Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Patients
2.2. Sandwich ELISA Technique
2.3. Perioperative Management
2.3.1. Anesthesia and Surgical Procedure
2.3.2. PGD Grading
2.3.3. Postoperative Immunosuppressive Regimen
2.3.4. Definition of SOFA
2.4. Statistical Analysis
3. Results
3.1. Demographic Data
3.1.1. Perioperative Course of CCL4, IL-23, LCN2, Leucocytes, and FSTL1 Serum Concentrations
3.1.2. Greater Relative Increase of FSTL1 in Patients with PGD
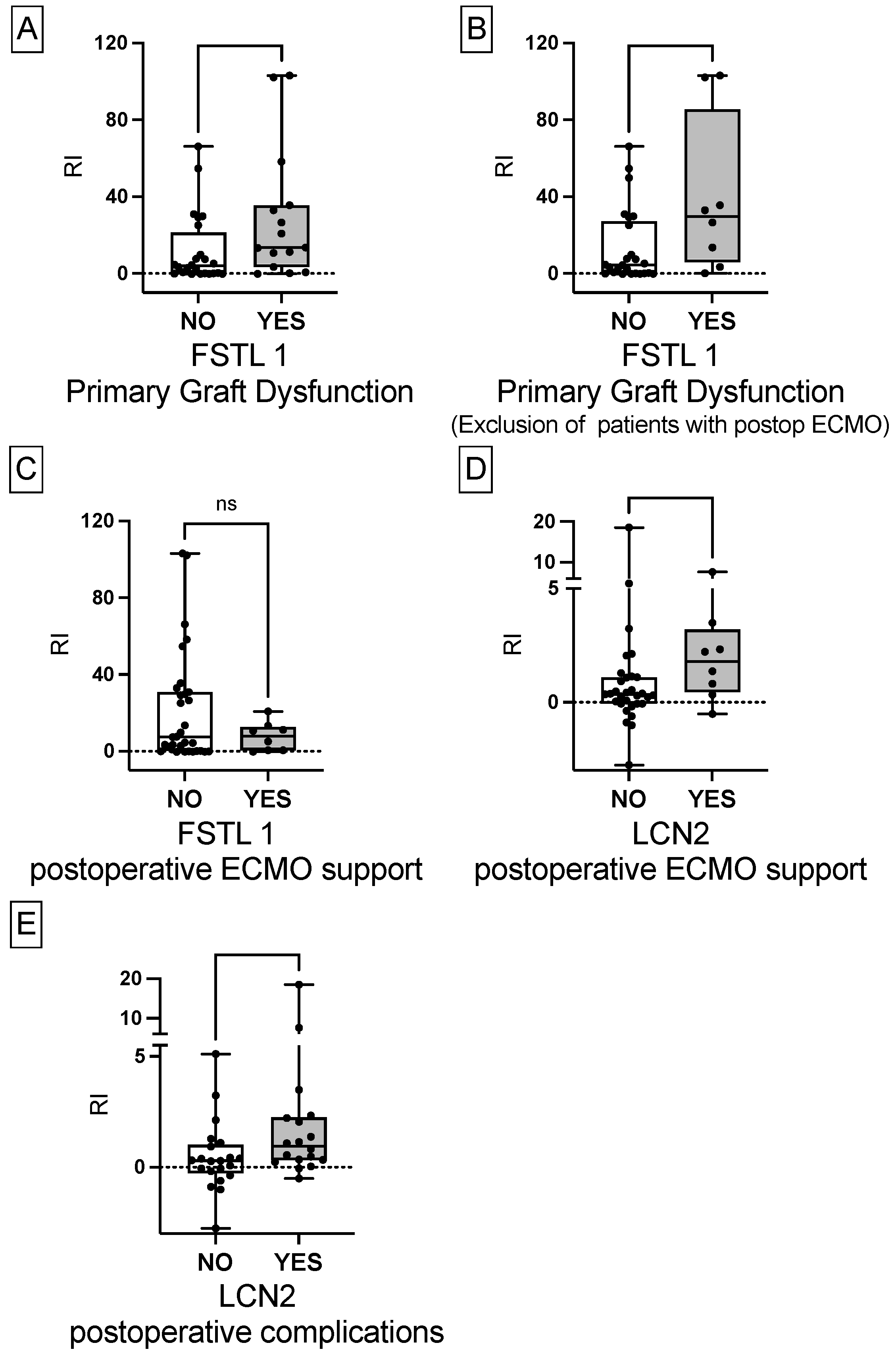
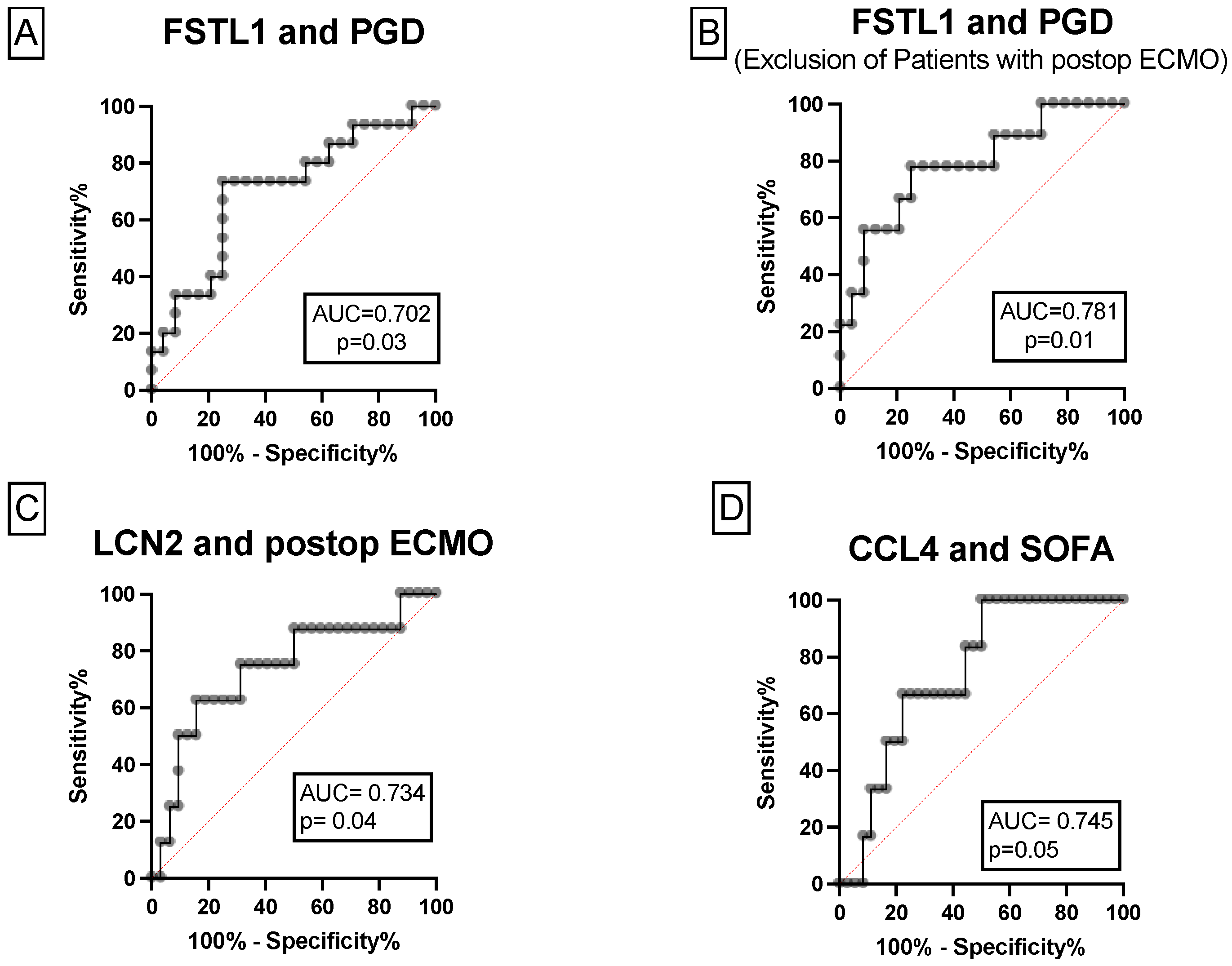
3.1.3. The Associations between FSTL1, CCL4, LCN2 and Postoperative Outcome
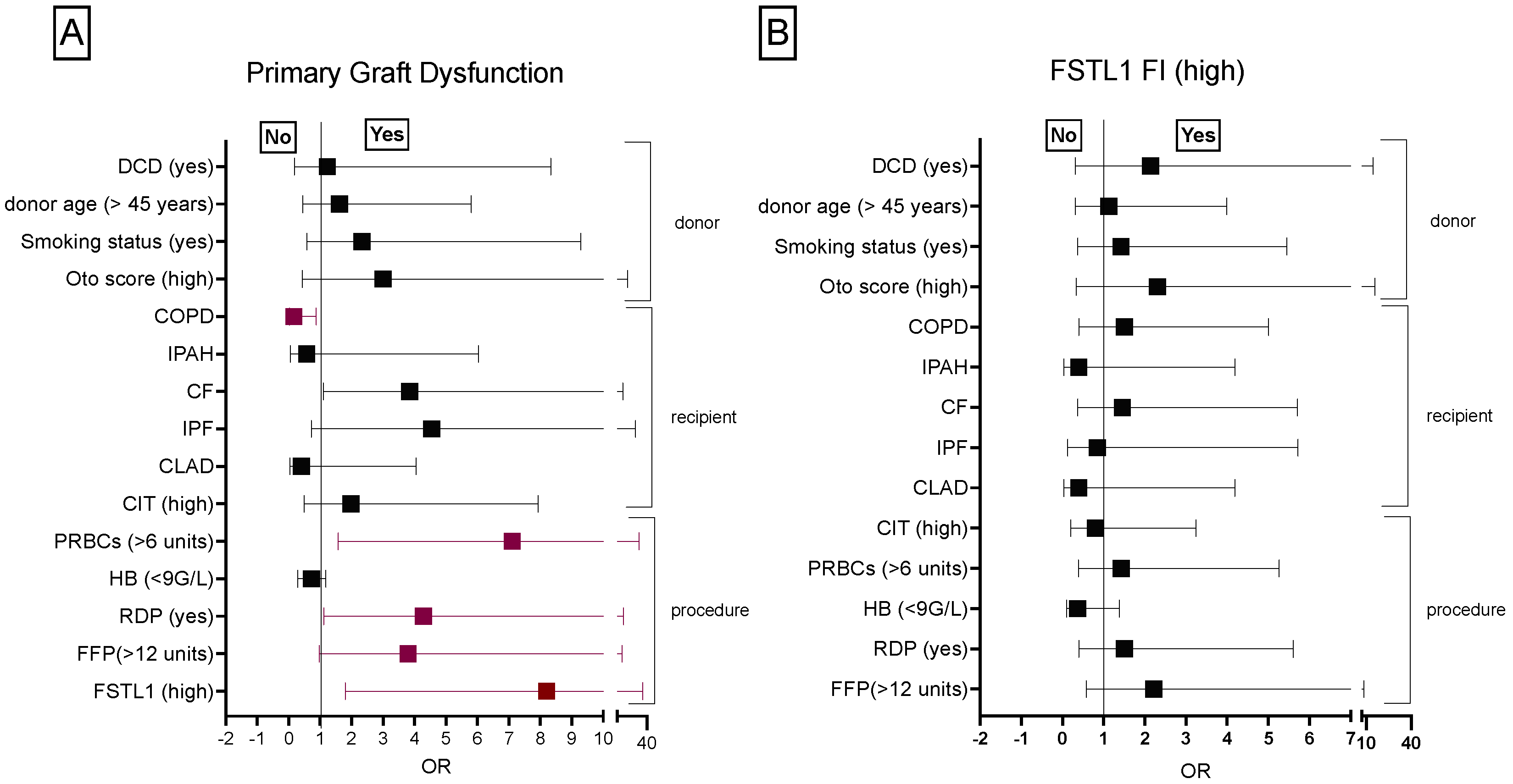
3.1.4. Donor, Recipient and Procedure Related Risk Factors Associated with PGD
3.1.5. FSTL1 Concentrations Measured in PRBCs and FFPs
3.1.6. Short-Term and Long-Term Outcome of Patients Undergoing LuTX
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, M.I.; Pither, T.L.; Fisher, A.J. Pathophysiology and classification of primary graft dysfunction after lung transplantation. J. Thorac. Dis. 2017, 9, 4084–4097. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Christie, J.D. Primary graft dysfunction. Proc. Am. Thorac Soc. 2009, 6, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shilling, R.A.; Wilkes, D.S. Immunobiology of Chronic Lung Allograft Dysfunction: New Insights from the Bench and Beyond. Am. J. Transplant. 2009, 9, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef]
- Christie, J.D.; Carby, M.; Bag, R.; Corris, P.; Hertz, M.; Weill, D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2005, 24, 1454–1459. [Google Scholar] [CrossRef]
- De Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef]
- Fisher, A.J.; Donnelly, S.C.; Hirani, N.; Haslett, C.; Strieter, R.M.; Dark, J.H.; Corris, P.A. Elevated Levels of Interleukin-8 in Donor Lungs Is Associated with Early Graft Failure after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2001, 163, 259–265. [Google Scholar] [CrossRef]
- Sayah, D.M.; Mallavia, B.; Liu, F.; Ortiz-Muñoz, G.; Caudrillier, A.; DerHovanessian, A.; Ross, D.J.; Iii, J.P.L.; Saggar, R.; Ardehali, A.; et al. Neutrophil Extracellular Traps Are Pathogenic in Primary Graft Dysfunction after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 455–463. [Google Scholar] [CrossRef]
