A TRICk to Improve the Effectiveness of RIC: Role of Limb Temperature in Enhancing the Effectiveness of Remote Ischemic Conditioning
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
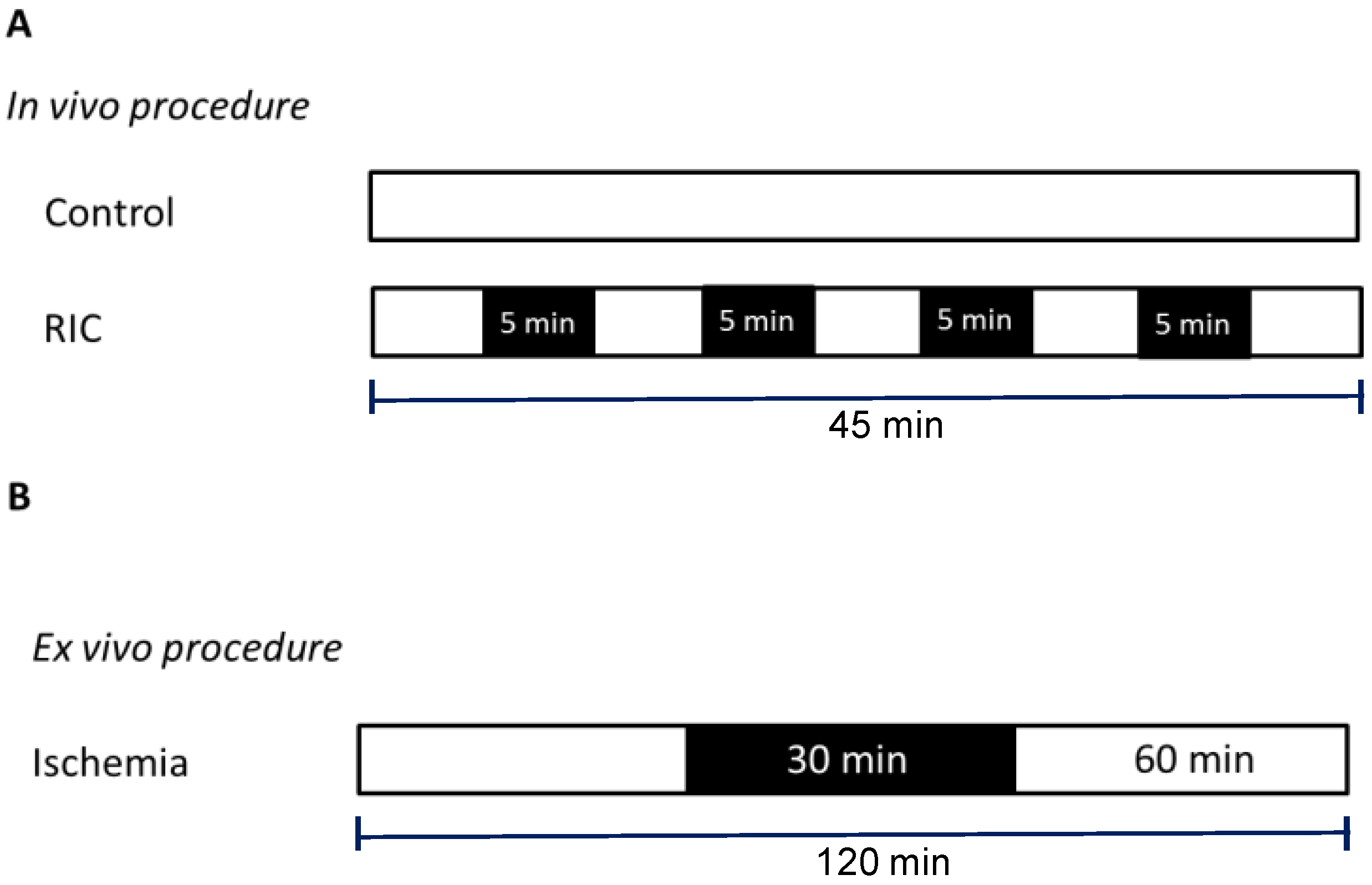
2.2. Remote Conditioning Protocol
- Cold-Controls (cold-CTR): left hind limb was maintained at a temperature of 20 °C for 45 min without cuff inflation;
- Warm-Controls (warm-CTR): left hind limb was maintained at a temperature of 40 °C for 45 min without cuff inflation;
- Cold-RIC (cold-RIC): RIC was performed while the left hind limb was maintained at a temperature of 20 °C;
- Warm-RIC (warm-RIC): RIC was performed while the left hind limb was maintained at a temperature of 40 °C.
