Current Trends in Cell-Free DNA Applications. Scoping Review of Clinical Trials
Abstract
:Simple Summary
Abstract
1. Introduction
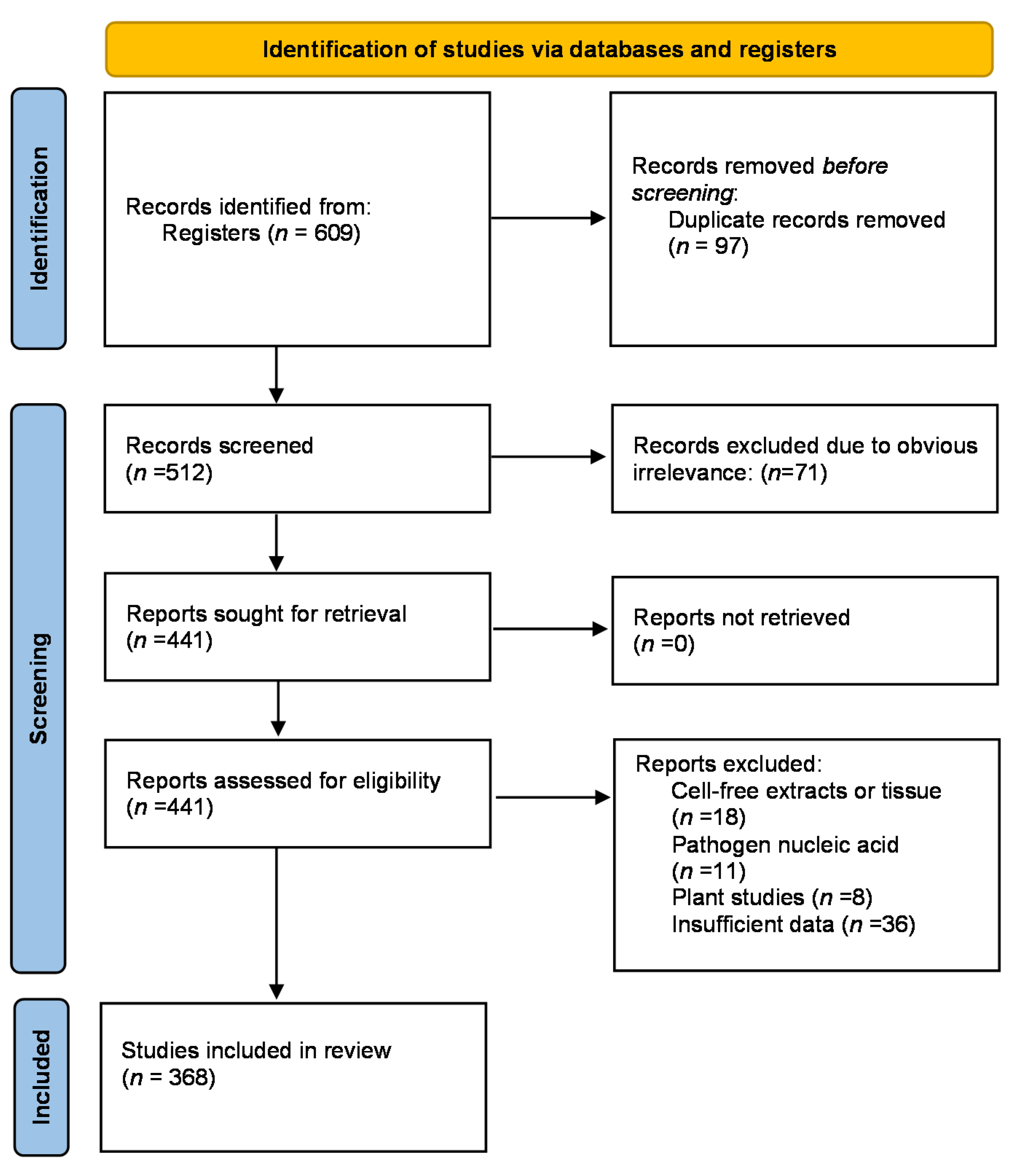
2. Methods
Study Selection
3. Results
4. Discussion
- (1)
- Projects with high application potential are subject to clinical trials, which does not necessarily reflect the real scientific/industrial interest.
