TNS1: Emerging Insights into Its Domain Function, Biological Roles, and Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Structure
2.1. FAB (Focal Adhesion Binding)-N Terminal
2.1.1. Protein Tyrosine Phosphatase (PTP)
2.1.2. C2
2.2. ABD II: Non-Conserved Zone in the Middle
2.3. FAB-C Terminal
2.3.1. Src Homology 2 (SH2)
2.3.2. Phosphotyrosine-Binding (PTB) Domain
3. Biological Processes in Which TNS1 Is Involved
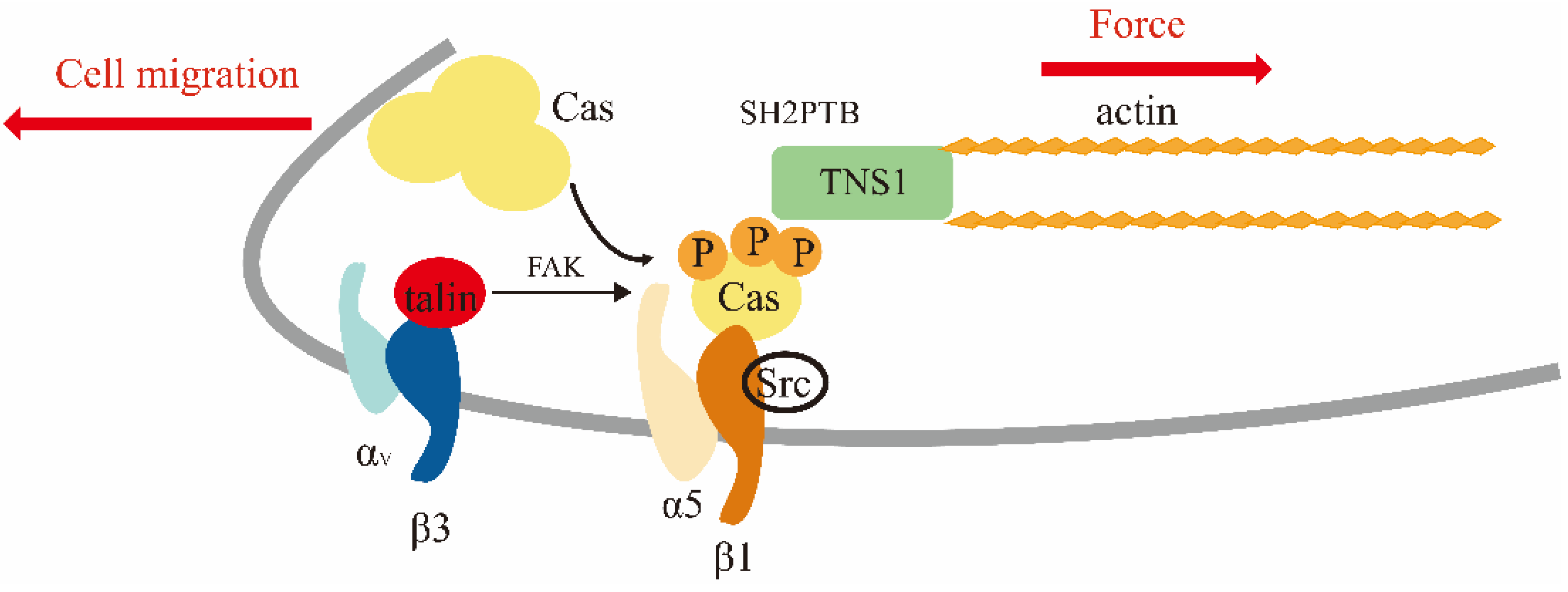
3.1. Cell Adhesion, Polarization, Migration, and Invasion
3.1.1. Cell Adhesion
3.1.2. Cell Polarization, Migration, and Invasion
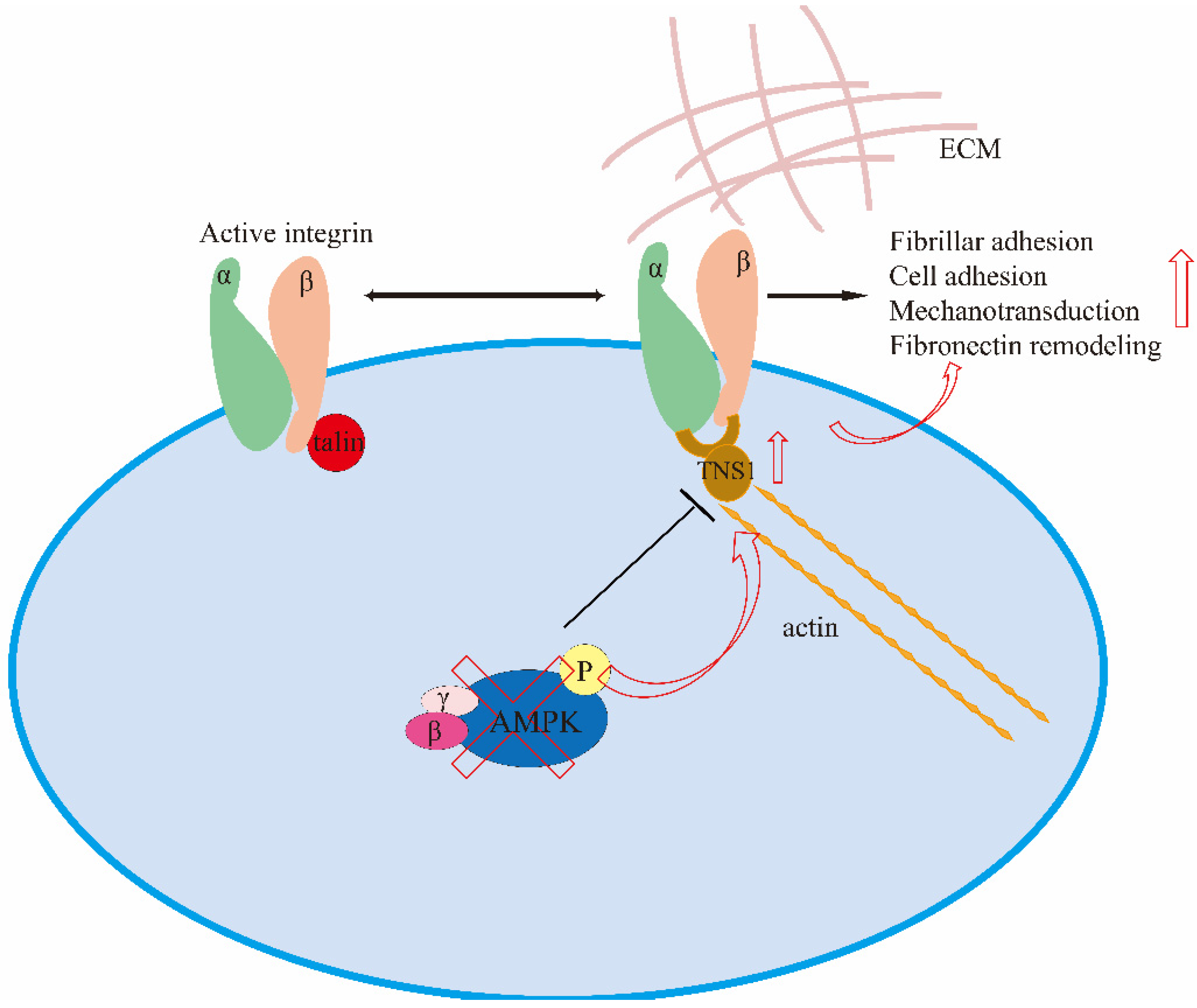
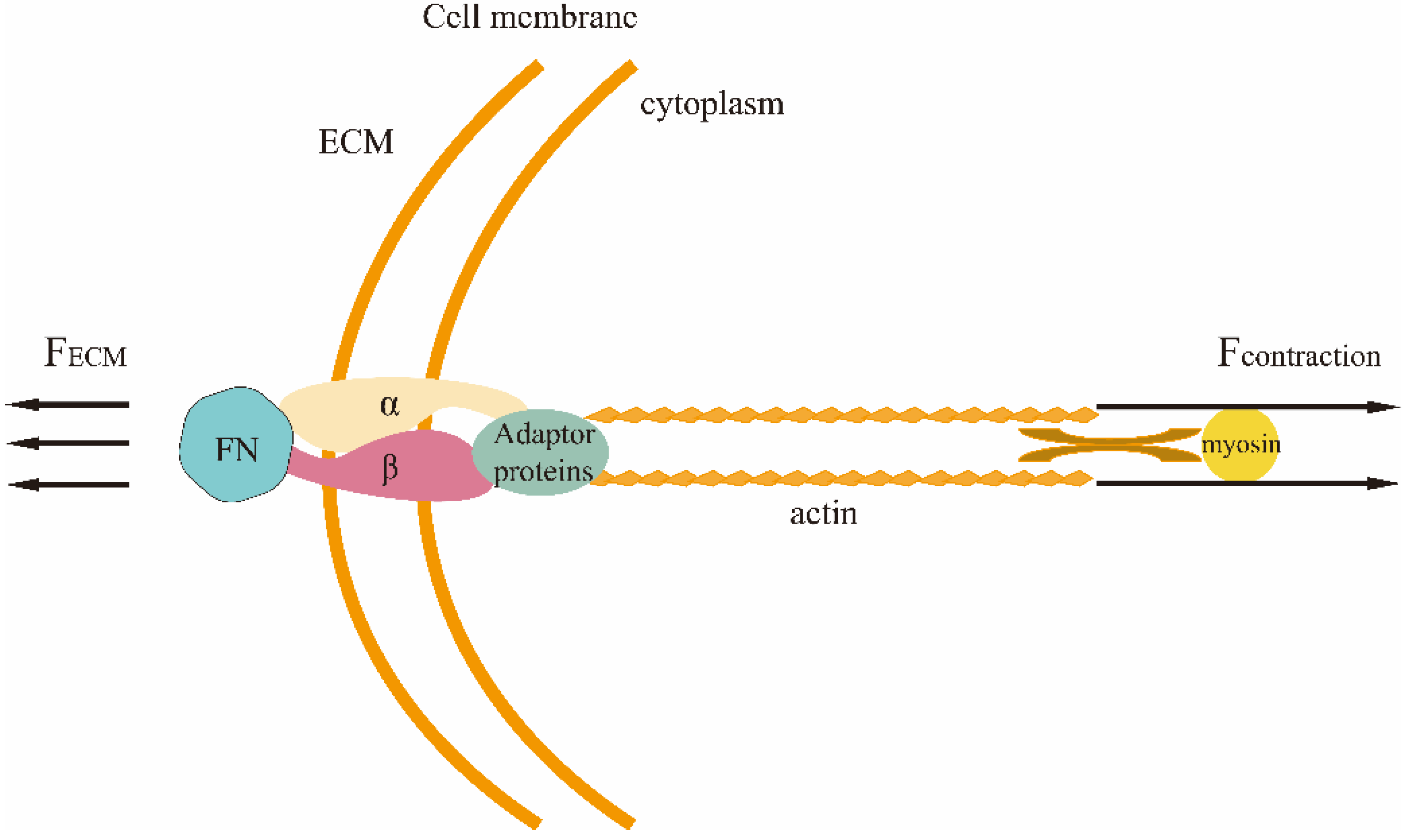
3.2. Mechano-Transduction
3.3. Cell Proliferation and Apoptosis
4. TNS1 and Tumors
4.1. The Role of TNS1 in Tumors
4.2. The Dual Role of TNS1 in Cancer
4.2.1. Colorectal Cancer
4.2.2. Gastric Cancer
4.2.3. Breast Cancer
4.2.4. Lung Cancer
4.2.5. Bladder Cancer
4.2.6. Renal Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, S.; Lu, M.L.; Lo, S.H.; Lin, S.; Butler, J.A.; Druker, B.J.; Roberts, T.M.; An, Q.; Chen, L.B. Presence of an SH2 domain in the actin-binding protein tensin. Science 1991, 252, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Duncan, I.C.; Bozorgchami, H.; Lo, S.H. Tensin1 and a previously undocumented family member, tensin2, positively regulate cell migration. Proc. Natl. Acad. Sci. USA 2002, 99, 733–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, S.H.; Janmey, P.A.; Hartwig, J.H.; Chen, L.B. Interactions of tensin with actin and identification of its three distinct actin-binding domains. J. Cell Biol. 1994, 125, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ishii, A.; Wong, W.K.; Chen, L.B.; Lo, S.H. Molecular characterization of human tensin. Biochem. J. 2000, 351 Pt 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H. Tensin. Int. J. Biochem. Cell Biol. 2004, 36, 31–34. [Google Scholar] [CrossRef]
- Legate, K.R.; Faessler, R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J. Cell Sci. 2009, 122, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Winograd-Katz, S.E.; Faessler, R.; Geiger, B.; Legate, K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 273–288. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Haynie, D.T. Molecular physiology of the tensin brotherhood of integrin adaptor proteins. Proteins-Struct. Funct. Bioinform. 2014, 82, 1113–1127. [Google Scholar] [CrossRef]
- Salgia, R.; Brunkhorst, B.; Pisick, E.; Li, J.L.; Lo, S.H.; Chen, L.B.; Griffin, J.D. increased tyrosine phosphorylation of focal adhesion proteins in myeloid cell-lines expressing p210(BCR/ABL). Oncogene 1995, 11, 1149–1155. [Google Scholar]
- Jiang, B.; Yamamura, S.; Nelson, P.R.; Mureebe, L.; Kent, K.C. Differential effects of platelet-derived growth factor isotypes on human smooth muscle cell proliferation and migration are mediated by distinct signaling pathways. Surgery 1996, 120, 427–432. [Google Scholar] [CrossRef]
- Ishida, T.; Ishida, M.; Suero, J.; Takahashi, M.; Berk, B.C. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J. Clin. Investig. 1999, 103, 789–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niziol, M.; Zinczuk, J.; Zareba, K.; Guzinska-Ustymowicz, K.; Pryczynicz, A. Immunohistochemical Analysis of the Expression of Adhesion Proteins: TNS1, TNS2 and TNS3 in Correlation with Clinicopathological Parameters in Gastric Cancer. Biomolecules 2021, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-P.; Sun, P.; Wang, A.; Lo, S.H. Tensin1 positively regulates RhoA activity through its interaction with DLC1. Biochim. Biophys. Acta-Mol. Cell Res. 2018, 1865, 1383. [Google Scholar] [CrossRef]
- Hall, E.H.; Daugherty, A.E.; Choi, C.K.; Horwitz, A.F.; Brautigan, D.L. Tensin1 Requires Protein Phosphatase-1 alpha in Addition to RhoGAP DLC-1 to Control Cell Polarization, Migration, and Invasion. J. Biol. Chem. 2009, 284, 34713–34722. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Sun, X.; Yang, S.; Song, W. Prazosin inhibits the proliferation and survival of acute myeloid leukaemia cells through down-regulating TNS1. Biomed. Pharmacother. 2020, 124, 109731. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lo, S.H. Regulation of tensin-promoted cell migration by its focal adhesion binding and Src homology domain 2. Biochem. J. 2003, 370, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Alonso, A.; Pulido, R. The extended human PTPome: A growing tyrosine phosphatase family. FEBS J. 2016, 283, 1404–1429. [Google Scholar] [CrossRef] [Green Version]
