Adding New Pieces to the Puzzle of Karyotype Evolution in Harttia (Siluriformes, Loricariidae): Investigation of Amazonian Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chromosome Obtainment and C-Banding
2.3. Fluorescence In Situ Hybridization (FISH)-Based Experiments
2.4. Image Analysis and Processing
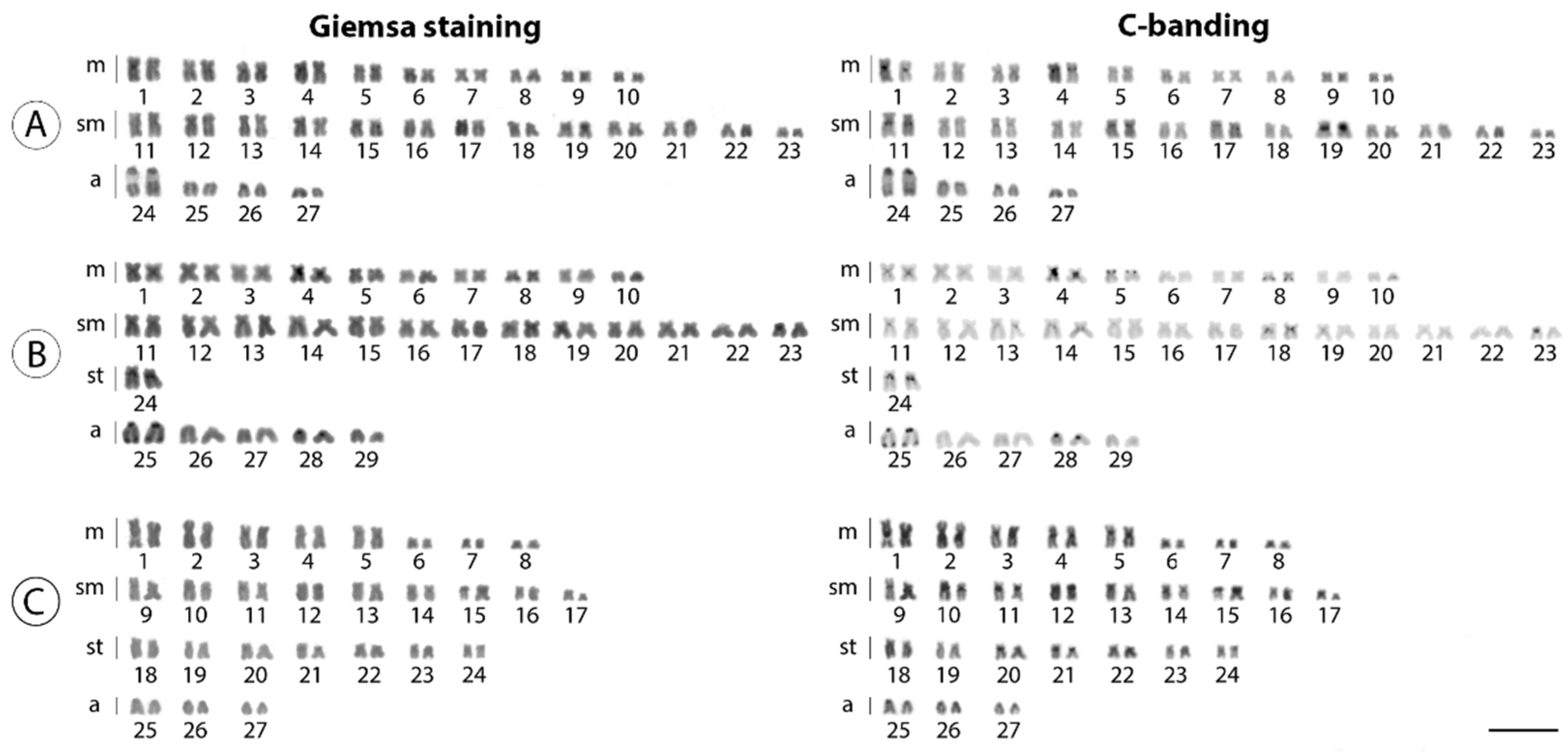
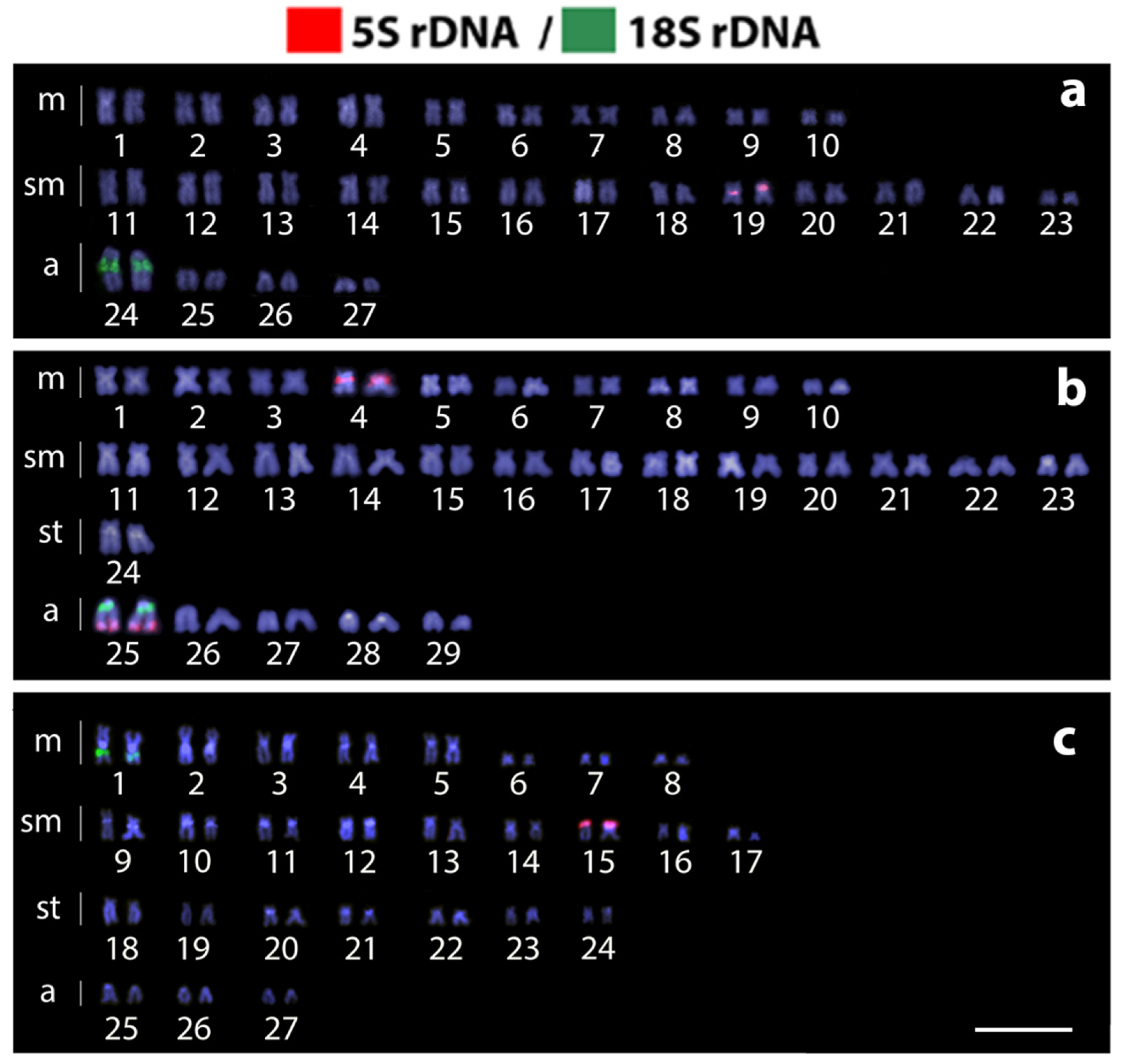
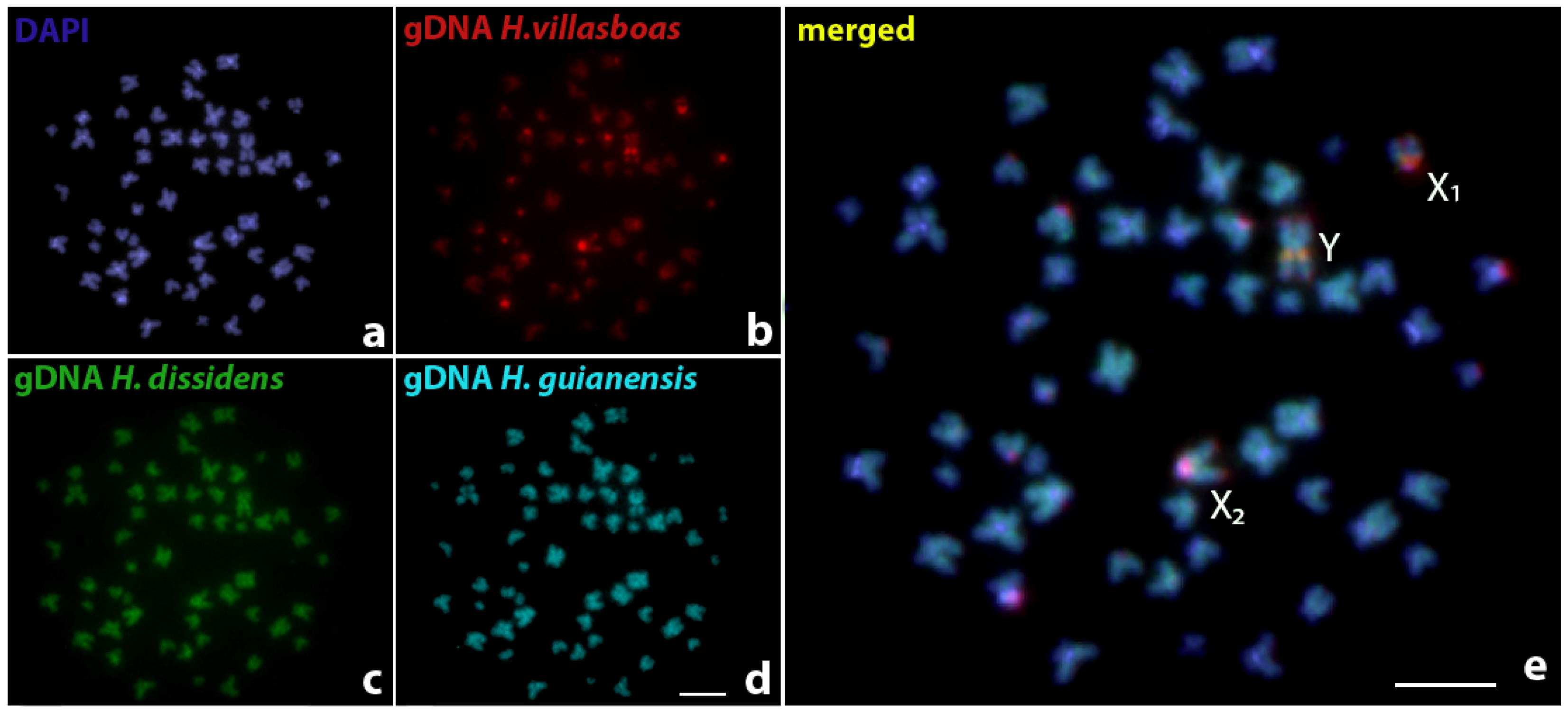
