Pathogens and Elicitors Induce Local and Systemic Changes in Triacylglycerol Metabolism in Roots and in Leaves of Arabidopsis thaliana
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Pathogen Treatment
2.3. Elicitor and Effector Treatment
2.4. Quantification of V.l.
2.5. Lipid Analysis
2.6. Statistical Analysis
3. Results
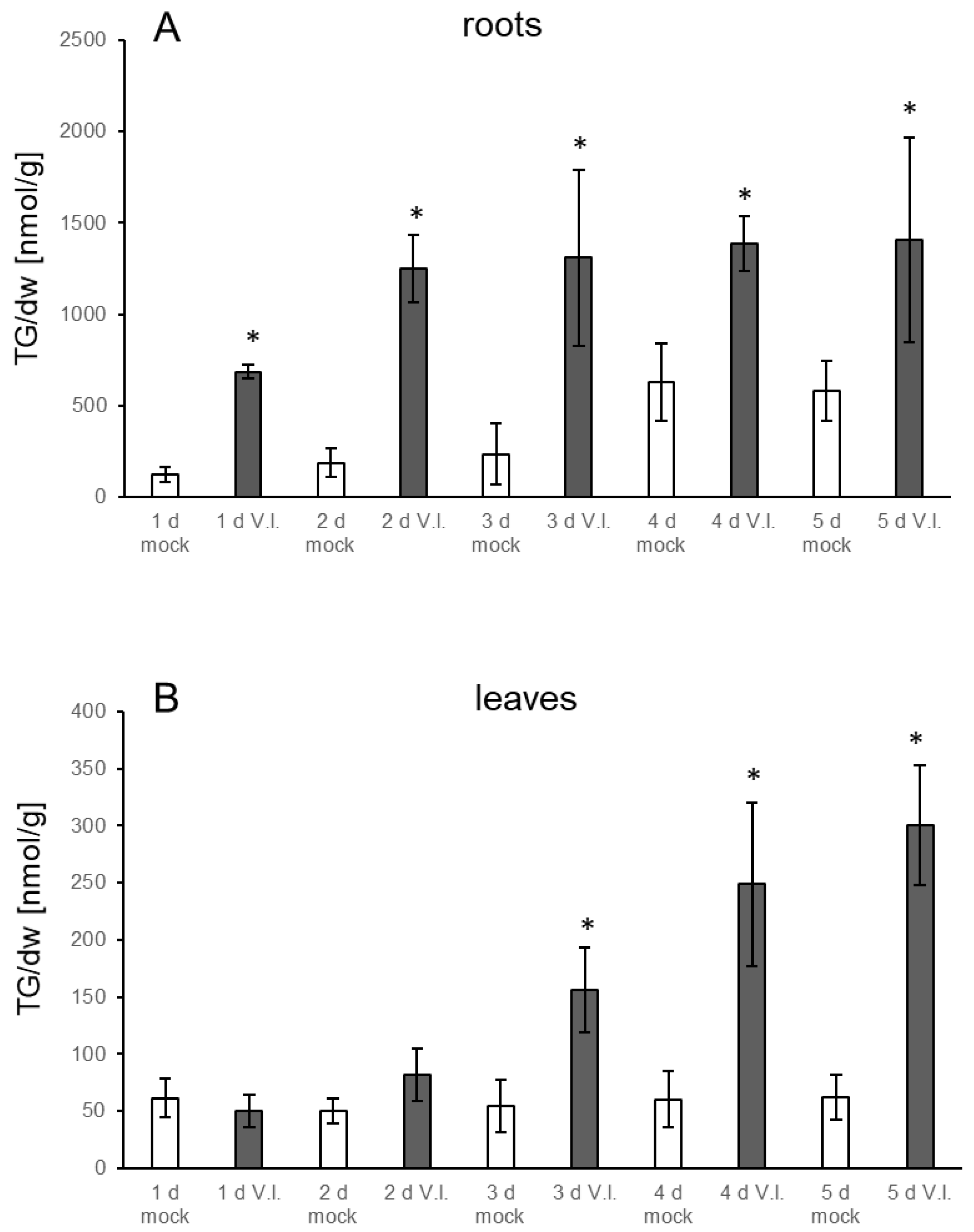
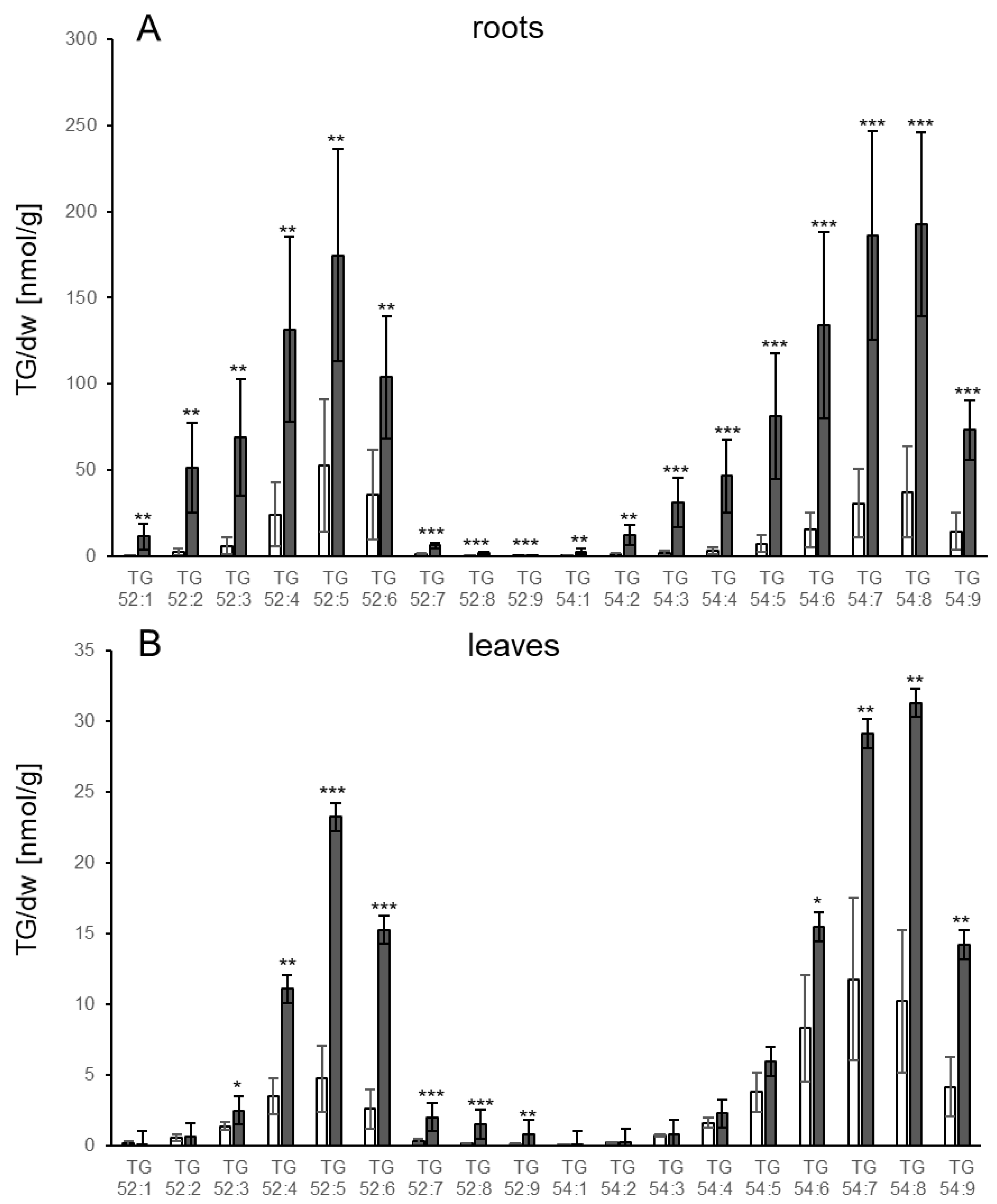
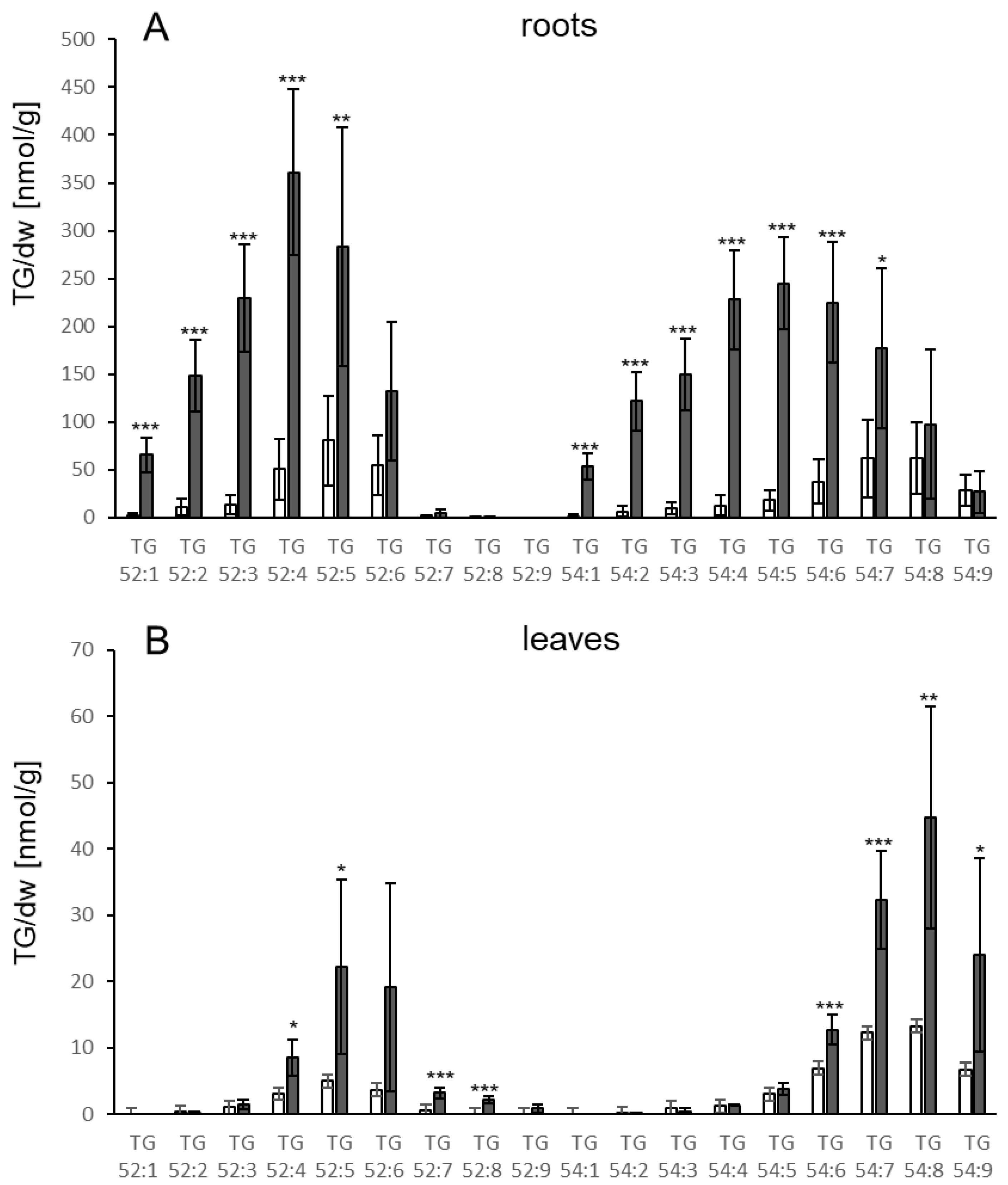
3.1. TGs Accumulate upon Root Infection with Verticillium in Roots and Systemically in Leaves
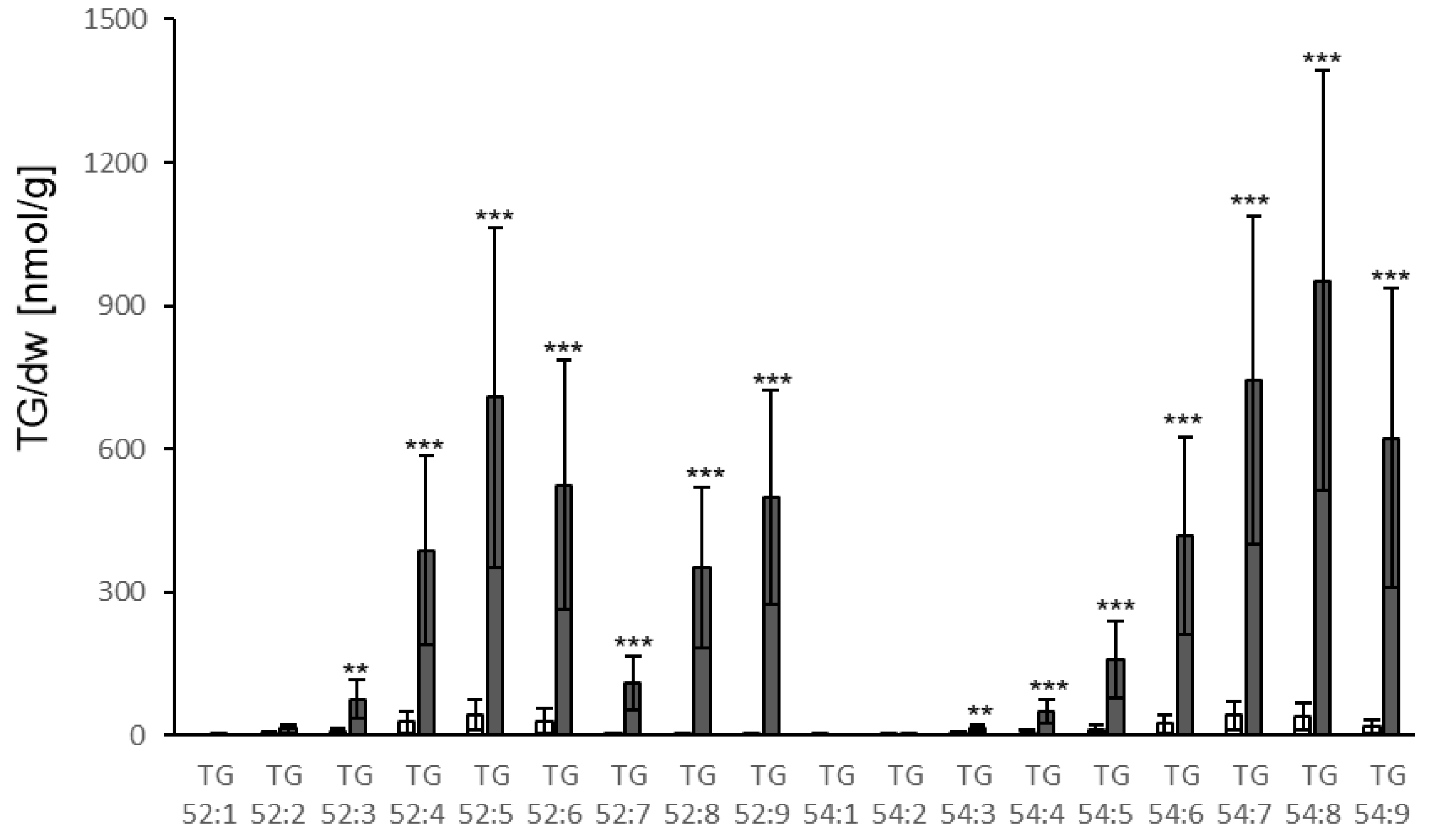
3.2. TG Accumulation in Response to P. syringae
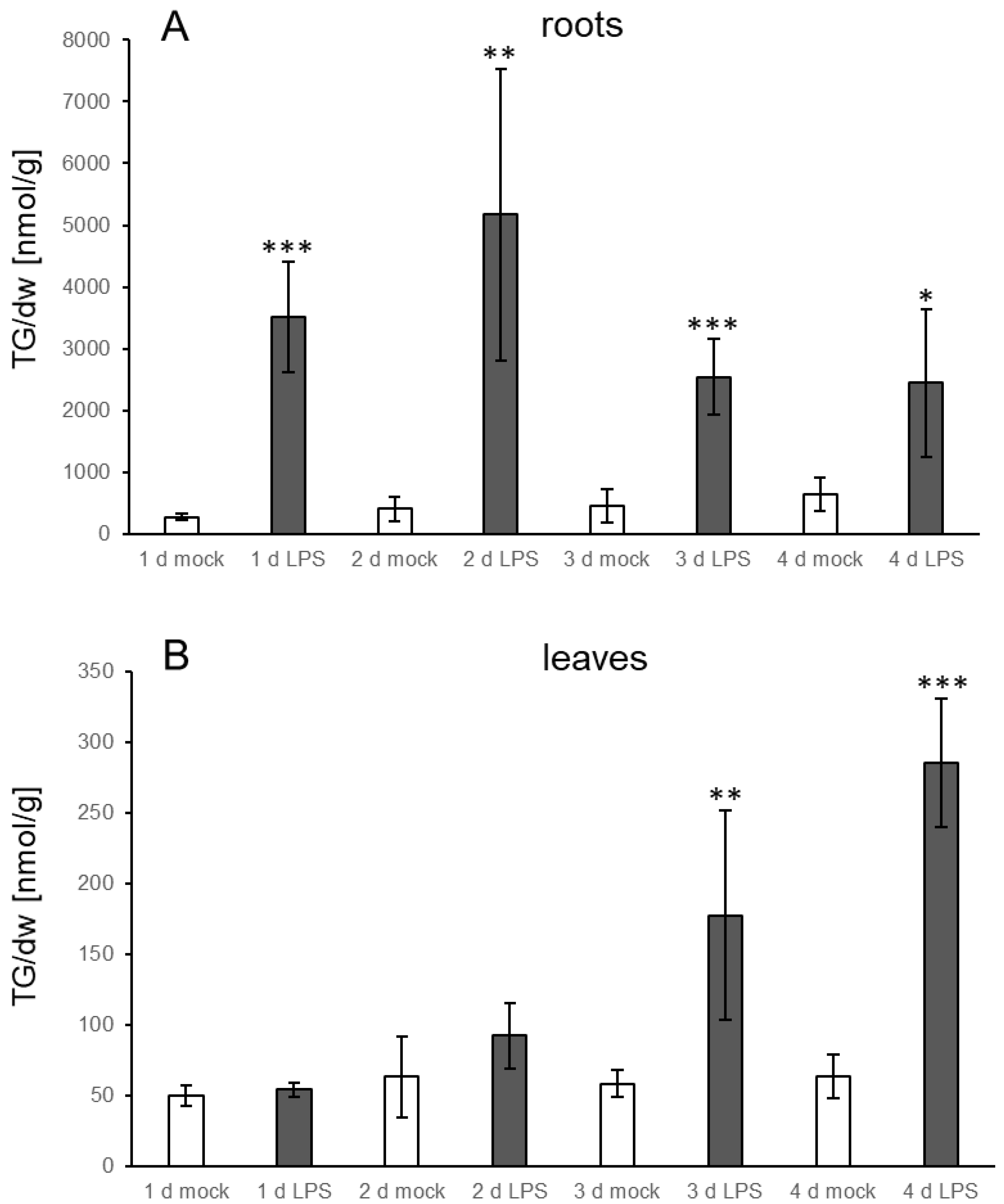
3.3. TG Accumulation Can Also Be Induced by Elicitors
3.4. TG Accumulation Can Also Be Induced by Effectors
4. Discussion
4.1. TG Levels Change in Response to Biotic Stress in Roots
4.2. Bacterial Elicitors and Effectors Induce Strong Accumulation of TG
4.3. Verticillium and LPS Also Induce Increases in TG Levels in Distal Leaves
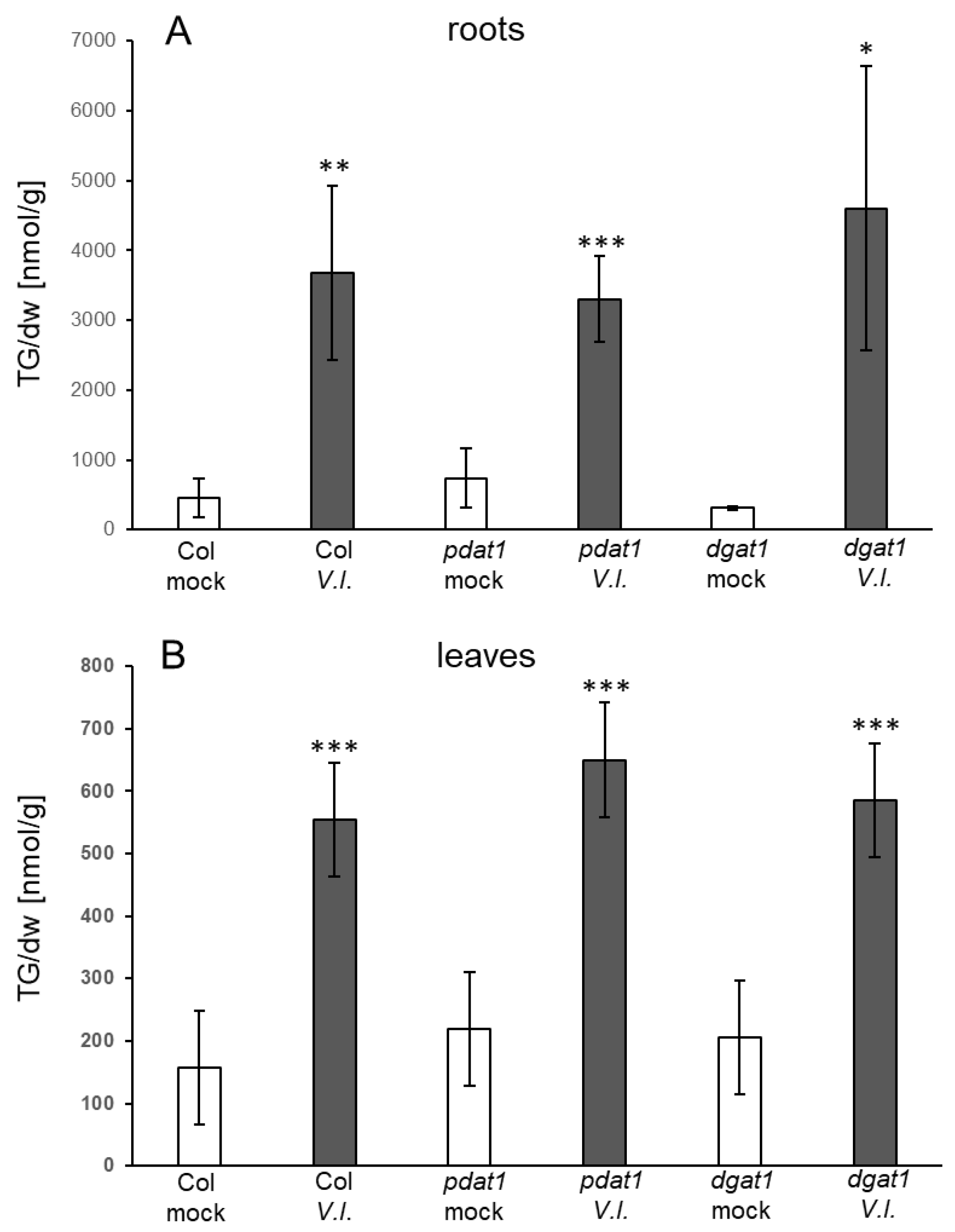
4.4. Which Pathways Contribute to TG Synthesis in Response to Fungal Infection?
4.5. What Is the Relevance of TG Accumulation in Response to Biotic Stress?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dhanapal, S.; Yadav, B.S. The dynamic responses of plant physiology and metabolism during environmental stress progression. Mol. Biol. Rep. 2020, 47, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Okazaki, Y.; Myouga, F.; Shinozaki, K.; Saito, K. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci. Rep. 2015, 5, 10533. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane Lipid Remodeling in Response to Salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Benning, C. Functions of triacylglycerols during plant development and stress. Curr. Opin. Biotechnol. 2018, 49, 191–198. [Google Scholar] [CrossRef]
- Xu, C.; Shanklin, J. Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu. Rev. Plant Biol. 2016, 67, 179–206. [Google Scholar] [CrossRef]
- Shimada, T.L.; Takano, Y.; Hara-Nishimura, I. Oil body-mediated defense against fungi: From tissues to ecology. Plant Signal. Behav. 2015, 10, e989036. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef]
- Mueller, S.P.; Krause, D.M.; Mueller, M.J.; Fekete, A. Accumulation of extra-chloroplastic triacylglycerols in Arabidopsis seedlings during heat acclimation. J. Exp. Bot. 2015, 66, 4517–4526. [Google Scholar] [CrossRef] [Green Version]
- Gaude, N.; Brehelin, C.; Tischendorf, G.; Kessler, F.; Dormann, P. Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant J. 2007, 49, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasulla, F.; Vom Dorp, K.; Dombrink, I.; Zahringer, U.; Gisch, N.; Dormann, P.; Bartels, D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: A comparative approach. Plant J. 2013, 75, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hersh, H.L.; Benning, C. SENSITIVE TO FREEZING2 aides in resilience to salt and drought in freezing-sensitive tomato. Plant Physiol. 2016, 172, 1432–1442. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Yu, L.; Xu, C. A central role for triacylglycerol in membrane lipid breakdown, fatty acid beta-oxidation, and plant survival under extended darkness. Plant Physiol. 