- Eto, M.; Kirkbride, J.; Elliott, E.; Lo, S.H.; Brautigan, D.L. Association of the tensin N-terminal protein-tyrosine phosphatase domain with the alpha isoform of protein phosphatase-1 in focal adhesions. J. Biol. Chem. 2007, 282, 17806–17815. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Wen, Y.; Xuan, C.; Chen, Q.; Xiang, Q.; Wang, J.; Liu, Y.; Luo, L.; Zhao, S.; Deng, Y.; et al. Identifying the key genes and microRNAs in prostate cancer bone metastasis by bioinformatics analysis. FEBS Open Bio 2020, 10, 674–688. [Google Scholar] [CrossRef]
- Muharram, G.; Sahgal, P.; Korpela, T.; De Franceschi, N.; Kaukonen, R.; Clark, K.; Tulasne, D.; Carpen, O.; Ivaska, J. Tensin-4-Dependent MET Stabilization Is Essential for Survival and Proliferation in Carcinoma Cells. Dev. Cell 2014, 29, 421–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafizi, S.; Alindri, F.; Karlsson, R.; Dahlback, B. Interaction of Axl receptor tyrosine kinase with C1-TEN, a novel C1 domain-containing protein with homology to tensin. Biochem. Biophys. Res. Commun. 2002, 299, 793–800. [Google Scholar] [CrossRef]
- Cui, Y.M.; Liao, Y.C.; Lo, S.H. Epidermal growth factor modulates tyrosine phosphorylation of a novel tensin family member, tensin3. Mol. Cancer Res. 2004, 2, 225–232. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Si, L.; White, R.W.D.; Lo, S.H. The phosphotyrosine-independent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J. Cell Biol. 2007, 176, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiadou, M.; Lilja, J.; Jacquemet, G.; Guzmán, C.; Rafaeva, M.; Alibert, C.; Yan, Y.; Sahgal, P.; Lerche, M.; Manneville, J.B.; et al. AMPK negatively regulates tensin-dependent integrin activity. J. Cell Biol. 2017, 216, 1107–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, M.; Yu, E.C.; Liddington, R.C.; Pasquale, E.B.; Pellecchia, M. The PTB domain of tensin: NMR solution structure and phosphoinositides binding studies. Biopolymers 2008, 89, 86–92. [Google Scholar] [CrossRef]
- Hao, L.; Yang, H.; Du, C.; Fu, X.; Zhao, N.; Xu, S.; Cui, F.; Mao, C.; Wang, Y. Directing the fate of human and mouse mesenchymal stem cells by hydroxyl-methyl mixed self-assembled monolayers with varying wettability. J. Mater. Chem. B 2014, 2, 4794–4801. [Google Scholar] [CrossRef] [Green Version]
- Tan, F.; Liu, J.; Liu, M.; Wang, J. Charge density is more important than charge polarity in enhancing osteoblast-like cell attachment on poly(ethylene glycol)-diacrylate hydrogel. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 76, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhu, X.; Li, M.; Shi, L.; Ong, J.L.T.; Janczewski, D.; Neoh, K.G. Parallel Control over Surface Charge and Wettability Using Polyelectrolyte Architecture: Effect on Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 30552–30563. [Google Scholar] [CrossRef] [PubMed]
- De Luca, I.; Di Salle, A.; Alessio, N.; Margarucci, S.; Simeone, M.; Galderisi, U.; Calarco, A.; Peluso, G. Positively charged polymers modulate the fate of human mesenchymal stromal cells via ephrinB2/EphB4 signaling. Stem Cell Res. 2016, 17, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Kao, W.-L.; You, Y.-W.; Chu, Y.-H.; Chu, K.-J.; Chen, P.-J.; Wu, C.-Y.; Lee, Y.-H.; Shyue, J.-J. Effect of surface potential on epithelial cell adhesion, proliferation and morphology. Colloids Surf. B-Biointerfaces 2016, 141, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, N.; Shi, H.; Liu, J.; Shi, L.; Zhang, B.; Wang, H.; Ji, J.; Chu, P.K. Upregulation of BMSCs Osteogenesis by Positively-Charged Tertiary Amines on Polymeric Implants via Charge/iNOS Signaling Pathway. Sci. Rep. 2015, 5, 9369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, F.; Xu, X.; Deng, T.; Yin, M.; Zhang, X.; Wang, J. Fabrication of positively charged poly(ethylene glycol)-diacrylate hydrogel as a bone tissue engineering scaffold. Biomed. Mater. 2012, 7, 055009. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.B.; English, A.; Abraham, M.; Zaharias, R.; Stanford, C.; Keller, J. The effect of hydrogel charge density on cell attachment. Biomaterials 2004, 25, 3023–3028. [Google Scholar] [CrossRef]