2.3. Isolated Heart Perfusion Technique
2.4. Infarct Size Assessment
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
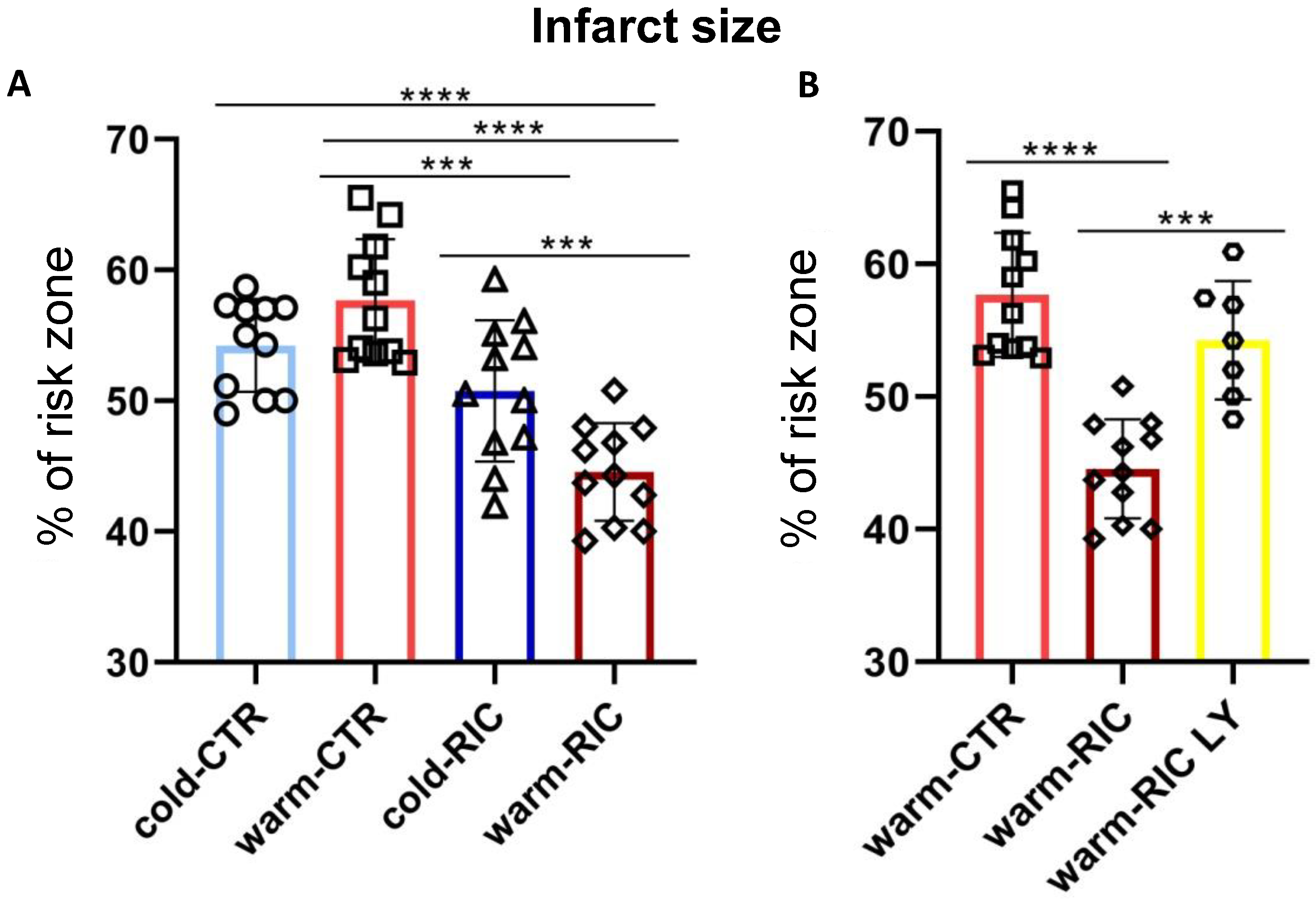
3.1. Warm-RIC Reduced the Extent of Myocardial Infarction More Effectively Than Cold-RIC
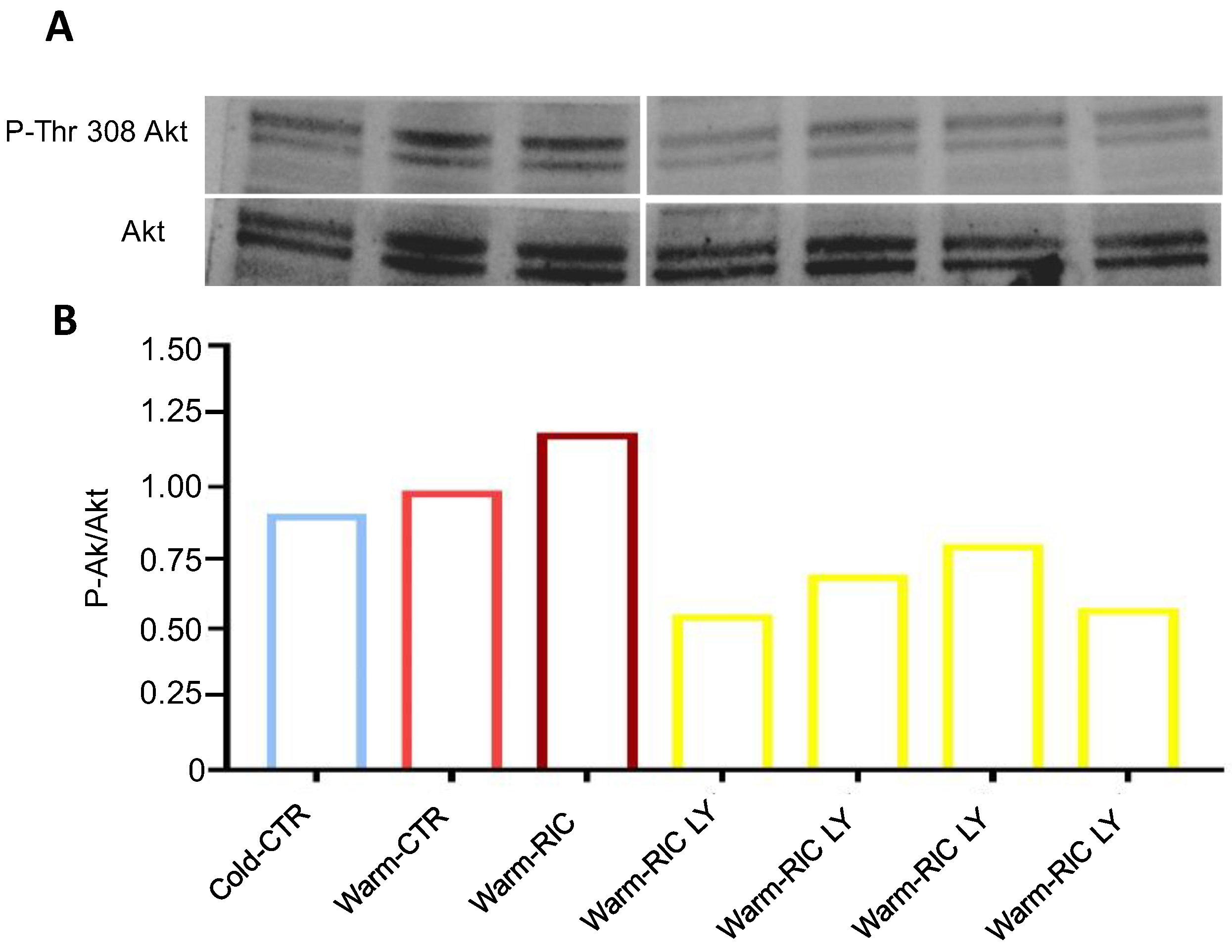
3.2. Warm-RIC Acted via the PI3K/Akt Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Penna, C.; Granata, R.; Tocchetti, C.G.; Gallo, M.P.; Alloatti, G.; Pagliaro, P. Endogenous Cardioprotective Agents: Role in Pre and Postconditioning. Curr. Drug. Targets 2015, 16, 843–867. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Skyschally, A.; Heusch, G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. 2017, 469, 159–181, Erratum in Pflugers Arch. 2017, 469, 843. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Kharbanda, R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J. Cardiol. 2017, 9, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Sun, R.; Zeng, Y.; Chen, S.; Zhang, P. Protective approaches against myocardial ischemia reperfusion injury. Exp. Ther. Med. 2016, 12, 3823–3829. [Google Scholar] [CrossRef]
- Ludman, A.J.; Yellon, D.M.; Hausenloy, D.J. Cardiac preconditioning for ischaemia: Lost in translation. Dis. Model Mech. 2010, 3, 35–38. [Google Scholar] [CrossRef][Green Version]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. CONDI-2/ERIC-PPCI Investigators. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet (Lond. Engl.) 2019, 394, 1415–1424. [Google Scholar] [CrossRef]
