Tomato Prosystemin Is Much More than a Simple Systemin Precursor
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Condition
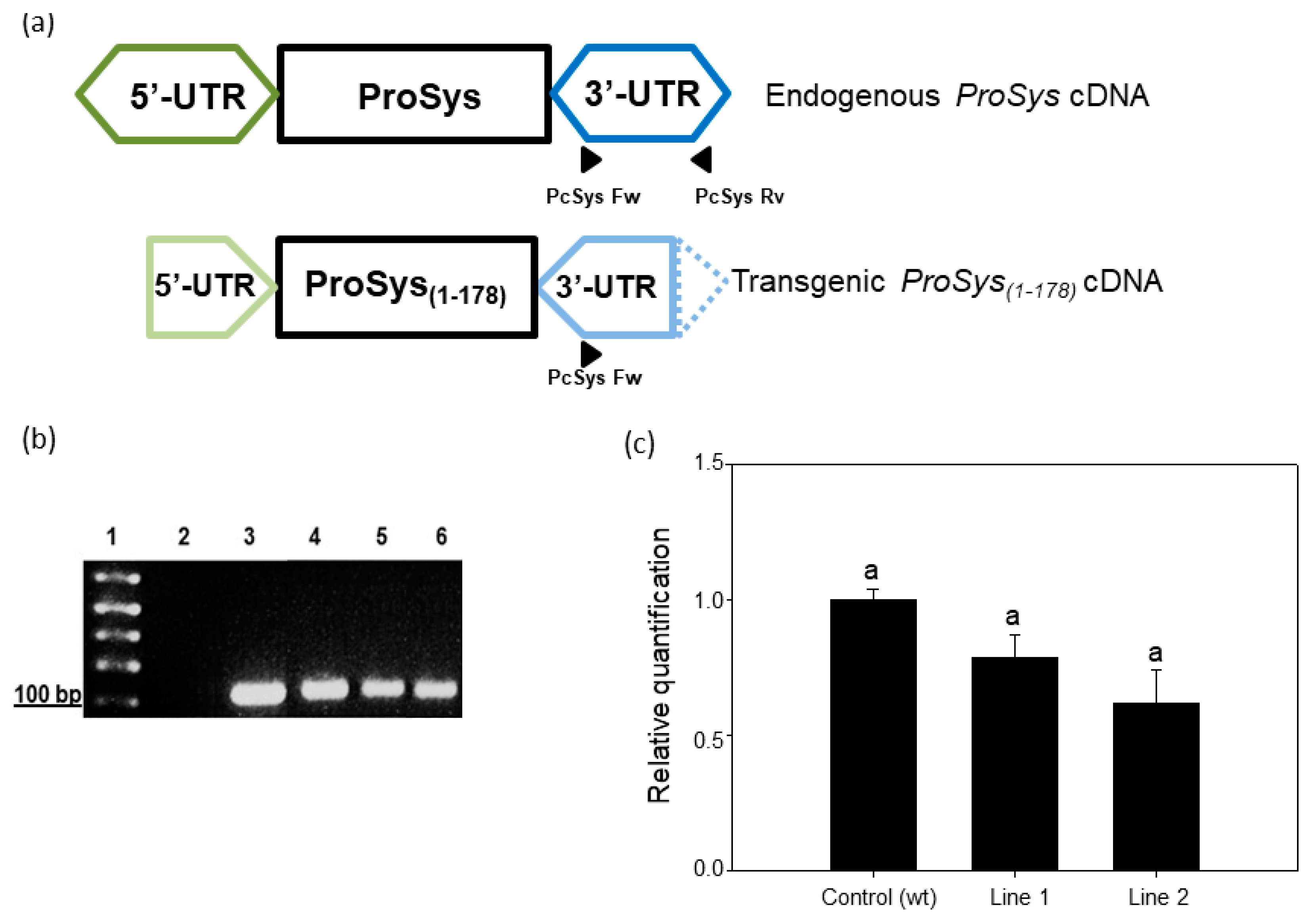
2.2. Tomato Transgenic Plants Production and Analysis
2.3. Molecular Cloning, Expression, and Purification of ProSys(1-178)
2.4. LC-ESI-MS, Circular Dichroism and Light Scattering Analyses
2.5. Plant Treatments with ProSys(1-178)
2.6. OGs Extraction by Chelating Agent
2.7. MALDI-TOF (Matrix Assisted Laser Desorption Ionization-Time of Flight) Mass Spectrometry
2.8. Bioassays
2.8.1. Herbivory by S. littoralis Larvae
2.8.2. Infection by the Necrotrophic Fungus B. cinerea
2.9. Two-Color Microarray-Based Gene Expression Analysis
2.10. Relative Quantification of Gene Expression
2.11. Statistical Analysis
3. Results
3.1. Production and Characterization of Transgenic Tomato Plants Expressing ProSys(1-178)
3.2. Production and Characterization of the Biochemical Features of ProSys(1-178)
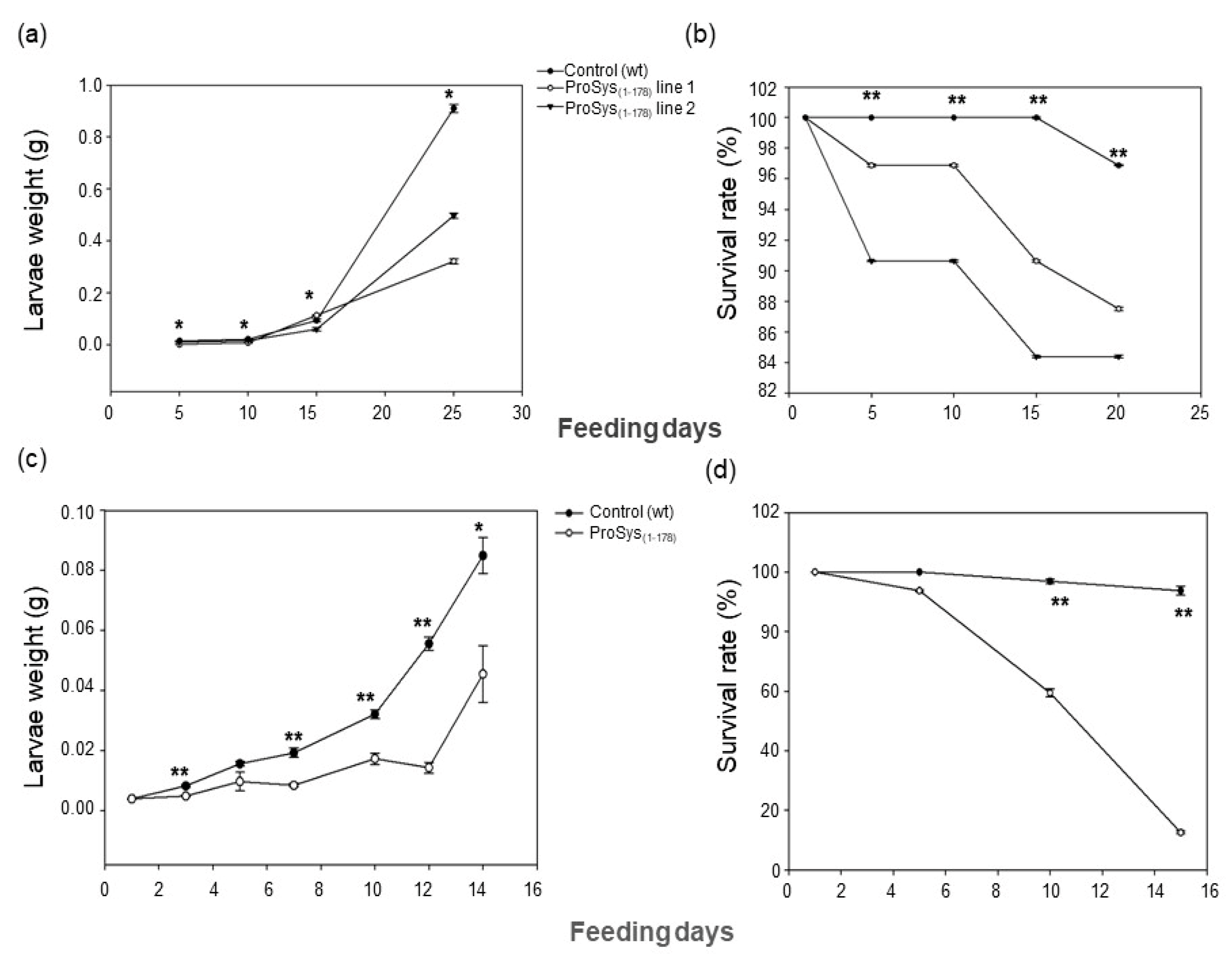
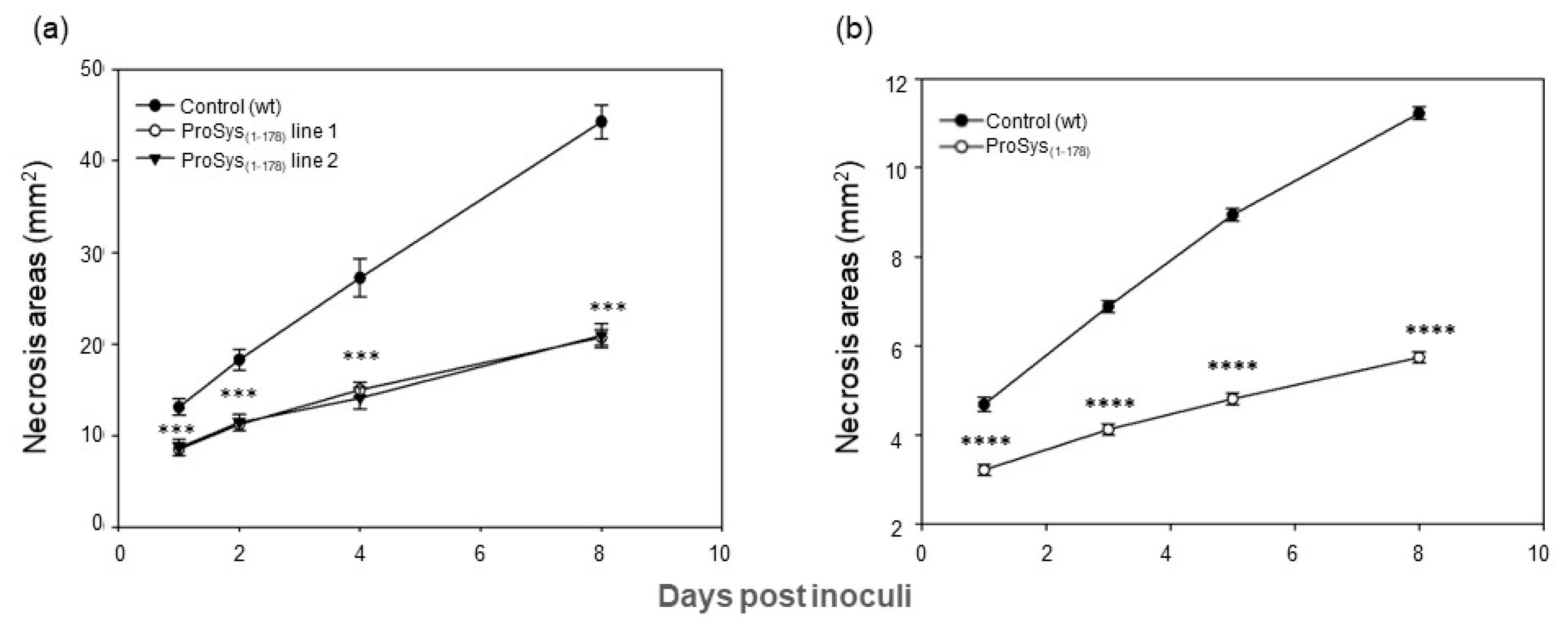
3.3. ProSys(1-178) Enhances Plant Resistance against S. littoralis and B. cinerea
3.3.1. Transgenic Plants Assays
3.3.2. Plant Treatments with Exogenous ProSys(1-178)
3.4. The Transcriptomic Profiles of Tomato Plants Is Strongly Influenced by ProSys(1-178) Expression
3.4.1. Defense-Related Genes
3.4.2. Anatomical Defensive Structure
3.4.3. Secondary Metabolism
3.4.4. Hormone-Related Pathways
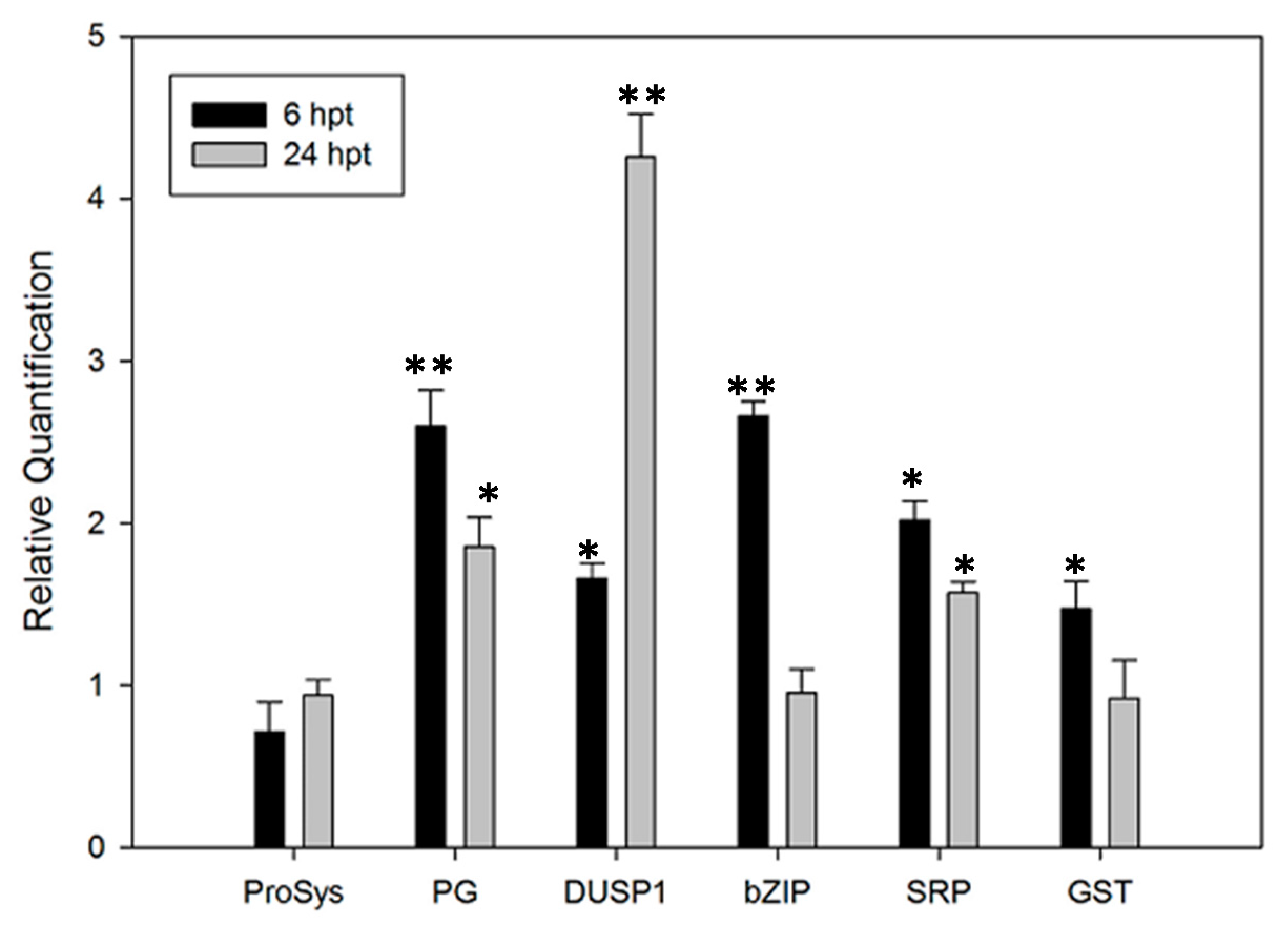
3.5. Defense-Related Genes Are Up-Regulated in ProSys(1-178)-Treated Plants
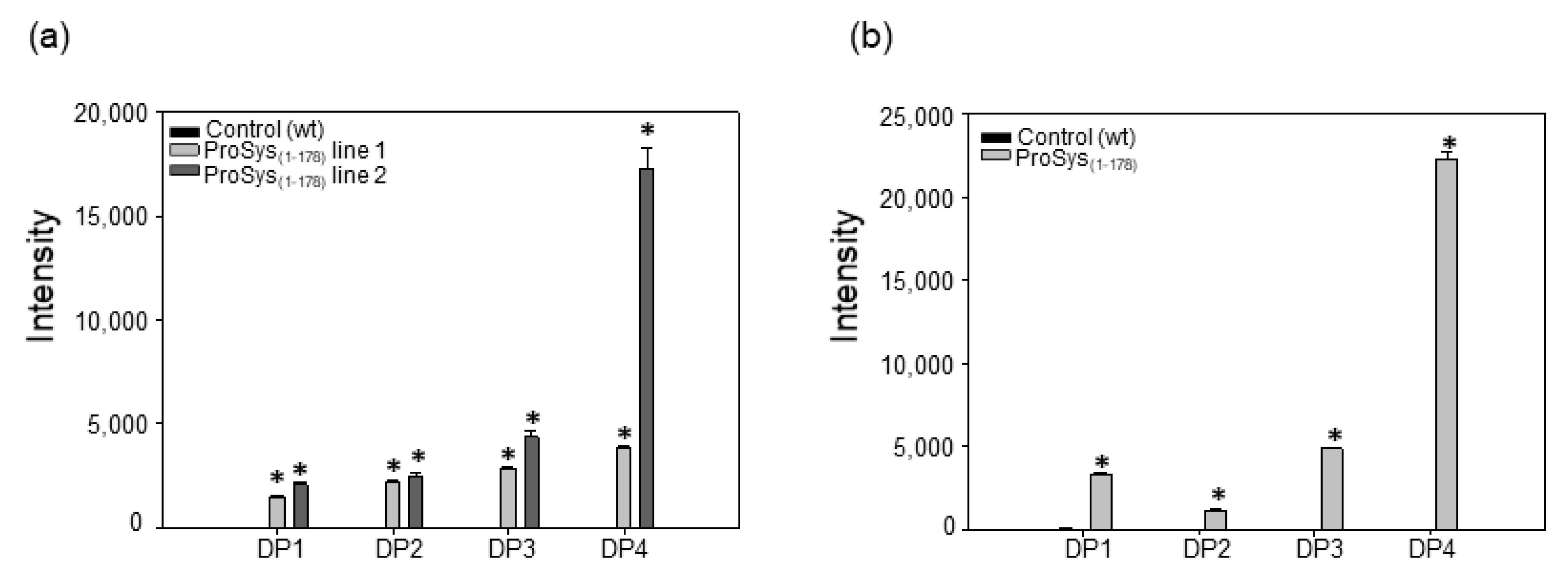
3.6. ProSys(1-178) Induces the Release of Oligogalacturonides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGurl, B.; Orozco-Cardenas, M.; Pearce, G.; Ryan, C.A. Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc. Natl. Acad. Sci. USA 1994, 91, 9799–9802. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Strydom, D.; Johnson, S.; Ryan, C.A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 1991, 253, 895–897. [Google Scholar] [CrossRef]
- Ryan, C.A. The systemin signaling pathway: Differential activation of plant defensive genes. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 112–121. [Google Scholar] [CrossRef]
- Beloshistov, R.E.; Dreizler, K.; Galiullina, R.A.; Tuzhikov, A.I.; Serebryakova, M.V.; Reichardt, S.; Shaw, J.; Taliansky, M.E.; Pfannstiel, J.; Chichkova, N.V. Phytaspase-mediated precursor processing and maturation of the wound hormone systemin. New Phytol. 2018, 218, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- McGurl, B.; Pearce, G.; Orozco-Cardenas, M.; Ryan, C.A. Structure, expression, and antisense inhibition of the systemin precursor gene. Science 1992, 255, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Howe, G.A. Systemic signaling in the wound response. Curr. Plant Biol. 2005, 8, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Bhattacharya, R.; Chen, Y.-C. Peptide signals for plant defense display a more universal role. Plant Signal. Behav. 2008, 3, 1091–1092. [Google Scholar] [CrossRef]