- (2)
- Only two biggest international clinical trial databases were used. Minor national databases were not taken into account.
5. Conclusions
- (1)
- The analysis was based on 368 trials attended by over 250,000 participants, where 233 are still active or recruiting.
- (2)
- The vast majority of 255 clinical trials are on oncology, 48 on NIPT, and 41 on transplant medicine.
- (3)
- The projects are performed using modern methods.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponti, G.; Manfredini, M.; Tomasi, A. Non-blood sources of cell-free DNA for cancer molecular profiling in clinical pathology and oncology. Crit. Rev. Oncol. 2019, 141, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Bronkhorst, A.J.; Ungerer, V.; Diehl, F.; Anker, P.; Dor, Y.; Fleischhacker, M.; Gahan, P.B.; Hui, L.; Holdenrieder, S.; Thierry, A.R. Towards systematic nomenclature for cell-free DNA. Qual. Life Res. 2021, 140, 565–578. [Google Scholar] [CrossRef]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- Grabuschnig, S.; Bronkhorst, A.J.; Holdenrieder, S.; Rodriguez, I.R.; Schliep, K.P.; Schwendenwein, D.; Ungerer, V.; Sensen, C.W. Putative Origins of Cell-Free DNA in Humans: A Review of Active and Passive Nucleic Acid Release Mechanisms. Int. J. Mol. Sci. 2020, 21, 8062. [Google Scholar] [CrossRef]
- Wang, W.; Kong, P.; Ma, G.; Li, L.; Zhu, J.; Xia, T.; Xie, H.; Zhou, W.; Wang, S. Characterization of the release and biological significance of cell-free DNA from breast cancer cell lines. Oncotarget 2017, 8, 43180–43191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachariah, R.R.; Schmid, S.; Buerki, N.; Radpour, R.; Holzgreve, W.; Zhong, X. Levels of Circulating Cell-Free Nuclear and Mitochondrial DNA in Benign and Malignant Ovarian Tumors. Obstet. Gynecol. 2008, 112, 843–850. [Google Scholar] [CrossRef]
- Kohler, C.; Radpour, R.; Barekati, Z.; Asadollahi, R.; Bitzer, J.; Wight, E.; Bürki, N.; Diesch, C.; Holzgreve, W.; Zhong, X.Y. Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Mol. Cancer 2009, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvianti, F.; Giuliani, C.; Petrone, L.; Mancini, I.; Vezzosi, V.; Pupilli, C.; Pinzani, P. Integrity and Quantity of Total Cell-Free DNA in the Diagnosis of Thyroid Cancer: Correlation with Cytological Classification. Int. J. Mol. Sci. 2017, 18, 1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Hann, H.-W.; Wan, S.; Hann, R.S.; Wang, C.; Lai, Y.; Ye, X.; Evans, A.; Myers, R.E.; Ye, Z.; et al. Cell-free circulating mitochondrial DNA content and risk of hepatocellular carcinoma in patients with chronic HBV infection. Sci. Rep. 2016, 6, 23992. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sampath, H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells 2019, 8, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stawski, R.; Walczak, K.; Perdas, E.; Wlodarczyk, A.; Sarniak, A.; Kosielski, P.; Meissner, P.; Budlewski, T.; Padula, G.; Nowak, D. Decreased integrity of exercise-induced plasma cell free nuclear DNA—Negative association with the increased oxidants production by circulating phagocytes. Sci. Rep. 2019, 9, 15970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamkovich, S.N.; Kirushina, N.A.; Voytsitskiy, V.E.; Tkachuk, V.A.; Laktionov, P.P. Features of Circulating DNA Fragmentation in Blood of Healthy Females and Breast Cancer Patients. Adv. Exp. Med. Biol. 2016, 924, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.R.; Thorne, A.; Faro, M.L.L. Donor-specific Cell-free DNA as a Biomarker in Solid Organ Transplantation. A Systematic Review. Transplantation 2019, 103, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Hidalgo, L.G.; Aziz, F.; Parajuli, S.; Mohamed, M.; Mandelbrot, D.A.; Djamali, A. Use of Donor-Derived Cell-Free DNA for Assessment of Allograft Injury in Kidney Transplant Recipients During the Time of the Coronavirus Disease 2019 Pandemic. Transplant. Proc. 2020, 52, 2592–2595. [Google Scholar] [CrossRef]
