Neurotherapeutic Potential of Cervus elaphus Sibericus on Axon Regeneration and Growth Cone Reformation after H2O2-Induced Injury in Rat Primary Cortical Neurons
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Culture of Cortical Neurons
2.2. Preparation of CES Extract
2.3. H2O2-Induced Oxidative Injury and CES Treatment
2.4. Laceration Injury
2.5. Neuronal Viability Assays
2.6. Immunocytochemistry
2.7. Axon and Growth Cone Quantification
2.8. Flow Cytometry
2.9. Real-Time PCR
2.10. Statistical Analysis
3. Results
3.1. CES Protect Neurons from H2O2-Induced Neuronal Death in Cortical Neurons
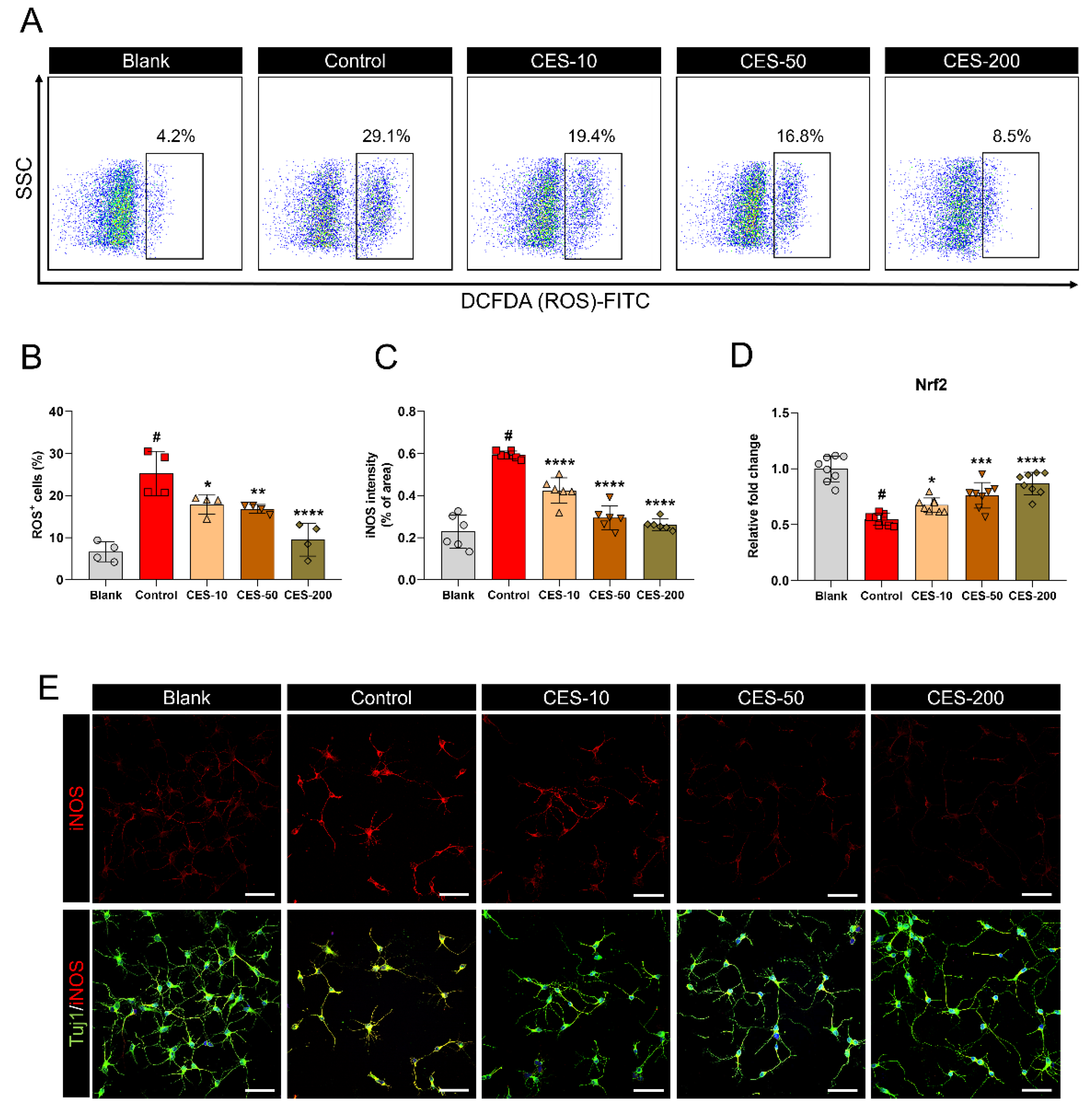
3.2. CES Suppresses H2O2-Promoted iNOS Expression and ROS Production in Cortical Neurons by Activating the Nrf2 Pathway
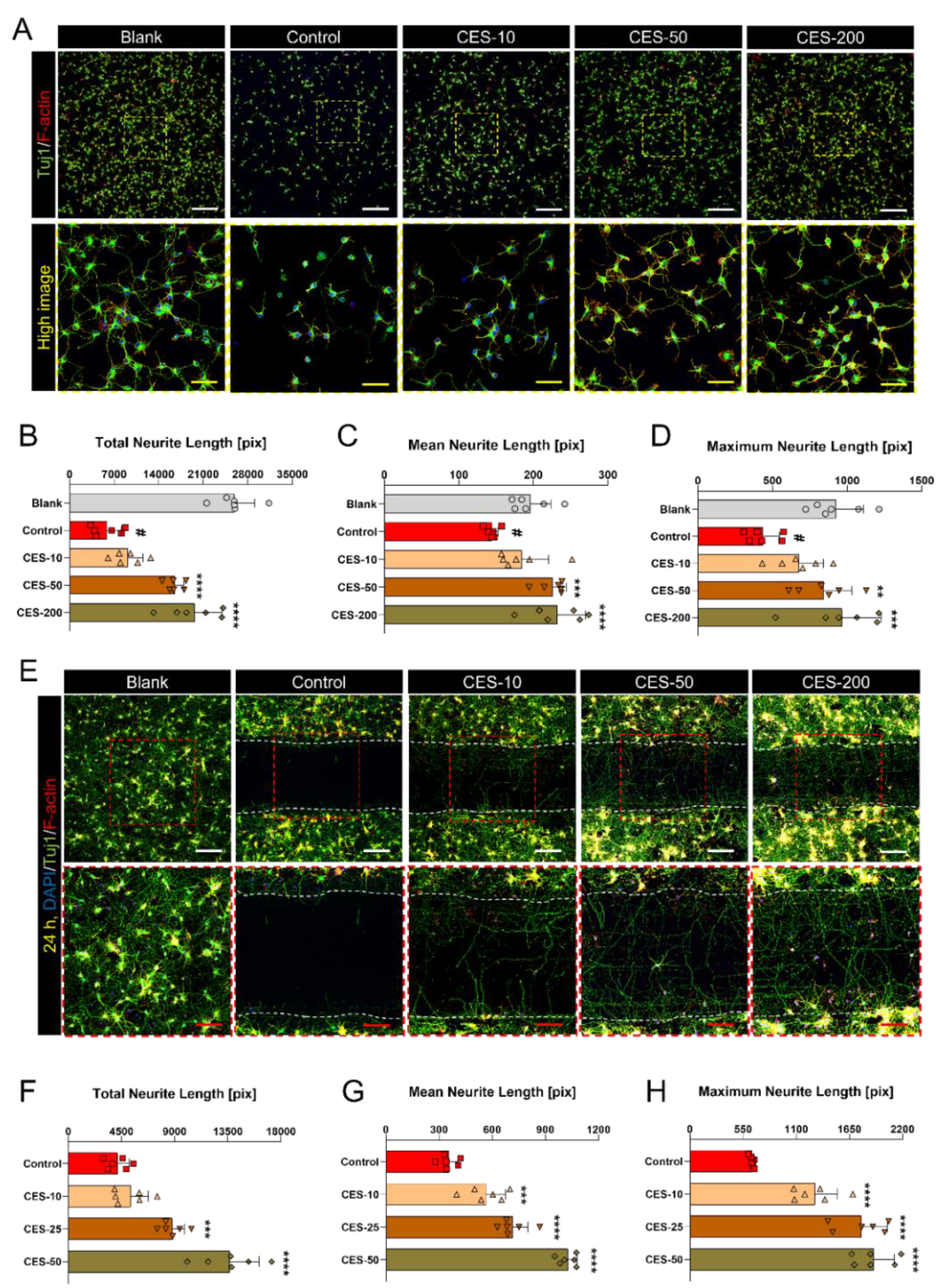
3.3. CES Not Only Promotes Re-Elongation of H2O2-Injured Axons, but also Accelerates Regenerative Axon Growth of Mature Cortical Neurons after Laceration Injury
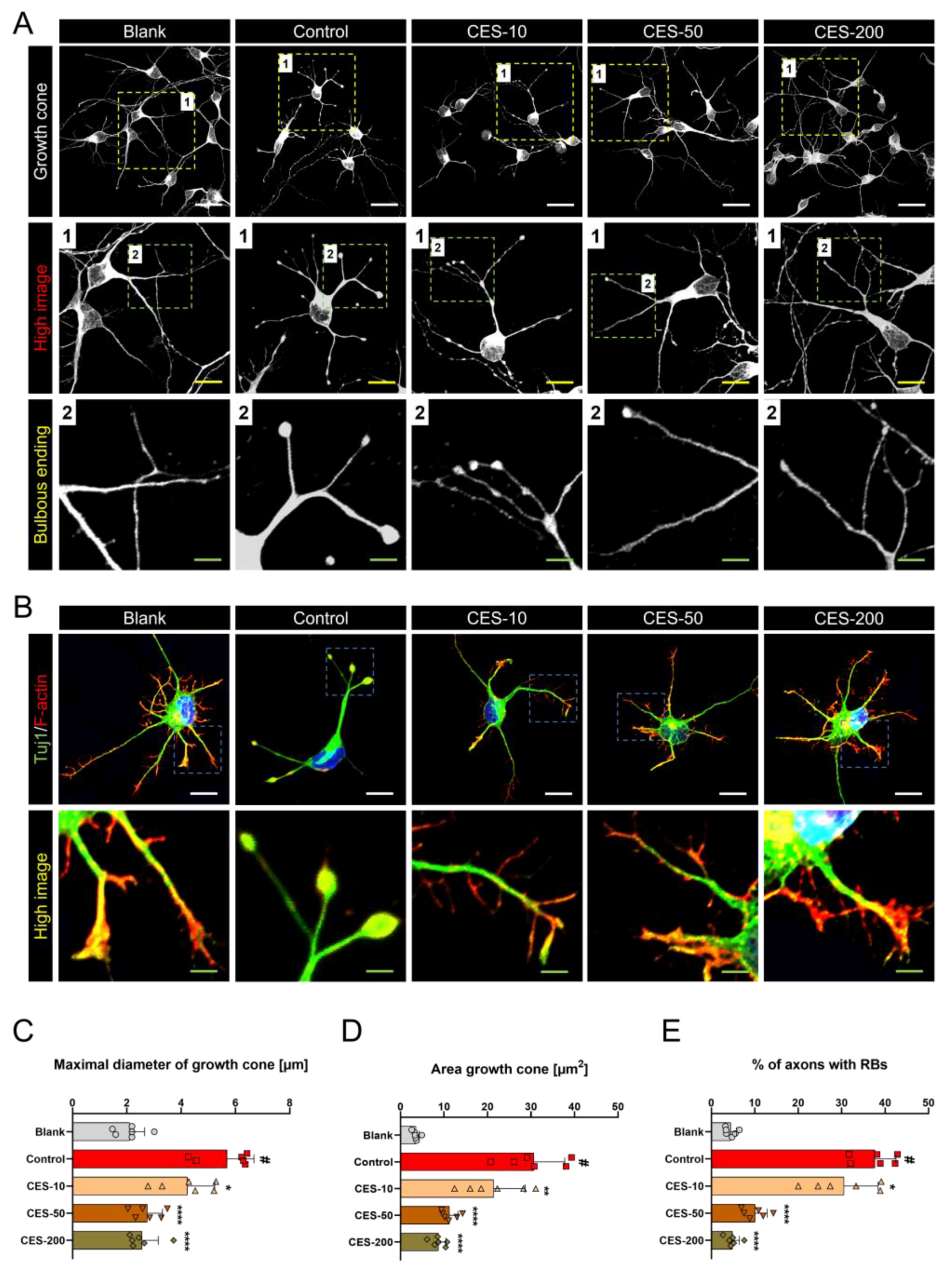
3.4. CES Inhibits Conversion of Growth Cone into a Retraction Bulb and Stabilizes Formation of F-Actin Rich Structures in Growth Cone of H2O2-Treated Cortical Neurons
3.5. CES Promotes Re-Elongation of H2O2-Injured Axons by Enhancing Brain-Derived Neurotrophic Factor and Nerve Growth Factor Expression in Cortical Neurons
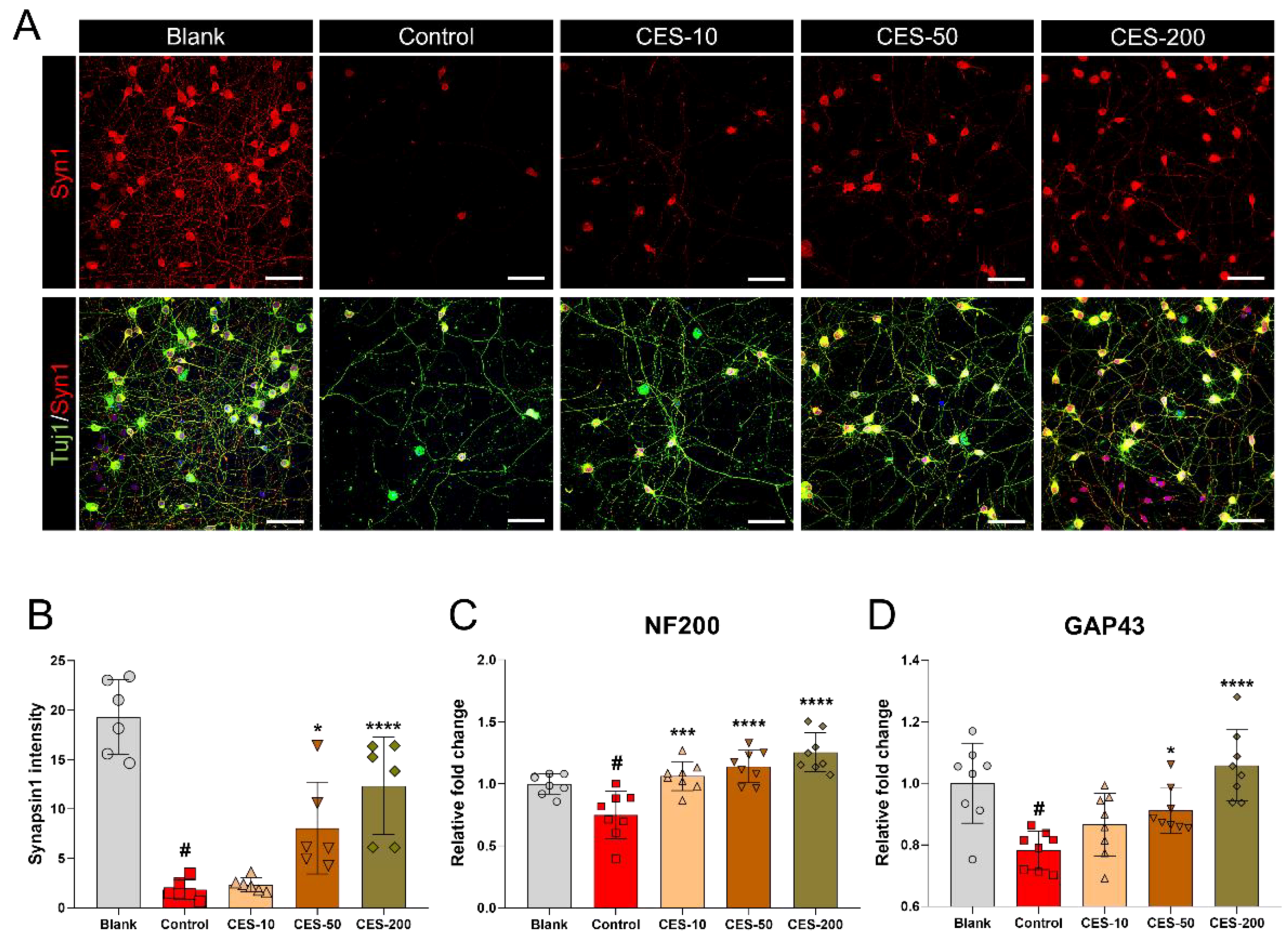
3.6. CES Attenuates H2O2-Induced Reduction of Synapsin1 in Cortical Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, H.; Shakkour, Z.; Tabet, M.; Abdelhady, S.; Kobaisi, A.; Abedi, R.; Nasrallah, L.; Pintus, G.; Al-Dhaheri, Y.; Mondello, S.; et al. Traumatic Brain Injury: Oxidative Stress and Novel Anti-Oxidants Such as Mitoquinone and Edaravone. Antioxidants 2020, 9, 943. