Invasive Urban Mammalian Predators: Distribution and Multi-Scale Habitat Selection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
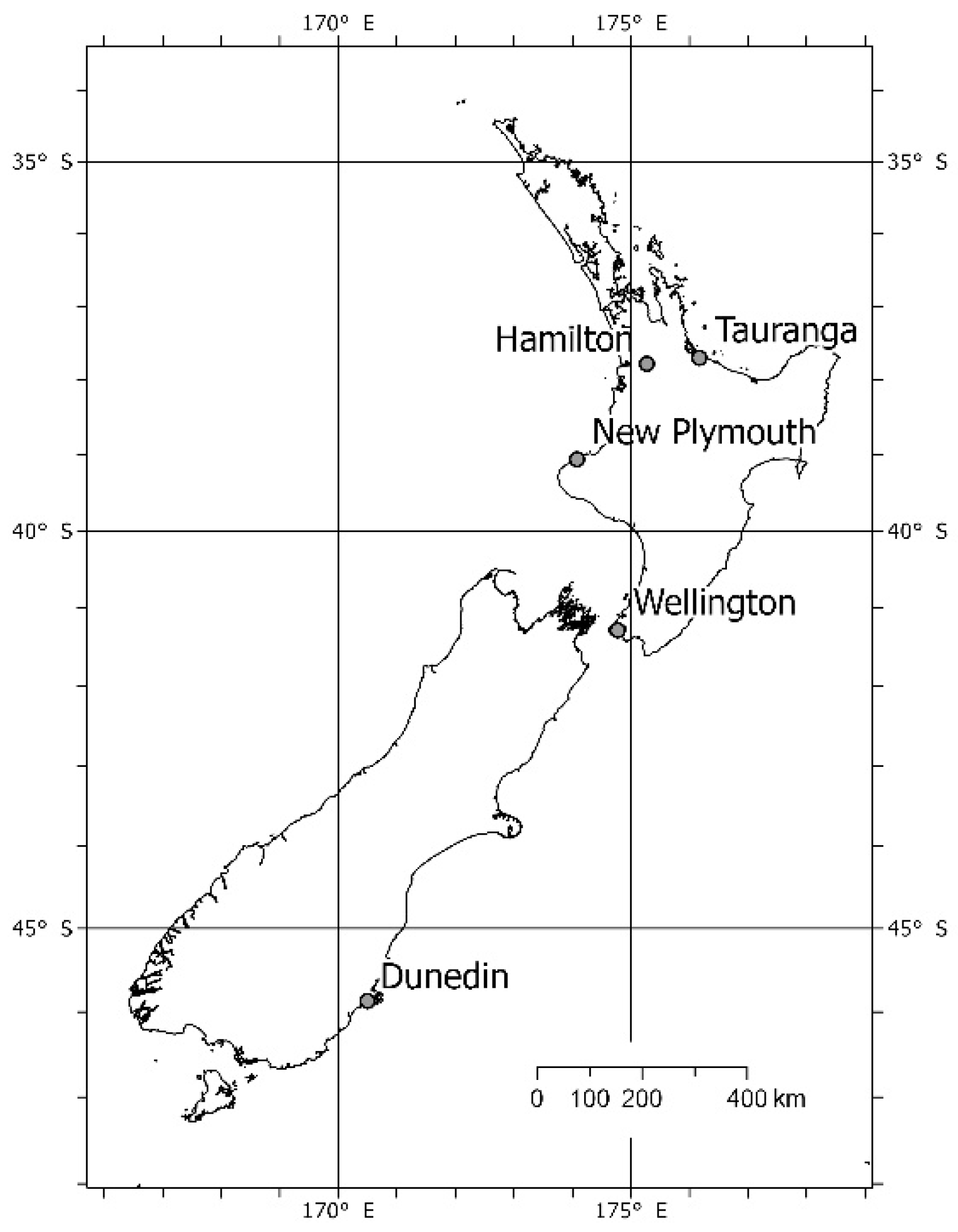
2.1. Study Design and Detection Devices
2.2. Identification of Predators
2.3. Habitat Surveys
2.4. Data Transformation
2.5. Statistical Analysis
2.5.1. Process of Variable Selection and Model Building
2.5.2. Main Model Presentation
3. Results
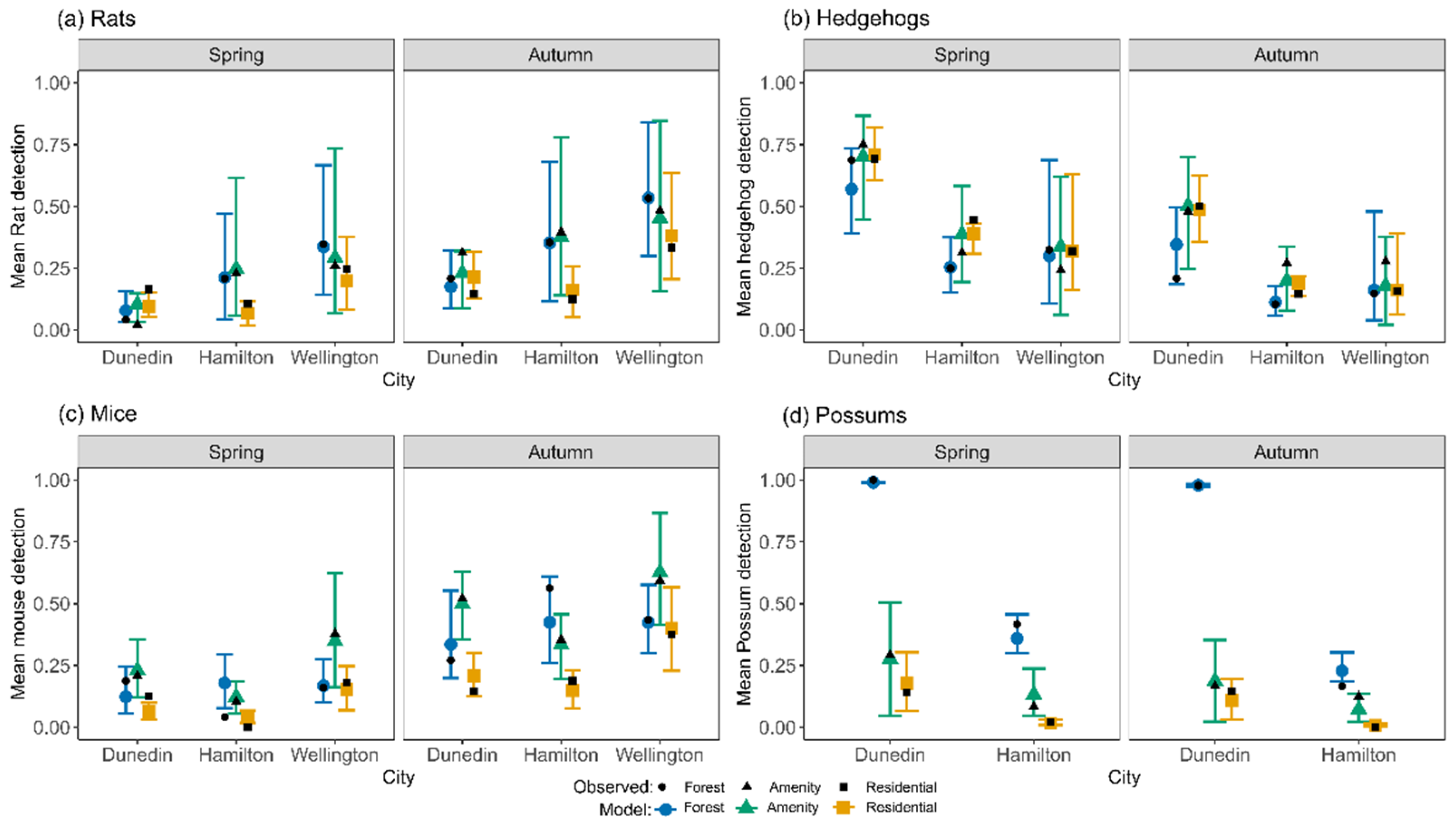
3.1. Detections in Hamilton, Wellington and Dunedin
3.2. Species Detection Models (=Main Models)
3.3. Effects of Season, City, Habitat Type, and Method on Odds of Detecting Mammalian Predators
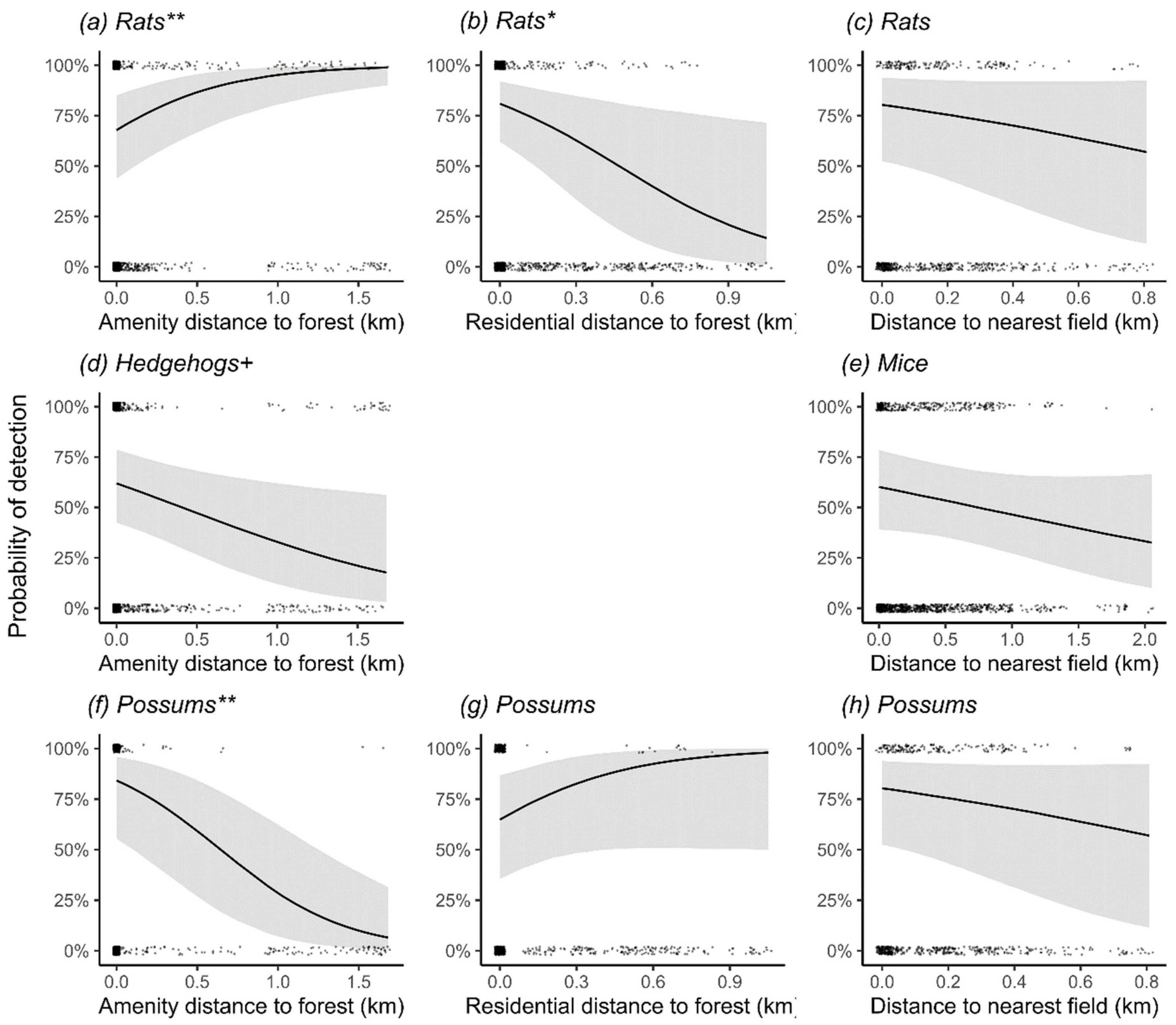
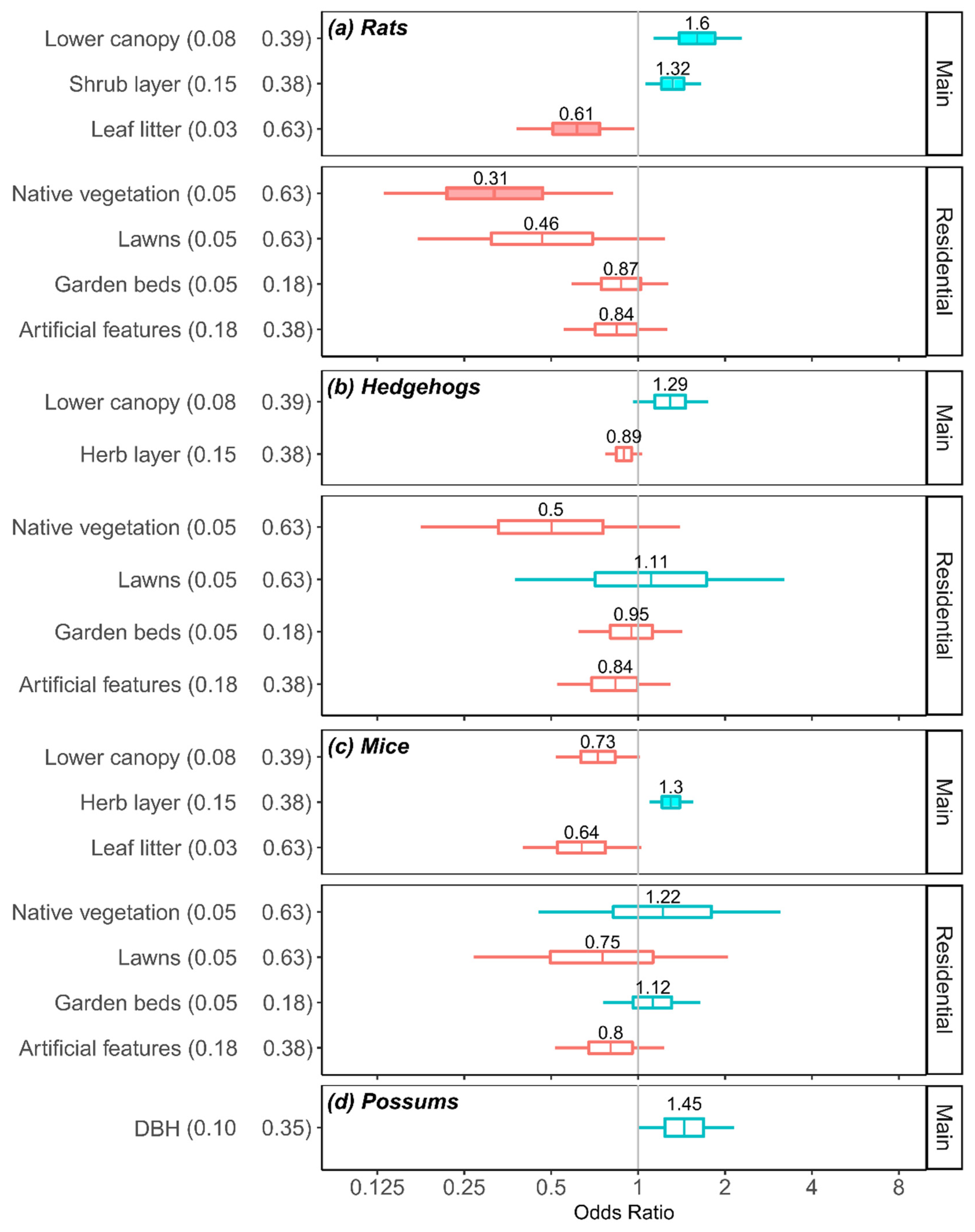
3.4. Effects of Habitat Covariates on Odds of Detecting Mammalian Predators in Three Main Cities
3.5. Practical Importance of Habitat Covariates
3.6. Two-City Models
4. Discussion
4.1. Broad-Scale Predictors of Mammal Presence
4.2. Habitat Associations: Rats
4.3. Habitat Associations: Hedgehogs
4.4. Habitat Associations: Mice
4.5. Habitat Associations: Possums
4.6. Application to Two Further Cities
4.7. Model Performance and Study Limitations
4.8. Towards More Efficient Urban Predator Control
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacGregor-Fors, I. Misconceptions or misunderstandings? On the standardization of basic terms and definitions in urban ecology. Landsc. Urban Plan. 2011, 100, 347–349. [Google Scholar] [CrossRef]
- McPhearson, T.; Pickett, S.T.A.; Grimm, N.B.; Niemelä, J.; Alberti, M.; Elmqvist, T.; Weber, C.; Haase, D.; Breuste, J.; Qureshi, S. Advancing urban ecology toward a science of cities. Bioscience 2016, 66, 198–212. [Google Scholar] [CrossRef]
- Savard, J.P.L.; Clergeau, P.; Mennechez, G. Biodiversity concepts and urban ecosystems. Landsc. Urban Plan. 2000, 48, 131–142. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, Biodiversity, and Conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- Watling, J.I.; Nowakowski, A.J.; Donnelly, M.A.; Orrock, J.L. Meta-analysis reveals the importance of matrix composition for animals in fragmented habitat. Global Ecol. Biogeogr. 2011, 20, 209–217. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the city: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. Bioscience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Wolch, J.R.; Byrne, J.; Newell, J.P. Urban green space, public health, and environmental justice: The challenge of making cities ‘just green enough’. Landsc. Urban Plan. 2014, 125, 234–244. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Wood, E.; Harsant, A.; Dallimer, M.; de Cronin, C.A.; McEachan, R.; Hassall, C. Not all green space is created equal: Biodiversity predicts psychological restorative benefits from urban green space. Front. Psychol. 2018, 9, 2320. [Google Scholar] [CrossRef] [PubMed]
- Garden, J.G.; McAlpine, C.A.; Possingham, H.P. Multi-scaled habitat considerations for conserving urban biodiversity: Native reptiles and small mammals in Brisbane, Australia. Lands. Ecol. 2010, 25, 1013–1028. [Google Scholar] [CrossRef]
- Braaker, S.; Moretti, M.; Boesch, R.; Ghazoul, J.; Obrist, M.K.; Bontadina, F. Assessing habitat connectivity for ground-dwelling animals in an urban environment. Ecol. Appl. 2014, 24, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Kambites, C.; Owen, S. Renewed prospects for green infrastructure planning in the UK. Plan. Pract. Res. 2006, 21, 483–496. [Google Scholar] [CrossRef]
- Haaland, C.; van den Bosch, C.K. Challenges and strategies for urban green-space planning in cities undergoing densification: A review. Urban For. Urban Green. 2015, 14, 760–771. [Google Scholar] [CrossRef]