- Dengu, F. Next-generation sequencing methods to detect donor-derived cell-free DNA after transplantation. Transplant. Rev. 2020, 34, 100542. [Google Scholar] [CrossRef] [PubMed]
- Goldwaser, T.; Klugman, S. Cell-free DNA for the detection of fetal aneuploidy. Fertil. Steril. 2018, 109, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.M.; Tsui, D.W. Circulating cell-free DNA for non-invasive cancer management. Cancer Genet. 2018, 228–229, 169–179. [Google Scholar] [CrossRef]
- Gahan, P.B.; Anker, P.; Stroun, M. Metabolic DNA as the Origin of Spontaneously Released DNA? Ann. N. Y. Acad. Sci. 2008, 1137, 7–17. [Google Scholar] [CrossRef]
- Perdas, E.; Stawski, R.; Nowak, D.; Zubrzycka, M. Potential of Liquid Biopsy in Papillary Thyroid Carcinoma in Context of miRNA, BRAF and p53 Mutation. Curr. Drug Targets 2018, 19, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Osumi, H.; Shinozaki, E.; Yamaguchi, K. Circulating Tumor DNA as a Novel Biomarker Optimizing Chemotherapy for Colorectal Cancer. Cancers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Oliveira, K.C.S.; Ramos, I.B.; Silva, J.M.C.; Barra, W.F.; Riggins, G.J.; Palande, V.; Pinho, C.T.; Frenkel-Morgenstern, M.; Santos, S.E.; Assumpcao, P.P.; et al. Current Perspectives on Circulating Tumor DNA, Precision Medicine, and Personalized Clinical Management of Cancer. Mol. Cancer Res. 2020, 18, 517–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischhacker, M.; Schmidt, B. Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys. Acta (BBA) Rev. Cancer 2007, 1775, 181–232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Choudhury, Y.; Ghosh, S.K.; Mondal, R. Application and optimization of minimally invasive cell-free DNA techniques in oncogenomics. Tumor Biol. 2018, 40, 1010428318760342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiala, C.; Diamandis, E.P. New approaches for detecting cancer with circulating cell-free DNA. BMC Med. 2019, 17, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [Green Version]
- Sacher, A.; Paweletz, C.; Dahlberg, S.; Alden, R.S.; O’Connell, A.; Feeney, N.; Mach, S.L.; Jänne, P.A.; Oxnard, G.R. Prospective Validation of Rapid Plasma Genotyping for the Detection ofEGFRandKRASMutations in Advanced Lung Cancer. JAMA Oncol. 2016, 2, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Rimelen, V.; Ahle, G.; Pencreach, E.; Zinniger, N.; Debliquis, A.; Zalmaï, L.; Harzallah, I.; Hurstel, R.; Alamome, I.; Lamy, F.; et al. Tumor cell-free DNA detection in CSF for primary CNS lymphoma diagnosis. Acta Neuropathol. Commun. 2019, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Oxnard, G.; Klein, E.; Swanton, C.; Seiden, M.; Smith, D.; Richards, D.; Yeatman, T.J.; Cohn, A.L.; Lapham, R.; et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Martuszewski, A.; Paluszkiewicz, P.; Król, M.; Banasik, M.; Kepinska, M. Donor-Derived Cell-Free DNA in Kidney Transplantation as a Potential Rejection Biomarker: A Systematic Literature Review. J. Clin. Med. 2021, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Schütz, E.; Fischer, A.; Beck, J.; Harden, M.; Koch, M.; Wuensch, T.; Stockmann, M.; Nashan, B.; Kollmar, O.; Matthaei, J.; et al. Graft-derived cell-free DNA, a noninvasive early rejection and graft damage marker in liver transplantation: A prospective, observational, multicenter cohort study. PLoS Med. 2017, 14, e1002286. [Google Scholar] [CrossRef]
