The First Survival Score for Patients Aged ≥80 Years Irradiated for Brain Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation therapy for brain metastases: An ASTRO clinical practice guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, T.; Yamamoto, M.; Higuchi, Y.; Sato, Y.; Shuto, T.; Akabane, A.; Jokura, H.; Yomo, S.; Nagano, O.; Kawagishi, J.; et al. Local tumor progression treated with Gamma Knife radiosurgery: Differences between patients with 2–4 versus 5–10 brain metastases based on an update of a multi-institutional prospective observational study (JLGK0901). J. Neurosurg. 2019, 132, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, S.; Dziggel, L.; Hornung, D.; Blanck, O.; Schild, S.E.; Rades, D. A new prognostic instrument to predict the probability of developing new cerebral metastases after radiosurgery alone. Radiat. Oncol. 2014, 9, 215. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., 2nd; Deming, R.; Burri, S.H.; et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Mulvenna, P.; Nankivell, M.; Barton, R.; Faivre-Finn, C.; Wilson, P.; McColl, E.; Moore, B.; Brisbane, I.; Ardron, D.; Holt, T.; et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet 2016, 388, 2004–2014. [Google Scholar]
- DeAngelis, L.M.; Delattre, J.Y.; Posner, J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989, 39, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, C.; Sugie, C.; Iwata, H. Radiotherapy for metastatic brain tumors. Int. J. Clin. Oncol. 2009, 14, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.E.; Prabhu, R.S.; Hdeib, A.; McCracken, D.J.; Lasker, G.F.; McDermott, M.W.; Kalkanis, S.N.; Olson, J.J. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of whole brain radiation therapy in adults with newly diagnosed metastatic brain tumors. Neurosurgery 2019, 84, E159–E162. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Panzner, A.; Dziggel, L.; Haatanen, T.; Lohynska, R.; Schild, S.E. Dose-escalation of whole-brain radiotherapy for brain metastasis in patients with a favorable survival prognosis. Cancer 2012, 118, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Evers, J.N.; Schild, S.E.; Segedin, B.; Nagy, V.; Khoa, M.T.; Trang, N.T.; Rades, D. A new score predicting survival prognosis after whole-brain radiotherapy alone for brain metastases in elderly patients. Anticancer Res. 2014, 34, 2455–2458. [Google Scholar] [PubMed]