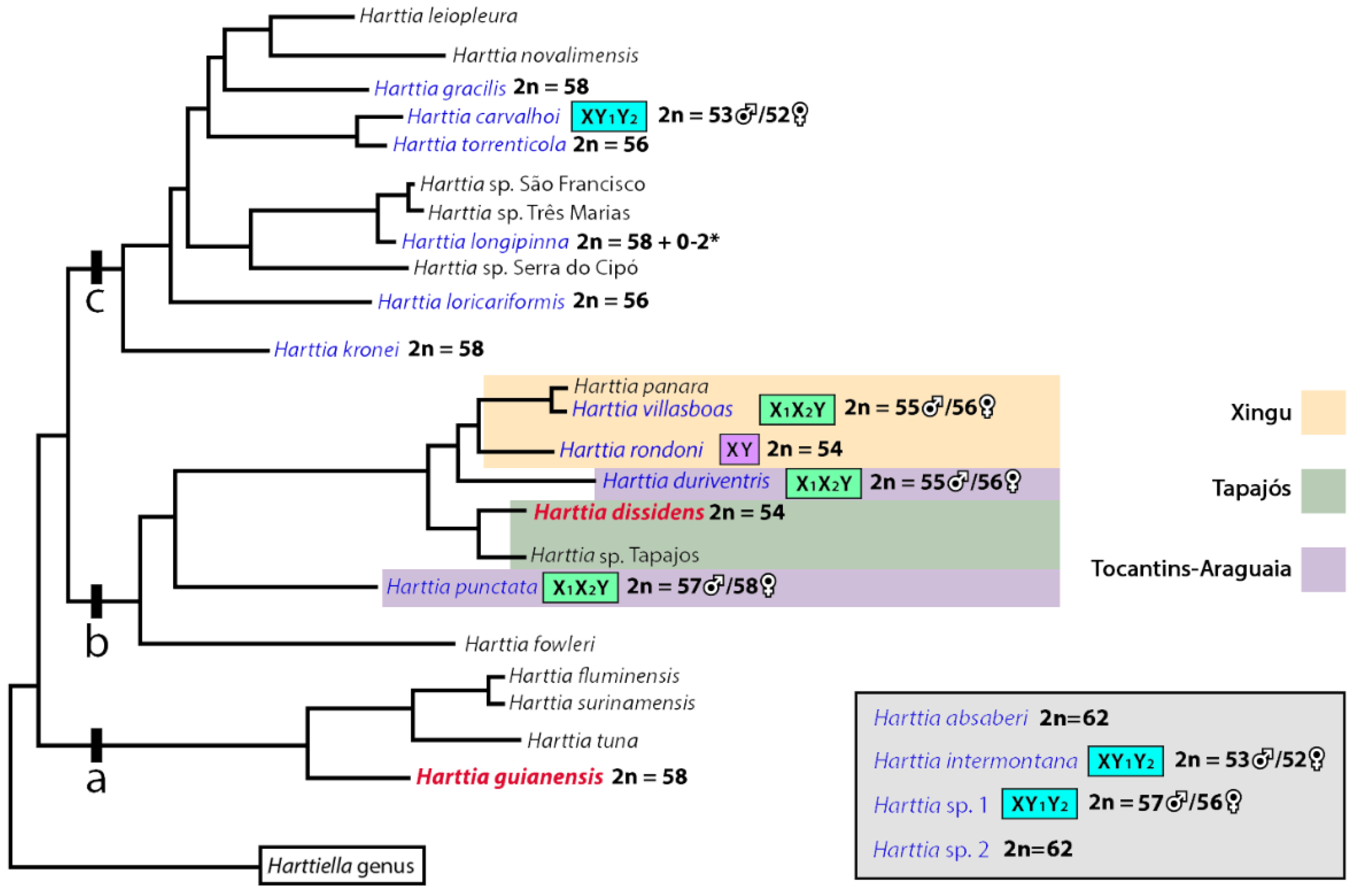
3. Results
4. Discussion
4.1. New Pieces into the Chromosomal Evolution of Harttia
4.2. Sex Chromosomes in Harttia species
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 24 August 2020).