- Clarkson, B.D.; Kirby, C.L. Ecological restoration in urban environments in New Zealand. Ecol. Manag. Restor. 2016, 17, 180–190. [Google Scholar] [CrossRef]
- Pauleit, S.; Ambrose-Oji, B.; Andersson, E.; Anton, B.; Buijs, A.; Haase, D.; Elands, B.; Hansen, R.; Kowarik, I.; Kronenberg, J. Advancing urban green infrastructure in Europe: Outcomes and reflections from the GREEN SURGE project. Urban For. Urban Green. 2019, 40, 4–16. [Google Scholar] [CrossRef]
- Sandström, U.G.; Angelstam, P.; Mikusiński, G. Ecological diversity of birds in relation to the structure of urban green space. Landsc. Urban Plan. 2006, 77, 39–53. [Google Scholar] [CrossRef]
- Ingram, M. Urban ecological restoration. Ecol. Restor. 2008, 26, 175–177. [Google Scholar] [CrossRef]
- Norton, B.A.; Evans, K.L.; Warren, P.H. Urban biodiversity and Landscape Ecology: Patterns, processes and planning. Curr. Landsc. Ecol. Rep. 2016, 1, 178–192. [Google Scholar] [CrossRef]
- Clout, M. Biodiversity conservation and the management of invasive animals in New Zealand. Invasive Species Biodivers. Manag. 2001, 24, 349. [Google Scholar]
- Norton, D.A. Species invasions and the limits to restoration: Learning from the New Zealand experience. Science 2009, 325, 569–571. [Google Scholar] [CrossRef]
- King, C.M.; Forsyth, D.M. (Eds.) The Handbook of New Zealand Mammals, 3rd ed.; CSIRO Publishing: Melbourne, Australia, 2021; p. 538. [Google Scholar]
- Doherty, T.S.; Glen, A.S.; Nimmo, D.G.; Ritchie, E.G.; Dickman, C.R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA 2016, 113, 11261–11265. [Google Scholar] [CrossRef] [PubMed]
- Towns, D.R.; Simberloff, D.; Atkinson, I.A.E. Restoration of New Zealand islands: Redressing the effects of introduced species. Pac. Conserv. Biol. 1997, 3, 99–124. [Google Scholar] [CrossRef]
- Russell, J.C.; Innes, J.G. Rattus Norvegicus. In The Handbook of New Zealand Mammals, 3rd ed.; King, C.M., Forsyth, D.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 161–240. [Google Scholar]
- Taylor, R.H.; Thomas, B.W. Rats eradicated from rugged Breaksea island (170 ha), Fiordland, New Zealand. Biol. Conserv. 1993, 65, 191–198. [Google Scholar] [CrossRef]
- Allen, R.B.; Lee, W.G.; Rance, B.D. Regeneration in indigenous forest after eradication of Norway rats, Breaksea Island, New Zealand. N. Z. J. Bot. 1994, 32, 429–439. [Google Scholar] [CrossRef]
- Byers, K.A.; Lee, M.J.; Patrick, D.M.; Himsworth, C.G. Rats about town: A systematic review of rat movement in urban ecosystems. Front. Ecol. Evol. 2019, 7, 13. [Google Scholar] [CrossRef]
- Morgan, D.K.J.; Waas, J.R.; Innes, J. An inventory of mammalian pests in a New Zealand city. N. Z. J. Zool. 2009, 36, 23–33. [Google Scholar] [CrossRef]
- Innes, J.G.; Russell, J.C. Rattus rattus. In The Handbook of New Zealand Mammals, 3rd ed.; King, C.M., Forsyth, D.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 161–240. [Google Scholar]
- Jones, C.; Moss, K.; Sanders, M. Diet of hedgehogs (Erinaceus europaeus) in the upper Waitaki Basin, New Zealand: Implications for conservation. N. Z. J. Ecol. 2005, 29, 29–35. [Google Scholar]
- Nottingham, C.M.; Glen, A.S.; Stanley, M.C. Snacks in the city: The diet of hedgehogs in Auckland urban forest fragments. N. Z. J. Ecol. 2019, 43, 3374. [Google Scholar] [CrossRef]
- Williams, P.A.; Karl, B.J.; Bannister, P.; Lee, W.G. Small mammals as potential seed dispersers in New Zealand. Austral. Ecol. 2000, 25, 523–532. [Google Scholar] [CrossRef]
- Wilson, D.J.; Wright, E.F.; Canham, C.D.; Ruscoe, W.A. Neighbourhood analyses of tree seed predation by introduced rodents in a New Zealand temperate rainforest. Ecography 2007, 30, 105–119. [Google Scholar] [CrossRef]