- Fujimoto, D.; Taniguchi, K.; Takashima, J.; Miura, F.; Kobayashi, H. Validity and safety of laparoscopic gastrectomy with D1+ lymphadenectomy for very elderly advanced gastric cancer patients; retrospective cohort study. Jpn. J. Clin. Oncol. 2022, 3, hyac126. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Oyamada, Y.; Ikemura, S.; Nukaga, S.; Inoue, T.; Arai, D.; Ohgino, K.; Kuroda, A.; Ishioka, K.; Sakamaki, F.; et al. Real-world clinical practice for advanced non-small-cell lung cancer in the very elderly: A retrospective multicenter analysis. Clin. Lung Cancer 2022, 23, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Depboylu, B. Treatment and patient related quality of life issues in elderly and very elderly breast cancer patients. Transl. Cancer Res. 2020, 9 (Suppl. S1), S146–S153. [Google Scholar] [CrossRef]
- Clérigo, V.; Hasmucrai, D.; Teixeira, E.; Alves, P.; Vilariça, A.S.; Sotto-Mayor, R. Characterization and management of elderly and very elderly patients with non-small cell lung cancer. Clin. Respir. J. 2020, 14, 683–686. [Google Scholar] [CrossRef]
- Wilson, T.; Fleischer, L.; Patel, S.; Bhatnagar, A.; Ahmad, N.; Kubicek, G. Outcomes of curative treatment for head and neck squamous cell carcinoma in very elderly adults ≥80 years old. Head Neck 2022. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yamamoto, M.; Sato, Y.; Kawabe, T.; Higuchi, Y.; Kasuya, H.; Yamamoto, T.; Matsumura, A.; Barfod, B.E. Stereotactic radiosurgery for brain metastases: A case-matched study comparing treatment results for patients 80 years of age or older versus patients 65–79 years of age. J. Neurosurg. 2014, 121, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Yomo, S.; Hayashi, M. Is upfront stereotactic radiosurgery a rational treatment option for very elderly patients with brain metastases? A retrospective analysis of 106 consecutive patients age 80 years and older. BMC Cancer 2016, 16, 948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peters, E.; Pritzkuleit, R.; Beske, F.; Katalinic, A. Demographic change and disease rates. A projection until 2050. Bundesgesundheitsblatt 2010, 53, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Strader, L.A.; Helmer, S.D.; Yates, C.L.; Tenofsky, P.L. Octogenarians: Noncompliance with breast cancer treatment recommendations. Am. Surg. 2014, 80, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for brain metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1960 patients in the RTOG database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Dziggel, L.; Nagy, V.; Segedin, B.; Lohynska, R.; Veninga, T.; Khoa, M.T.; Trang, N.T.; Schild, S.E. A new survival score for patients with brain metastases who received whole-brain radiotherapy (WBRT) alone. Radiother. Oncol. 