- Pearce, G. Systemin, hydroxyproline-rich systemin and the induction of protease inhibitors. Curr. Protein Pep. Sci. 2011, 12, 399–408. [Google Scholar] [CrossRef]
- Narváez-Vásquez, J.; Orozco-Cárdenas, M.L.; Ryan, C.A. Systemic wound signaling in tomato leaves is cooperatively regulated by systemin and hydroxyproline-rich glycopeptide signals. Plant Mol. Biol. 2007, 65, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Sasso, R.; Pasquariello, M.; Iodice, L.; Carretta, A.; Cascone, P.; Ariati, L.; Digilio, M.; Guerrieri, E.; Rao, R. Systemin regulates both systemic and volatile signaling in tomato plants. J. Chem. Ecol. 2007, 33, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, D.C.; Refi-Hind, S.; Stratmann, J.W.; Lincoln, D.E. Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry 2010, 71, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Corrado, G.; Coppola, V.; Cascone, P.; Martinelli, R.; Digilio, M.C.; Pennacchio, F.; Rao, R. Prosystemin overexpression in tomato enhances resistance to different biotic stresses by activating genes of multiple signaling pathways. Plant Mol. Biol. Rep. 2015, 33, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, J.E. Salt stress activation of wound-related genes in tomato plants. Plant Physiol. 2003, 132, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Lin, J. Systemin-mediated long-distance systemic defense responses. New Phytol. 2020, 226, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Cascone, P.; De Pascale, S.; Barbieri, G.; Corrado, G.; Rao, R.; Maggio, A. Systemin-dependent salinity tolerance in tomato: Evidence of specific convergence of abiotic and biotic stress responses. Physiol. Plant 2010, 138, 10–21. [Google Scholar] [CrossRef]
- El Oirdi, M.; El Rahman, T.A.; Rigano, L.; El Hadrami, A.; Rodriguez, M.C.; Daayf, F.; Vojnov, A.; Bouarab, K. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell 2011, 23, 2405–2421. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Lelio, I.D.; Romanelli, A.; Gualtieri, L.; Molisso, D.; Ruocco, M.; Avitabile, C.; Natale, R.; Cascone, P.; Guerrieri, E. Tomato plants treated with systemin peptide show enhanced levels of direct and indirect defense associated with increased expression of defense-related genes. Plants 2019, 8, 395. [Google Scholar] [CrossRef]
- Dombrowski, J.E.; Pearce, G.; Ryan, C.A. Proteinase inhibitor-inducing activity of the prohormone prosystemin resides exclusively in the C-terminal systemin domain. Proc. Natl. Acad. Sci. USA 1999, 96, 12947–12952. [Google Scholar] [CrossRef]
- Ryan, C.A.; Pearce, G. Systemins: A functionally defined family of peptide signals that regulate defensive genes in Solanaceae species. Proc. Natl. Acad. Sci. USA 2003, 100, 14577–14580. [Google Scholar] [CrossRef]
- Buonanno, M.; Coppola, M.; Di Lelio, I.; Molisso, D.; Leone, M.; Pennacchio, F.; Langella, E.; Rao, R.; Monti, S.M. Prosystemin, a prohormone that modulates plant defense barriers, is an intrinsically disordered protein. Protein. Sci. 2018, 27, 620–632. [Google Scholar] [CrossRef]