- Miller, C.J.; Miller, T.K. Population dynamics and diet of rodents on Rangitoto Island, New Zealand, including the effect of a 1080 poison operation. N. Z. J. Ecol. 1995, 19, 19–27. [Google Scholar]
- Miller, A.P.; Webb, P.I. Diet of house mice (Mus musculus L.) on coastal sand dunes, Otago, New Zealand. N. Z. J. Zool. 2001, 28, 49–55. [Google Scholar] [CrossRef]
- Newman, D.G. Effects of a mouse, Mus musculus, eradication programme and habitat change on lizard populations of Mana Island, New Zealand, with special reference to McGregor’s skink, Cyclodina macgregori. N. Z. J. Zool. 1994, 21, 443–456. [Google Scholar] [CrossRef]
- Norbury, G.; Van Den Munckhof, M.; Neitzel, S.; Hutcheon, A.; Reardon, J.; Ludwig, K. Impacts of invasive house mice on post-release survival of translocated lizards. N. Z. J. Ecol. 2014, 38, 322–327. [Google Scholar]
- Angel, A.; Wanless, R.M.; Cooper, J. Review of impacts of the introduced house mouse on islands in the Southern Ocean: Are mice equivalent to rats? Biol. Invasions 2009, 11, 1743–1754. [Google Scholar] [CrossRef]
- Leathwick, J.R.; Hay, J.R.; Fitzgerald, A.E. The influence of browsing by introduced mammals on the decline of North Island kokako. N. Z. J. Ecol. 1983, 6, 55–70. [Google Scholar]
- Innes, J.; Crook, B.; Jansen, P. A time-lapse video camera system for detecting predators at nests of forest birds: A trial with North Island kokako. In Proceedings of the Resource Technology 1994 Conference, Melbourne, Australia, 26–30 September 1994; University of Melbourne: Melbourne, Australia, 1994; p. 439. [Google Scholar]
- Innes, J. The impacts of possums on native fauna. In Possums as Conservation Pests; O’Donnell, C.F.J., Ed.; Department of Conservation: Wellington, New Zealand, 1995; pp. 11–15. [Google Scholar]
- Wilson, P.R.; Karl, B.J.; Toft, R.J.; Beggs, J.R.; Taylor, R.H. The role of introduced predators and competitors in the decline of kaka (Nestor meridionalis) populations in New Zealand. Biol. Conserv. 1998, 83, 175–185. [Google Scholar] [CrossRef]
- Aarts, G.; MacKenzie, M.; McConnell, B.; Fedak, M.; Matthiopoulos, J. Estimating space-use and habitat preference from wildlife telemetry data. Ecography 2008, 31, 140–160. [Google Scholar] [CrossRef]
- McIntyre, N.E. Scale-dependent habitat selection by the darkling beetle Eleodes hispilabris (Coleoptera: Tenebrionidae). Am. Midl. Nat. 1997, 138, 230–235. [Google Scholar]
- Razgour, O.; Hanmer, J.; Jones, G. Using multi-scale modeling to predict habitat suitability for species of conservation concern: The grey long-eared bat as a case study. Biol. Conserv. 2011, 144, 2922–2930. [Google Scholar] [CrossRef]
- Masi, E.; Pino, F.A.; Santos, M.D.G.S.; Genehr, L.; Albuquerque, J.O.M.; Bancher, A.M.; Alves, J.C.M. Socioeconomic and environmental risk factors for urban rodent infestation in São Paulo, Brazil. J. Pest Sci. 2010, 83, 231–241. [Google Scholar] [CrossRef]
- Sacchi R, Gentilli A, Pilon N, Bernini F GIS-modeling the distribution of Rattus norvegicus in urban areas using non toxic attractive baits. Hystrix 2008, 19, 13–22.
- Harris, S. An estimation of the number of foxes (Vulpes vulpes) in the city of Bristol, and some possible factors affecting their distribution. J. Appl. Ecol. 1981, 18, 455–465. [Google Scholar] [CrossRef]
- Harris, S.; Raynor, J.M.V. Urban fox (Vulpes vulpes) population estimates and habitat requirements in several British cities. J. Anim. Ecol. 1986, 55, 575–591. [Google Scholar] [CrossRef]
- Marks, C.A.; Bloomfield, T.W. Home-range size and selection of natal den and diurnal shelter sites by urban red foxes (Vulpes vulpes) in Melbourne. Wild. Res. 2006, 33, 339–347. [Google Scholar] [CrossRef]
- Gehrt, S.D.; Anchor, C.; White, L.A. Home range and landscape use of coyotes in a metropolitan landscape: Conflict or coexistence? J. Mammal. 2009, 90, 1045–1057. [Google Scholar] [CrossRef]