- Veraar, C.; Kliman, J.; Benazzo, A.; Oberndorfer, F.; Laggner, M.; Hacker, P.; Raunegger, T.; Janik, S.; Jaksch, P.; Klepetko, W.; et al. Potential novel biomarkers for chronic lung allograft dysfunction and azithromycin responsive allograft dysfunction. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Chao, J.; Donham, P.; van Rooijen, N.; Wood, J.G.; Gonzalez, N.C. Monocyte Chemoattractant Protein–1 Released from Alveolar Macrophages Mediates the Systemic Inflammation of Acute Alveolar Hypoxia. Am. J. Respir. Cell Mol. Biol. 2011, 45, 53–61. [Google Scholar] [CrossRef]
- Cantu, E.; Lederer, D.; Meyer, K.; Milewski, K.; Suzuki, Y.; Shah, R.J.; Diamond, J.M.; Meyer, N.J.; Tobias, J.W.; Baldwin, D.A.; et al. Gene Set Enrichment Analysis Identifies Key Innate Immune Pathways in Primary Graft Dysfunction After Lung Transplantation. Am. J. Transplant. 2013, 13, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Szerafin, T.; Mitterbauer, A.; Debreceni, T.; Maros, T.; Dworschak, M.; Roth, G.A.; Ankersmit, H.J. Low tidal volume ventilation during cardiopulmonary bypass reduces postoperative chemokine serum concentrations. Thorac. Cardiovasc. Surg. 2014, 62, 677–682. [Google Scholar] [PubMed]
- Hoetzenecker, K.; Benazzo, A.; Stork, T.; Sinn, K.; Schwarz, S.; Schweiger, T.; Klepetko, W.; Kifjak, D.; Baron, D.M.; Hager, H.; et al. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J. Thorac. Cardiovasc. Surg. 2020, 160, 320–327.e321. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Warszawska, J.M.; Schenk, P.; Debreceni, T.; Dworschak, M.; Roth, G.; Szerafin, T.; Ankersmit, H.J. Intraoperative ventilation strategy during cardiopulmonary bypass attenuates the release of matrix metalloproteinases and improves oxygenation. J. Surg. Res. 2015, 195, 294–302. [Google Scholar] [CrossRef]
- Lubberts, E.; Koenders, I.M.; Berg, W.B.V.D. The role of T cell interleukin-17 in conducting destructive arthritis: Lessons from animal models. Arthritis Res. Ther. 2005, 7, 29–37. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef]
- Bobadilla, J.L.; Love, R.B.; Jankowska-Gan, E.; Xu, Q.; Haynes, L.D.; Braun, R.K.; Hayney, M.S.; Munoz del Rio, A.; Meyer, K.; Greenspan, D.S.; et al. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am. J. Respir. Crit. Care Med. 2008, 177, 660–668. [Google Scholar] [CrossRef]