- Oldfield, C.J.; Meng, J.; Yang, J.Y.; Yang, M.Q.; Uversky, V.N.; Dunker, A.K. Flexible nets: Disorder and induced fit in the associations of p53 and 14-3-3 with their partners. BMC Genom. 2008, 9 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, K.; Salladini, E.; O’Brien, D.P.; Brier, S.; Chenal, A.; Yacoubi, I.; Longhi, S. Structural disorder and induced folding within two cereal, ABA stress and ripening (ASR) proteins. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Tompa, P. Intrinsically unstructured proteins. Trends Biochem. Sci. 2002, 27, 527–533. [Google Scholar] [CrossRef]
- Corrado, G.; Arena, S.; Araujo-Burgos, T.; Coppola, M.; Rocco, M.; Scaloni, A.; Rao, R. The expression of the tomato prosystemin in tobacco induces alterations irrespective of its functional domain. Plant Cell Tissue Organ Cult. 2016, 125, 509–519. [Google Scholar] [CrossRef]
- Corrado, G.; Alagna, F.; Rocco, M.; Renzone, G.; Varricchio, P.; Coppola, V.; Coppola, M.; Garonna, A.; Baldoni, L.; Scaloni, A. Molecular interactions between the olive and the fruit fly Bactrocera oleae. BMC Plant Biol. 2012, 12, 86. [Google Scholar] [CrossRef]
- Truppo, E.; Supuran, C.T.; Sandomenico, A.; Vullo, D.; Innocenti, A.; Di Fiore, A.; Alterio, V.; De Simone, G.; Monti, S.M. Carbonic anhydrase VII is S-glutathionylated without loss of catalytic activity and affinity for sulfonamide inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Pontiggia, D.; Ciarcianelli, J.; Salvi, G.; Cervone, F.; De Lorenzo, G.; Mattei, B. Sensitive detection and measurement of oligogalacturonides in Arabidopsis. Front. Plant Sci. 2015, 6, 258. [Google Scholar] [CrossRef]
- Di Lelio, I.; Varricchio, P.; Di Prisco, G.; Marinelli, A.; Lasco, V.; Caccia, S.; Casartelli, M.; Giordana, B.; Rao, R.; Gigliotti, S. Functional analysis of an immune gene of Spodoptera littoralis by RNAi. J. Insect Physiol. 2014, 64, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Bovi, P.D.; Ciliento, R.; Gaudio, L.; Di Maro, A.; Aceto, S.; Lorito, M.; Rao, R. Inducible expression of a Phytolacca heterotepala ribosome-inactivating protein leads to enhanced resistance against major fungal pathogens in tobacco. Phytopathology 2005, 95, 206–215. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Underwood, W. The plant cell wall: A dynamic barrier against pathogen invasion. Front. Plant Sci. 2012, 3, 85. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef]
- Leon-Reyes, A.; Spoel, S.H.; De Lange, E.S.; Abe, H.; Kobayashi, M.; Tsuda, S.; Millenaar, F.F.; Welschen, R.A.; Ritsema, T.; Pieterse, C.M. Ethylene modulates the role of nonexpressor of pathogenesis-related genes1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 2009, 149, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.; Van Wees, S.C. Shifting from priming of salicylic acid-to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef]