- Sayah, D.; Weigt, S.S.; Ramsey, A.; Ardehali, A.; Golden, J.; Ross, D.J. Plasma Donor-derived Cell-free DNA Levels Are Increased During Acute Cellular Rejection After Lung Transplant: Pilot Data. Transplant. Direct 2020, 6, e608. [Google Scholar] [CrossRef]
- Richmond, M.E.; Zangwill, S.D.; Kindel, S.J.; Deshpande, S.R.; Schroder, J.N.; Bichell, D.P.; Knecht, K.R.; Mahle, W.T.; Wigger, M.A.; Gaglianello, N.A.; et al. Donor fraction cell-free DNA and rejection in adult and pediatric heart transplantation. J. Heart Lung Transplant. 2020, 39, 454–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, E.; Sethi, S.; Peng, A.; Najjar, R.; Mirocha, J.; Haas, M.; Vo, A.; Jordan, S.C. Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am. J. Transplant. 2019, 19, 1663–1670. [Google Scholar] [CrossRef]
- Sigdel, T.K.; Archila, F.A.; Constantin, T.; Prins, S.A.; Liberto, J.; Damm, I.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; Demko, Z.; et al. Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR. J. Clin. Med. 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gielis, E.M.; Ledeganck, K.J.; Dendooven, A.; Meysman, P.; Beirnaert, C.; Laukens, K.; De Schrijver, J.; Van Laecke, S.; Van Biesen, W.; Emonds, M.-P.; et al. The use of plasma donor-derived, cell-free DNA to monitor acute rejection after kidney transplantation. Nephrol. Dial. Transplant. 2019, 35, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Knüttgen, F.; Beck, J.; Dittrich, M.; Oellerich, M.; Zittermann, A.; Schulz, U.; Fuchs, U.; Knabbe, C.; Schütz, E.; Gummert, J.; et al. Graft-Derived Cell-Free DNA as a Noninvasive Biomarker of Cardiac Allograft Rejection: A Cohort Study on Clinical Validity and Confounding Factors. Transplantation 2021. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; De Vlaminck, I.; Luikart, H.; Ross, D.J.; Nicolls, M.R. Donor-derived, cell-free DNA levels by next-generation targeted sequencing are elevated in allograft rejection after lung transplantation. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef]
- Goh, S.K.; Do, H.; Testro, A.; Pavlovic, J.; Vago, A.; Lokan, J.; Jones, R.M.; Christophi, C.; Dobrovic, A.; Muralidharan, V. The Measurement of Donor-Specific Cell-Free DNA Identifies Recipients With Biopsy-Proven Acute Rejection Requiring Treatment After Liver Transplantation. Transplant. Direct 2019, 5, e462. [Google Scholar] [CrossRef]
- Carbone, L.; Cariati, F.; Sarno, L.; Conforti, A.; Bagnulo, F.; Strina, I.; Pastore, L.; Maruotti, G.M.; Alviggi, C. Non-Invasive Prenatal Testing: Current Perspectives and Future Challenges. Genes 2021, 12, 15. [Google Scholar] [CrossRef]
- Kruckow, S.; Schelde, P.; Hatt, L.; Ravn, K.; Petersen, O.B.; Uldbjerg, N.; Vogel, I.; Singh, R. Does Maternal Body Mass Index Affect the Quantity of Circulating Fetal Cells Available to Use for Cell-Based Noninvasive Prenatal Test in High-Risk Pregnancies? Fetal Diagn. Ther. 2019, 45, 353–356. [Google Scholar] [CrossRef]