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 111834233X. [Google Scholar]
- Volff, J.N. Genome evolution and biodiversity in teleost fish. Heredity 2005, 94, 280–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, V.; Venkatesh, B. Rapidly evolving fish genomes and teleost diversity. Curr. Opin. Genet. Dev. 2008, 18, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Kerk, S.Y.; Tan, Y.Y.; Brenner, S.; Venkatesh, B. Ancient vertebrate conserved noncoding elements have been evolving rapidly in teleost fishes. Mol. Biol. Evol. 2011, 28, 1205–1215. [Google Scholar] [CrossRef] [Green Version]
- Sallan, L.C. Major issues in the origins of ray-finned fish (Actinopterygii) biodiversity. Biol. Rev. 2014, 89, 950–971. [Google Scholar] [CrossRef] [PubMed]
- Mable, B.K.; Alexandrou, M.A.; Taylor, M.I. Genome duplication in amphibians and fish: An extended synthesis. J. Zool. 2011, 284, 151–182. [Google Scholar] [CrossRef]
- Braasch, I.; Postlethwait, J.H. Polyploidy in fish and the teleost genome duplication. In Polyploidy and Genome Evolution; Springer: Berlin/Heidelberg, Germany, 2012; pp. 341–383. [Google Scholar]
- Bernardi, G. Speciation in fishes. Mol. Ecol. 2013, 22, 5487–5502. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Schulte, P.M.; Wood, C.M.; Schiemer, F. Niche dimensions in fishes: An integrative view. Physiol. Biochem. Zool. 2010, 83, 808–826. [Google Scholar] [CrossRef] [Green Version]
- Reis, R.E.; Albert, J.S.; Di Dario, F.; Mincarone, M.M.; Petry, P.; Rocha, L.A. Fish biodiversity and conservation in South America. J. Fish Biol. 2016, 89, 12–47. [Google Scholar] [CrossRef] [Green Version]
- Albert, J.S.; Reis, R. Historical Biogeography of Neotropical Freshwater Fishes; Univ of California Press: Berkeley, CA, USA, 2011; ISBN 0520948505. [Google Scholar]
- Albert, J.S.; Tagliacollo, V.A.; Dagosta, F. Diversification of Neotropical freshwater fishes. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 27–53. [Google Scholar] [CrossRef]
- Arai, R. Fish Karyotypes: A Check List; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Gregory, T.R. Animal Genome Size Database. Available online: http://www.genomesize.com (accessed on 19 August 2021).
- Yoshida, K.; Kitano, J. Tempo and mode in karyotype evolution revealed by a probabilistic model incorporating both chromosome number and morphology. PLoS Genet. 2021, 17, e1009502. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Schartl, M.; Schmid, M.; Nanda, I. Dynamics of vertebrate sex chromosome evolution: From equal size to giants and dwarfs. Chromosoma 2016, 125, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Guiguen, Y.; Fostier, A.; Herpin, A. Sex determination and differentiation in fish: Genetic, genomic, and endocrine aspects. In Sex Control in Aquaculture, 1st ed.; Wang, H.-P., Piferrer, F., Chen, S.-L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 35–63. [Google Scholar]
- Shen, Z.; Wang, H. Environmental sex determination and sex differentiation in teleost—How sex is established. In Sex Control in Aquaculture, 1st ed.; Wang, H.-P., Piferrer, F., Chen, S.-L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 85–115. [Google Scholar]
- Kuwamura, T.; Sunobe, T.; Sakai, Y.; Kadota, T.; Sawada, K. Hermaphroditism in fishes: An annotated list of species, phylogeny, and mating system. Ichthyol. Res. 2020, 67, 341–360. [Google Scholar] [CrossRef]
- Schaefer, S.A.; Lauder, G.V. Historical transformation of functional design: Evolutionary morphology of feeding mechanisms in loricarioid catfishes. Syst. Zool. 1986, 35, 489–508. [Google Scholar] [CrossRef]
- Reis, R.E.; Kullander, S.; Ferraris, C.J. Check List of the Freshwater Fishes of South and Central America; Edipucrs: Porto Alegre, Brazil, 2003; ISBN 8574303615. [Google Scholar]
- Kavalco, K.F.; Pazza, R.; Bertollo, L.A.C.; Moreira-Filho, O. Karyotypic diversity and evolution of Loricariidae (Pisces, Siluriformes). Heredity 2005, 94, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Takagui, F.H.; Baumgärtner, L.; Venturelli, N.B.; Paiz, L.M.; Viana, P.; Dionísio, J.F.; Pompeo, L.R.S.; Margarido, V.P.; Fenocchio, A.S.; da Rosa, R. Unrevealing the karyotypic evolution and cytotaxonomy of armored catfishes (Loricariinae) with emphasis in Sturisoma, Loricariichthys, Loricaria, Proloricaria, Pyxiloricaria, and Rineloricaria. Zebrafish 2020, 17, 319–332. [Google Scholar] [CrossRef]