2013, 108, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Kieckebusch, S.; Lohynska, R.; Veninga, T.; Stalpers, L.J.; Dunst, J.; Schild, S.E. Reduction of overall treatment time in patients irradiated for more than three brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Trifiletti, D.M.; Ballman, K.V.; Brown, P.D.; Anderson, S.K.; Carrero, X.W.; Cerhan, J.H.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.; et al. Optimizing whole brain radiation therapy dose and fractionation: Results from a prospective phase 3 trial (NCCTG N107C [Alliance]/CEC.3). Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 255–260. [Google Scholar] [CrossRef]
| Characteristic | Number of Subjects | Proportion (%) |
|---|---|---|
| Age | ||
| 80–84 years | 68 | 72 |
| 85–90 years | 26 | 28 |
| Gender | ||
| Female | 39 | 41 |
| Male | 55 | 59 |
| ECOG performance score | ||
| 0–1 | 30 | 32 |
| 2 | 36 | 38 |
| 3 | 28 | 30 |
| Tumor type | ||
| Breast cancer | 10 | 11 |
| Non-small cell lung cancer | 42 | 45 |
| Small-cell lung cancer | 9 | 10 |
| Less radiosensitive tumors | 6 | 6 |
| Cancer of unknown primary | 6 | 6 |
| Gastrointestinal cancers | 9 | 10 |
| Other malignancies | 12 | 13 |
| Number of brain metastases | ||
| 1 | 18 | 19 |
| 2–3 | 24 | 26 |
| ≥4 | 52 | 55 |
| Extra-cranial metastases | ||
| No | 20 | 21 |
| Yes | 74 | 79 |
| Interval tumor diagnosis to WBRT | ||
| ≤12 months | 65 | 69 |
| >12 months | 29 | 31 |
| Treatment period (years) | ||
| 2000–2012 | 48 | 51 |
| 2013–2021 | 46 | 49 |
| WBRT regimen | ||
| 20 Gy in 5 fractions | 12 | 13 |
| 30 Gy in 10 fractions | 40 | 43 |
| >30 Gy in 11–20 fractions | 42 | 45 |
| Characteristic | Survival Rates (%) | p-Value | |||
|---|---|---|---|---|---|
| At 1 Month | At 2 Months | At 3 Months | At 6 Months | ||
| Age | 0.54 | ||||
| 80–84 years | 50 | 44 | 35 | 22 | |
| 85–90 years | 69 | 42 | 23 | 15 | |
| Gender | 0.83 | ||||
| Female | 51 | 46 | 31 | 23 | |
| Male | 58 | 42 | 33 | 18 | |
| ECOG performance score | 0.003 | ||||
| 0–1 | 57 | 50 | 40 | 33 | |
| 2 | 61 | 53 | 42 | 25 | |
| 3 | 46 | 25 | 11 | 0 | |
| ECOG performance score | <0.001 | ||||
| 0–2 | 59 | 52 | 41 | 29 | |
| 3 | 46 | 25 | 11 | 0 | |
| Tumor type | 0.73 | ||||
| Breast cancer | 50 | 40 | 40 | 30 | |
| Non-small cell lung cancer | 55 | 45 | 29 | 24 | |
| Small-cell lung cancer | 44 | 44 | 22 | 11 | |
| Less radiosensitive tumors | 67 | 50 | 33 | 17 | |
| Cancer of unknown primary | 100 | 83 | 67 | 17 | |
| Gastrointestinal cancers | 44 | 22 | 22 | 11 | |
| Other malignancies | 50 | 33 | 33 | 17 | |
| Number of brain metastases | 0.014 | ||||
| 1 | 94 | 78 | 56 | 44 | |
| 2–3 | 50 | 38 | 29 | 25 | |