- Albert, M. Peptides as triggers of plant defence. J. Exp. Bot. 2013, 64, 5269–5279. [Google Scholar] [CrossRef][Green Version]
- Farrokhi, N.; Whitelegge, J.P.; Brusslan, J.A. Plant peptides and peptidomics. Plant Biotechnol. J. 2008, 6, 105–134. [Google Scholar] [CrossRef]
- Wang, L.; Einig, E.; Almeida-Trapp, M.; Albert, M.; Fliegmann, J.; Mithöfer, A.; Kalbacher, H.; Felix, G. The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants 2018, 4, 152–156. [Google Scholar] [CrossRef]
- Kim, P.M.; Sboner, A.; Xia, Y.; Gerstein, M. The role of disorder in interaction networks: A structural analysis. Mol. Syst. Biol. 2008, 4, 179. [Google Scholar] [CrossRef] [PubMed]
- Wallmann, A.; Kesten, C. Common functions of disordered proteins across evolutionary distant organisms. Int. J. Mol. Sci. 2020, 21, 2105. [Google Scholar] [CrossRef] [PubMed]
- Cairns, N.G.; Pasternak, M.; Wachter, A.; Cobbett, C.S.; Meyer, A.J. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006, 141, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Gallé, Á.; Czékus, Z.; Bela, K.; Horváth, E.; Ördög, A.; Csiszár, J.; Poór, P. Plant glutathione transferases and light. Front. Plant Sci. 2019, 9, 1944. [Google Scholar] [CrossRef]
- Gulyás, Z.; Boldizsár, Á.; Novák, A.; Szalai, G.; Pál, M.; Galiba, G.; Kocsy, G. Central role of the flowering repressor ZCCT2 in the redox control of freezing tolerance and the initial development of flower primordia in wheat. BMC Plant Biol. 2014, 14, 91. [Google Scholar] [CrossRef]
- Vernoux, T.; Wilson, R.C.; Seeley, K.A.; Reichheld, J.-P.; Muroy, S.; Brown, S.; Maughan, S.C.; Cobbett, C.S.; Van Montagu, M.; Inzé, D. The root meristemless1/cadmium sensitive2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 2000, 12, 97–109. [Google Scholar] [CrossRef]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Walling, L.L. Adaptive defense responses to pathogens and insects. Adv. Bot. Res. 2009, 51, 551–612. [Google Scholar] [CrossRef]
- Camps, M.; Nichols, A.; Arkinstall, S. Dual specificity phosphatases: A gene family for control of MAP kinase function. FASEB J. 2000, 14, 6–16. [Google Scholar] [CrossRef]
- Gao, X.; Cox, K.L., Jr.; He, P. Functions of calcium-dependent protein kinases in plant innate immunity. Plants 2014, 3, 160–176. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Li, M.; He, P.; Zhang, Y. Loss-of-function of Arabidopsis receptor-like kinase BIR 1 activates cell death and defense responses mediated by BAK 1 and SOBIR 1. New Phytol. 2016, 212, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Cassin, A.M.; Lonsdale, A.; Bacic, A.; Doblin, M.S.; Schultz, C.J. Pipeline to identify hydroxyproline-rich glycoproteins. Plant Physiol. 2017, 174, 886–903. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Ryan, C.A. Systemic signaling in tomato plants for defense against herbivores: Isolation and characterization of three novel defense-signaling glycopeptide hormones coded in a single precursor gene. J. Biol. Chem. 2003, 278, 30044–30050. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, D.; Voigt, C.A. Callose biosynthesis in Arabidopsis with a focus on pathogen response: What we have learned within the last decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef]