2017, 174, 1517–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurchenko, O.; Kimberlin, A.; Mehling, M.; Koo, A.J.; Chapman, K.D.; Mullen, R.T.; Dyer, J.M. Response of high leaf-oil Arabidopsis thaliana plant lines to biotic or abiotic stress. Plant Signal. Behav. 2018, 13, e1464361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, S.P.; Unger, M.; Guender, L.; Fekete, A.; Mueller, M.J. Phospholipid:diacylglycerol acyltransferase-mediated triacylglyerol synthesis augments basal thermotolerance. Plant Physiol. 2017, 175, 486–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoeller, M.; Stingl, N.; Krischke, M.; Fekete, A.; Waller, F.; Berger, S.; Mueller, M.J. Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: Biogenesis of pimelic and azelaic acid. Plant Physiol. 2012, 160, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.L.; Takano, Y.; Shimada, T.; Fujiwara, M.; Fukao, Y.; Mori, M.; Okazaki, Y.; Saito, K.; Sasaki, R.; Aoki, K.; et al. Leaf oil body functions as a subcellular factory for the production of a phytoalexin in Arabidopsis. Plant Physiol. 2014, 164, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [Green Version]
- Newman, M.A.; Sundelin, T.; Nielsen, J.T.; Erbs, G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 2013, 4, 139. [Google Scholar] [CrossRef] [Green Version]
- Alexander, C.; Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef]