- Giuliano-Caetano, L. Polimorfismo Cromossômico Robertsoniano Em Populações de Rineloricaria latirostris (Pisces, Loricariinae); Universidade Federal de Sâo Carlos: São Carlo, Brazil, 1998. [Google Scholar]
- de Lara Kamei, M.C.S.; Baumgärtner, L.; Paiva, S.; Zawadzki, C.H.; Martins-Santos, I.C.; de Portela-Castro, A.L.B. Chromosomal diversity of three species of Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae), from the Paraná River Basin, Brazil: A species complex in Hypostomus Ancistroides reinforced by a ZZ/ZW sex chromosome system. Zebrafish 2017, 14, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Takagui, F.H.; de Moura, L.F.; Ferreira, D.C.; Centofante, L.; de A. Vitorino, C.; Bueno, V.; Margarido, V.P.; Venere, P.C. Karyotype diversity in Doradidae (Siluriformes, Doradoidea) and presence of the heteromorphic ZZ/ZW sex chromosome system in the family. Zebrafish 2017, 14, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Reis, D.A.; de Oliveira Brandão, K.; de Almeida-Toledo, L.F.; Pazza, R.; Kavalco, K.F. The persevering cytotaxonomy: Discovery of a unique XX/XY sex chromosome system in catfishes suggests the existence of a new, endemic and rare species. Cytogenet. Genome Res. 2018, 156, 45–55. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.C.; Ribeiro, M.O.; de Medeiros Costa, G.; Zawadzki, C.H.; Prizon-Nakajima, A.C.; Borin-Carvalho, L.A.; Martins-Santos, I.C.; de Brito Portela-Castro, A.L. Cytogenetic characterization of Hypostomus soniae Hollanda-Carvalho & Weber, 2004 from the Teles Pires River, southern Amazon basin: Evidence of an early stage of an XX/XY sex chromosome system. Comp. Cytogenet. 2019, 13, 411. [Google Scholar]
- Glugoski, L.; Deon, G.; Schott, S.; Vicari, M.R.; Nogaroto, V.; Moreira-Filho, O. Comparative cytogenetic analyses in Ancistrus species (Siluriformes: Loricariidae). Neotrop. Ichthyol. 2020, 18, e200013. [Google Scholar] [CrossRef]
- Centofante, L.; Bertollo, L.A.C.; Moreira-Filho, O. Cytogenetic characterization and description of an XX/XY1Y2 sex chromosome system in catfish Harttia carvalhoi (Siluriformes, Loricariidae). Cytogenet. Genome Res. 2006, 320–324. [Google Scholar] [CrossRef]
- Blanco, D.R.; Vicari, M.R.; Lui, R.L.; Traldi, J.B.; Bueno, V.; de Martinez, J.F.; Brandão, H.; Oyakawa, O.T.; Moreira Filho, O. Karyotype diversity and evolutionary trends in armored catfish species of the genus Harttia (Siluriformes: Loricariidae). Zebrafish 2017, 14, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Deon, G.A.; Glugoski, L.; Vicari, M.R.; Nogaroto, V.; Sassi, F.M.C.; Cioffi, M.B.; Liehr, T.; Bertollo, L.A.C.; Moreira-Filho, O. Highly rearranged karyotypes and multiple sex chromosome systems in armored catfishes from the genus Harttia (Teleostei, Siluriformes). Genes 2020, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.M.C.; Deon, G.A.; Moreira-Filho, O.; Vicari, M.R.; Bertollo, L.A.C.; Liehr, T.; de Oliveira, E.A.; Cioffi, M.B. Multiple sex chromosomes and evolutionary relationships in Amazonian catfishes: The outstanding model of the genus Harttia (Siluriformes: Loricariidae). Genes 2020, 11, 1179. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Oyakawa, O.T. New loricariid fishes from headwaters on Serra da Mantiqueira and Complexo do Espinhaço, Minas Gerais State, Brazil (Teleostei: Siluriformes: Loricariidae). Zootaxa 2019, 4586, 401–424. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M. Estudos Cromossômicos e Moleculares em Loricariinae Com Ênfase em Espécies de Rineloricaria (Siluriformes, Loricariidae): Uma Perspectiva Evolutiva. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2010. [Google Scholar]