- Voxeur, A.; Habrylo, O.; Guénin, S.; Miart, F.; Soulié, M.-C.; Rihouey, C.; Pau-Roblot, C.; Domon, J.-M.; Gutierrez, L.; Pelloux, J. Oligogalacturonide production upon Arabidopsis thaliana–Botrytis cinerea interaction. Proc. Natl. Acad. Sci. USA 2019, 116, 19743–19752. [Google Scholar] [CrossRef]
- Hardham, A.R.; Jones, D.A.; Takemoto, D. Cytoskeleton and cell wall function in penetration resistance. Curr. Opin. Plant Biol. 2007, 10, 342–348. [Google Scholar] [CrossRef]
- Hardham, A.R. Microtubules and biotic interactions. Plant J. 2013, 75, 278–289. [Google Scholar] [CrossRef]
- Moral, J.; Montilla-Bascón, G.; Canales, F.J.; Rubiales, D.; Prats, E. Cytoskeleton reorganization/disorganization is a key feature of induced inaccessibility for defence to successive pathogen attacks. Mol. Plant Pathol. 2017, 18, 662–671. [Google Scholar] [CrossRef]
- Schmelzer, E. Cell polarization, a crucial process in fungal defence. Trends Plant Sci. 2002, 7, 411–415. [Google Scholar] [CrossRef]
- Janda, M.; Matoušková, J.; Burketová, L.; Valentová, O. Interconnection between actin cytoskeleton and plant defense signaling. Plant Signal. Behav. 2014, 9, e976486. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Luo, Z.B. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Biotechnol. Isoprenoids 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Parizad, S.; Dizadji, A.; Habibi, M.K.; Winter, S.; Kalantari, S.; Movi, S.; Tendero, C.L.; Alonso, G.L.; Moratalla-Lopez, N. The effects of geographical origin and virus infection on the saffron (Crocus sativus L.) quality. Food Chem. 2019, 295, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Bernards, M.A.; Båstrup-Spohr, L. Phenylpropanoid metabolism induced by wounding and insect herbivory. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: New York, NY, USA, 2008; pp. 189–211. [Google Scholar] [CrossRef]
- Gallie, D.R. Appearance and elaboration of the ethylene receptor family during land plant evolution. Plant Mol. Biol. 2015, 87, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; De Lorenzo, G. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef]
- Savatin, D.V.; Ferrari, S.; Sicilia, F.; De Lorenzo, G. Oligogalacturonide-auxin antagonism does not require posttranscriptional gene silencing or stabilization of auxin response repressors in Arabidopsis. Plant Physiol. 2011, 157, 1163–1174. [Google Scholar] [CrossRef]