- Cao, H.; Lan, Q.; Shi, Q.; Zhou, X.; Liu, G.; Liu, J.; Tang, G.; Qiu, C.; Qiu, C.; Xu, J.; et al. Anti-IL-23 antibody blockade of IL-23/IL-17 pathway attenuates airway obliteration in rat orthotopic tracheal transplantation. Int. Immunopharmacol. 2011, 11, 569–575. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Liu, T.; Wu, J.; Zhao, J.; Wang, J.; Zou, M.; Cao, L.; Liu, X.; Pan, Y.; et al. FSTL1 aggravates cigarette smoke-induced airway inflammation and airway remodeling by regulating autophagy. BMC Pulm. Med. 2021, 21, 45. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Li, X.; Zhao, J.; Geng, Y.; Ning, W. Follistatin like-1 (Fstl1) is required for the normal formation of lung airway and vascular smooth muscle at birth. PLoS ONE 2017, 12, e0177899. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Miller, M.; Cao, L.; Zhao, J.; Wu, J.; Wang, J.; Liu, L.; Li, S.; Zou, M.; et al. Autophagy plays a role in FSTL1-induced epithelial mesenchymal transition and airway remodeling in asthma. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, L27–L40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, W.; Liu, J.; Li, J.; Wang, J.; Zhang, Y.; Zhang, Z.; Liu, Y.; Jin, Y.; Li, J.; et al. Follistatin-like 1 protects against hypoxia-induced pulmonary hypertension in mice. Sci. Rep. 2017, 7, srep45820. [Google Scholar] [CrossRef] [PubMed]
- Veraar, C.; Schwarz, S.; Thanner, J.; Direder, M.; Boehm, P.M.; Harnoncourt, L.; Ortmayr, J.; Veraar, C.; Mascherbauer, J.; Klepetko, W.; et al. Transient perioperative inflammation following lung transplantation and major thoracic surgery with elective extracorporeal support: A prospective observational study. Ann. Transl. Med. 2021, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Oto, T.; Levvey, B.J.; Whitford, H.; Griffiths, A.P.; Kotsimbos, T.; Williams, T.J.; Snell, G.I. Feasibility and Utility of a Lung Donor Score: Correlation with Early Post-Transplant Outcomes. Ann. Thorac. Surg. 2007, 83, 257–263. [Google Scholar] [CrossRef]
- Benazzo, A.; Schwarz, S.; Frommlet, F.; Schweiger, T.; Jaksch, P.; Schellongowski, P.; Staudinger, T.; Klepetko, W.; Lang, G.; Hoetzenecker, K.; et al. Twenty-year experience with extracorporeal life support as bridge to lung trans-plantation. J. Thorac. Cardiovasc. Surg. 2019, 157, 2515–2525.e2510. [Google Scholar] [CrossRef]