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012, 50, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwak, Y.S.; Hassler, S.E.; Hulsebosch, C.E. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain 2013, 154, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 1–21. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Lin, Q.; Liang, Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, H.; Lee, J.; Jeon, W.J.; Baek, S.H.; Ha, I.H. Neurotherapeutic Effect of Inula britannica var. Chinensis against H2O2-Induced Oxidative Stress and Mitochondrial Dysfunction in Cortical Neurons. Antioxidants 2021, 10, 375. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, S.; Zhu, J.; Shang, J.; Gao, X. Hypoglycemic, hypolipidemic and antioxidant effects of peptides from red deer antlers in streptozotocin-induced diabetic mice. Tohoku J. Exp. Med. 2015, 236, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W.; Kim, K.S.; Park, S.D.; Kim, J.K.; Chung, K.H.; Kim, D.S.; Lee, Y.C.; Kim, C.H. Effect of Cervus korean TEMMINCK var. mantchuricus Swinhoe on protease activities, antioxidant and free radical damages in rheumatis arthritis rats. Toxicol. Vitro 2008, 22, 80–86. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Z.; Ma, L.; Yang, L.; Wu, T.; Fu, Z. Pilose antler polypeptides ameliorate inflammation and oxidative stress and improves gut microbiota in hypoxic-ischemic injured rats. Nutr. Res. 2019, 64, 93–108. [Google Scholar] [CrossRef]
- Wu, T.; Yang, L.; Chen, Y.; Ni, Y.; Jiang, J.; Zhang, W.; Zhou, Q.; Zheng, X.; Wang, Q.; Fu, Z.; et al. Pilose antler polypeptides ameliorates hypoxic-ischemic encephalopathy by activated neurotrophic factors and SDF1/CXCR4 axis in rats. Acta Biochim. Biophys. Sin. 