Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sea Bass
2.2. Experimental Design
2.2.1. Pre-Experiment: Determination of the Use of MOEO and Anaesthetic
2.2.2. Experiment: Simulation of Live Transport of Sea Bass
2.3. Pre-Treatment of Sample and Determination of Indicators
2.3.1. Light Microscopy of Gill Tissue
2.3.2. Scanning Electron Microscopy of Gill Tissue
2.3.3. Determination of Liver Injury Indices
2.3.4. Determination of Blood Immune Indices
3. Statistical Analysis
4. Results and Discussion
4.1. Survival Rates of Sea Bass
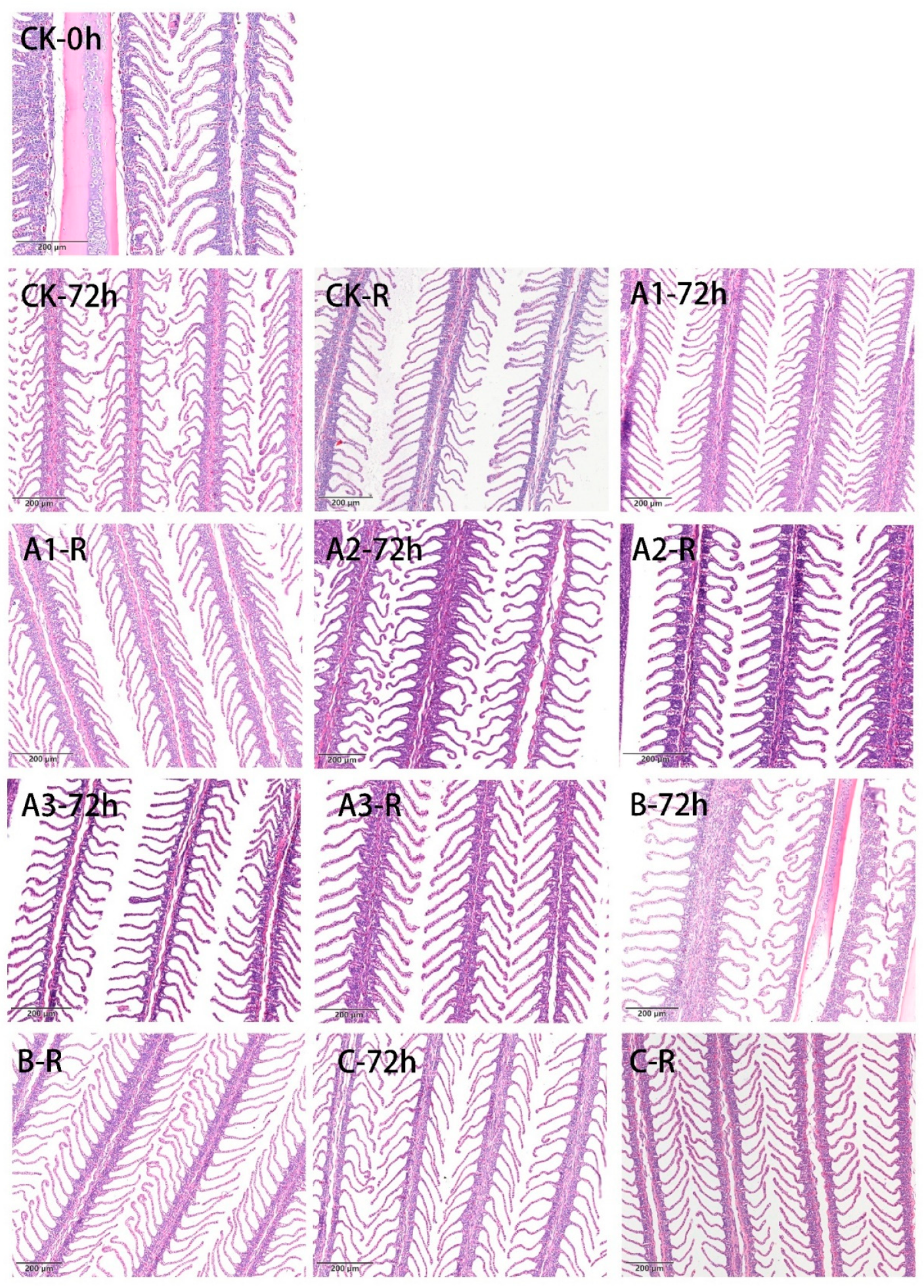
4.2. Light Microscopy of Gill Tissue
4.3. Scanning Electron Microscopy of Gill Tissue
4.4. Determination of Liver Injury Indices
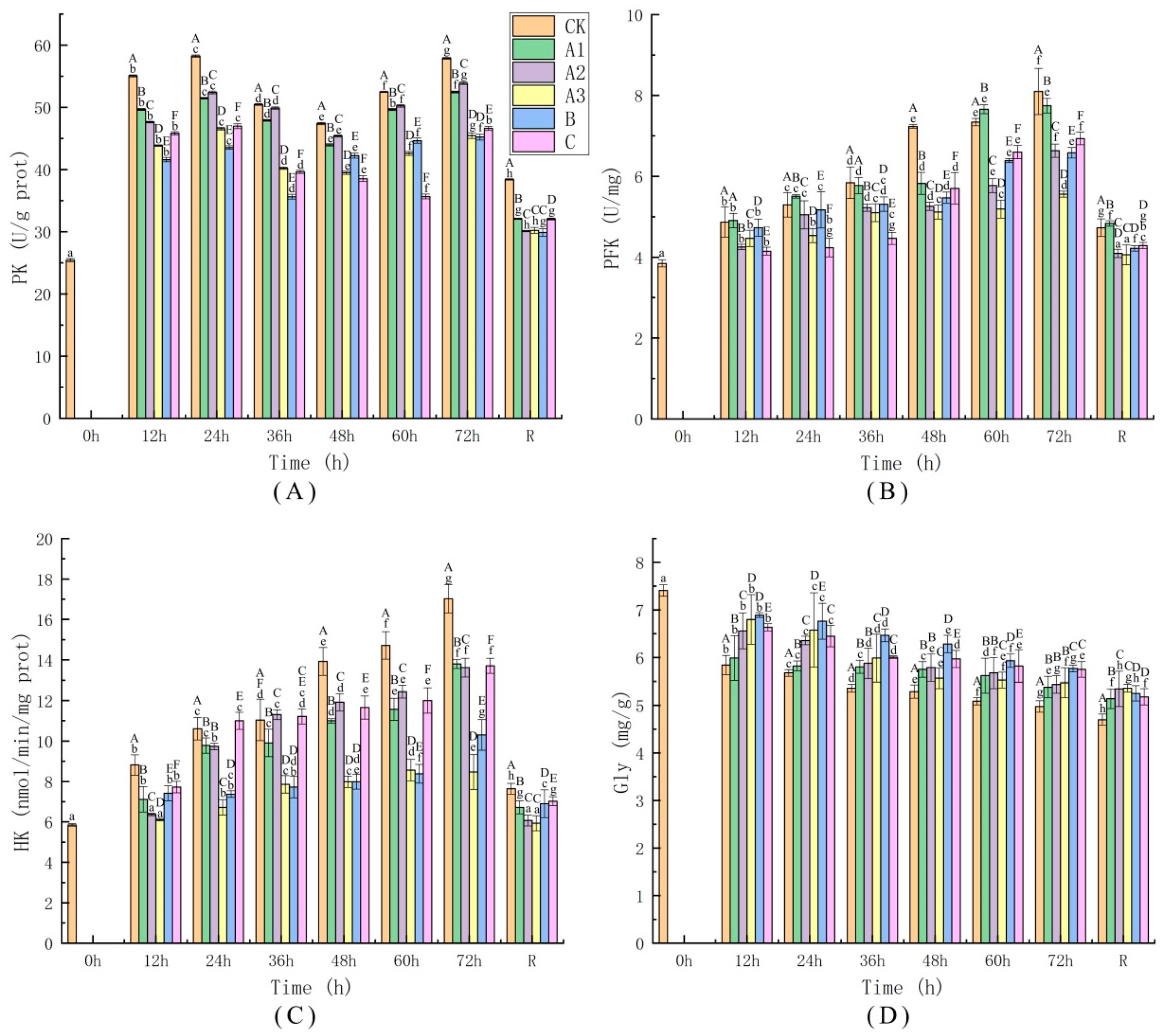
4.4.1. Determination of Glycolytic Pathway Activity
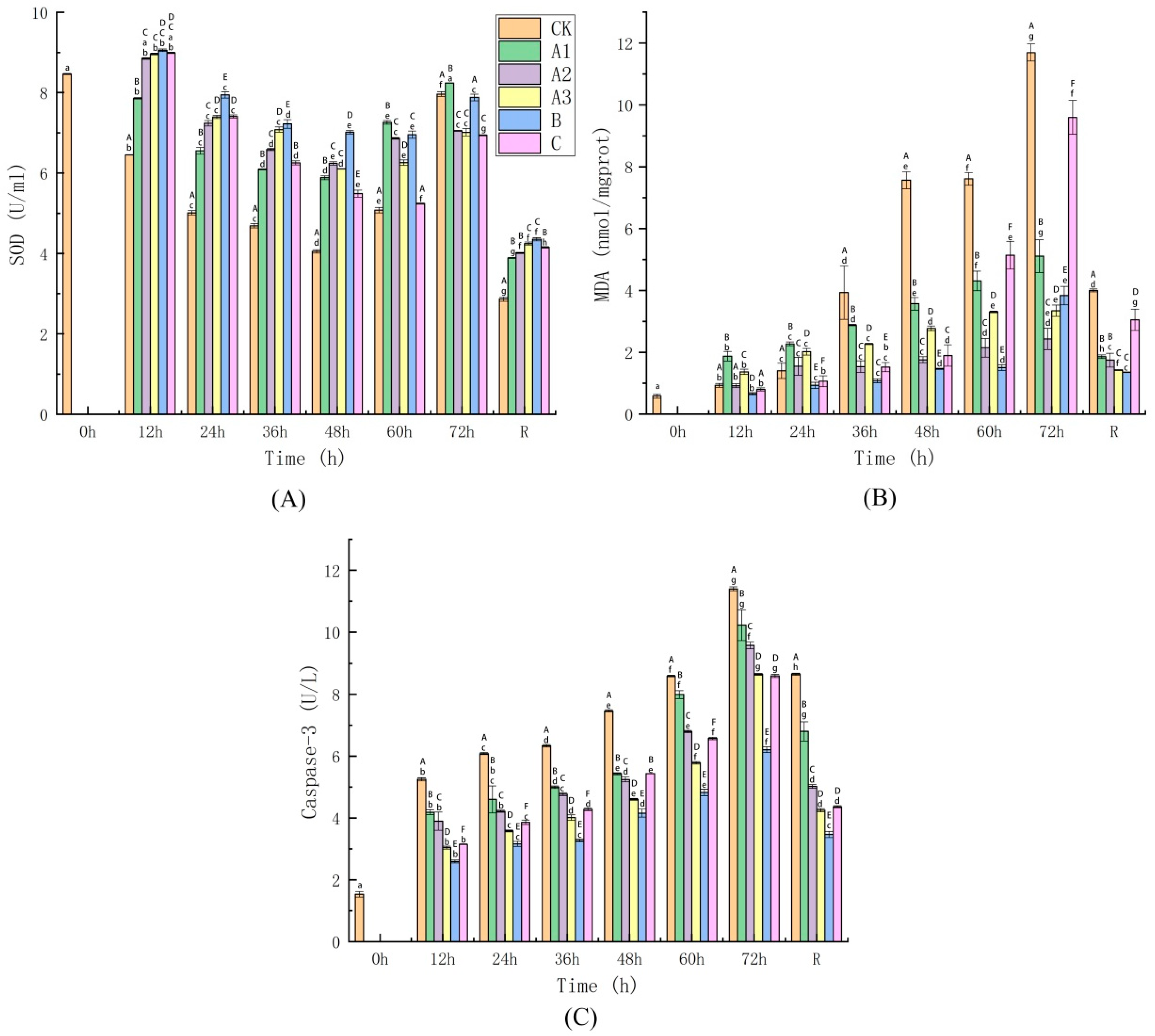
4.4.2. Determination of Oxidative Stress Injury Indices
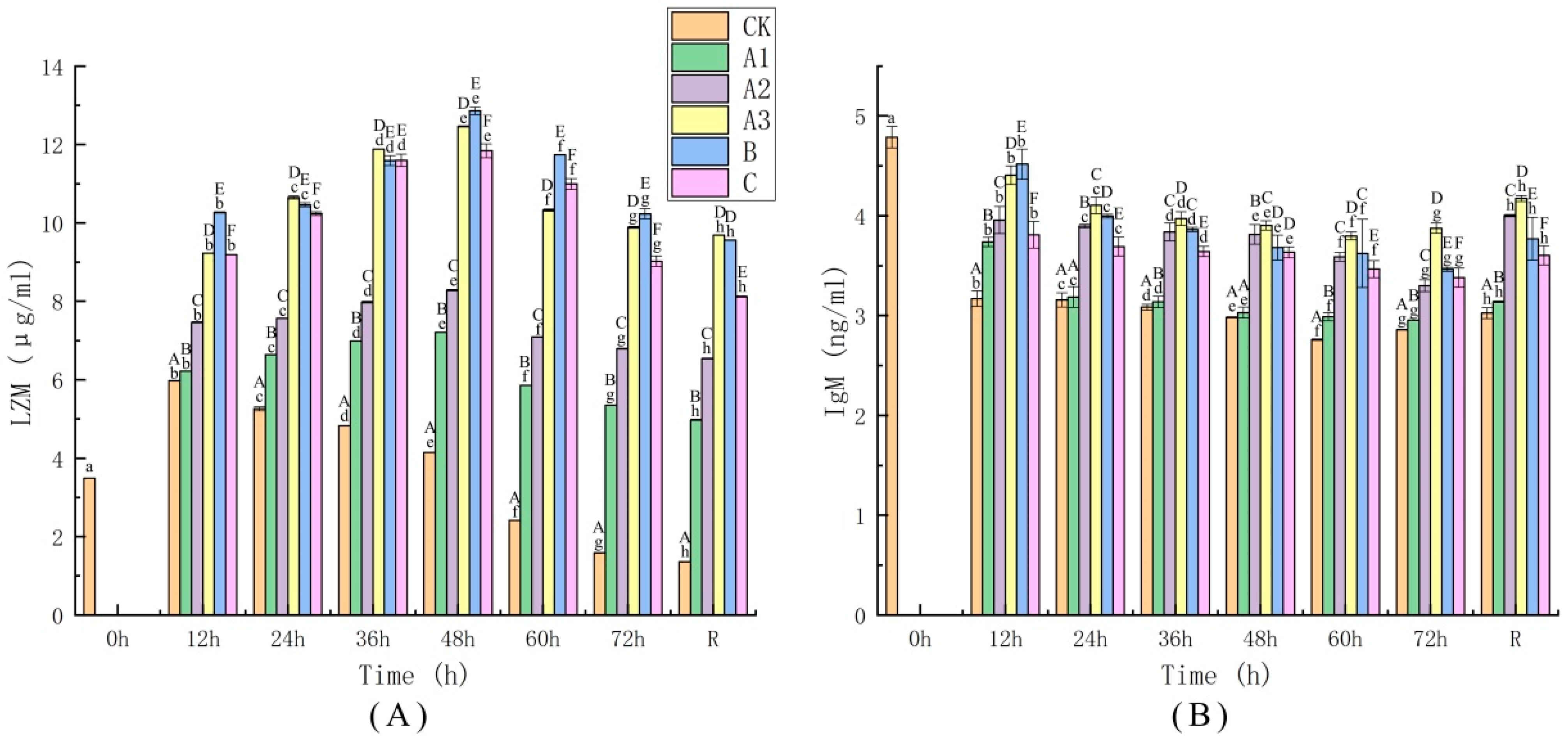
4.5. Determination of Immune Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Lan, W.; Sun, X.; Xie, J. Effects of chitosan grafted phenolic acid coating on microbiological, physicochemical and protein changes of sea bass (Lateolabrax japonicus) during refrigerated storage. J. Food Sci. 2020, 85, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Cordiner, S.; Egginton, S.J.F.P. Biochemistry, Effects of seasonal temperature acclimatization on muscle metabolism in rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 1997, 16, 333–343. [Google Scholar] [CrossRef]
- Li, P.; Chen, Z.; Tan, M.; Mei, J.; Xie, J. Evaluation of weakly acidic electrolyzed water and modified atmosphere packaging on the shelf life and quality of farmed puffer fish (Takifugu obscurus) during cold storage. J. Food Saf. 2020, 40, e12773. [Google Scholar] [CrossRef]
- Picchietti, S.; Miccoli, A.; Fausto, A.M. Gut immunity in European sea bass (Dicentrarchus labrax): A review. Fish Shellfish. Immunol. 2021, 108, 94–108. [Google Scholar] [CrossRef]
- Paterson, B.D.; Rimmer, M.A.; Meikle, G.M.; Semmens, G.L. Physiological responses of the Asian sea bass, Lates calcarifer to water quality deterioration during simulated live transport: Acidosis, red-cell swelling, and levels of ions and ammonia in the plasma. Aquaculture 2003, 218, 717–728. [Google Scholar] [CrossRef]