- Moser, B.; Megerle, A.; Bekos, C.; Janik, S.; Szerafin, T.; Birner, P.; Schiefer, A.-I.; Mildner, M.; Lang, I.; Skoro-Sajer, N.; et al. Local and Systemic RAGE Axis Changes in Pulmonary Hypertension: CTEPH and iPAH. PLoS ONE 2014, 9, e106440. [Google Scholar] [CrossRef]
- Schwarz, S.; Benazzo, A.; Dunkler, D.; Muckenhuber, M.; del Sorbo, L.; di Nardo, M.; Sinn, K.; Moser, B.; Matilla, J.R.; Lang, G.; et al. Ventilation parameters and early graft function in double lung transplantation. J. Heart Lung Transplant. 2020, 40, 4–11. [Google Scholar] [CrossRef]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V.; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults with Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Jiang, D.; Dong, Y.; Liu, Y.; Zhang, S.; Guo, J.; Qi, C.; Zhao, C.; Jiang, F.; et al. Targeting FSTL1 for Multiple Fibrotic and Systemic Autoimmune Diseases. Mol. Ther. 2020, 29, 347–364. [Google Scholar] [CrossRef]
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534. [Google Scholar] [CrossRef]
- Menger, J.; Koch, S.; Mouhieddine, M.; Schwarz, S.; Hoetzenecker, K.; Jaksch, P.; Steinlechner, B.; Dworschak, M. Initial Postoperative Hemoglobin Values Are Independently Associated with One-Year Mortality in Patients Undergoing Double-Lung Transplantation Requiring Intraoperative Transfusion. J. Cardiothorac. Vasc. Anesthesia 2021, 35, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Le Luduec, J.B.; Condamine, T.; Louvet, C.; Thebault, P.; Heslan, J.M.; Heslan, M.; Chiffoleau, E.; Cuturi, M.C. An immunomodulatory role for follistatin-like 1 in heart allograft transplantation. Am. J. Transplant. 2008, 8, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Kahli, A.; Guenancia, C.; Zeller, M.; Grosjean, S.; Stamboul, K.; Rochette, L.; Girard, C.; Vergely, C. Growth Differentiation Factor-15 (GDF-15) Levels Are Associated with Cardiac and Renal Injury in Patients Undergoing Coronary Artery Bypass Grafting with Cardiopulmonary Bypass. PLoS ONE 2014, 9, e105759. [Google Scholar] [CrossRef] [PubMed]