- Bromage, D.I.; Pickard, J.M.; Rossello, X.; Ziff, O.J.; Burke, N.; Yellon, D.M.; Davidson, S.M. Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: A systematic review and meta-analysis. Cardiovasc. Res. 2017, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Lecour, S.; Andreadou, I.; Bøtker, H.E.; Davidson, S.M.; Heusch, G.; Ruiz-Meana, M.; Schulz, R.; Zuurbier, C.J.; Ferdinandy, P.; Hausenloy, D.J.; et al. IMproving Preclinical Assessment of Cardioprotective Therapies (IMPACT) criteria: Guidelines of the EU-CARDIOPROTECTION COST Action. Basic Res. Cardiol. 2021, 116, 52. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Davidson, S.M.; Hausenloy, D.J.; Yellon, D.M. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res. Cardiol. 2016, 111, 50. [Google Scholar] [CrossRef]
- Sbroggiò, M.; Bertero, A.; Velasco, S.; Fusella, F.; De Blasio, E.; Bahou, W.F.; Silengo, L.; Turco, E.; Brancaccio, M.; Tarone, G. ERK1/2 activation in heart is controlled by melusin, focal adhesion kinase and the scaffold protein IQGAP1. J. Cell. Sci. 2011, 124, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Brancaccio, M.; Tullio, F.; Rubinetto, C.; Perrelli, M.G.; Angotti, C.; Pagliaro, P.; Tarone, G. Overexpression of the muscle-specific protein, melusin, protects from cardiac ischemia/reperfusion injury. Basic Res. Cardiol. 2014, 109, 418. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.R.; Batchu, S.N.; Das, D.; Suresh, M.R.; Falck, J.R.; Graves, J.P.; Zeldin, D.C.; Seubert, J.M. Role of B-type natriuretic peptide in epoxyeicosatrienoic acid-mediated improved post-ischaemic recovery of heart contractile function. Cardiovasc. Res. 2009, 83, 362–370. [Google Scholar] [CrossRef]