- Abdel-Naime, W.A.; Fahim, J.R.; Fouad, M.A.; Kamel, M.S. Antibacterial, antifungal, and GC–MS studies of Melissa officinalis. S. Afr. J. Bot. 2019, 124, 228–234. [Google Scholar] [CrossRef]
- Wu, S.M.; Chen, J.-R.; Chang, C.-Y.; Tseng, Y.-J.; Pan, B.S. Potential benefit of I-Tiao-Gung (Glycine tomentella) extract to enhance ornamental fish welfare during live transport. Aquaculture 2021, 534. [Google Scholar] [CrossRef]
- Fan, X.; Qin, X.; Zhang, C.; Zhu, Q.; Chen, J.; Chen, P. Metabolic and anti-oxidative stress responses to low temperatures during the waterless preservation of the hybrid grouper (Epinephelus fuscogutatus♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 508, 10–18. [Google Scholar] [CrossRef]
- Tacchi, L.; Lowrey, L.; Musharrafieh, R.; Crossey, K.; Larragoite, E.T.; Salinas, I. Effects of transportation stress and addition of salt to transport water on the skin mucosal homeostasis of rainbow trout (Oncorhynchus mykiss). Aquaculture 2015, 435, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, X.; Zhang, F.; Wang, T.; Zheng, X.; Li, Y.; Huang, B.; Zhang, C. Physiological and morphological changes in Turbot (Psetta maxima) gill tissue during waterless storage. Aquaculture 2019, 508, 30–35. [Google Scholar] [CrossRef]
- Urbinati, E.C.; Zanuzzo, F.S.; Biller, J.D. Stress and immune system in fish. In Biology and Physiology of Freshwater Neotropical Fish; Academic Press: Cambridge, MA, USA, 2020; pp. 93–114. [Google Scholar]
- Nardocci, G.; Navarro, C.; Cortes, P.P.; Imarai, M.; Montoya, M.; Valenzuela, B.; Jara, P.; Acuna-Castillo, C.; Fernandez, R. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellfish Immunol. 2014, 40, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, X.; Yang, J.; Zhang, R.; Zhang, Q.; Yang, J. The bacteriolytic mechanism of an invertebrate-type lysozyme from mollusk Octopus ocellatus. Fish Shellfish Immunol. 2019, 93, 232–239. [Google Scholar] [CrossRef]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. Natural versus synthetic anesthetic for transport of live fish: A review. Aquac. Fish. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Berka, R. The Transport of Live Fish. A. Review. 1986. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs144p2_044511.pdf (accessed on 22 December 2021).