- Ranf, S.; Gisch, N.; Schaffer, M.; Illig, T.; Westphal, L.; Knirel, Y.A.; Sanchez-Carballo, P.M.; Zahringer, U.; Huckelhoven, R.; Lee, J.; et al. A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 2015, 16, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Tinte, M.M.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Lipopolysaccharide perception in Arabidopsis thaliana: Diverse LPS chemotypes from Burkholderia cepacia, Pseudomonas syringae and Xanthomonas campestris trigger differential defence-related perturbations in the metabolome. Plant Physiol. Biochem. 2020, 156, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Mackey, D. Dose-response to and systemic movement of dexamethasone in the GVG-inducible transgene system in Arabidopsis. Methods Mol. Biol. 2011, 712, 59–68. [Google Scholar]
- Xu, J.; Carlsson, A.S.; Francis, T.; Zhang, M.; Hoffman, T.; Giblin, M.E.; Taylor, D.C. Triacylglycerol synthesis by PDAT1 in the absence of DGAT1 activity is dependent on re-acylation of LPC by LPCAT2. BMC Plant Biol. 2012, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Grebner, W.; Stingl, N.E.; Oenel, A.; Mueller, M.J.; Berger, S. Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol. 2013, 161, 2159–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tocquin, P.C.L.; Havelange, A.; Pieltain, A.; Kurtem, E.; Bernier, G.; Perilleux, C. A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol. 2003, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Ralhan, A.; Schottle, S.; Thurow, C.; Iven, T.; Feussner, I.; Polle, A.; Gatz, C. The vascular pathogen Verticillium longisporum requires a jasmonic acid-independent COI1 function in roots to elicit disease symptoms in Arabidopsis shoots. Plant Physiol. 