- Whitson, B.A.; Prekker, M.E.; Herrington, C.S.; Whelan, T.P.; Radosevich, D.M.; Hertz, M.I.; Dahlberg, P.S. Primary Graft Dysfunction and Long-term Pulmonary Function After Lung Transplantation. J. Heart Lung Transplant. 2007, 26, 1004–1011. [Google Scholar] [CrossRef]
- Shah, R.; Diamond, J.; Kawut, S.; Cantu, E.; Wickersham, N.; Ware, L.; Christie, J. 207 Plasma RAGE Levels Measured 24 Hours after Lung Transplantation Are Associated with Bronchiolitis Obliterans Syndrome (BOS). J. Heart Lung Transplant. 2012, 31, S77–S78. [Google Scholar] [CrossRef]
- Ferrari, R.S.; Andrade, C.F. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.J.; Granger, D.N. Mechanisms of Reperfusion Injury. Am. J. Med. Sci. 1994, 307, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Carratelli, M.; Cornelli, U.; de Sanctis, M.T.; Incandela, L.; Barsotti, A.; Terranova, R.; Nicolaides, A. A simple test to monitor oxidative stress. Int. Angiol. 1999, 18, 127–130. [Google Scholar]
- Hayakawa, S.; Ohashi, K.; Shibata, R.; Takahashi, R.; Otaka, N.; Ogawa, H.; Ito, M.; Kanemura, N.; Hiramatsu-Ito, M.; Ikeda, N.; et al. Association of Circulating Follistatin-Like 1 Levels with Inflammatory and Oxidative Stress Markers in Healthy Men. PLoS ONE 2016, 11, e0153619. [Google Scholar] [CrossRef]
- Murakami, K.; Tanaka, M.; Usui, T.; Kawabata, D.; Shiomi, A.; Iguchi-Hashimoto, M.; Shimizu, M.; Yukawa, N.; Yoshifuji, H.; Nojima, T.; et al. Follistatin-related protein/follistatin-like 1 evokes an innate immune response via CD14 and toll-like receptor 4. FEBS Lett. 2012, 586, 319–324. [Google Scholar] [CrossRef]
- Ogura, Y.; Ouchi, N.; Ohashi, K.; Shibata, R.; Kataoka, Y.; Kambara, T.; Kito, T.; Maruyama, S.; Yuasa, D.; Matsuo, K.; et al. Therapeutic Impact of Follistatin-like 1 on Myocardial Ischemic Injury in Preclinical Models. Circulation 2012, 126, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, T.; Wu, J.; Li, T.; Jiao, X.; Zhang, H.; Zhao, J.; Wang, J.; Liu, L.; Cao, L.; et al. The Correlation between FSTL1 Expression and Airway Remodeling in Asthmatics. Mediat. Inflamm. 2017, 2017, 7918472. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Beppu, A.; Rosenthal, P.; Pham, A.; Das, S.; Karta, M.; Song, D.J.; Vuong, C.; Doherty, T.; Croft, M.; et al. Fstl1 Promotes Asthmatic Airway Remodeling by Inducing Oncostatin M. J. Immunol. 2015, 195, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Esnault, S.; Kurten, R.C.; Kelly, E.A.; Beppu, A.; Das, S.; Rosenthal, P.; Ramsdell, J.; Croft, M.; Zuraw, B.; et al. Segmental allergen challenge increases levels of airway follistatin-like 1 in patients with asthma. J. Allergy Clin. Immunol. 2016, 138, 596–599.e4. [Google Scholar] [CrossRef][Green Version]