- Claesson, D.; Wang, T.; Malte, H. Maximal oxygen consumption increases with temperature in the European eel (Anguilla anguilla) through increased heart rate and arteriovenous extraction. Conserv. Physiol. 2016, 4, cow027. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, H. The effect of 2-phenoxyethanol and transport packing density on the post-transport survival rate and metabolic activity in the goldfish. Carassius Auratus. Aquar. Sci. Conserv. 1998, 2, 1–7. [Google Scholar] [CrossRef]
- Ayson, F.G.; Parazo, M.M.; Reyes, D.M., Jr. Survival of young rabbitfish (Siganus guttatus Bloch) under simulated transport conditions. J. Appl. Ichthyol. 2010, 6, 161–166. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Mirghaed, A.T.; Yousefi, M. Application of herbal anaesthetics in aquaculture. Rev. Aquac. 2019, 11, 550–564. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Ghelichpour, M.; Hoseini, S.M. Myrcene and linalool as new anesthetic and sedative agents in common carp, Cyprinus carpio—Comparison with eugenol. Aquaculture 2016, 464, 165–170. [Google Scholar] [CrossRef]
- Ke, C.; Liu, Q.; Li, L.; Chen, J.; Zhao, C.; Xu, J.; Huang, K.; Mengsong, M.; Li, L. Residual levels and risk assessment of eugenol and its isomers in fish from China markets. Aquaculture 2018, 484, 338–342. [Google Scholar] [CrossRef]
- Schnick, R.R. Zero Withdrawal Anesthetic for All Finfish and Shellfish. Fisheries 2006, 31, 122–126. [Google Scholar] [CrossRef]
- Taherpour, A.A.; Maroofi, H.; Rafie, Z.; Larijani, K. Chemical composition analysis of the essential oil of Melissa officinalis L. from Kurdistan, Iran by HS/SPME method and calculation of the biophysicochemical coefficients of the components. Nat. Prod. Res. 2012, 26, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, C.; Petersen, M. Cloning and characterisation of rosmarinic acid synthase from Melissa officinalis L. Phytochemistry 2011, 72, 572–578. [Google Scholar] [CrossRef]
- Awad, R.; Muhammad, A.; Durst, T.; Trudeau, V.L.; Arnason, J.T. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother. Res. 2009, 23, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.D.F.; Baldissera, M.D.; Bianchini, A.E.; da Silva, E.G.; Veraz Mourao, R.H.; Flores da Silva, L.V.; Schmidt, D.; Heinzmann, B.M.; Baldisserotto, B. Citral and linalool chemotypes of Lippia alba essential oil as anesthetics for fish: A detailed physiological analysis of side effects during anesthetic recovery in silver catfish (Rhamdia quelen). Fish Physiol. Biochem. 2018, 44, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Cho, S.Y.; Bhuiyan, M.J.; Kim, K.H.; Lee, S.J. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br. J. Nutr. 2010, 104, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topal, U.; Sasaki, M.; Goto, M.; Otles, S. Chemical compositions and antioxidant properties of essential oils from nine species of Turkish plants obtained by supercritical carbon dioxide extraction and steam distillation. Int. J. Food Sci. Nutr. 2008, 59, 619–634. [Google Scholar] [CrossRef]
- Jeusette, I.; Tami, G.; Fernandez, A.; Torre, C.; Tvarijonaviciute, A.; Ceron, J.; Salas-Mani, A.; Fatjò, J. Evaluation of a new prescription diet with lemon balm, fish peptides, oligofructose and L-tryptophan to reduce urinary cortisol, used as a marker of stress, in cats. J. Vet. Behav. 2021, 42, 30–36. [Google Scholar] [CrossRef]
- Boaventura, T.P.; Souza, C.F.; Ferreira, A.L.; Favero, G.C.; Baldissera, M.D.; Heinzmann, B.M.; Baldisserotto, B.; Luz, R.K. The use of Ocimum gratissimum L. essential oil during the transport of Lophiosilurus alexandri: Water quality, hematology, blood biochemistry and oxidative stress. Aquaculture 2021, 531, 8. [Google Scholar] [CrossRef]
- Khumpirapang, N.; Pikulkaew, S.; Anuchapreeda, S.; Okonogi, S. Alpinia galanga. oil-A new natural source of fish anaesthetic. Aquac. Res. 2018, 49, 1546–1556. [Google Scholar] [CrossRef]
- Rodrigues, P.; Ferrari, F.T.; Barbosa, L.B.; Righi, A.; Laporta, L.; Garlet, Q.I.; Baldisserotto, B.; Heinzmann, B.M. Nanoemulsion boosts anesthetic activity and reduces the side effects of Nectandra grandiflora Nees essential oil in fish. Aquaculture 2021, 545, 737146. [Google Scholar] [CrossRef]
- Hong, J.; Chen, X.; Liu, S.; Fu, Z.; Han, M.; Wang, Y.; Gu, Z.; Ma, Z. Impact of fish density on water quality and physiological response of golden pompano (Trachinotus ovatus) flingerlings during transportation. Aquaculture 2019, 507, 260–265. [Google Scholar] [CrossRef]
- Sullivan, M.L.; Tomasso, J.R. Limiting and Optimal Temperatures for the Northern Atlantic Population of Black Sea Bass. N. Am. J. Aquac. 2010, 72, 258–260. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Q.; Mei, J.; Xie, J. Effect of 3-Aminobenzoic Acid Ethyl Ester Methanesulfonate (MS-222) on Quality of Marine Cultured Turbot (Scophthalmus maximus) during Simulated Transport in Water. Fishes 2021, 6, 20. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Q.; Cao, J.; Mei, J.; Xie, J. Effects of Ascorbic Acid and beta-1,3-Glucan on Survival, Physiological Response and Flesh Quality of Cultured Tiger Grouper (Epinephelus fuscoguttatus) during Simulated Transport in Water. Biology (Basel) 2020, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Salbego, J.; Toni, C.; Becker, A.G.; Zeppenfeld, C.C.; Menezes, C.C.; Loro, V.L.; Heinzmann, B.M.; Baldisserotto, B. Biochemical parameters of silver catfish (Rhamdia quelen) after transport with eugenol or essential oil of Lippia alba added to the water. Braz. J. Biol. 2017, 77, 696–702. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.P.B.; Lemos, C.H.D.P.; Felix e Silva, A.; de Souza, S.A.; Albinati, A.C.L.; Lima, A.O.; Copatti, C.E. Use of eugenol for the anaesthesia and transportation of freshwater angelfish (Pterophyllum scalare). Aquaculture 2019, 513, 734409. [Google Scholar] [CrossRef]
- Fotedar, S.; Evans, L. Health management during handling and live transport of crustaceans: A review. J. Invertebr. Pathol. 2011, 106, 143–152. [Google Scholar] [CrossRef]
- Small, B.C. Anesthetic efficacy of metomidate and comparison of plasma cortisol responses to tricaine methanesulfonate, quinaldine and clove oil anesthetized channel catfish Ictalurus punctatus. Aquaculture 2003, 218, 177–185. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Santos, R.C.V.; Stefani, L.M.; Moreira, K.L.S.; da Veiga, M.L.; da Rocha, M.I.U.M.; Baldisserotto, B. Pseudomonas aeruginosa strain PA01 impairs enzymes of the phosphotransfer network in the gills of Rhamdia quelen. Vet. Microbiol. 2017, 201, 121–125. [Google Scholar] [CrossRef]
- Blair, S.D.; Matheson, D.; Goss, G.G. Physiological and morphological investigation of Arctic grayling (Thymallus arcticus) gill filaments with high salinity exposure and recovery. Conserv. Physiol. 2017, 5, cox040. [Google Scholar] [CrossRef] [Green Version]
- Kropf, C.; Fent, K.; Segner, H. ABC transporters in fish gill tissue. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, S31. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Dymowska, A.; Stecyk, J.A.W. New insights into the plasticity of gill structure. Respir. Physiol. Neurobiol. 2012, 184, 214–222. [Google Scholar] [CrossRef]
- Mata, C.; Salazar-Lugo, R.; Oliveros, A.; Rojas, L. Histological analysis of gills, liver and kidney of neotropical freshwater fish Colossoma macropomum exposed to three temperatures. Toxicol. Lett. 2009, 189, S191. [Google Scholar] [CrossRef]