2012, 159, 1192–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, P.D. Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. Biochim. Biophys. Acta 2016, 1861, 1214–1225. [Google Scholar] [CrossRef]
- Kim, M.G.; Geng, X.; Lee, S.Y.; Mackey, D. The Pseudomonas syringae type III effector AvrRpm1 induces significant defenses by activating the Arabidopsis nucleotide-binding leucine-rich repeat protein RPS2. Plant J. 2009, 57, 645–653. [Google Scholar] [CrossRef]
- Tan, W.J.; Yang, Y.C.; Zhou, Y.; Huang, L.P.; Xu, L.; Chen, Q.F.; Yu, L.J.; Xiao, S. Diacylglycerol acyltransferase and DIACYLGLYCEROL KINASE Modulate Triacylglycerol and Phosphatidic Acid Production in the Plant Response to Freezing Stress. Plant Physiol. 2018, 177, 1303–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaff, J.; Denton, A.K.; Usadel, B.; Pfaff, C. Phosphate starvation causes different stress responses in the lipid metabolism of tomato leaves and roots. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158763. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kou, M.; Gao, Z.; Liu, Y.; Xuan, Y.; Liu, Y.; Tang, Z.; Cao, Q.; Li, Z.; Sun, J. Involvement of Phosphatidylserine and Triacylglycerol in the Response of Sweet Potato Leaves to Salt Stress. Front. Plant Sci. 2019, 10, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Gao, Y.; Liang, Y.; Dong, Y.; Yang, X.; Yuan, J.; Qiu, D. The Verticillium dahliae SnodProt1-Like Protein VdCP1 Contributes to Virulence and Triggers the Plant Immune System. Front. Plant Sci. 2017, 8, 1880. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Khan, N.U.; Wang, N.; Yang, X.; Qiu, D. The Protein Elicitor PevD1 Enhances Resistance to Pathogens and Promotes Growth in Arabidopsis. Int. J. Biol. Sci. 2016, 12, 931–943. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.; Li, B.; Yang, X.; Dong, Y.; Qiu, D. A Verticillium dahliae Pectate Lyase Induces Plant Immune Responses and Contributes to Virulence. Front. Plant Sci. 2018, 9, 1271. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zhou, C.; Fan, J.; Shanklin, J.; Xu, C. Mechanisms and functions of membrane lipid remodeling in plants. Plant J. 2021, 107, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Moellering, E.R.; Muthan, B.; Benning, C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 2010, 330, 226–228. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Xu, Y.; Wang, J.; Singer, S.D.; Chen, G. The Role of Triacylglycerol in Plant Stress Response. Plants 2020, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Arisz, S.A.; Heo, J.Y.; Koevoets, I.T.; Zhao, T.; van Egmond, P.; Meyer, A.J.; Zeng, W.; Niu, X.; Wang, B.; Mitchell-Olds, T.; et al. Diacylglycerol acyltransferase1 Contributes to Freezing Tolerance. Plant Physiol. 2018, 177, 1410–1424. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.R.; Shrestha, P.; Yin, F.; Petrie, J.R.; Singh, S.P. AtDGAT2 is a functional acyl-CoA:diacylglycerol acyltransferase and displays different acyl-CoA substrate preferences than AtDGAT1. FEBS Lett. 2013, 587, 2371–2376. [Google Scholar] [CrossRef] [Green Version]