- Fink-Neuboeck, N.; Lindenmann, J.; Bajric, S.; Maier, A.; Riedl, R.; Weinberg, A.M.; Smolle-Juettner, F.M. Clinical impact of interleukin 6 as a predictive biomarker in the early diagnosis of postoperative systemic inflammatory response syndrome after major thoracic surgery: A prospective clinical trial. Surgery 2016, 160, 443–453. [Google Scholar] [CrossRef]
- Chang, T.-T.; Yang, H.-Y.; Chen, C.; Chen, J.-W. CCL4 Inhibition in Atherosclerosis: Effects on Plaque Stability, Endothelial Cell Adhesiveness, and Macrophages Activation. Int. J. Mol. Sci. 2020, 21, 6567. [Google Scholar] [CrossRef]
- Pesonen, E.J.; Korpela, R.; Leijala, M.; Sairanen, H.; Pitkänen, O.M.; Ravio, K.O.; Venge, P.; Andersson, S. Prolonged granulocyte activation, as well as hypoxanthine and free radical production after open heart surgery in children. Intensive Care Med. 1996, 22, 500–506. [Google Scholar] [CrossRef]
- Passov, A.; Petäjä, L.; Pihlajoki, M.; Salminen, U.-S.; Suojaranta, R.; Vento, A.; Andersson, S.; Pettilä, V.; Schramko, A.; Pesonen, E. The origin of plasma neutrophil gelatinase-associated lipocalin in cardiac surgery. BMC Nephrol. 2019, 20, 182. [Google Scholar] [CrossRef]
- Dent, C.L.; Ma, Q.; Dastrala, S.; Bennett, M.; Mitsnefes, M.M.; Barasch, J.; Devarajan, P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: A prospective uncontrolled cohort study. Crit. Care 2007, 11, R127–R128. [Google Scholar] [CrossRef]
- Al-Ruzzeh, S.; Hoare, G.; Marczin, N.; Asimakopoulos, G.; George, S.; Taylor, K.; Amrani, M. Off-Pump Coronary Artery Bypass Surgery Is Associated with Reduced Neutrophil Activation as Measured by the Expression of CD11b: A Prospective Randomized Study. Heart Surg. Forum 2003, 6, 89–93. [Google Scholar] [CrossRef]
- Mishra, J.; Ma, Q.; Kelly, C.; Mitsnefes, M.; Mori, K.; Barasch, J.; Devarajan, P. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr. Nephrol. 2006, 21, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]