- Egnew, N.; Renukdas, N.; Ramena, Y.; Yadav, A.K.; Kelly, A.M.; Lochmann, R.T.; Sinha, A.K. Physiological insights into largemouth bass (Micropterus salmoides) survival during long-term exposure to high environmental ammonia. Aquat. Toxicol. 2019, 207, 72–82. [Google Scholar] [CrossRef]
- Mangang, Y.A.; Pandey, P.K. Hemato-biochemical responses and histopathological alterations in the gill and kidney tissues of Osteobrama belangeri (Valenciennes, 1844) exposed to different sub-lethal unionized ammonia. Aquaculture 2021, 542. [Google Scholar] [CrossRef]
- Liu, M.-J.; Guo, H.-Y.; Liu, B.; Zhu, K.-C.; Guo, L.; Liu, B.-S.; Zhang, N.; Yang, J.-W.; Jiang, S.-G.; Zhang, D.-C. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2021, 222, 112504. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grings, M.; Parmeggiani, B.; Moura, A.P.; de Moura Alvorcem, L.; Wyse, A.T.S.; Wajner, M.; Leipnitz, G. Evidence that Thiosulfate Inhibits Creatine Kinase Activity in Rat Striatum via Thiol Group Oxidation. Neurotox. Res. 2018, 34, 693–705. [Google Scholar] [CrossRef]
- Serafini, S.; de Freitas Souza, C.; Baldissera, M.D.; Baldisserotto, B.; Picoli, F.; Segat, J.C.; Baretta, D.; da Silva, A.S. Fish exposed to eprinomectin show hepatic oxidative stress and impairment in enzymes of the phosphotransfer network. Aquaculture 2019, 508, 199–205. [Google Scholar] [CrossRef]
- Fudge, D.S.; Stevens, E.D.; Ballantyne, J.S. Enzyme adaptation along a heterothermic tissue: The visceral retia mirabilia of the bluefin tuna. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 272, R1834. [Google Scholar] [CrossRef] [PubMed]
- Trenzado, C.E.; Morales, A.E.; Higuera, M.J.A. Physiological effects of crowding in rainbow trout, Oncorhynchus mykiss, selected for low and high stress responsiveness. Aquaculture 2006, 258, 583–593. [Google Scholar] [CrossRef]
- Moreira, I.S.; Peres, H.; Couto, A.; Enes, P.; Oliva-Teles, A.J.A. Temperature and dietary carbohydrate level effects on performance and metabolic utilisation of diets in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2008, 274, 153–160. [Google Scholar] [CrossRef]
- Santos, R.D.; Diniz, L.P.; Galina, A.; Da-Silva, W.S.J.B.R. Characterization of non-cytosolic hexokinase activity in white skeletal muscle from goldfish (Carassius auratus L.) and the effect of cold acclimation. Biosci. Rep. 2010, 30, 413–423. [Google Scholar] [CrossRef]