- Bøtker, H.E.; Hausenloy, D.; Andreadou, I.; Antonucci, S.; Boengler, K.; Davidson, S.M.; Deshwal, S.; Devaux, Y.; Di Lisa, F.; Di Sante, M.; et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res. Cardiol. 2018, 113, 39. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Alloatti, G.; Crisafulli, A. Mechanisms Involved in Cardioprotection Induced by Physical Exercise. Antioxid. Redox Signal. 2020, 32, 1115–1134. [Google Scholar] [CrossRef]
- Verdouw, P.D.; Gho, B.C.; Koning, M.M.; Schoemaker, R.G.; Duncker, D.J. Cardioprotection by ischemic and nonischemic myocardial stress and ischemia in remote organs. Implications for the concept of ischemic preconditioning. Ann. N. Y. Acad. Sci. 1996, 793, 27–42. [Google Scholar] [CrossRef]
- Comità, S.; Femmino, S.; Thairi, C.; Alloatti, G.; Boengler, K.; Pagliaro, P.; Penna, C. Regulation of STAT3 and its role in cardioprotection by conditioning: Focus on non-genomic roles targeting mitochondrial function. Basic Res. Cardiol. 2021, 116, 56. [Google Scholar] [CrossRef]
- Chen, H.; Jing, X.Y.; Shen, Y.J.; Wang, T.L.; Ou, C.; Lu, S.F.; Cai, Y.; Li, Q.; Chen, X.; Ding, Y.J.; et al. Stat5-dependent cardioprotection in late remote ischaemia preconditioning. Cardiovasc. Res. 2018, 114, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef]
- Guo, Y.; Flaherty, M.P.; Wu, W.J.; Tan, W.; Zhu, X.; Li, Q.; Bolli, R. Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: Results of a comprehensive analysis of determinants of infarct size in 1074 mice. Basic Res. Cardiol. 2012, 107, 288. [Google Scholar] [CrossRef]
- Chien, G.L.; Wolff, R.A.; Davis, R.F.; van Winkle, D.M. “Normothermic range” temperature affects myocardial infarct size. Cardiovasc. Res. 1994, 28, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.L.; Kloner, R.A. Myocardial temperature in acute myocardial infarction: Protection with mild regional hypothermia. Am. J. Physiol. 1997, 273, H220–H227. [Google Scholar] [CrossRef]