2018, 50, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, T.Y.; Wang, C.H.; Chen, K.N.; Huang, I.N.; Hong, W.S.; Wang, S.Y.; Chen, Y.P.; Kuo, C.Y.; Chen, M.J. The Antiinfective Effects of Velvet Antler of Formosan Sambar Deer (Cervus unicolor swinhoei) on Staphylococcus aureus-Infected Mice. Evid.-Based Complement. Alternat. Med. 2011, 2011, 534069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.Y.; Wang, T.; Dai, T.Y.; Wang, C.H.; Chen, K.N.; Chen, Y.P.; Chen, M.J. Effect of the Velvet Antler of Formosan Sambar Deer (Cervus unicolor swinhoei) on the Prevention of an Allergic Airway Response in Mice. Evid.-Based Complement. Alternat. Med. 2012, 2012, 481318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Jeon, B.T.; Wang, Y.; Choi, E.J.; Park, P.J.; Seong, H.J.; Moon, S.H.; Kim, E.K. First Evalulation of the Biologically Active Substances and Antioxidant Potential of Regrowth Velvet Antler by mean of Multiple Biochemical Assays. J. Chem. 2015, 2015, 975292. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.R.; Jeon, H.L.; Shin, S.K.; Kim, H.J.; Ahn, C.W.; Jung, S.U.; Park, S.H.; Kim, M.R. Neuroprotective action of deer bone extract against glutamate or Abeta(1)(-)(4)(2)-induced oxidative stress in mouse hippocampal cells. J. Med. Food 2014, 17, 226–235. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Li, Y.; Yang, M.; Wang, X.; Peng, Y. Velvet Antler Methanol Extracts Ameliorate Parkinson’s Disease by Inhibiting Oxidative Stress and Neuroinflammation: From C. elegans to Mice. Oxid. Med. Cell Longev. 2021, 2021, 8864395. [Google Scholar] [CrossRef]