- Alves, A.L.; Oliveira, C.; Foresti, F. Karyotype variability in eight species of the subfamilies Loricariinae and Ancistrinae (Teleostei, Siluriformes, Loricariidae). Caryologia 2003, 56, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Bertollo, L.A.C.; Cioffi, M.B.; Moreira-Filho, O. Direct chromosome preparation from freshwater teleost fishes. In Fish Cytogenetic Techniques (Chondrichthyans and Teleosts); Ozouf-Costaz, C., Pisano, E., Foresti, F., Almeida Toledo, L.F., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 21–26. [Google Scholar]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Pendás, A.M.; Móran, P.; Freije, J.P.; Garcia-Vásquez, E. Chromosomal location and nucleotide sequence of two tandem repeats of the Atlantic salmon 5S rDNA. Cytogenet. Cell Genet. 1994, 67, 31–36. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Martins, C.; Centofante, L.; Jacobina, U.P.; Bertollo, L.A.C. Chromosomal variability among allopatric populations of Erythrinidae fish Hoplias malabaricus: Mapping of three classes of repetitive DNAs. Cytogenet. Genome Res. 2009, 125, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Phimphan, S.; Chaiyasan, P.; Suwannapoom, C.; Reungsing, M.; Juntaree, S.; Tanomtong, A.; Supiwong, W. Comparative karyotype study of three Cyprinids (Cyprinidae, Cyprininae) in Thailand by classical cytogenetic and FISH techniques. Comp. Cytogenet. 2020, 14, 597. [Google Scholar] [CrossRef] [PubMed]
- Kubat, Z.; Hobza, R.; Vyskot, B.; Kejnovský, E. Microsatellite accumulation in the Y chromosome of Silene latifolia. Genome 2008, 51, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Yano, C.F.; Bertollo, L.A.C.; Cioffi, M.B. Fish-FISH: Molecular cytogenetics in fish species. In Fluorescence In Situ Hybridization (FISH)—Application Guide; Liehr, T., Ed.; Springer: Berlin, Germany, 2017; pp. 429–444. [Google Scholar]
- Sember, A.; Bertollo, L.A.C.; Ráb, P.; Yano, C.F.; Hatanaka, T.; de Oliveira, E.A.; Cioffi, M.B. Sex chromosome evolution and genomic divergence in the fish Hoplias malabaricus (Characiformes, Erythrinidae). Front. Genet. 2018, 9, 71. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 855. [Google Scholar]
- Zwick, M.S.; Hanson, R.E.; Mcknight, T.D.; Islam-Faridi, M.H.; Stelly, D.M.; Wing, R.A.; Price, H.J. A rprocedure for the isolation of C0t−1 DNA from plants. Genome 1997, 40, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Londoño-Burbano, A.; Reis, R.E. A combined molecular and morphological phylogeny of the Loricariinae (Siluriformes: Loricariidae), with emphasis on the Harttiini and Farlowellini. PLoS ONE 2021, 16, e0247747. [Google Scholar] [CrossRef] [PubMed]
- Covain, R.; Fisch-Muller, S.; Oliveira, C.; Mol, J.H.; Montoya-Burgos, J.I.; Dray, S. Molecular phylogeny of the highly diversified catfish subfamily Loricariinae (Siluriformes, Loricariidae) reveals incongruences with morphological classification. Mol. Phylogenet. Evol. 2016, 94, 492–517. [Google Scholar] [CrossRef]
- Ziemniczak, K.; Barros, A.V.; Rosa, K.O.; Nogaroto, V.; Almeida, M.C.; Cestari, M.M.; Moreira-Filho, O.; Artoni, R.F.; Vicari, M.R. Comparative cytogenetics of Loricariidae (Actinopterygii: Siluriformes): Emphasis in Neoplecostominae and Hypoptopomatinae. Ital. J. Zool. 2012, 79, 492–501. [Google Scholar] [CrossRef]
- Barros, A.V.; Wolski, M.A.V.; Nogaroto, V.; Almeida, M.C.; Moreira-Filho, O.; Vicari, M.R. Fragile sites, dysfunctional telomere and chromosome fusions: What is 5S rDNA role? Gene 2017, 608, 20–27. [Google Scholar] [CrossRef]
- Glugoski, L.; Giuliano-Caetano, L.; Moreira-Filho, O.; Vicari, M.R.; Nogaroto, V. Co-located hAT transposable element and 5S rDNA in an interstitial telomeric sequence suggest the formation of Robertsonian fusion in armored catfish. Gene 2018, 650, 49–54. [Google Scholar] [CrossRef]
- Molina, W.F.; Galetti-Jr, P.M. Robertsonian rearrangements in the reef fish Chromis (Perciformes, Pomacentridae) involving chromosomes bearing 5S rRNA genes. Genet. Mol. Biol. 2002, 25, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Rosa, K.O.; Ziemniczak, K.; de Barros, A.V.; Nogaroto, V.; Almeida, M.C.; Cestari, M.M.; Artoni, R.F.; Vicari, M.R. Numeric and structural chromosome polymorphism in Rineloricaria lima (Siluriformes: Loricariidae): Fusion points carrying 5S rDNA or telomere sequence vestiges. Rev. Fish. Biol. Fish. 2012, 22, 739–749. [Google Scholar] [CrossRef]
- Kushwaha, B.; Baisvar, V.S.; Kumar, R. 18S rDNA mapping revealed conservation and rearrangements of chromosome segments in two Channa species. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 1–5. [Google Scholar] [CrossRef]