- Rodnick, K.J.; Sidell, B.D. Structural and biochemical analyses of cardiac ventricular enlargement in cold-acclimated striped bass. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 273, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Pritsos, K.L.; Perez, C.R.; Muthumalage, T.; Dean, K.M.; Cacela, D.; Hanson-Dorr, K.; Cunningham, F.; Bursian, S.J.; Link, J.E.; Shriner, S.; et al. Dietary intake of Deepwater Horizon oil-injected live food fish by double-crested cormorants resulted in oxidative stress. Ecotoxicol. Environ. Saf. 2017, 146, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Paital, B. Antioxidant and oxidative stress parameters in brain of Heteropneustes fossilis under air exposure condition; role of mitochondrial electron transport chain. Ecotoxicol. Environ. Saf. 2013, 95, 69–77. [Google Scholar] [CrossRef]
- Wu, S.-M.; Tseng, Y.-J.; Lin, J.-J.; Pan, B.S. Mitigation of stress and water deterioration with a root extract of Glycine tomentella during simulated transport of orange-spotted grouper (Epinephelus coioides). Aquaculture 2020, 514, 734485. [Google Scholar] [CrossRef]
- Sirbulescu, R.F.; Ilies, I.; Zupanc, G.K. Structural and functional regeneration after spinal cord injury in the weakly electric teleost fish, Apteronotus leptorhynchus. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2009, 195, 699–714. [Google Scholar] [CrossRef]
- Hegazy, A.M.; Chen, N.; Lin, H.; Babu, V.S.; Li, F.; Yang, Y.; Qin, Z.; Shi, F.; Li, J.; Lin, L. Induction of apoptosis in SSN-1cells by Snakehead Fish Vesiculovirus (SHVV) via Matrix protein dependent intrinsic pathway. Fish Shellfish Immunol. 2021, 113, 24–34. [Google Scholar] [CrossRef]
- Guo, H.; Dixon, B. Understanding acute stress-mediated immunity in teleost fish. Fish Shellfish. Immunol. Rep. 2021, 2, 100010. [Google Scholar] [CrossRef]
- Raberg, L.; Grahn, M.; Hasselquist, D.; Svensson, E. On the adaptive significance of stress-induced immunosuppression. Proc. Biol. Sci. 1998, 265, 1637–1641. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, M.; Xie, J.; Xu, P.; Pan, L.J.A.E.S. Effects of acute cold stress onserum biochemical and immune parameters and liver HSP70 gene expression in GIFT strain of Nile tilapia (Oreochromis niloticus). Acta Ecol. Sin. 2011, 31, 4866–4873. [Google Scholar]