- Qi, L.; Yan, J.; Li, Y.; Jiang, H.; Sun, J.; Chen, Q.; Li, H.; Chu, J.; Yan, C.; Sun, X. Arabidopsis thaliana plants differentially modulate auxin biosynthesis and transport during defense responses to the necrotrophic pathogen Alternaria brassicicola. New Phytol. 2012, 195, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Cervone, F.; Hahn, M.G.; De Lorenzo, G.; Darvill, A.; Albersheim, P. Host-pathogen interactions: XXXIII. A plant protein converts a fungal pathogenesis factor into an elicitor of plant defense responses. Plant Physiol. 1989, 90, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Cardenas, M.; Ryan, C.A. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 6553–6557. [Google Scholar] [CrossRef] [PubMed]
- Denoux, C.; Galletti, R.; Mammarella, N.; Gopalan, S.; Werck, D.; De Lorenzo, G.; Ferrari, S.; Ausubel, F.M.; Dewdney, J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant. 2008, 1, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Federici, L.; Di Matteo, A.; Fernandez-Recio, J.; Tsernoglou, D.; Cervone, F. Polygalacturonase inhibiting proteins: Players in plant innate immunity? Trends Plant Sci. 2006, 11, 65–70. [Google Scholar] [CrossRef]
- Ferrari, S.; Galletti, R.; Denoux, C.; De Lorenzo, G.; Ausubel, F.M.; Dewdney, J. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires phytoalexin deficient3. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, P.; Broberg, M.; Kariola, T.; Sipari, N.; Pirhonen, M.; Palva, E.T. Short oligogalacturonides induce pathogen resistance-associated gene expression in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Montesano, M.; Kõiv, V.; Mäe, A.; Palva, E.T. Novel receptor-like protein kinases induced by Erwinia carotovora and short oligogalacturonides in potato. Mol. Plant Pathol. 2001, 2, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Simpson, S.; Ashford, D.; Harvey, D.; Bowles, D. Short chain oligogalacturonides induce ethylene production and expression of the gene encoding aminocyclopropane 1-carboxylic acid oxidase in tomato plants. Glycobiology 1998, 8, 579–583. [Google Scholar] [CrossRef]
- Thain, J.; Gubb, I.; Wildon, D. Depolarization of tomato leaf cells by oligogalacturonide elicitors. Plant Cell Environ. 1995, 18, 211–214. [Google Scholar] [CrossRef]
- Weber, J.; Olsen, O.; Wegener, C.; Von Wettstein, D. Digalacturonates from pectin degradation induce tissue responses against potato soft rot. Physiol. Mol. Plant Path. 1996, 48, 389–401. [Google Scholar] [CrossRef]
- Li, B.; Meng, X.; Shan, L.; He, P. Transcriptional regulation of pattern-triggered immunity in plants. Cell Host Microbe 2016, 19, 641–650. [Google Scholar] [CrossRef]
- Norman-Setterblad, C.; Vidal, S.; Palva, E.T. Interacting signal pathways control defense gene expression in Arabidopsis in response to cell wall-degrading enzymes from Erwinia carotovora. Mol. Plant Microbe Interact. 2000, 13, 430–438. [Google Scholar] [CrossRef] [PubMed]

| Gene ID | logFC | Description |
|---|---|---|
| Solyc09g011630.2.1 | 2,097,059 | Glutathione S-transferase |
| Solyc09g011500.2.1 | 36,160,662 | Glutathione S-transferase |
| Solyc02g092270.2.1 | 24,208,286 | NADH dehydrogenase |
| Solyc03g083900.2.1 | 23,965,964 | Laccase-22 |
| Solyc09g098150.2.1 | 2,361,944 | Metacaspase 7 |
| Solyc08g082170.2.1 | 6,065,851 | Polygalacturonase |