- De Rosa, M.; Carteni, M.; Petillo, O.; Calarco, A.; Margarucci, S.; Rosso, F.; De Rosa, A.; Farina, E.; Grippo, P.; Peluso, G. Cationic polyelectrolyte hydrogel fosters fibroblast spreading, proliferation, and extracellular matrix production: Implications for tissue engineering. J. Cell. Physiol. 2004, 198, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bondar, O.V.; Saifullina, D.V.; Shakhmaeva, I.I.; Mavlyutova, I.I.; Abdullin, T.I. Monitoring of the Zeta Potential of Human Cells upon Reduction in Their Viability and Interaction with Polymers. Acta Nat. 2012, 4, 78–81. [Google Scholar] [CrossRef]
- Wei, Q.; Becherer, T.; Angioletti-Uberti, S.; Dzubiella, J.; Wischke, C.; Neffe, A.T.; Lendlein, A.; Ballauff, M.; Haag, R. Protein Interactions with Polymer Coatings and Biomaterials. Angew. Chem.-Int. Ed. 2014, 53, 8004–8031. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, I.; Vuori, K.; Liddington, R.C. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat. Struct. Biol. 2002, 9, 101–106. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix–the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guan, J.L.; Chien, S. Biochemistry and biomechanics of cell motility. Annu. Rev. Biomed. Eng. 2005, 7, 105–150. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H. Tensins. Curr. Biol. 2017, 27, R331–R332. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.A.; Lin, S. High-affinity interaction of vinculin with actin-filaments In Vitro. Cell 1982, 28, 83–90. [Google Scholar] [CrossRef]
- Wilkins, J.A.; Lin, S. A re-examination of the interaction of vinculin with actin. J. Cell Biol. 1986, 102, 1085–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, J.A.; Risinger, M.A.; Lin, S. Studies on proteins that copurify with smooth-muscle vinculin—Identification of immunologically related species in focal adhesions of nonmuscle and Z-lines of muscle-cells. J. Cell Biol. 1986, 103, 1483–1494. [Google Scholar] [CrossRef]
- Nishino, T.; Sasaki, N.; Chihara, M.; Nagasaki, K.-i.; Torigoe, D.; Kon, Y.; Agui, T. Distinct Distribution of the Tensin Family in the Mouse Kidney and Small Intestine. Exp. Anim. 2012, 61, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Horton, E.R.; Byron, A.; Askari, J.A.; Ng, D.H.J.; Millon-Fremillon, A.; Robertson, J.; Koper, E.J.; Paul, N.R.; Warwood, S.; Knight, D.; et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 2015, 17, 1577–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapley, P.; Horwitz, A.; Buck, C.; Duggan, K.; Rohrschneider, L. Integrins isolated from Rous-sarcoma virus-transformed chicken-embryo fibroblasts. Oncogene 1989, 4, 325–333. [Google Scholar]
- Zhang, Z.T.; Lee, C.H.; Mandiyan, V.; Borg, J.P.; Margolis, B.; Schlessinger, J.; Kuriyan, J. Sequence-specific recognition of the internalization motif of the Alzheimer’s amyloid precursor protein by the X11 PTB domain. EMBO J. 1997, 16, 6141–6150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwahlen, C.; Li, S.C.; Kay, L.E.; Pawson, T.; Forman-Kay, J.D. Multiple modes of peptide recognition by the PTB domain of the cell fate determinant Numb. EMBO J. 2000, 19, 1505–1515. [Google Scholar] [CrossRef]
- Stolt, P.C.; Jeon, H.; Song, H.K.; Herz, J.; Eck, M.J.; Blacklow, S.C. Origins of peptide selectivity and phosphoinositide binding revealed by structures of disabled-1 PTB domain complexes. Structure 2003, 11, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Calderwood, D.A.; Fujioka, Y.; de Pereda, J.M.; Garcia-Alvarez, B.; Nakamoto, T.; Margolis, B.; McGlade, C.J.; Liddington, R.C.; Ginsberg, M.H. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 2272–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadokoro, S.; Shattil, S.J.; Eto, K.; Tai, V.; Liddington, R.C.; de Pereda, J.M.; Ginsberg, M.H.; Calderwood, D.A. Talin binding to integrin beta tails: A final common step in integrin activation. Science 2003, 302, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Wang, P.; Streuli, C.; Geiger, B.; Humphries, M.J.; Ballestrem, C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007, 179, 1043–1057. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.E.; Kamm, R.D.; Mofrad, M.R.K. Force-induced activation of Talin and its possible role in focal adhesion mechanotransduction. J. Biomech. 2007, 40, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- McCleverty, C.J.; Lin, D.C.; Liddington, R.C. Structure of the PTB domain of tensin1 and a model for its recruitment to fibrillar adhesions. Protein Sci. 2007, 16, 1223–1229. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Alvarez, B.; de Pereda, J.M.; Calderwood, D.A.; Ulmer, T.S.; Critchley, D.; Campbell, I.D.; Ginsberg, M.H.; Liddington, R.C. Structural determinants of integrin recognition by Talin. Mol. Cell 2003, 11, 49–58. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol 2020, 30, 720–735. [Google Scholar] [CrossRef]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legerstee, K.; Houtsmuller, A.B. A Layered View on Focal Adhesions. Biology 2021, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Paez, J.S.; Libring, S.; Hopkins, K.; Solorio, L.; Wendt, M.K. Transglutaminase-2 facilitates extracellular vesicle-mediated establishment of the metastatic niche. Oncogenesis 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, E.H.; Balsbaugh, J.L.; Rose, K.L.; Shabanowitz, J.; Hunt, D.F.; Brautigan, D.L. Comprehensive Analysis of Phosphorylation Sites in Tensin1 Reveals Regulation by p38MAPK. Mol. Cell. Proteom. 2010, 9, 2853–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, M.; Artym, V.V.; Green, J.A.; Yamada, K.M. The matrix reorganized: Extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 2006, 18, 463–471. [Google Scholar] [CrossRef]
- Zamir, E.; Katz, B.Z.; Aota, S.; Yamada, K.M.; Geiger, B.; Kam, Z. Molecular diversity of cell-matrix adhesions. J. Cell Sci. 1999, 112 Pt 11, 1655–1669. [Google Scholar] [CrossRef]
- Zamir, E.; Katz, M.; Posen, Y.; Erez, N.; Yamada, K.M.; Katz, B.Z.; Lin, S.; Lin, D.C.; Bershadsky, A.; Kam, Z.; et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2000, 2, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Ballestrem, C.; Kam, Z.; Geiger, B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 2003, 116 Pt 22, 4605–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klapholz, B.; Brown, N.H. Talin–the master of integrin adhesions. J. Cell. Sci. 2017, 130, 2435–2446. [Google Scholar]
- Tachibana, K.; Sato, T.; D’Avirro, N.; Morimoto, C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J. Exp. Med. 1995, 182, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.D.; Schaller, M.D.; Parsons, J.T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol. Biol. Cell 1995, 6, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.C.; Appeddu, P.A.; Parsons, J.T.; Hildebrand, J.D.; Schaller, M.D.; Guan, J.L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 1995, 270, 16995–16999. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.A.; Schaller, M.D.; Ginsberg, M.H. Integrins: Emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 1995, 11, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.L. Focal adhesion kinase in integrin signaling. Matrix Biol. 1997, 16, 195–200. [Google Scholar] [CrossRef]
- Arias-Salgado, E.G.; Lizano, S.; Shattil, S.J.; Ginsberg, M.H. Specification of the direction of adhesive signaling by the integrin beta cytoplasmic domain. J. Biol. Chem. 2005, 280, 29699–29707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Salgado, E.G.; Lizano, S.; Sarkar, S.; Brugge, J.S.; Ginsberg, M.H.; Shattil, S.J. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. USA 2003, 100, 13298–13302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.W.; Ellis, B.; Boerner, R.J.; Knight, W.B.; White, G.C., 2nd; Schaller, M.D. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J. Biol. Chem. 1998, 273, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Schlaepfer, D.D.; Hunter, T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Biol. Chem. 1997, 272, 13189–13195. [Google Scholar] [CrossRef] [Green Version]
- Schlaepfer, D.D.; Hanks, S.K.; Hunter, T.; van der Geer, P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 1994, 372, 786–791. [Google Scholar] [CrossRef]