- Morris, D.W. Patterns and scale of habitat use in two temperate-zone, small mammal faunas. Can. J. Zoolog. 1984, 62, 1540–1547. [Google Scholar] [CrossRef]
- Murphy, E.C.; Nathan, H.W. Mus musculus. In The Handbook of New Zealand Mammals, 3rd ed.; King, C.M., Forsyth, D.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 161–240. [Google Scholar]
- Dickman, C.R.; Doncaster, C.P. The ecology of small mammals in urban habitats. I. Populations in a patchy environment. J. Anim. Ecol. 1987, 56, 629–640. [Google Scholar] [CrossRef]
- Traweger, D.; Slotta-Bachmayr, L. Introducing GIS-modeling into the management of a brown rat (Rattus norvegicus Berk.)(Mamm. Rodentia Muridae) population in an urban habitat. J. Pest Sci. 2005, 78, 17–24. [Google Scholar] [CrossRef]
- King, C.M.; Innes, J.G.; Flux, M.; Kimberley, M.O.; Leathwick, J.R.; Williams, D.S. Distribution and abundance of small mammals in relation to habitat in Pureora Forest Park. N. Z. J. Ecol. 1996, 20, 215–240. [Google Scholar]
- Ragg, J.R.; Moller, H. Microhabitat selection by feral ferrets (Mustela furo) in a pastoral habitat, East Otago, New Zealand. N. Z. J. Ecol. 2000, 24, 39–46. [Google Scholar]
- Christie, J.E.; Kemp, J.; Rickard, C.; Murphy, E.C. Measuring stoat (Mustela erminea) and ship rat (Rattus rattus) capture success against micro-habitat factors. N. Z. J. Ecol. 2006, 30, 43–51. [Google Scholar]
- Harper, G.A. Habitat selection of feral cats (Felis catus) on a temperate, forested island. Austral. Ecol. 2007, 32, 305–314. [Google Scholar] [CrossRef]
- Pickerell, G.A.; O’Donnell, C.F.J.; Wilson, D.J.; Seddon, P.J. How can we detect introduced mammalian predators in non-forest habitats? A comparison of techniques. N. Z. J. Ecol. 2014, 38, 86–102. [Google Scholar]
- Morgan, D.K.J.; Waas, J.R.; Innes, J.; Fitzgerald, N. Identification of nest predators using continuous time-lapse recording in a New Zealand city. N. Z. J. Zool. 2011, 38, 343–347. [Google Scholar] [CrossRef]
- Adams, A.L.; Dickinson, K.J.M.; Robertson, B.C.; van Heezik, Y. Predicting summer site occupancy for an invasive species, the common brushtail possum (Trichosurus vulpecula), in an urban environment. PLoS ONE 2013, 8, e58422. [Google Scholar] [CrossRef]
- Anton, V. Understanding Distributions of Invasive Mammals in Urban Environments Using Remote Cameras and Citizen Science. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2019. [Google Scholar]
- Wroot, A.J. Feeding ecology of the European hedgehog Erinaceus europaeus. Ph.D. Thesis, Royal Holloway, University of London, London, UK, 1984. [Google Scholar]
- Doncaster, C.P. Factors regulating local variations in abundance: Field tests on hedgehogs, Erinaceus europaeus. Oikos 1994, 69, 182–192. [Google Scholar] [CrossRef]
- Dowding, C.V.; Harris, S.; Poulton, S.; Baker, P.J. Nocturnal ranging behaviour of urban hedgehogs, Erinaceus europaeus, in relation to risk and reward. Anim. Behav. 2010, 80, 13–21. [Google Scholar] [CrossRef]
- Pirnat, J. Conservation and management of forest patches and corridors in suburban landscapes. Landsc. Urban Plan. 2000, 52, 135–143. [Google Scholar] [CrossRef]
- Livesley, S.J.; McPherson, E.G.; Calfapietra, C. The urban forest and ecosystem services: Impacts on urban water, heat, and pollution cycles at the tree, street, and city scale. J. Environ. Qual. 2016, 45, 119–124. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Mata, L.; Mackie, J.A.; Hahs, A.K.; Stork, N.E.; Williams, N.S.G.; Livesley, S.J. Increasing biodiversity in urban green spaces through simple vegetation interventions. J. Appl. Ecol. 2017, 54, 1874–1883. [Google Scholar] [CrossRef]