- Demers, N.E.; Bayne, C.J. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 1997, 21, 363. [Google Scholar] [CrossRef]
- Mu, Y.; Li, W.; Wei, Z.; He, L.; Zhang, W.; Chen, X. Transcriptome analysis reveals molecular strategies in gills and heart of large yellow croaker (Larimichthys crocea) under hypoxia stress. Fish Shellfish Immunol. 2020, 104, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.; Fierro-Castro, C.; Teles, M.; Tridico, R.; Tort, L.; Sunyer, J.O. Effect of acute stress on IgM and IgT responses in vaccinated fish. Fish Shellfish. Immunol. 2013, 34, 1727–1728. [Google Scholar] [CrossRef]

| Method | Keep-Alive Transportation Time/h | ||||||
|---|---|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | 60 | 72 | R-12 | |
| 10 mg/L MOEO | 100 | 100 | 100 | 100 | 92 | 84 | 80 |
| 20 mg/L MOEO | 100 | 100 | 100 | 100 | 96 | 96 | 96 |
| 40 mg/L MOEO | 100 | 100 | 100 | 100 | 100 | 96 | 96 |
| 60 mg/L MOEO | 100 | 100 | 90 | 90 | 80 | 75 | 65 |
| 80 mg/L MOEO | 100 | 100 | 95 | 90 | 75 | 60 | 45 |
| 10 mg/L MS-222 | 100 | 100 | 100 | 100 | 95 | 80 | 70 |
| 20 mg/L MS-222 | 100 | 100 | 100 | 100 | 100 | 85 | 75 |
| 30 mg/L MS-222 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 40 mg/L MS-222 | 100 | 100 | 100 | 100 | 95 | 90 | 90 |
| 50 mg/L MS-222 | 100 | 95 | 90 | 90 | 85 | 80 | 80 |
| 10 mg/L Eugenol | 100 | 100 | 100 | 95 | 90 | 80 | 75 |
| 15 mg/L Eugenol | 100 | 100 | 100 | 100 | 95 | 80 | 70 |
| 20 mg/L Eugenol | 100 | 100 | 100 | 100 | 100 | 100 | 94 |
| 25 mg/L Eugenol | 100 | 100 | 100 | 100 | 100 | 90 | 90 |
| 30 mg/L Eugenol | 100 | 100 | 100 | 90 | 90 | 85 | 80 |
| CK | 100 | 100 | 90 | 84 | 76 | 60 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Mei, J.; Cao, J.; Xie, J. Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport. Biology 2022, 11, 11. https://doi.org/10.3390/biology11010011
Wang Q, Mei J, Cao J, Xie J. Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport. Biology. 2022; 11(1):11. https://doi.org/10.3390/biology11010011
Chicago/Turabian StyleWang, Qi, Jun Mei, Jie Cao, and Jing Xie. 2022. "Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport" Biology 11, no. 1: 11. https://doi.org/10.3390/biology11010011
APA StyleWang, Q., Mei, J., Cao, J., & Xie, J. (2022). Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport. Biology, 11(1), 11. https://doi.org/10.3390/biology11010011