- Burridge, K.; Turner, C.E.; Romer, L.H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: A role in cytoskeletal assembly. J. Cell Biol. 1992, 119, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Nojima, Y.; Morino, N.; Mimura, T.; Hamasaki, K.; Furuya, H.; Sakai, R.; Sato, T.; Tachibana, K.; Morimoto, C.; Yazaki, Y.; et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J. Biol. Chem. 1995, 270, 15398–15402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuori, K.; Ruoslahti, E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 1995, 270, 22259–22262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlaepfer, D.D.; Broome, M.A.; Hunter, T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: Involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol. Cell. Biol. 1997, 17, 1702–1713. [Google Scholar] [CrossRef]
- Vuori, K.; Hirai, H.; Aizawa, S.; Ruoslahti, E. Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: A role for Src family kinases. Mol. Cell. Biol. 1996, 16, 2606–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wavreille, A.S.; Pei, D. A chemical approach to the identification of tensin-binding proteins. ACS Chem. Biol. 2007, 2, 109–118. [Google Scholar] [CrossRef]
- Polte, T.R.; Hanks, S.K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc. Natl. Acad. Sci. USA 1995, 92, 10678–10682. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, T.; Sakai, R.; Honda, H.; Ogawa, S.; Ueno, H.; Suzuki, T.; Aizawa, S.; Yazaki, Y.; Hirai, H. Requirements for localization of p130cas to focal adhesions. Mol. Cell. Biol. 1997, 17, 3884–3897. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Tan, S.H.; Machiyama, H.; Kawauchi, K.; Araki, K.; Hirata, H.; Sawada, Y. Association between tensin 1 and p130Cas at focal adhesions links actin inward flux to cell migration. Biol. Open 2016, 5, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.-C.; Lo, S.H. Deleted in liver cancer-1 (DLC-1): A tumor suppressor not just for liver. Int. J. Biochem. Cell Biol. 2008, 40, 843–847. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Li, G.; Asmussen, H.K.; Asnaghi, L.; Vass, W.C.; Braverman, R.; Yamada, K.M.; Popescu, N.C.; Papageorge, A.G.; Lowy, D.R. Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc. Natl. Acad. Sci. USA 2007, 104, 9012–9017. [Google Scholar] [CrossRef] [Green Version]
- Narumiya, S.; Tanji, M.; Ishizaki, T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009, 28, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Gómez del Pulgar, T.; Benitah, S.A.; Valerón, P.F.; Espina, C.; Lacal, J.C. Rho GTPase expression in tumourigenesis: Evidence for a significant link. Bioessays 2005, 27, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J. Rho proteins and cancer. Breast Cancer Res. Treat. 2004, 84, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Symons, M.; Campbell, S.L.; Der, C.J. Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res. Treat. 2004, 84, 61–71. [Google Scholar] [CrossRef]
- Joshi, R.; Qin, L.; Cao, X.; Zhong, S.; Voss, C.; Min, W.; Li, S.S.C. DLC1 SAM domain-binding peptides inhibit cancer cell growth and migration by inactivating RhoA. J. Biol. Chem. 2020, 295, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M. Cell and tissue mechanics: The new cell biology frontier. Mol. Biol. Cell 2017, 28, 1815–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeGoff, L.; Lecuit, T. Mechanical Forces and Growth in Animal Tissues. Cold Spring Harb. Perspect. Biol. 2016, 8, a019232. [Google Scholar] [CrossRef] [Green Version]
- Jansen, K.A.; Donato, D.M.; Balcioglu, H.E.; Schmidt, T.; Danen, E.H.J.; Koenderink, G.H. A guide to mechanobiology: Where biology and physics meet. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 3043–3052. [Google Scholar] [CrossRef] [Green Version]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating force and shape—The rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef]
- Wang, N. Review of cellular mechanotransduction. J. Phys. D-Appl. Phys. 2017, 50, 233002. [Google Scholar] [CrossRef]