- Xin, J.L.; Zhang, Y.; Li, Y.; Zhang, L.Z.; Lin, Y.; Zheng, L.W. Protective effects of Cervus nippon Temminck velvet antler polypeptides against MPP+induced cytotoxicity in SHSY5Y neuroblastoma cells. Mol. Med. Rep. 2017, 16, 5143–5150. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Wang, Y.; Jeon, B.T.; Moon, S.H.; Lee, S.H. Enzymatic hydrolysate from velvet antler suppresses adipogenesis in 3T3-L1 cells and attenuates obesity in high-fat diet-fed mice. EXCLI J. 2017, 16, 328–339. [Google Scholar] [CrossRef]
- Li, L.; Yang, F.; Jia, R.; Yan, P.; Ma, L. Velvet antler polypeptide prevents the disruption of hepatic tight junctions via inhibiting oxidative stress in cholestatic mice and liver cell lines. Food Funct. 2020, 11, 9752–9763. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Wang, J.; Luo, J.; Ruan, H.; Zhang, J. Purified Sika deer antler protein attenuates GM-induced nephrotoxicity by activating Nrf2 pathway and inhibiting NF-kappaB pathway. Sci. Rep. 2020, 10, 15601. [Google Scholar] [CrossRef]
- Popova, D.; Karlsson, J.; Jacobsson, S.O.P. Comparison of neurons derived from mouse P19, rat PC12 and human SH-SY5Y cells in the assessment of chemical- and toxin-induced neurotoxicity. BMC Pharmacol. Toxicol. 2017, 18, 42. [Google Scholar] [CrossRef]
- Yang, Y.; Herrup, K. Cell division in the CNS: Protective response or lethal event in post-mitotic neurons? Biochim. Biophys. Acta 2007, 1772, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Dent, E.W.; Gupton, S.L.; Gertler, F.B. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erturk, A.; Hellal, F.; Enes, J.; Bradke, F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J. Neurosci. 2007, 27, 9169–9180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Hong, J.Y.; Jeon, W.J.; Lee, J.; Baek, S.H.; Ha, I.H. Lycopus lucidus Turcz Exerts Neuroprotective Effects Against H2O2-Induced Neuroinflammation by Inhibiting NLRP3 Inflammasome Activation in Cortical Neurons. J. Inflamm. Res. 2021, 14, 1759–1773. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, B.; Huh, H.W.; Na, Y.G.; Kim, M.; Han, M.; Lee, H.; Pham, T.M.A.; Lee, H.K.; Lee, J.Y.; et al. Achyranthis radix Extract-Loaded Eye Drop Formulation Development and Novel Evaluation Method for Dry Eye Treatment. Pharmaceutics 2020, 12, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupack, J.; Xiong, X.P.; Jiang, L.L.; Zhang, T.; Zhou, L.; Campos, A.; Ranscht, B.; Mobley, W.; Pasquale, E.B.; Xu, H.; et al. Soluble SORLA Enhances Neurite Outgrowth and Regeneration through Activation of the EGF Receptor/ERK Signaling Axis. J. Neurosci. 2020, 40, 5908–5921. [Google Scholar] [CrossRef]
- Latremoliere, A.; Cheng, L.; DeLisle, M.; Wu, C.; Chew, S.; Hutchinson, E.B.; Sheridan, A.; Alexandre, C.; Latremoliere, F.; Sheu, S.H.; et al. Neuronal-Specific TUBB3 Is Not Required for Normal Neuronal Function but Is Essential for Timely Axon Regeneration. Cell Rep. 2018, 24, 1865–1879.e1869. [Google Scholar] [CrossRef] [Green Version]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Bayir, H.; Kagan, V.E.; Borisenko, G.G.; Tyurina, Y.Y.; Janesko, K.L.; Vagni, V.A.; Billiar, T.R.; Williams, D.L.; Kochanek, P.M. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: Support for a neuroprotective role of iNOS. J. Cereb. Blood Flow Metab. 2005, 25, 673–684. [Google Scholar] [CrossRef]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Hill, C.E.; Beattie, M.S.; Bresnahan, J.C. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp. Neurol. 