01 ab |
| λ | 0.17 ± 0.00 ab | 0.11 ± 0.01 b | 0.20 ± 0.03 a | 0.18 ± 0.03 a | 0.16 ± 0.02 ab |
| MI | 3.08 ± 0.05 ab | 3.28 ± 0.05 b | 3.07 ± 0.17 b | 3.12 ± 0.16 b | 3.98 ± 0.07 a |
| PPI | 2.25 ± 0.08 a | 2.38 ± 0.09 a | 2.54 ± 0.10 a | 2.47 ± 0.18 a | 2.02 ± 0.10 b |
| NCR | 0.75 ± 0.01 a | 0.74 ± 0.04 a | 0.80 ± 0.02 a | 0.79 ± 0.02 a | 0.76 ± 0.02 a |
| WI | 1.93 ± 0.31 b | 1.22 ± 0.23 b | 1.02 ± 0.07 b | 1.44 ± 0.34 b | 5.11 ± 1.52 a |
| EI | 40.56 ± 0.56 abc | 44.81 ± 2.89 a | 36.74 ± 0.38 c | 42.86 ± 0.00 ab | 39.72 ± 1.21 bc |
| SI | 80.87 ± 0.81 a | 84.31 ± 1.53 a | 79.24 ± 1.59 a | 82.6 ± 3.47 a | 80.29 ± 2.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, W.; Liu, P.; Zhou, H.; Chen, Z.; Suonan, J. Plant-Soil Mediated Effects of Long-Term Warming on Soil Nematodes of Alpine Meadows on the Qinghai–Tibetan Plateau. Biology 2022, 11, 1596. https://doi.org/10.3390/biology11111596
Liu Y, Wang W, Liu P, Zhou H, Chen Z, Suonan J. Plant-Soil Mediated Effects of Long-Term Warming on Soil Nematodes of Alpine Meadows on the Qinghai–Tibetan Plateau. Biology. 2022; 11(11):1596. https://doi.org/10.3390/biology11111596
Chicago/Turabian StyleLiu, Yanfang, Wenying Wang, Pan Liu, Huakun Zhou, Zhe Chen, and Ji Suonan. 2022. "Plant-Soil Mediated Effects of Long-Term Warming on Soil Nematodes of Alpine Meadows on the Qinghai–Tibetan Plateau" Biology 11, no. 11: 1596. https://doi.org/10.3390/biology11111596
APA StyleLiu, Y., Wang, W., Liu, P., Zhou, H., Chen, Z., & Suonan, J. (2022). Plant-Soil Mediated Effects of Long-Term Warming on Soil Nematodes of Alpine Meadows on the Qinghai–Tibetan Plateau. Biology, 11(11), 1596. https://doi.org/10.3390/biology11111596