- Ayme, L.; Arragain, S.; Canonge, M.; Baud, S.; Touati, N.; Bimai, O.; Jagic, F.; Louis-Mondesir, C.; Briozzo, P.; Fontecave, M.; et al. Arabidopsis thaliana DGAT3 is a [2Fe-2S] protein involved in TAG biosynthesis. Sci. Rep. 2018, 8, 17254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.A.; van Erp, H.; Quettier, A.L.; Shaw, E.; Menard, G.; Kurup, S.; Eastmond, P.J. The sugar-dependent1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol. 2013, 162, 1282–1289. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Okazaki, Y.; Takano, K.; Myouga, F.; Shinozaki, K.; Knoch, E.; Fukushima, A.; Saito, K. Heat inducible lipase1 Remodels Chloroplastic Monogalactosyldiacylglycerol by Liberating alpha-Linolenic Acid in Arabidopsis Leaves under Heat Stress. Plant. Cell 2018, 30, 1887–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.G.; Park, M.E.; Park, B.Y.; Kim, H.U.; Seo, P.J. The Arabidopsis MYB96 Transcription Factor Mediates ABA-Dependent Triacylglycerol Accumulation in Vegetative Tissues under Drought Stress Conditions. Plants 2019, 8, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Jaillais, Y.; Ott, T. The Nanoscale Organization of the Plasma Membrane and Its Importance in Signaling: A Proteolipid Perspective. Plant Physiol. 2020, 182, 1682–1696. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.M.; Ma, Z.; Triebl, A.; Nath, S.; Cheng, Y.; Gong, B.Q.; Han, X.; Wang, J.; Li, J.F.; Wenk, M.R.; et al. The bacterial quorum sensing signal DSF hijacks Arabidopsis thaliana sterol biosynthesis to suppress plant innate immunity. Life Sci. Alliance 2020, 3, e202000720. [Google Scholar] [CrossRef]