- Kirby, T.J.; Lammerding, J. Cell Mechanotransduction Stretch to express. Nat. Mater. 2016, 15, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.K.; Nelson, C.M.; Biais, N.; Fabry, B.; Moeller, J.; Pruitt, B.L.; Wollnik, C.; Kudryasheva, G.; Rehfeldt, F.; Federle, W. Mechanotransduction: Use the force(s). BMC Biol. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.S. Mechanotransduction—A field pulling together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ju, L.; Rushdi, M.; Ge, C.; Zhu, C. Receptor-mediated cell mechanosensing. Mol. Biol. Cell 2017, 28, 3134–3155. [Google Scholar] [CrossRef]
- De Felice, D.; Alaimo, A. Mechanosensitive Piezo Channels in Cancer: Focus on altered Calcium Signaling in Cancer Cells and in Tumor Progression. Cancers 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011, 124 Pt 8, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
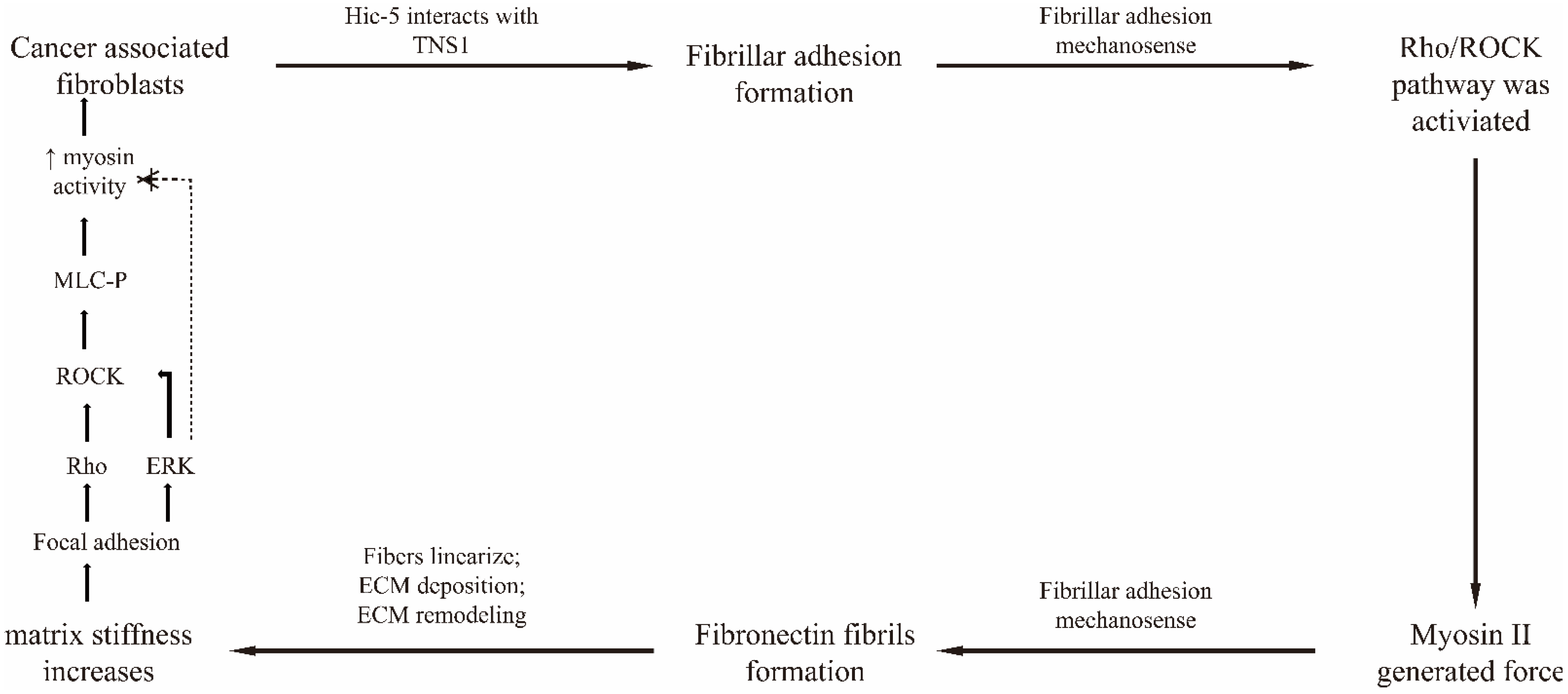
- Goreczny, G.J.; Forsythe, I.J.; Turner, C.E. Hic-5 regulates fibrillar adhesion formation to control tumor extracellular matrix remodeling through interaction with tensin1. Oncogene 2018, 37, 1699–1713. [Google Scholar] [CrossRef]
- Udan, R.S.; Kango-Singh, M.; Nolo, R.; Tao, C.; Halder, G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003, 5, 914–920. [Google Scholar] [CrossRef]
- Pantalacci, S.; Tapon, N.; Léopold, P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 2003, 5, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.F.; Pfleger, C.M.; Hariharan, I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 2003, 114, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.X.; Guan, K.L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Li, L.; Wang, L.; Wang, C.Y.; Yu, J.; Guan, K.L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012, 26, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Wada, K.; Itoga, K.; Okano, T.; Yonemura, S.; Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011, 138, 3907–3914. [Google Scholar] [CrossRef] [Green Version]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.C.; Meng, Z. Mechanoregulation of YAP and TAZ in Cellular Homeostasis and Disease Progression. Front. Cell Dev. Biol. 2021, 9, 673599. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Lu, Y.; Li, P.; Yin, M.X.; Lv, D.; Zhang, W.; Wang, H.; Zhou, Z.; Ji, H.; Zhao, Y.; et al. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res. 2013, 23, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Koontz, L.M.; Liu-Chittenden, Y.; Yin, F.; Zheng, Y.; Yu, J.; Huang, B.; Chen, Q.; Wu, S.; Pan, D. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev. Cell 2013, 25, 388–401. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Kim, J. Regulation of Hippo signaling by actin remodeling. BMB Rep. 2018, 51, 151–156. [Google Scholar] [CrossRef]
- DeRan, M.; Yang, J.; Shen, C.H.; Peters, E.C.; Fitamant, J.; Chan, P.; Hsieh, M.; Zhu, S.; Asara, J.M.; Zheng, B.; et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014, 9, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Gailite, I.; Aerne, B.L.; Tapon, N. Differential control of Yorkie activity by LKB1/AMPK and the Hippo/Warts cascade in the central nervous system. Proc. Natl. Acad. Sci. USA 2015, 112, E5169–E5178. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.S.; Meng, Z.; Kim, Y.C.; Park, H.W.; Hansen, C.G.; Kim, S.; Lim, D.S.; Guan, K.L. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 2015, 17, 500–510. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.D.; Li, X.; Aziz, K.E.; Gan, B.; Johnson, R.L.; Chen, J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 2015, 17, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Ishii, A.; Lo, S.H. A role of tensin in skeletal-muscle regeneration. Biochem. J. 2001, 356 Pt 3, 737–745. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Wu, L.; Xie, W.; Li, L.; Yuan, Y.; Chen, Y.; Lin, Y.; He, X. Elevated transgelin/TNS1 expression is a potential biomarker in human colorectal. Oncotarget 2018, 9, 1107–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auger, K.R.; Songyang, Z.; Lo, S.H.; Roberts, T.M.; Chen, L.B. Platelet-derived growth factor-induced formation of tensin and phosphoinositide 3-kinase complexes. J. Biol. Chem. 1996, 271, 23452–23457. [Google Scholar] [CrossRef] [Green Version]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Direkze, N.C.; Forbes, S.J.; Brittan, M.; Hunt, T.; Jeffery, R.; Preston, S.L.; Poulsom, R.; Hodivala-Dilke, K.; Alison, M.R.; Wright, N.A. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-tranplanted mice. Stem Cells 2003, 21, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, L.M.; Miller, N.; Nicklee, T.; Woo, J.; Hulse-Smith, L.; Tsao, M.S.; Khokha, R.; Martin, L.; Boyd, N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.P.; Martin, L.J.; Hanna, W.; Banerjee, D.; Miller, N.; Fishell, E.; Khokha, R.; Boyd, N.F. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol. Biomark. Prev. 2001, 10, 243–248. [Google Scholar]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003, 19, 677–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Juliano, R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol. Cells 2004, 17, 188–202. [Google Scholar] [PubMed]
- Huang, H.D.; Kamm, R.D.; Lee, R.T. Cell mechanics and mechanotransduction: Pathways, probes, and physiology. Am. J. Physiol.-Cell Physiol. 2004, 287, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- Vial, E.; Sahai, E.; Marshall, C.J. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 2003, 4, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stutchbury, B.; Atherton, P.; Tsang, R.; Wang, D.-Y.; Ballestrem, C. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J. Cell Sci. 2017, 130, 1612–1624. [Google Scholar] [CrossRef] [Green Version]
- Kim-Kaneyama, J.; Suzuki, W.; Ichikawa, K.; Ohki, T.; Kohno, Y.; Sata, M.; Nose, K.; Shibanuma, M. Uni-axial stretching regulates intracellular localization of Hic-5 expressed in smooth-muscle cells In Vivo. J. Cell Sci. 2005, 118, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 2003, 14, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, L.; Chen, D.; Fagotto-Kaufmann, C.; Hubmacher, D.; McKee, M.D.; Annis, D.S.; Mosher, D.F.; Reinhardt, D.P. Fibrillin Assembly Requires Fibronectin. Mol. Biol. Cell 2009, 20, 846–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadler, K.E.; Hill, A.; Canty-Laird, E.G. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008, 20, 495–501. [Google Scholar] [CrossRef]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinković, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef] [Green Version]
- Mannaerts, I.; Leite, S.B.; Verhulst, S.; Claerhout, S.; Eysackers, N.; Thoen, L.F.; Hoorens, A.; Reynaert, H.; Halder, G.; van Grunsven, L.A. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol. 2015, 63, 679–688. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Warren, J.S.A.; Xiao, Y.; Lamar, J.M. YAP/TAZ Activation as a Target for Treating Metastatic Cancer. Cancers 2018, 10, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Singh, M.P.; Rai, S.; Singh, N.K.; Srivastava, S. Transcriptomic landscape of early age onset of colorectal cancer identifies novel genes and pathways in Indian CRC patients. Sci. Rep. 2021, 11, 11765. [Google Scholar] [CrossRef]
- Zhou, H.-m.; Fang, Y.-y.; Weinberger, P.M.; Ding, L.-l.; Cowell, J.K.; Hudson, F.Z.; Ren, M.; Lee, J.R.; Chen, Q.-k.; Su, H.; et al. Transgelin increases metastatic potential of colorectal cancer cells In Vivo and alters expression of genes involved in cell motility. BMC Cancer 2016, 16, 55. [Google Scholar] [CrossRef] [Green Version]
- Mi, B.; Li, Q.; Li, T.; Liu, G.; Sai, J. High miR-31-5p expression promotes colon adenocarcinoma progression by targeting TNS1. Aging-Us 2020, 12, 7480–7490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-w.; Ming, X.-l.; Rong, Y.; Huang, C.-q.; Weng, H.; Chen, H.; Bian, J.-m.; Wang, F.-b. Diagnostic Value Investigation and Bioinformatics Analysis of miR-31 in Patients with Lymph Node Metastasis of Colorectal Cancer. Anal. Cell. Pathol. 2019, 2019, 9740475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Chen, F.; Ren, X.; Yang, Y.; Luo, J.; Yuan, J.; Yuan, J.; Tong, Q. RNA-Binding Protein COL14A1, TNS1, NUSAP1 and YWHAE Are Valid Biomarkers to Predict Peritoneal Metastasis in Gastric Cancer. Front. Oncol. 2022, 12, 830688. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, Y.; Meyer, D.S.; Zhang, Z.; Shokat, K.M.; Akhurst, R.J.; Miyazono, K.; Derynck, R. Chronic TGF-beta exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci. Signal. 2019, 12, eaau8544. [Google Scholar] [CrossRef]
- Dongre, A.; Rashidian, M.; Reinhardt, F.; Bagnato, A.; Keckesova, Z.; Ploegh, H.L.; Weinberg, R.A. Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas. Cancer Res. 2017, 77, 3982–3989. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [Green Version]