| Solyc05g054700.2.1 | 31,435,359 | Dual-specificity phosphatase 1 |
| Solyc01g081250.2.1 | −43,141,214 | Glutathione-S-transferase |
| Solyc01g104860.2.1 | −5,063,558 | Peroxidase 43 |
| Solyc01g105070.2.1 | −3,189,162 | Peroxidase |
| Solyc07g017880.2.1 | −23,453,817 | Peroxidase |
| Solyc09g007270.2.1 | −2,231,321 | Ascorbate peroxidase |
| Solyc12g094620.1.1 | −27,919,602 | Catalase |
| Solyc03g115930.1.1 | −221,963 | Calmodulin-like protein |
| Solyc01g010020.2.1 | −21,430,988 | Calmodulin |
| Solyc01g103450.2.1 | 22,321,863 | Chaperone DnaK |
| Solyc07g006540.2.1 | 21,124,113 | Chaperone protein ClpB |
| Solyc04g081570.2.1 | 39,538,085 | Chaperone protein htpG |
| Solyc11g065260.1.1 | 25,225,124 | Chaperone protein dnaJ |
| Solyc02g080470.2.1 | 2,050,839 | Heat shock protein 4 |
| Solyc12g043110.1.1 | 23,375 | Heat shock protein 70 |
| Solyc09g074930.2.1 | 52,265,615 | Stress-related protein |
| Solyc04g081070.2.1 | 21,339,486 | Heat shock protein DnaJ domain protein |
| Solyc04g063230.2.1 | 20,030,978 | Dehydration-responsive family protein |
| Solyc06g069870.2.1 | 22,527,552 | Dehydration-responsive family protein |
| Solyc03g005600.2.1 | 23,916,254 | Dehydration-responsive protein |
| Gene ID | logFC | Description |
|---|---|---|
| Solyc02g078230.1.1 | 25,605,323 | Callose synthase 11 |
| Solyc12g056580.1.1 | 23,134,887 | Cellulose synthase |
| Solyc04g071650.2.1 | 22,216,854 | Cellulose synthase |
| Solyc03g097050.2.1 | 2,168,395 | Cellulose synthase |
| Solyc08g061100.2.1 | 20,450,845 | Cellulose synthase |
| Solyc01g087210.2.1 | 2,039,101 | Cellulose synthase |
| Solyc05g009930.2.1 | 2,154,703 | Hydroxyproline-rich glycoprotein family protein |
| Solyc08g082170.2.1 | 6,065,851 | Polygalacturonase |
| Solyc02g084390.2.1 | 2,097,367 | Kinesin protein nack1 |
| Solyc06g009780.2.1 | 22,364,771 | Kinesin |
| Solyc11g005330.1.1 | 20,083,592 | Actin |
| Solyc04g015830.2.1 | 20,667,365 | Villin 2 |
| Solyc02g021420.2.1 | 28,023,307 | Villin-4 |
| Gene ID | logFC | Description |
|---|---|---|
| Solyc12g098590.1.1 | 37,859,063 | Crocetin chloroplastic-like |
| Solyc02g085020.2.1 | 36,648,946 | Dihydroflavonol 4-reductase |
| Solyc05g053550.2.1 | 25,119,457 | Chalcone synthase |
| Solyc09g091510.2.1 | 21,222,255 | Chalcone synthase |
| Solyc02g083860.2.1 | 2,006,604 | Flavanone 3 beta-hydroxylase |
| Solyc08g005860.2.1 | 22,907,643 | Putrescine-binding periplasmic protein |
| Solyc04g082030.1.1 | −2,169,399 | Ornithine decarboxylase |
| Gene ID | logFC | Description |
|---|---|---|
| Solyc01g099210.2.1 | −20,045,187 | Lipoxygenase |
| Solyc01g111960.2.1 | −52,605,577 | GDSL esterase/lipase |
| Solyc05g043320.1.1 | −22,984,564 | GDSL esterase/lipase |
| Solyc11g051060.1.1 | −2,308,117 | GDSL esterase/lipase 2 |
| Solyc02g090940.2.1 | −20,605,335 | Lipase |
| Solyc03g093360.2.1 | −21,316,884 | Wound/stress protein |
| Solyc03g098740.1.1 | −24,175,534 | Kunitz trypsin inhibitor |
| Solyc09g084470.2.1 | −62,815,356 | Proteinase inhibitor I |
| Solyc07g007250.2.1 | −14,125,946 | Metallocarboxypeptidase inhibitor |