- Nielsen, A.B.; Van Den Bosch, M.; Maruthaveeran, S.; van den Bosch, C. Species richness in urban parks and its drivers: A review of empirical evidence. Urban Ecosyst. 2014, 17, 305–327. [Google Scholar] [CrossRef]
- Cameron, R.W.F.; Blanuša, T.; Taylor, J.E.; Salisbury, A.; Halstead, A.J.; Henricot, B.; Thompson, K. The domestic garden–Its contribution to urban green infrastructure. Urban For. Urban Green. 2012, 11, 129–137. [Google Scholar] [CrossRef]
- van Heezik, Y.; Freeman, C.; Porter, S.; Dickinson, K.J.M. Garden size, householder knowledge, and socio-economic status influence plant and bird diversity at the scale of individual gardens. Ecosystems 2013, 16, 1442–1454. [Google Scholar] [CrossRef]
- Kendal, D.; Williams, N.S.G.; Williams, K.J.H. Drivers of diversity and tree cover in gardens, parks and streetscapes in an Australian city. Urban For. Urban Green. 2012, 11, 257–265. [Google Scholar] [CrossRef]
- van Heezik, Y.M.; Dickinson, K.J.M.; Freeman, C. Closing the gap: Communicating to change gardening practices in support of native biodiversity in urban private gardens. Ecol. Soc. 2012, 17, 34. [Google Scholar] [CrossRef]
- Kikillus, K.H.; Chambers, G.K.; Farnworth, M.J.; Hare, K.M. Research challenges and conservation implications for urban cat management in New Zealand. Pac. Conserv. Biol. 2016, 23, 15–24. [Google Scholar] [CrossRef]
- van Heezik, Y.; Smyth, A.; Adams, A.; Gordon, J. Do domestic cats impose an unsustainable harvest on urban bird populations? Biol. Conserv. 2010, 143, 121–130. [Google Scholar] [CrossRef]
- Stats, N.Z. Geographic Boundary Viewer. 2019. Available online: http://statsnz.maps.arcgis.com/apps/webappviewer/index.html?id=6f49867abe464f86ac7526552fe19787 (accessed on 24 May 2020).
- Anton, V.; Hartley, S.; Wittmer, H.U. Evaluation of remote cameras for monitoring multiple invasive mammals in New Zealand. N. Z. J. Ecol. 2018, 42, 74–79. [Google Scholar]
- Moller, H.; Alterio, N. Home range and spatial organisation of stoats (Mustela erminea), ferrets (Mustela furo) and feral house cats (Felis catus) on coastal grasslands, Otago Peninsula, New Zealand: Implications for yellow-eyed penguin (Megadyptes antipodes) conservation. N. Z. J. Zool. 1999, 26, 165–174. [Google Scholar] [CrossRef]
- Smith, D.H.; Weston, K.A. Capturing the cryptic: A comparison of detection methods for stoats (Mustela erminea) in alpine habitats. Wildl. Res. 2017, 44, 418–426. [Google Scholar] [CrossRef]
- Allen, R.B. RECCE: An Inventory Method for Describing New Zealand Vegetation; No. 181; Forest Research Institute: Christchurch, New Zealand, 1992. [Google Scholar]
- Manel, S.; Williams, H.C.; Ormerod, S.J. Evaluating presence–absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Makowski, D.; Ben-Shachar, M.S.; Chen, S.A.; Lüdecke, D. Indices of effect existence and significance in the Bayesian framework. Front. Psychol. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Kruschke, J. Doing Bayesian data analysis: A tutorial with R, JAGS, and Stan; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Gelman, A.; Goodrich, B.; Gabry, J.; Vehtari, A. R-squared for Bayesian regression models. Am. Stat. 2019, 73, 307–309. [Google Scholar] [CrossRef]
- Gelman, A.; Vehtari, A.; Simpson, D.; Margossian, C.C.; Carpenter, B.; Yao, Y.; Kennedy, L.; Gabry, J.; Bürkner, P.-C.; Modrák, M. Bayesian workflow. arXiv 2020, arXiv:2011.01808v1.2020. [Google Scholar]
- McAlpine, M.; Helson, R. Otari Native Botanic Garden and Johnston Hill Reserve/Karori Cemetery; Operational Report No. 99/7; Wellington Regional Council: Wellington, New Zealand, 1999.
- Biosecurity Department—Pest Animals. Wrights Hill Key Native Ecosystem Management Area (KNEMA) Possum Control; Operation Report No. 03/03; Greater Wellington Regional Council: Wellington, New Zealand, 2003.