- Schwartz, L.M.; Verbinski, S.G.; Vander Heide, R.S.; Reimer, K.A. Epicardial temperature is a major predictor of myocardial infarct size in dogs. J. Mol. Cell. Cardiol. 1997, 29, 1577–1583. [Google Scholar] [CrossRef]
- Pagliaro, P.; Aragno, M.; Penna, C. Role of temperature in myocardial ischemic injury and protection by conditioning. Cond. Med. 2020, 3, 31–46. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penna, C.; Sorge, M.; Tullio, F.; Comità, S.; Femminò, S.; Brancaccio, M.; Pagliaro, P. A TRICk to Improve the Effectiveness of RIC: Role of Limb Temperature in Enhancing the Effectiveness of Remote Ischemic Conditioning. Biology 2022, 11, 146. https://doi.org/10.3390/biology11010146
Penna C, Sorge M, Tullio F, Comità S, Femminò S, Brancaccio M, Pagliaro P. A TRICk to Improve the Effectiveness of RIC: Role of Limb Temperature in Enhancing the Effectiveness of Remote Ischemic Conditioning. Biology. 2022; 11(1):146. https://doi.org/10.3390/biology11010146
Chicago/Turabian StylePenna, Claudia, Matteo Sorge, Francesca Tullio, Stefano Comità, Saveria Femminò, Mara Brancaccio, and Pasquale Pagliaro. 2022. "A TRICk to Improve the Effectiveness of RIC: Role of Limb Temperature in Enhancing the Effectiveness of Remote Ischemic Conditioning" Biology 11, no. 1: 146. https://doi.org/10.3390/biology11010146
APA StylePenna, C., Sorge, M., Tullio, F., Comità, S., Femminò, S., Brancaccio, M., & Pagliaro, P. (2022). A TRICk to Improve the Effectiveness of RIC: Role of Limb Temperature in Enhancing the Effectiveness of Remote Ischemic Conditioning. Biology, 11(1), 146. https://doi.org/10.3390/biology11010146