- Barkan, D.; El Touny, L.H.; Michalowski, A.M.; Smith, J.A.; Chu, I.; Davis, A.S.; Webster, J.D.; Hoover, S.; Simpson, R.M.; Gauldie, J.; et al. Metastatic Growth from Dormant Cells Induced by a Col-I-Enriched Fibrotic Environment. Cancer Res. 2010, 70, 5706–5716. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.-C.; Diermeier, S.D.; Yu, A.T.; Brine, L.D.; Russo, S.; Bhatia, S.; Alsudani, H.; Kostroff, K.; Bhuiya, T.; Brogi, E.; et al. MaTAR25 lncRNA regulates the Tensin1 gene to impact breast cancer progression. Nat. Commun. 2020, 11, 6438. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, S.; Zheng, M.; Chen, Z.; Wang, G.; Ma, J.; Zhang, B.; Huang, W.; Sun, X.; Wang, C. miR-31-5p modulates cell progression in lung adenocarcinoma through TNS1/p53 axis. Strahlenther Onkol. 2022, 198, 304–314. [Google Scholar] [CrossRef]
- Chen, L.; Ren, Z.; Cai, Y. Construction and Analysis of Survival-Associated Competing Endogenous RNA Network in Lung Adenocarcinoma. BioMed Res. Int. 2021, 2021, 4093426. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cai, Y.; Jiang, H.; Lv, Z.; Yang, C.; Xu, H.; Li, Z.; Li, Y. LncRNA MAGI2-AS3 inhibits bladder cancer progression by targeting the miR-31-5p/TNS1 axis. Aging-Us 2020, 12, 25547–25563. [Google Scholar] [CrossRef] [PubMed]
- Martuszewska, D.; Ljungberg, B.; Johansson, M.; Landberg, G.; Oslakovic, C.; Dahlback, B.; Hafizi, S. Tensin3 Is a Negative Regulator of Cell Migration and All Four Tensin Family Members Are Downregulated in Human Kidney Cancer. PLoS ONE 2009, 4, e4350. [Google Scholar] [CrossRef] [PubMed]

| Tumor | Tumor Background | Positive Regulation | Negative Regulation |
|---|---|---|---|
| Colorectal cancer | Early-age-onset colorectal cancer Colorectal cancer Lymph node metastasis of colorectal cancer Colon adenocarcinoma | TNS1 affects MAPK signaling pathway and promotes cellular metastasis Pseudopod formation promotes cell proliferation and invasion TNS1 regulates cell movement to promote lymphatic metastasis of tumors Regulation of Hippo signaling TNS1 regulates EMT and accelerates tumor metastasis | TNS1 inhibits the production of the primary tumor TNS1 promotes Macrophage2 polarization and inhibits COAD tumorigenesis |
| Gastric cancer | TNS1 enhances cell contraction to promote proliferation and metastasis | ||
| Breast cancer | Breast cancer extracellular vesicle (EV) fractions derived from metastatic breast cancer cells | TNS1 promotes the formation of focal adhesions; participates in EMT of cancer cells (4T1); and promotes proliferation, invasion, and migration of breast cancer cells Phosphorylation of TNS1 promotes EMT, promotes EV FN fiber formation, and promotes transfer | TNS1 inhibits CDC42 (Rho-GTPase) expression and inhibits cancer cells (MCF-7, SKBR3, MDA-MB-231) invasion and migration but does not affect proliferation |
| Lung cancer | Lung adenocarcinoma | TNS1 promotes p53 expression, promotes LUAD cell apoptosis, and inhibits LUAD cell proliferation, migration, and invasion | |
| Bladder cancer | LncRNA MAGI2-AS3 promotes TNS1 expression by targeting miR-31-5p/TNS1 axis to inhibit bladder cancer generation, proliferation, migration, and invasion | ||
| Renal cancer | The mRNA level of TNS1 in RCCs negatively correlates with tumor grade |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ye, J.; Dong, F.; Cao, L.; Wang, M.; Sun, G. TNS1: Emerging Insights into Its Domain Function, Biological Roles, and Tumors. Biology 2022, 11, 1571. https://doi.org/10.3390/biology11111571
Wang Z, Ye J, Dong F, Cao L, Wang M, Sun G. TNS1: Emerging Insights into Its Domain Function, Biological Roles, and Tumors. Biology. 2022; 11(11):1571. https://doi.org/10.3390/biology11111571
Chicago/Turabian StyleWang, Zhihui, Jingxue Ye, Fengrui Dong, Li Cao, Min Wang, and Guibo Sun. 2022. "TNS1: Emerging Insights into Its Domain Function, Biological Roles, and Tumors" Biology 11, no. 11: 1571. https://doi.org/10.3390/biology11111571
APA StyleWang, Z., Ye, J., Dong, F., Cao, L., Wang, M., & Sun, G. (2022). TNS1: Emerging Insights into Its Domain Function, Biological Roles, and Tumors. Biology, 11(11), 1571. https://doi.org/10.3390/biology11111571