- Sweetapple, P.J.; Nugent, G. Ship rat demography and diet following possum control in a mixed podocarp—Hardwood forest. N. Z. J. Ecol. 2007, 31, 186–201. [Google Scholar]
- Ruscoe, W.A.; Ramsey, D.S.; Pech, R.P.; Sweetapple, P.J.; Yockney, I.; Barron, M.C.; Perry, M.; Nugent, G.; Carran, R.; Warne, R.; et al. Unexpected consequences of control: Competitive vs. predator release in a four-species assemblage of invasive mammals. Ecol. Lett. 2011, 14, 1035–1042. [Google Scholar] [CrossRef]
- Russell, J.C.; Clout, M.N. Modeling the distribution and interaction of introduced rodents on New Zealand offshore islands. Glob. Ecol. Biogeogr. 2004, 13, 497–507. [Google Scholar] [CrossRef]
- Walker, S.; Kemp, J.R.; Elliott, G.P.; Mosen, C.C.; Innes, J.G. Spatial patterns and drivers of invasive rodent dynamics in New Zealand forests. Biol. Invasions 2019, 21, 1627–1642. [Google Scholar] [CrossRef]
- Wilson, D.J.; Innes, J.G.; Fitzgerald, N.B.; Bartlam, S.; Watts, C.; Smale, M.C. Population dynamics of house mice without mammalian predators and competitors. N. Z. J. Ecol. 2018, 42, 192–203. [Google Scholar] [CrossRef]
- Baker, P.J.; Ansell, R.J.; Dodds, P.A.; Webber, C.E.; Harris, S. Factors affecting the distribution of small mammals in an urban area. Mammal Rev. 2003, 33, 95–100. [Google Scholar] [CrossRef]
- FitzGibbon, S.I.; Putland, D.A.; Goldizen, A.W. The importance of functional connectivity in the conservation of a ground-dwelling mammal in an urban Australian landscape. Lands. Ecol. 2007, 22, 1513–1525. [Google Scholar] [CrossRef]
- Bray, M. Habitat Use by the Black Rat, Rattus rattus (Rodentia: Muridae) at North Head, NSW. Bachelor’s Thesis, University of Sydney, Sydney, Australia, 1994. [Google Scholar]
- Daniel, M. Seasonal diet of the ship rat (Rattus r. rattus) in lowland forest in New Zealand. Proc. N. Z. Ecol. Soc. 1973, 20, 21–30. [Google Scholar]
- Grant-Hoffman, M.N.; Barboza, P.S. Herbivory in invasive rats: Criteria for food selection. Biol. Invasion 2010, 12, 805–825. [Google Scholar] [CrossRef]
- Foster, S.; King, C.; Patty, B.; Miller, S. Tree-climbing capabilities of Norway and ship rats. N. Z. J. Zool. 2011, 34, 285–296. [Google Scholar] [CrossRef]
- Cox, M.P.; Dickman, C.R.; Cox, W.G. Use of habitat by the black rat (Rattus rattus) at North Head, New South Wales: An observational and experimental study. Austral. Ecol. 2000, 25, 375–385. [Google Scholar] [CrossRef]
- Ruffell, J.; Didham, R.K.; Barrett, P.; Gorman, N.; Pike, R.; Hickey-Elliott, A.; Sievwright, K.; Armstrong, D.P. Discriminating the drivers of edge effects on nest predation: Forest edges reduce capture rates of ship rats (Rattus rattus), a globally invasive nest predator, by altering vegetation structure. PLoS ONE. 2014, 9, e113098. [Google Scholar] [CrossRef]
- Colvin, B.A.; Degregorio, R.; Fleetwood, C. (1996) Norway rat infestation of urban landscaping and preventative design criteria. In Proceedings of the 17th Vertebrate Pest Conference; Timm, R.M., Crabb, A.C., Eds.; University of California: Davis, CA, USA, 1996. [Google Scholar]
- Cavia, R.; Cueto, G.R.; Suárez, O.V. Changes in rodent communities according to the landscape structure in an urban ecosystem. Landsc. Urban Plan. 2009, 90, 11–19. [Google Scholar] [CrossRef]
- Madden, H.; Van Andel, T.; Miller, J.; Stech, M.; Verdel, K.; Eggermont, E. Vegetation associations and relative abundance of rodents on St. Eustatius, Caribbean Netherlands. Glob. Ecol. Conserv. 2019, 20, e00743. [Google Scholar] [CrossRef]
- Traweger, D.; Travnitzky, R.; Moser, C.; Walzer, C.; Bernatzky, G. Habitat preferences and distribution of the brown rat (Rattus norvegicus Berk.) in the city of Salzburg (Austria): Implications for an urban rat management. J. Pest Sci. 2006, 79, 113–125. [Google Scholar] [CrossRef]
- Himsworth, C.G.; Parsons, K.L.; Jardine, C.; Patrick, D.M. Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector-Borne Zoonot. 2013, 13, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.G.; King, C.M.; Flux, M.; Kimberley, M.O. Population biology of the ship rat and Norway rat in Pureora Forest Park, 1983–1987. N. Z. J. Zool. 2001, 28, 57–78. [Google Scholar] [CrossRef]
- Woolley, C.K.; Hartley, S.; Nelson, N.J.; Shanahan, D.F. Public willingness to engage in backyard conservation in New Zealand: Exploring motivations and barriers for participation. People Nat. 2021, 3, 929–940. [Google Scholar] [CrossRef]
- Hubert, P.; Julliard, R.; Biagianti, S.; Poulle, M. Ecological factors driving the higher hedgehog (Erinaceus europeaus) density in an urban area compared to the adjacent rural area. Landsc. Urban Plan. 2011, 103, 34–43. [Google Scholar] [CrossRef]
- Rondinini, C.; Doncaster, C.P. Roads as barriers to movement for hedgehogs. Funct. Ecol. 2002, 16, 504–509. [Google Scholar] [CrossRef]
- Williams, R.L.; Stafford, R.; Goodenough, A.E. Biodiversity in urban gardens: Assessing the accuracy of citizen science data on garden hedgehogs. Urban Ecosyst. 2015, 18, 819–833. [Google Scholar] [CrossRef]
- Barnett, S.A.; Spencer, M.M. Feeding, social behaviour and interspecific competition in wild rats. Behaviour 1951, 3, 229–242. [Google Scholar]
- Yom-Tov, Y.; Yom-Tov, S.; Moller, H. Competition, coexistence, and adaptation amongst rodent invaders to Pacific and New Zealand islands. J. Biogeogr. 1999, 26, 947–958. [Google Scholar] [CrossRef]
- Bridgman, L.J.; Innes, J.; Gillies, C.; Fitzgerald, N.B.; Miller, S.; King, C.M. Do ship rats display predatory behaviour towards house mice? Anim. Behav. 2013, 86, 257–268. [Google Scholar] [CrossRef]
- Dickman, C.R. Predation and habitat shift in the house mouse, Mus domesticus. Ecology 1992, 73, 313–322. [Google Scholar] [CrossRef]
- Fitzgerald, B.M.; Daniel, M.J.; Fitzgerald, A.E.; Karl, B.J.; Meads, M.J.; Notman, P.R. Factors affecting the numbers of house mice (Mus musculus) in hard beech (Nothofagus truncata) forest. J. R. Soc. N. Z. 1996, 26, 237–249. [Google Scholar] [CrossRef][Green Version]
- Adams, A.L.; Recio, M.R.; Robertson, B.C.; Dickinson, K.J.M.; van Heezik, Y. Understanding home range behaviour and resource selection of invasive common brushtail possums (Trichosurus vulpecula) in urban environments. Biol. Invasions 2014, 16, 1791–1804. [Google Scholar] [CrossRef]
- Fitzgerald, A.E. Diet of the opossum Trichosurus vulpecula (Kerr) in the Orongorongo Valley, Wellington, New Zealand, in relation to food-plant availability. N. Z. J. Zool. 1976, 3, 399–419. [Google Scholar] [CrossRef]