2001, 171, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.J.; Zahid, S. The Role of Synapsins in Neurological Disorders. Neurosci. Bull. 2018, 34, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.A.; Coppola, D.; Palma Esposito, F.; Buonocore, C.; Ausuri, J.; Tortorella, E.; de Pascale, D. Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- McChesney, J.D. Natural products in drug discovery--organizing for success. P. R. Health Sci. J. 2002, 21, 91–95. [Google Scholar]
- Jhon, G.J.; Park, S.Y.; Han, S.Y.; Lee, S.; Kim, Y.; Chang, Y.S. Studies of the chemical structure of gangliosides in deer antler, Cervus nippon. Chem. Pharm. Bull. 1999, 47, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Palmano, K.; Rowan, A.; Guillermo, R.; Guan, J.; McJarrow, P. The role of gangliosides in neurodevelopment. Nutrients 2015, 7, 3891–3913. [Google Scholar] [CrossRef] [Green Version]
- Nawabi, H.; Belin, S.; Cartoni, R.; Williams, P.R.; Wang, C.; Latremoliere, A.; Wang, X.; Zhu, J.; Taub, D.G.; Fu, X.; et al. Doublecortin-Like Kinases Promote Neuronal Survival and Induce Growth Cone Reformation via Distinct Mechanisms. Neuron 2015, 88, 704–719. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Kim, M.G.; Leem, K.H. Comparison of the Effect of Velvet Antler from Different Sections on Longitudinal Bone Growth of Adolescent Rats. Evid.-Based Complement. Alternat. Med. 2016, 2016, 1927534. [Google Scholar] [CrossRef] [Green Version]
- Samejima, Y.; Matsuoka, H. A new viewpoint on antlers reveals the evolutionary history of deer (Cervidae, Mammalia). Sci. Rep. 2020, 10, 8910. [Google Scholar] [CrossRef]




| Gene | 5′-3′ | Primer Sequence |
|---|---|---|
| Nrf2 | Forward | GATCTGTCAGCTACTCCCAG |
| Reverse | GCAAGCGACTCATGGTCATC | |
| BDNF | Forward | CTTGGAGAAGGAAACCGCCT |
| Reverse | GTCCACACAAAGCTCTCGGA | |
| NGF | Forward | CCAAGGACGCAGCTTTCTATC |