- Robatzek, S.; Chinchilla, D.; Boller, T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006, 20, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.N.; Liu, H.; Wang, Y.P.; Seematti, J.; Grenville-Briggs, L.J.; Wang, Z.; Zhan, J. Pathogen-Mediated Stomatal Opening: A Previously Overlooked Pathogenicity Strategy in the Oomycete Pathogen Phytophthora infestans. Front. Plant Sci. 2021, 12, 668797. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schieferle, S.; Tappe, B.; Korte, P.; Mueller, M.J.; Berger, S. Pathogens and Elicitors Induce Local and Systemic Changes in Triacylglycerol Metabolism in Roots and in Leaves of Arabidopsis thaliana. Biology 2021, 10, 920. https://doi.org/10.3390/biology10090920
Schieferle S, Tappe B, Korte P, Mueller MJ, Berger S. Pathogens and Elicitors Induce Local and Systemic Changes in Triacylglycerol Metabolism in Roots and in Leaves of Arabidopsis thaliana. Biology. 2021; 10(9):920. https://doi.org/10.3390/biology10090920
Chicago/Turabian StyleSchieferle, Sebastian, Beeke Tappe, Pamela Korte, Martin J. Mueller, and Susanne Berger. 2021. "Pathogens and Elicitors Induce Local and Systemic Changes in Triacylglycerol Metabolism in Roots and in Leaves of Arabidopsis thaliana" Biology 10, no. 9: 920. https://doi.org/10.3390/biology10090920
APA StyleSchieferle, S., Tappe, B., Korte, P., Mueller, M. J., & Berger, S. (2021). Pathogens and Elicitors Induce Local and Systemic Changes in Triacylglycerol Metabolism in Roots and in Leaves of Arabidopsis thaliana. Biology, 10(9), 920. https://doi.org/10.3390/biology10090920