| Diagnosis | COPD | CF | IPF | IPAH | CLAD |
|---|---|---|---|---|---|
| Basic demographic data | |||||
| n (%) | 15 (100) | 12 (100) | 6 (100) | 4 (100) | 5 (100) |
| age (yrs), mean ± SD | 59 ± 6 | 27 ± 7 | 54 ± 12 | 39 ± 12 | 37 ± 5 |
| female:male ratio n | 4:11 | 7:5 | 2:4 | 2:2 | 3:2 |
| ECMO support | |||||
| ECMO preoperative n (%) | - | 2 (17) | 2 (33) | - | 1 (20) |
| ECMO Intraoperative n (%) | 15 (100) | 12 (100) | 6 (100) | 4 (100) | 5 (100) |
| ECMO postoperative n (%) | 2 (13) | 2 (17) | 2 (33) | 1 (25) | 1 (20) |
| Intraoperative characteristics | |||||
| Time (min) | |||||
| Length of ECC, mean ± SD | 183 ± 31 | 193 ± 50 | 174 ± 60 | 236 ± 39 | 237 ± 77 |
| Length of surgery, mean ± SD | 315 ± 70 | 312 ± 74 | 357 ± 57 | 380 ± 47 | 369 ± 131 |
| Vasoactive administration | |||||
| Norepinephrine n (%) | - | ||||
| <0.1 μg/kg/min | 11 (73) | 6 (50) | 1 (17) | 2 (50) | 4 (80) |
| >0.1–0.5 μg/kg/min | 4 (27) | 6 (50) | 5 (83) | - | 1 (20) |
| >0.5 μg/kg/min | - | - | - | 2 (50) | - |
| Blood and coagulation products | |||||
| RDP n (%) | 13 (86) | 6 (50) | 2 (33) | 1 (25) | 4 (80) |
| PRBCs, mean ± SD | 5 ± 3 | 9 ± 9 | 6 ± 3 | 12 ± 12 | 7 ± 3 |
| FFPs, mean ± SD | 11 ± 5 | 14 ± 12 | 9 ± 5 | 20 ± 18 | 10 ± 3 |
| Max. BL (mg/dl), mean ± SD | 3.1 ± 1.2 | 3.5 ± 1.3 | 3.8 ± 0.9 | 4.2 ± 2.1 | 3.6 ± 0.3 |
| Min. HB (g/dl), mean ± SD | 9.1 ± 0.9 | 9.2 ± 1.5 | 9.6 ± 1.2 | 8.5 ± 2.2 | 9.3 ± 1.3 |
| COPD | CF | IPF | IPAH | CLAD | |
|---|---|---|---|---|---|
| PGD-Grading (0 h) n (%) | |||||
| 0 | 13 (86) | 5 (41) | 2 (33) | 3 (75) | 5 (100) |
| 1 | - | 1 (8) | - | - | |
| 2 | - | 1 (8) | 2 (33) | - | - |
| 3 | - | 2 (16) | - | - | - |
| ECMO (3) | 1 (7) | 1 (8) | 1 (16) | 1 (25) | - |
| ECMO (ungradable) | 1 (7) | 2 (16) | 1 (16) | - | |
| Extubated | - | - | - | - | - |
| PGD-Grading (24 h) n (%) | |||||
| 0 | 7 (47) | 5 (41) | 3 (50) | 3 (75) | 2 (60) |
| 1 | - | 1 (8) | - | - | 1 (20) |
| 2 | - | 1 (8) | - | - | |
| 3 | - | 1 (8) | - | ||
| ECMO (3) | 1 (7) | 1 (8) | 1 (16) | 1 (25) | |
| ECMO (ungradable) | 1 (7) | - | 1 (16) | - | |
| Extubated | 6 (40) | 4 (33) | 1 (16) | - | 1 (20) |
| PGD-Grading (48 h) n (%) | |||||
| 0 | 3 (20) | 3 (25) | 1 (16) | 4 (80) | 1 (20) |
| 1 | 1 (7) | - | 1 (16) | - | |
| 2 | - | - | 1 (16) | - | |