- Getlekha, N.; Molina, W.F.; Cioffi, M.B.; Yano, C.F.; Maneechot, N.; Bertollo, L.A.C.; Supiwong, W.; Tanomtong, A. Repetitive DNAs highlight the role of chromosomal fusions in the karyotype evolution of Dascyllus species (Pomacentridae, Perciformes). Genetica 2016, 144, 203–211. [Google Scholar] [CrossRef]
- Cazaux, B.; Catalan, J.; Veyrunes, F.; Douzery, E.J.P.; Britton-Davidian, J. Are ribosomal DNA clusters rearrangement hotspots? A case study in the genus Mus (Rodentia, Muridae). BMC Evol. Biol. 2011, 11, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchurikov, N.A.; Uroshlev, L.A.; Klushevskaya, E.S.; Alembekov, I.R.; Lagarkova, M.A.; Kravatskaya, G.I.; Makeev, V.Y.; Kravatsky, Y. V Chromosomal translocations in NK-cell lymphomas originate from inter-chromosomal contacts of active rDNA clusters possessing hot spots of DSBs. Cancers 2021, 13, 3889. [Google Scholar] [CrossRef]
- Schöfer, C.; Weipoltshammer, K. Nucleolus and chromatin. Histochem. Cell Biol. 2018, 150, 209–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthelot, C.; Muffato, M.; Abecassis, J.; Crollius, H.R. The 3D organization of chromatin explains evolutionary fragile genomic regions. Cell Rep. 2015, 10, 1913–1924. [Google Scholar] [CrossRef] [Green Version]
- Warmerdam, D.O.; Wolthuis, R.M.F. Keeping ribosomal DNA intact: A repeating challenge. Chromosome Res. 2019, 27, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Slijepcevic, P. Telomeres and mechanisms of Robertsonian fusion. Chromosoma 1998, 107, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ocalewicz, K. Telomeres in fishes. Cytogenet. Genome Res. 2013, 141, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Rapp Py-Daniel, L.H.; Oliveira, E.C. Seven new species of Harttia from the Amazonian-Guyana region (Siluriformes: Loricariidae). Ichthyol. Explor. Freshw. 2001, 12, 79–96. [Google Scholar]
- Oyakawa, O.T.; Fichberg, I.; Py-Daniel, L.R. Three new species of Harttia (Loricariidae: Loricariinae) from Serra do Cachimbo, Rio Xingu basin, Pará, Northern Brazil. Zootaxa 2018, 4387, 75–90. [Google Scholar] [CrossRef]
- Lahn, B.T.; Page, D.C. Retroposition of autosomal mRNA yielded testis-specific gene family on human Y chromosome. Nat. Genet. 1999, 21, 429–433. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B.; Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 2005, 95, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergero, R.; Charlesworth, D. The evolution of restricted recombination in sex chromosomes. Trends Ecol. Evol. 2009, 24, 94–102. [Google Scholar] [CrossRef]
- Ortiz-Barrientos, D.; Engelstädter, J.; Rieseberg, L.H. Recombination rate evolution and the origin of species. Trends Ecol. Evol. 2016, 31, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Charlesworth, D. The guppy sex chromosome system and the sexually antagonistic polymorphism hypothesis for Y chromosome recombination suppression. Genes 2018, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Wellenreuther, M.; Bernatchez, L. Eco-evolutionary genomics of chromosomal inversions. Trends Ecol. Evol. 2018, 33, 427–440. [Google Scholar] [CrossRef]
- Connallon, T.; Olito, C.; Dutoit, L.; Papoli, H.; Ruzicka, F.; Yong, L. Local adaptation and the evolution of inversions on sex chromosomes and autosomes. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natri, H.M.; Merilä, J.; Shikano, T. The evolution of sex determination associated with a chromosomal inversion. Nat. Commun. 2019, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Peichel, C.L.; McCann, S.R.; Ross, J.A.; Naftaly, A.F.S.; Urton, J.R.; Cech, J.N.; Grimwood, J.; Schmutz, J.; Myers, R.M.; Kingsley, D.M. Assembly of the threespine stickleback Y chromosome reveals convergent signatures of sex chromosome evolution. Genome Biol. 2020, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.B.; Bertollo, L.A.C. Chromosomal distribution and evolution of repetitive DNAs in fish. In Repetitive DNA; Garrido-Ramos, M.A., Ed.; Karger: Basel, Switzerland, 2012; pp. 197–221. ISBN 9783318021509. [Google Scholar]
- Blommaert, J.; Riss, S.; Hecox-Lea, B.; Welch, D.B.M.; Stelzer, C.-P. Small, but surprisingly repetitive genomes: Transposon expansion and not polyploidy has driven a doubling in genome size in a metazoan species complex. BMC Genom. 2019, 20, 466. [Google Scholar] [CrossRef] [Green Version]