- Taylor-Phillips, S.; Freeman, K.; Geppert, J.; Agbebiyi, A.; Uthman, A.O.; Madan, J.; Clarke, A.; Quenby, S.; Clarke, A. Accuracy of non-invasive prenatal testing using cell-free DNA for detection of Down, Edwards and Patau syndromes: A systematic review and meta-analysis. BMJ Open 2016, 6, e010002. [Google Scholar] [CrossRef] [Green Version]
- McCullough, R.M.; Almasri, E.A.; Guan, X.; Geis, J.A.; Hicks, S.C.; Mazloom, A.R.; Deciu, C.; Oeth, P.; Bombard, A.T.; Paxton, B.; et al. Non-Invasive Prenatal Chromosomal Aneuploidy Testing—Clinical Experience: 100,000 Clinical Samples. PLoS ONE 2014, 9, e109173. [Google Scholar] [CrossRef]
- Clementi, A.; Virzì, G.M.; Brocca, A.; Pastori, S.; De Cal, M.; Marcante, S.; Granata, A.; Ronco, C. The Role of Cell-Free Plasma DNA in Critically Ill Patients with Sepsis. Blood Purif. 2016, 41, 34–40. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, Y.; Gong, Y.; Sun, R.; Su, L.; Lin, X.; Shen, A.; Zhou, J.; Caiji, Z.; Wang, X.; et al. Diagnosis of Sepsis with Cell-free DNA by Next-Generation Sequencing Technology in ICU Patients. Arch. Med. Res. 2016, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Stawski, R.; Walczak, K.; Kosielski, P.; Meissner, P.; Budlewski, T.; Padula, G.; Nowak, D. Repeated bouts of exhaustive exercise increase circulating cell free nuclear and mitochondrial DNA without development of tolerance in healthy men. PLoS ONE 2017, 12, e0178216. [Google Scholar] [CrossRef]
- Breitbach, S.; Tug, S.; Simon, P. Circulating Cell-Free DNA. Sports Med. 2012, 42, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Dauber, E.; Kollmann, D.; Kozakowski, N.; Rasoul-Rockenschaub, S.; Soliman, T.; Berlakovich, G.A.; Mayr, W.R. Quantitative PCR of INDELs to measure donor-derived cell-free DNA—A potential method to detect acute rejection in kidney transplantation: A pilot study. Transpl. Int. 2019, 33, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Perdas, E.; Stawski, R.; Kaczka, K.; Nowak, D.; Zubrzycka, M. Altered levels of circulating nuclear and mitochondrial DNA in patients with Papillary Thyroid Cancer. Sci. Rep. 2019, 9, 14438. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chiu, P.-F.; Wu, C.-L.; Kuo, C.-L.; Huang, C.-S.; Liu, C.-S. Urinary cell-free mitochondrial and nuclear deoxyribonucleic acid correlates with the prognosis of chronic kidney diseases. BMC Nephrol. 2019, 20, 391. [Google Scholar] [CrossRef] [PubMed]
- Trumpff, C.; Marsland, A.L.; Basualto-Alarcón, C.; Martin, J.L.; Carroll, J.E.; Sturm, G.; Vincent, A.E.; Mosharov, E.V.; Gu, Z.; Kaufman, B.A.; et al. Acute psychological stress increases serum circulating cell-free mitochondrial DNA. Psychoneuroendocrinology 2019, 106, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Day, S.; Jonker, A.H.; Lau, L.P.L.; Hilgers, R.-D.; Irony, I.; Larsson, K.; Roes, K.C.; Stallard, N. Recommendations for the design of small population clinical trials. Orphanet J. Rare Dis. 2018, 13, 195. [Google Scholar] [CrossRef] [PubMed]
| Cell-Free DNA | Abbreviation | Application |
|---|---|---|
| Total cfDNA | cfDNA, ccfDNA | General marker of physiological well-being, elevated in sepsis or under stress, temporarily increases during exercise |
| Circulating cell-free DNA | ||
| Serum/Plasma cell-free DNA | ||
| Circulating free DNA | ||
| Tumoral plasma DNA | ctDNA | Used in cancer therapy management, marker of cancer relapse or metastasis |
| (Circulating tumoral DNA) | ||
| Donor Derived cell-free DNA | dd-cfDNA | Used in detection of allograft rejection in transplantation |
| (Donor specific cell-free DNA) | dscfDNA | |