- Nugent, G.; Fraser, W.; Sweetapple, P. Top down or bottom up? Comparing the impacts of introduced arboreal possums and ‘terrestrial’ ruminants on native forests in New Zealand. Biol. Conserv. 2001, 99, 65–79. [Google Scholar] [CrossRef]
- Patterson, C.R.; Seddon, P.J.; Wilson, D.J.; van Heezik, Y. Habitat-specific densities of urban brushtail possums. N. Z. J. Ecol. 2021, 45, 1–9. [Google Scholar] [CrossRef]
- Harper, M.J. Home range and den use of common brushtail possums (Trichosurus vulpecula) in urban forest remnants. Wildl. Res. 2006, 32, 681–687. [Google Scholar] [CrossRef]
- Carthew, S.M.; Yáñez, B.M.; Ruykys, L. Straddling the divide: Den use by brushtail possums (Trichosurus vulpecula) in urban parklands. Urban Ecosyst. 2015, 18, 525–538. [Google Scholar] [CrossRef]
- Warburton, B.; Yockney, I. Comparison of two luring methods for trapping brushtail possums in non-forest habitats of New Zealand. N. Z. J. Zool. 2009, 36, 401–405. [Google Scholar] [CrossRef]
- Glen, A.S.; Byrom, A.E.; Pech, R.P.; Cruz, J.; Schwab, A.; Sweetapple, P.J.; Yockney, I.; Nugent, G.; Coleman, M.; Whitford, J. Ecology of brushtail possums in a New Zealand dryland ecosystem. N.Z. J. Ecol. 2012, 36, 29–37. [Google Scholar]
- Feng, A.Y.T.; Himsworth, C.G. The secret life of the city rat: A review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosyst. 2014, 17, 149–162. [Google Scholar] [CrossRef]
- Jolly, J.N. Habitat use and movements of the opossum (Trichosurus vulpecula) in a pastoral habitat on Banks Peninsula. In Proceedings (New Zealand Ecological Society); New Zealand Ecological Society (Inc.): Northland, New Zealand, 1976; pp. 70–78. [Google Scholar]
- Garvey, P.M.; Byrom, A.E. Mustela furo. In The Handbook of New Zealand Mammals, 3rd ed.; King, C.M., Forsyth, D.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 285–341. [Google Scholar]
- King, C.M.; Veale, A.J. Mustela erminea. In The Handbook of New Zealand Mammals, 3rd ed.; King, C.M., Forsyth, D.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 285–341. [Google Scholar]
- Garvey, P.M.; Glen, A.S.; Clout, M.N.; Nichols, M.; Pech, R.P. Niche partitioning in a guild of invasive mammalian predators. Ecol. Appl. 2022, 32, e2566. [Google Scholar] [CrossRef]
- Gillies, C. Interim DOC trail. In Camera Guide v1.0.3: Using Camera Traps to Monitor Feral Cats, Mustelids and Rats; Unpublished report; New Zealand Department of Conservation: Hamilton, New Zealand, 2019. [Google Scholar]
- Balls, C. Understanding the Distribution of Introduced Mammalian Predators in an Urban Environment Using Monitoring Tools and Community Trapping. Master’s Thesis, Victoria University of Wellington, Wellington, New Zealand, 2019. [Google Scholar]
- Wilson, D.J.; Lee, W.G.; Webster, R.A.; Allen, R.B. Effects of possums and rats on seedling establishment at two forest sites in New Zealand. N. Z. J. Ecol. 2003, 27, 147–155. [Google Scholar]
- Cowan, P.E.; Glen, A.S. Trichosurus vulpecula, In The Handbook of New Zealand Mammals, 3rd ed.; King, C.M., Forsyth, D.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 43–77. [Google Scholar]
- GWRC. Greater Wellington Regional Council Regional Pest Management Strategy 2002–2022 Pest Plants and Pest Animals, Operational Plan 2005–2006 Wellington. 2005; p. 17. Available online: https://www.gw.govt.nz/assets/Documents/2005/09/v1-Operational_Plan_2005_06.pdf (accessed on 14 April 2020).
| Parameter Name | Description |
|---|---|
| Season | Sampling season: spring or autumn (2 levels, categorical) |
| City | City: Hamilton, Wellington, Dunedin (3 levels, categorical) |
| Habitat type | Forest fragment, Amenity park, Residential garden (3 levels, categorical) |
| Method | Method of detection: Cards (tracking tunnels, chew cards) or camera (2 levels, categorical) |
| Distance to nearest field | Distance from sampling station to nearest grassy field > 1 ha (km) |
| Distance to coast (Dunedin) | Distance to the coast in Dunedin. (Rat and mouse models only) (km) |
| Distance to coast (Wellington) | Distance to the coast in Wellington. (Rat and mouse models only) (km) |
| Distance to freshwater | Distance to nearest freshwater body. (Rat and mouse models only) (km) |
| Residential distance to forest | Distance of residential sites to nearest forest fragment > 1 ha (km) |
| Amenity distance to forest | Distance from amenity park sites to nearest forest fragment > 1 ha (km) |
| DBH | Diameter at breast height of the largest tree in sampling plot (m) |
| Leaf litter cover | Proportion of ground area covered by leaf litter. |
| Herb layer cover | Total vegetation cover within the herb layer (0–0.3 m high) |
| Shrub layer cover | Total vegetation cover within the shrub layer (0.3–2 m high) |
| Lower canopy cover | Average total vegetation cover in sub- and lower canopy layers (2–12 m high) |
| Line | Transect line identifier (random effect, 48 levels, categorical) |
| Station | Station within each line (random effect, 10 levels, categorical) |
| Residential only parameters | |
| Compost | Presence/absence compost heap/bin (2 levels, categorical) |
| Level of property maintain | Level of property maintenance: low/medium/high (3 levels, categorical) |
| Native vegetation | Proportion native vegetation cover |
| Lawns | Proportion cover by lawns |
| Garden bed cover | Proportion cover of regularly turned soil beds i.e., flower & vegetable beds |
| Artificial features | Proportion cover of artificial hard landscaping features i.e., buildings, decking, paving, fences |
| Parameter | OR | CI | MPE | Significance |
|---|---|---|---|---|
| Intercept | 0.46 | 0.13–1.54 | 0.857 | |
| Season = Autumn | 3.22 | 2.36–4.27 | 1 | ** |
| City = Hamilton | 0.18 | 0.04–0.80 | 0.966 | + |
| City = Dunedin | 0.14 | 0.03–0.67 | 0.982 | * |
| Habitat = Amenity | 0.46 | 0.13–1.67 | 0.845 | |
| Habitat = Residential | 0.63 | 0.16–2.29 | 0.714 | |
| Method= Cards | 0.34 | 0.23–0.50 | 1 | ** |
| Hamilton: Amenity | 1.55 | 0.20–13.7 | 0.632 | |
| Dunedin: Amenity | 0.49 | 0.05–4.80 | 0.699 | |
| Hamilton: Residential | 4.46 | 0.47–50.2 | 0.853 | |
| Dunedin: Residential | 6.61 | 0.75–55.4 | 0.930 | |
| Distance to nearest field | 2.08 | 0.76–5.89 | 0.881 | |
| Residential distance to forest | 0.05 | 0–0.58 | 0.982 | * |
| Amenity distance to forest | 9.29 | 2.63–34.43 | 0.998 | ** |
| Shrub layer cover | 3.42 | 1.27–9.03 | 0.981 | * |