- Bracewell, R.; Chatla, K.; Nalley, M.J.; Bachtrog, D. Dynamic turnover of centromeres drives karyotype evolution in Drosophila. eLife 2019, 8, e49002. [Google Scholar] [CrossRef] [PubMed]
- Ávila Robledillo, L.; Neumann, P.; Koblížková, A.; Novák, P.; Vrbová, I.; Macas, J. Extraordinary sequence diversity and promiscuity of Ccentromeric Ssatellites in the Llegume tribe Fabeae. Mol. Biol. Evol. 2020, 37, 2341–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, D.R.; Vicari, M.R.; Artoni, R.F.; Traldi, J.B.; Moreira-Filho, O. Chromosomal characterization of armored catfish Harttia longipinna (Siluriformes, Loricariidae): First report of B chromosomes in the genus. Zoolog. Sci. 2012, 29, 604–609. [Google Scholar] [CrossRef]
- Sember, A.; Nguyen, P.; Perez, M.F.; Altmanová, M.; Ráb, P.; Cioffi, M.B. Multiple sex chromosomes in teleost fishes from a cytogenetic perspective: State of the art and future challenges. Philos. Trans. R. Soc. B 2021, 376, 20200098. [Google Scholar] [CrossRef]
- Vara, C.; Paytuví-Gallart, A.; Cuartero, Y.; Álvarez-González, L.; Marín-Gual, L.; Garcia, F.; Florit-Sabater, B.; Capilla, L.; Sanchéz-Guillén, R.A.; Sarrate, Z. The impact of chromosomal fusions on 3D genome folding and recombination in the germ line. Nat. Commun. 2021, 12, 2981. [Google Scholar] [CrossRef]
- Traut, W.; Winking, H. Meiotic chromosomes and stages of sex chromosome evolution in fish: Zebrafish, platyfish and guppy. Chromosome Res. 2001, 9, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Gamble, T. Using RAD-seq to recognize sex-specific markers and sex chromosome systems. Mol. Ecol. 2016, 25, 2114–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhl, H.; Guiguen, Y.; Höhne, C.; Kreuz, E.; Du, K.; Klopp, C.; Lopez-Roques, C.; Yebra-Pimentel, E.S.; Ciorpac, M.; Gessner, J. A 180 Myr-old female-specific genome region in sturgeon reveals the oldest known vertebrate sex determining system with undifferentiated sex chromosomes. Philos. Trans. R. Soc. B 2021, 376, 20200089. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, D.; Volff, J.-N.; Galiana, D.; Anderson, J.L.; Schartl, M. Transposable elements and early evolution of sex chromosomes in fish. Chromosome Res. 2015, 23, 545–560. [Google Scholar] [CrossRef] [PubMed]
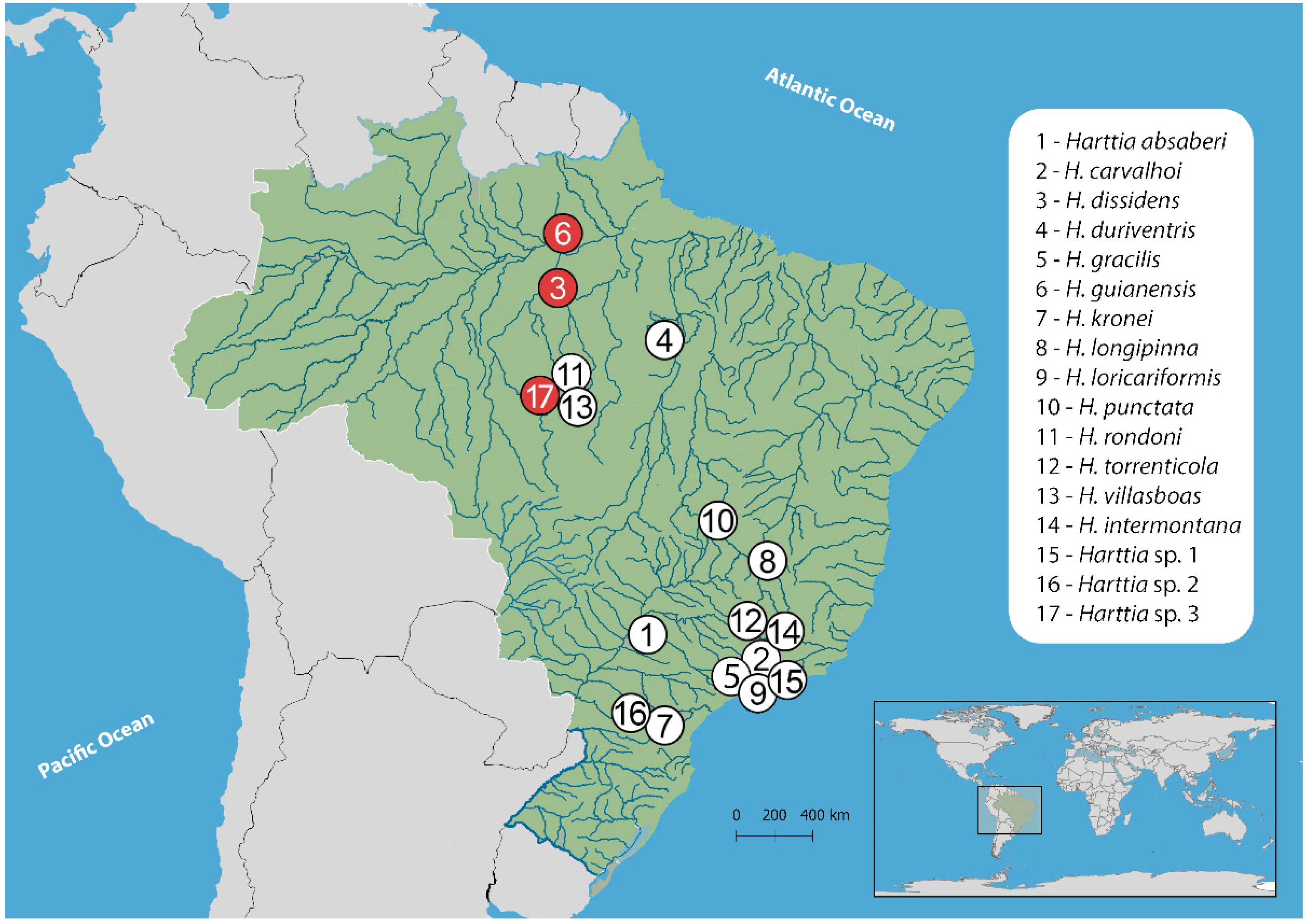


| Species | Locality | n |
|---|---|---|
| 3-Harttia dissidens | Upper section of Grim waterfall, Tambor stream, Rurópolis–PA, Brazil (4°5′37.8″ S 55°0′30.2″ W) | 25♂, 07♀ |
| 6-Harttia guianensis | Paraíso stream (formerly known as Inferno stream), Alenquer–PA, Brazil (1°29′02.2″ S 54°50′31.2″ W) | 10♂, 06♀ |
| 17-Harttia sp. 3 | Rio do Peixe, Cachoeira da Serra, Altamira–PA (08°39′20.7″ S 55°09′24.1″ W) | 15♂,11♀ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassi, F.d.M.C.; Moreira-Filho, O.; Deon, G.A.; Sember, A.; Bertollo, L.A.C.; Liehr, T.; Oliveira, V.C.S.; Viana, P.F.; Feldberg, E.; Vicari, M.R.; et al. Adding New Pieces to the Puzzle of Karyotype Evolution in Harttia (Siluriformes, Loricariidae): Investigation of Amazonian Species. Biology 2021, 10, 922. https://doi.org/10.3390/biology10090922
Sassi FdMC, Moreira-Filho O, Deon GA, Sember A, Bertollo LAC, Liehr T, Oliveira VCS, Viana PF, Feldberg E, Vicari MR, et al. Adding New Pieces to the Puzzle of Karyotype Evolution in Harttia (Siluriformes, Loricariidae): Investigation of Amazonian Species. Biology. 2021; 10(9):922. https://doi.org/10.3390/biology10090922
Chicago/Turabian StyleSassi, Francisco de M. C., Orlando Moreira-Filho, Geize A. Deon, Alexandr Sember, Luiz A. C. Bertollo, Thomas Liehr, Vanessa C. S. Oliveira, Patrik F. Viana, Eliana Feldberg, Marcelo R. Vicari, and et al. 2021. "Adding New Pieces to the Puzzle of Karyotype Evolution in Harttia (Siluriformes, Loricariidae): Investigation of Amazonian Species" Biology 10, no. 9: 922. https://doi.org/10.3390/biology10090922
APA StyleSassi, F. d. M. C., Moreira-Filho, O., Deon, G. A., Sember, A., Bertollo, L. A. C., Liehr, T., Oliveira, V. C. S., Viana, P. F., Feldberg, E., Vicari, M. R., & Cioffi, M. d. B. (2021). Adding New Pieces to the Puzzle of Karyotype Evolution in Harttia (Siluriformes, Loricariidae): Investigation of Amazonian Species. Biology, 10(9), 922. https://doi.org/10.3390/biology10090922