| Solyc07g007260.2.1 | −25,874,014 | Metallocarboxypeptidase inhibitor |
| Solyc04g040180.2.1 | −32,112,498 | S-adenosylmethionine-dependent methyltransferase |
| Solyc01g097270.2.1 | −23,048,432 | Chitinase |
| Solyc08g080650.1.1 | −30,336,623 | Osmotin |
| Solyc06g065370.2.1 | −2,287,122 | Subtilisin |
| Solyc09g006010.2.1 | −39,370,556 | Pathogenesis related protein PR-1 |
| Solyc00g174340.1.1 | −31,596,904 | Pathogenesis-related protein 1b |
| Solyc09g089580.2.1 | −2,396,617 | 1-aminocyclopropane-1-carboxylate oxidase |
| Solyc01g059860.2.1 | 21,835,773 | Serine threonine-protein kinase |
| Solyc11g006180.1.1 | 24,757,214 | Ethylene receptor |
| Solyc01g110800.2.1 | −33,291,337 | Auxin-induced SAUR-like protein |
| Solyc01g110940.2.1 | −21,495,044 | Auxin-induced SAUR-like protein |
| Solyc02g077880.2.1 | −32,065,403 | Auxin-repressed protein |
| Solyc02g082450.2.1 | −2,151,276 | Auxin efflux carrier family protein |
| Solyc04g082830.2.1 | −2,753,833 | Auxin efflux carrier family protein |
| Solyc03g082510.1.1 | −2,208,86 | Auxin-responsive family protein |
| Solyc05g008850.2.1 | −4,863,605 | Auxin responsive protein |
| Solyc10g052530.1.1 | −24,981,644 | Auxin-responsive protein |
| Solyc06g053260.1.1 | −47,414,575 | Auxin-responsive family protein |
| Solyc05g051660.1.1 | −2,158,873 | Gibberellin receptor GID1L2 |
| Solyc09g075670.1.1 | −2,292,731 | Gibberellin receptor GID1L2 |
| Solyc06g007890.2.1 | −2,320,646 | Gibberellin-regulated protein |
| Solyc11g017440.1.1 | −55,233,316 | Gibberellin-regulated protein 9 |
| Solyc11g011210.1.1 | −34,478,865 | Gibberellin regulated protein |
| Solyc07g056670.2.1 | −20,980,172 | Gibberellin 2-oxidase 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molisso, D.; Coppola, M.; Buonanno, M.; Di Lelio, I.; Monti, S.M.; Melchiorre, C.; Amoresano, A.; Corrado, G.; Delano-Frier, J.P.; Becchimanzi, A.; et al. Tomato Prosystemin Is Much More than a Simple Systemin Precursor. Biology 2022, 11, 124. https://doi.org/10.3390/biology11010124
Molisso D, Coppola M, Buonanno M, Di Lelio I, Monti SM, Melchiorre C, Amoresano A, Corrado G, Delano-Frier JP, Becchimanzi A, et al. Tomato Prosystemin Is Much More than a Simple Systemin Precursor. Biology. 2022; 11(1):124. https://doi.org/10.3390/biology11010124
Chicago/Turabian StyleMolisso, Donata, Mariangela Coppola, Martina Buonanno, Ilaria Di Lelio, Simona Maria Monti, Chiara Melchiorre, Angela Amoresano, Giandomenico Corrado, John Paul Delano-Frier, Andrea Becchimanzi, and et al. 2022. "Tomato Prosystemin Is Much More than a Simple Systemin Precursor" Biology 11, no. 1: 124. https://doi.org/10.3390/biology11010124
APA StyleMolisso, D., Coppola, M., Buonanno, M., Di Lelio, I., Monti, S. M., Melchiorre, C., Amoresano, A., Corrado, G., Delano-Frier, J. P., Becchimanzi, A., Pennacchio, F., & Rao, R. (2022). Tomato Prosystemin Is Much More than a Simple Systemin Precursor. Biology, 11(1), 124. https://doi.org/10.3390/biology11010124