| Lower canopy cover | 4.51 | 1.43–13.54 | 0.988 | * |
| Leaf litter | 0.44 | 0.20–0.95 | 0.960 | + |
| Residential Model | ||||
| Level of maintenance = medium | 0.43 | 0.14–1.16 | 0.918 | |
| Level of maintenance = high | 0.39 | 0.12–1.31 | 0.909 | |
| Compost = Yes | 2.77 | 1.19–6.25 | 0.986 | * |
| Proportion of native vegetation | 0.13 | 0.02–0.71 | 0.977 | * |
| Mown lawn cover | 0.26 | 0.04–1.41 | 0.902 | |
| Garden bed cover | 0.34 | 0.01–6.58 | 0.716 | |
| Artificial characteristics | 0.43 | 0.05–3.03 | 0.760 |
| Parameter | OR | CI | MPE | Significance |
|---|---|---|---|---|
| Intercept | 0.33 | 0.13–0.78 | 0.982 | * |
| Season = Autumn | 0.31 | 0.23–0.41 | 1 | ** |
| City = Hamilton | 1.23 | 0.55–2.89 | 0.666 | |
| City= Dunedin | 8.24 | 3.61–19.7 | 1 | ** |
| Habitat = Amenity | 2.86 | 1.11–7.10 | 0.969 | + |
| Habitat = Residential | 1.89 | 0.84–4.47 | 0.902 | |
| Method = Cards | 0.56 | 0.40–0.81 | 1 | ** |
| Amenity distance to forest | 0.30 | 0.10–0.87 | 0.973 | + |
| Lower canopy layer | 2.31 | 0.91–6.31 | 0.922 | |
| Herb layer | 0.61 | 0.30–1.17 | 0.898 | |
| Residential Model | ||||
| Level of maintenance = medium | 1.14 | 0.32–4.23 | 0.555 | |
| Level of maintenance = high | 0.98 | 0.24–3.98 | 0.512 | |
| Compost = Yes | 0.67 | 0.26–1.63 | 0.770 | |
| Proportion of native vegetation | 0.29 | 0.05–1.70 | 0.874 | |
| Lawn cover | 1.20 | 0.17–7.16 | 0.562 | |
| Garden bed cover | 0.64 | 0.03–18.6 | 0.583 | |
| Artificial characteristics | 0.40 | 0.04–3.92 | 0.754 |
| Parameter | OR | CI | MPE | Significance |
|---|---|---|---|---|
| Intercept | 0.35 | 0.12–1.04 | 0.947 | |
| Season = Autumn | 5.26 | 3.8–7.01 | 1 | ** |
| City = Hamilton | 0.70 | 0.23–2.14 | 0.712 | |
| City = Dunedin | 0.52 | 0.17–1.59 | 0.838 | |
| Habitat = Amenity | 1.35 | 0.52–3.49 | 0.702 | |
| Habitat = Residential | 0.59 | 0.23–1.52 | 0.824 | |
| Method = Cards | 0.83 | 0.55–1.22 | 0.784 | |
| Hamilton: Amenity | 0.45 | 0.10–2.00 | 0.812 | |
| Dunedin: Amenity | 1.21 | 0.27–5.50 | 0.588 | |
| Hamilton: Residential | 0.14 | 0.03–0.73 | 0.978 | * |
| Dunedin: Residential | 0.44 | 0.09–2.07 | 0.814 | |
| Distance to nearest field | 0.58 | 0.25–1.25 | 0.877 | |
| Lower canopy cover | 0.36 | 0.12–1.03 | 0.943 | |
| Leaf litter cover | 0.47 | 0.22–1.07 | 0.941 | |
| Herb layer cover | 3.18 | 1.47–6.89 | 0.994 | * |
| Residential Model | ||||
| Level of maintenance: medium | 0.85 | 0.29–2.65 | 0.598 | |
| Level of maintenance: high | 0.45 | 0.12–1.60 | 0.844 | |
| Compost: Yes | 0.97 | 0.43–2.17 | 0.52 | |
| Proportion of native vegetation | 1.40 | 0.26–7.24 | 0.63 | |
| Lawn cover | 0.61 | 0.11–3.68 | 0.68 | |
| Garden bed cover | 2.54 | 0.11–54.1 | 0.69 | |
| Artificial characteristics | 0.34 | 0.05–3.13 | 0.80 |
| Parameter | OR | CI | MPE | Significance |
|---|---|---|---|---|
| Intercept | 526 | 82.3–3577 | 1 | ** |
| Season = Autumn | 0.41 | 0.24–0.70 | 0.998 | ** |
| City = Hamilton | 0 | 0–0.01 | 0.994 | * |
| Habitat = Amenity | 0.01 | 0–0.04 | 1 | ** |
| Habitat = Residential | 0 | 0–0.00 | 1 | ** |
| Hamilton: Amenity | 43.1 | 5.11–373 | 0.992 | * |
| Hamilton: Residential Method = Cards | 4.69 0.21 | 0.23–83.2 0.10–0.43 | 0.805 1 | ** |
| Distance to nearest field | 0.25 | 0.03–2.34 | 0.854 | |
| Amenity distance to forest | 0.07 | 0.02–0.27 | 1 | ** |
| Residential distance to forest | 22.9 | 0.87–784 | 0.933 | |
| DBH | 4.36 | 0.95–19.9 | 0.949 |
| Parameter | OR | CI | MPE | Significance |
|---|---|---|---|---|
| Intercept | 0.47 | 0–38.3 | 0.62 | |
| City = Tauranga | 1.34 | 0.02–96.8 | 0.543 | |
| Habitat = Amenity | 19.17 | 0.15–2707 | 0.855 | |
| Habitat = Residential | 0 | 0–0.13 | 0.99 | * |
| Tauranga: Amenity | 1.55 | 0.01–564 | 0.552 | |
| Tauranga: Residential Method = Cards | 0.81 0.04 | 0–284 0–0.24 | 0.524 1 | ** |
| Distance to nearest field | 0.02 | 0–10.3 | 0.856 | |
| Residential distance to forest | 0.65 | 0–1001 | 0.54 | |
| Amenity distance to forest | 0.8 | 0–505 | 0.524 |
| Parameter | OR | CI | MPE | Significance |
|---|---|---|---|---|
| Intercept | 0 | 0–0.01 | 1 | ** |
| City = Tauranga | 11.4 | 0.16–777 | 0.858 | |
| Habitat = Amenity | 7.41 | 0.04–1361 | 0.738 | |
| Habitat = Residential | 85.6 | 0.94–9633 | 0.949 | |
| Method = Cards | 0.08 | 0.01–0.58 | 0.992 | * |
| Distance to nearest field | 2.57 | 0.01–930 | 0.6 |
| Parameter | OR | CI | MPE | Significance |
|---|---|---|---|---|
| Intercept | 0.02 | 0–0.59 | 0.976 | * |
| City = Tauranga | 14.1 | 0.2–695 | 0.848 | |
| Habitat = Amenity | 98.7 | 1.45–7806 | 0.962 | + |
| Habitat = Residential | 0.05 | 0–4.33 | 0.876 | |
| Tauranga: Amenity | 0.09 | 0–28.4 | 0.768 | |
| Tauranga: Residential Method = Cards | 0.01 2.5 | 0–1.17 0.62–9.72 | 0.943 0.87 | |
| Distance to nearest field | 1.7 | 0–664 | 0.558 |
| Parameter | OR | CI | MPE | Significance |
|---|---|---|---|---|
| Intercept | 0 | 0–0.11 | 0.999 | ** |
| City = Tauranga | 1.88 | 0.03–142.32 | 0.605 | |
| Habitat = Amenity | 8.32 | 0.08–841.47 | 0.781 | |
| Habitat = Residential | 0.01 | 0–3.39 | 0.911 | |
| Tauranga: Amenity | 0.08 | 0–21.81 | 0.778 | |
| Tauranga: Residential Method = Cards | 0.13 0.11 | 0–176 0.02–0.52 | 0.685 1 | ** |
| Distance to nearest field | 526.39 | 3.17–134,313.31 | 0.972 | + |
| Residential distance to forest | 0.16 | 0–393.44 | 0.654 | |
| Amenity distance to forest | 0.84 | 0–470.29 | 0.519 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, K.F.; Wilson, D.J.; Hartley, S.; Innes, J.G.; Fitzgerald, N.B.; Miller, P.; van Heezik, Y. Invasive Urban Mammalian Predators: Distribution and Multi-Scale Habitat Selection. Biology 2022, 11, 1527. https://doi.org/10.3390/biology11101527
Miller KF, Wilson DJ, Hartley S, Innes JG, Fitzgerald NB, Miller P, van Heezik Y. Invasive Urban Mammalian Predators: Distribution and Multi-Scale Habitat Selection. Biology. 2022; 11(10):1527. https://doi.org/10.3390/biology11101527
Chicago/Turabian StyleMiller, Kim F., Deborah J. Wilson, Stephen Hartley, John G. Innes, Neil B. Fitzgerald, Poppy Miller, and Yolanda van Heezik. 2022. "Invasive Urban Mammalian Predators: Distribution and Multi-Scale Habitat Selection" Biology 11, no. 10: 1527. https://doi.org/10.3390/biology11101527
APA StyleMiller, K. F., Wilson, D. J., Hartley, S., Innes, J. G., Fitzgerald, N. B., Miller, P., & van Heezik, Y. (2022). Invasive Urban Mammalian Predators: Distribution and Multi-Scale Habitat Selection. Biology, 11(10), 1527. https://doi.org/10.3390/biology11101527