| cell-free fetal DNA | cff-DNA | Used in non-invasive prenatal diagnostics, usually aneuploidy |
| circulating cell-free fetal DNA | ccff-DNA | |
| Mitochondrial cfDNA | cf mtDNA | Releases during mitochondrial dysfunction, elevated under physiological stress or in some cancers |
| Circulating mitochondrial DNA | ||
| Methylated cell-free DNA | m-cfDNA | Mostly applied in oncology as a marker for cancer screening. Could be done specifically for a selected gene or globally |
| cfDNA integrity index | cfDI | Allows to distinguish if cfDNA is released from apoptotic or necrotic cells |
| Condition | Specific Condition |
|---|---|
| Cancer (n = 255) | Lung Cancer (n = 64) |
| Colorectal cancer (n = 43) | |
| Breast (n = 33) | |
| Prostate (n = 18) | |
| Other (including all cancer diagnoses) (n = 97) | |
| NIPT (n = 50) | Prenatal Testing (n = 50) |
| Organ Transplant (n = 41) | Kidney (n = 24) |
| Lung (n = 7) | |
| Heart (n = 5) | |
| Other (n = 5) | |
| Other (n = 22) | Sepsis (n = 4) |
| Other (n = 18) |
| Recruitment Status | Number of Studies in Total | Number of Studies Cancer | Number of Studies Non-Cancer |
|---|---|---|---|
| Recruiting | 156 | 109 | 47 |
| Completed | 69 | 39 | 30 |
| Not yet recruiting | 55 | 41 | 14 |
| Suspended/Unknown status | 52 | 40 | 12 |
| Active, not recruiting | 22 | 16 | 6 |
| Terminated | 8 | 7 | 1 |
| Enrolling by invitation | 3 | 1 | 2 |
| Withdrawn | 3 | 3 | 0 |
| Type | Number of Studies in Total | Number of Studies Cancer | Number of Studies Non-Cancer |
|---|---|---|---|
| Interventional | 148 | 114 | 34 |
| Observational Study | 219 | 141 | 78 |
| ID Number | Title | Status | Primary Outcomes |
|---|---|---|---|
| ChiCTR-OOC-15006382 | Prognostic value of circulating cell-free DNA in patientsadmitted to Emergency Intensive Care Unit (EICU) | Active/recruiting | Serum cfDNA level; Prognose; |
| NCT03356249 | Next-Generation Sequencing Diagnostics of Bacteremia in Sepsis (Next GeneSiS-Trial) | Active/Recruiting | Sensitivity; Specificity; Positive predictive value |
| NCT00919685 | Investigations of New Markers in Patients with Shock | Completed | Demonstrate the superiority of at least one of 3 markers (HIF, MPs, cDNA) with regard to plasma lactate level for evaluating the treatment response in patients with shock |
| NCT04189549 | Preclinical Detection of Sepsis Early in Hospitalized Patients Following Surgery, Injury or Severe Illness (Pre-SEPSIS Trial) | Not yet recruiting | Clinical development of sepsis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawski, R.; Stec-Martyna, E.; Chmielecki, A.; Nowak, D.; Perdas, E. Current Trends in Cell-Free DNA Applications. Scoping Review of Clinical Trials. Biology 2021, 10, 906. https://doi.org/10.3390/biology10090906
Stawski R, Stec-Martyna E, Chmielecki A, Nowak D, Perdas E. Current Trends in Cell-Free DNA Applications. Scoping Review of Clinical Trials. Biology. 2021; 10(9):906. https://doi.org/10.3390/biology10090906
Chicago/Turabian StyleStawski, Robert, Emilia Stec-Martyna, Adam Chmielecki, Dariusz Nowak, and Ewelina Perdas. 2021. "Current Trends in Cell-Free DNA Applications. Scoping Review of Clinical Trials" Biology 10, no. 9: 906. https://doi.org/10.3390/biology10090906
APA StyleStawski, R., Stec-Martyna, E., Chmielecki, A., Nowak, D., & Perdas, E. (2021). Current Trends in Cell-Free DNA Applications. Scoping Review of Clinical Trials. Biology, 10(9), 906. https://doi.org/10.3390/biology10090906