| ≥4 | 44 | 35 | 25 | 10 | |
| Extra-cranial metastases | 0.034 | ||||
| No | 80 | 65 | 55 | 30 | |
| Yes | 49 | 38 | 26 | 18 | |
| Interval from tumor diagnosis to WBRT | 0.11 | ||||
| ≤12 months | 54 | 42 | 28 | 14 | |
| >12 months | 59 | 48 | 41 | 34 | |
| Treatment period (years) | 0.29 | ||||
| 2000–2012 | 63 | 48 | 35 | 21 | |
| 2013–2021 | 48 | 39 | 28 | 20 | |
| Dose-fractionation regimen | 0.66 | ||||
| 20 Gy in 5 fractions | 83 | 58 | 50 | 8 | |
| 30 Gy in 10 fractions | 55 | 40 | 30 | 20 | |
| >30 Gy in 11–20 fractions | 48 | 43 | 29 | 24 | |
| Characteristic | Survival Rate at 3 Months (%) | Scoring Points |
|---|---|---|
| ECOG performance score | ||
| 0–2 | 41 | 4 |
| 3 | 11 | 1 |
| Number of brain metastases | ||
| 1 | 56 | 6 |
| 2–3 | 29 | 3 |
| ≥4 | 25 | 3 |
| Extra-cranial metastases | ||
| No | 55 | 6 |
| Yes | 26 | 3 |
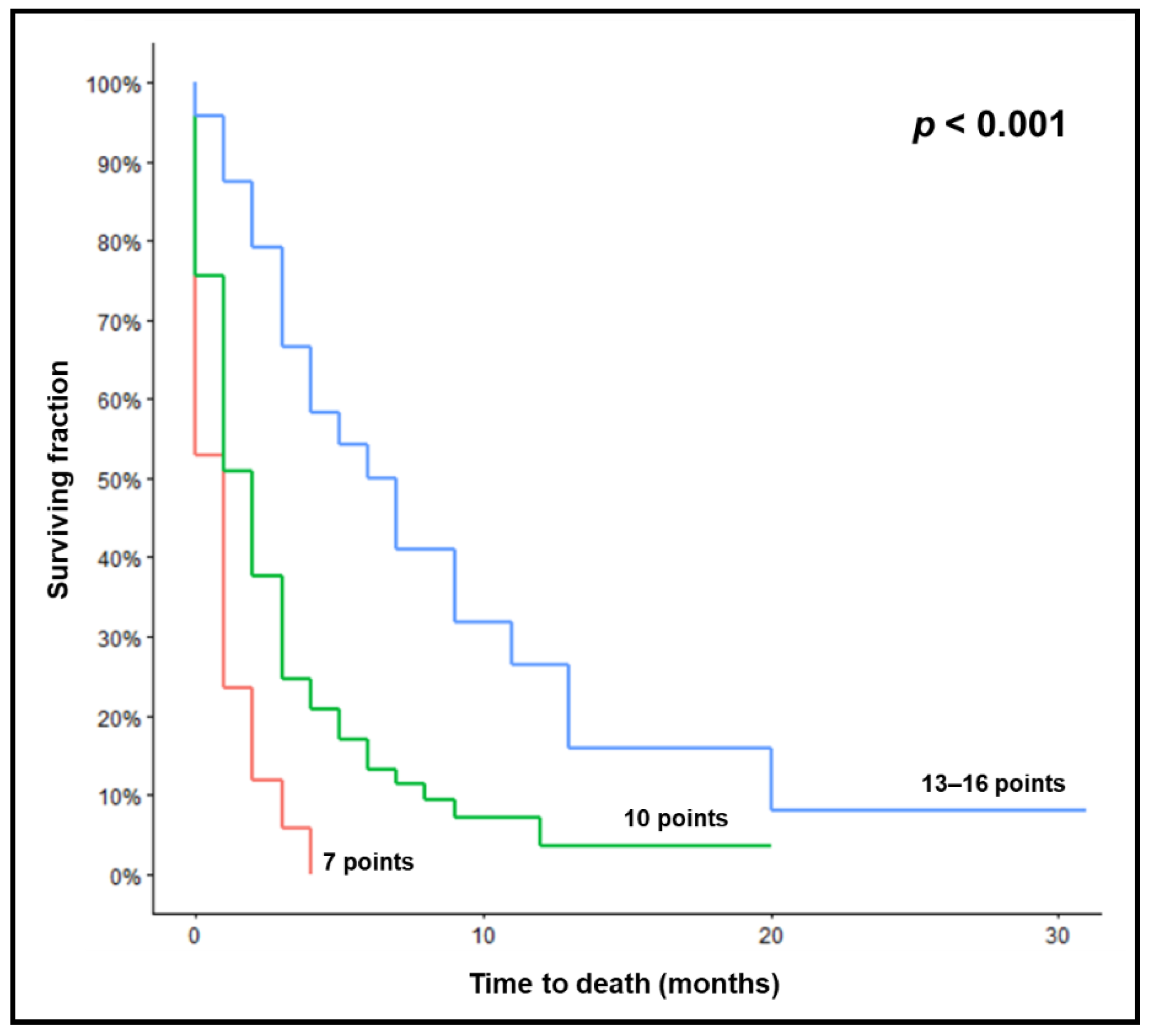
| Survival Rates (%) | p-Value | ||||
|---|---|---|---|---|---|
| At 1 Month | At 2 Months | At 3 Months | At 6 Months | ||
| Prognostic group | <0.001 | ||||
| 7 points | 24 | 12 | 6 | 0 | |
| 10 points | 51 | 38 | 25 | 13 | |
| 13–16 points | 88 | 79 | 67 | 50 | |
| Endpoint | PPVs of the New Score | PPVs of the Evers-Score |
|---|---|---|
| Death | ||
| within 1 month | 76% | 56% |
| within 2 months | 88% | 80% |
| within 3 months | 94% | 96% |
| within 6 months | 100% | 100% |
| Survival | ||
| for at least 1 month | 88% | 63% |
| for at least 2 months | 79% | 58% |
| for at least 3 months | 67% | 48% |
| for at least 6 months | 50% | 35% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rades, D.; Delikanli, C.; Schild, S.E.; Kristiansen, C.; Tvilsted, S.; Janssen, S. The First Survival Score for Patients Aged ≥80 Years Irradiated for Brain Metastases. Biology 2022, 11, 1434. https://doi.org/10.3390/biology11101434
Rades D, Delikanli C, Schild SE, Kristiansen C, Tvilsted S, Janssen S. The First Survival Score for Patients Aged ≥80 Years Irradiated for Brain Metastases. Biology. 2022; 11(10):1434. https://doi.org/10.3390/biology11101434
Chicago/Turabian StyleRades, Dirk, Cansu Delikanli, Steven E. Schild, Charlotte Kristiansen, Søren Tvilsted, and Stefan Janssen. 2022. "The First Survival Score for Patients Aged ≥80 Years Irradiated for Brain Metastases" Biology 11, no. 10: 1434. https://doi.org/10.3390/biology11101434
APA StyleRades, D., Delikanli, C., Schild, S. E., Kristiansen, C., Tvilsted, S., & Janssen, S. (2022). The First Survival Score for Patients Aged ≥80 Years Irradiated for Brain Metastases. Biology, 11(10), 1434. https://doi.org/10.3390/biology11101434