| Reverse | CTGTGTCAAGGGAATGCTGAAG | |
| NF200 | Forward | AACACCACTTAGATGGCGGG |
| Reverse | ACGTGGAGCGTTCAGCAATA | |
| GAP43 | Forward | TGCCCTTTCTCAGATCCACT |
| Reverse | TTGCCACACAGAGAGAGAGG | |
| GAPDH | Forward | CCCCCAATGTATCCGTTGTG |
| Reverse | TAGCCCAGGATGCCCTTTAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.Y.; Lee, J.; Kim, H.; Jeon, W.-J.; Yeo, C.; Choi, B.R.; Yoon, J.E.; Shin, J.Y.; Kim, J.-Y.; Ha, I.-H. Neurotherapeutic Potential of Cervus elaphus Sibericus on Axon Regeneration and Growth Cone Reformation after H2O2-Induced Injury in Rat Primary Cortical Neurons. Biology 2021, 10, 833. https://doi.org/10.3390/biology10090833
Hong JY, Lee J, Kim H, Jeon W-J, Yeo C, Choi BR, Yoon JE, Shin JY, Kim J-Y, Ha I-H. Neurotherapeutic Potential of Cervus elaphus Sibericus on Axon Regeneration and Growth Cone Reformation after H2O2-Induced Injury in Rat Primary Cortical Neurons. Biology. 2021; 10(9):833. https://doi.org/10.3390/biology10090833
Chicago/Turabian StyleHong, Jin Young, Junseon Lee, Hyunseong Kim, Wan-Jin Jeon, Changhwan Yeo, Bo Ram Choi, Jee Eun Yoon, Ji Yun Shin, Jeom-Yong Kim, and In-Hyuk Ha. 2021. "Neurotherapeutic Potential of Cervus elaphus Sibericus on Axon Regeneration and Growth Cone Reformation after H2O2-Induced Injury in Rat Primary Cortical Neurons" Biology 10, no. 9: 833. https://doi.org/10.3390/biology10090833
APA StyleHong, J. Y., Lee, J., Kim, H., Jeon, W.-J., Yeo, C., Choi, B. R., Yoon, J. E., Shin, J. Y., Kim, J.-Y., & Ha, I.-H. (2021). Neurotherapeutic Potential of Cervus elaphus Sibericus on Axon Regeneration and Growth Cone Reformation after H2O2-Induced Injury in Rat Primary Cortical Neurons. Biology, 10(9), 833. https://doi.org/10.3390/biology10090833