| 3 | - | - | - | - | |
| ECMO (3) | - | 1 (8) | - | - | |
| ECMO (ungradable) | - | - | 1 (16) | - | |
| Extubated | 11 (73) | 8 (66) | 2 (33) | - | 4 (80) |
| PGD-Grading (72 h) n (%) | |||||
| 0 | 2 (13) | 2 (16) | 2 (33) | 2 (50) | - |
| 1 | - | - | - | - | - |
| 2 | - | 1 (8) | - | - | - |
| 3 | - | - | - | - | - |
| ECMO (3) | - | - | - | - | - |
| ECMO (ungradable) | - | - | 1 (16) | - | - |
| Extubated | 13 (87) | 9 (75) | 4 (50) | 2 (50) | 5 (100) |
| Diagnosis | COPD | CF | IPF | IPAH | CLAD |
|---|---|---|---|---|---|
| High SOFA, n (%) | - | 1 (8) | 1 (16) | 2 (50) | 2 (20) |
| PGD, n (%) | 2 (13) | 7 (58) | 4 (66) | 1 (25) | - |
| AMR, n (%) | - | 1 (8) | - | - | - |
| Re-intubation, n (%) | 2 (13) | - | - | - | - |
| OBS, n (%) | 2 (13) | - | 2 (33) | 1 (25) | 1 (20) |
| HF, n (%) | 1 (6) | 1 (8) | - | 1 (25) | 2 (20) |
| CLAD, n (%) | 2 (13) | 2 (16) | 1 (16) | 1 (25) | 1 (20) |
| BOS, n (%) | 2 (13) | 1 (8) | - | - | - |
| RAS, n (%) | - | - | - | - | - |
| Mixed, n (%) | - | 1 (8) | 1 (16) | 1 (25) | 1 (20) |
| 30-day mortality, n (%) | 1(6) | - | - | - | 1 (20) |
| 3-year mortality, n (%) | 6 (40) | 2 (16) | 2 (33) | - | 4 (80) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veraar, C.; Kirschner, E.; Schwarz, S.; Jaksch, P.; Hoetzenecker, K.; Tschernko, E.; Dworschak, M.; Ankersmit, H.J.; Moser, B. Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO. Biology 2022, 11, 1475. https://doi.org/10.3390/biology11101475
Veraar C, Kirschner E, Schwarz S, Jaksch P, Hoetzenecker K, Tschernko E, Dworschak M, Ankersmit HJ, Moser B. Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO. Biology. 2022; 11(10):1475. https://doi.org/10.3390/biology11101475
Chicago/Turabian StyleVeraar, Cecilia, Enzo Kirschner, Stefan Schwarz, Peter Jaksch, Konrad Hoetzenecker, Edda Tschernko, Martin Dworschak, Hendrik J. Ankersmit, and Bernhard Moser. 2022. "Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO" Biology 11, no. 10: 1475. https://doi.org/10.3390/biology11101475
APA StyleVeraar, C., Kirschner, E., Schwarz, S., Jaksch, P., Hoetzenecker, K., Tschernko, E., Dworschak, M., Ankersmit, H. J., & Moser, B. (2022). Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO. Biology, 11(10), 1475. https://doi.org/10.3390/biology11101475








