Impact of Forest Management on the Temporal Dynamics of Herbaceous Plant Diversity in the Carpathian Beech Forests over 40 Years
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
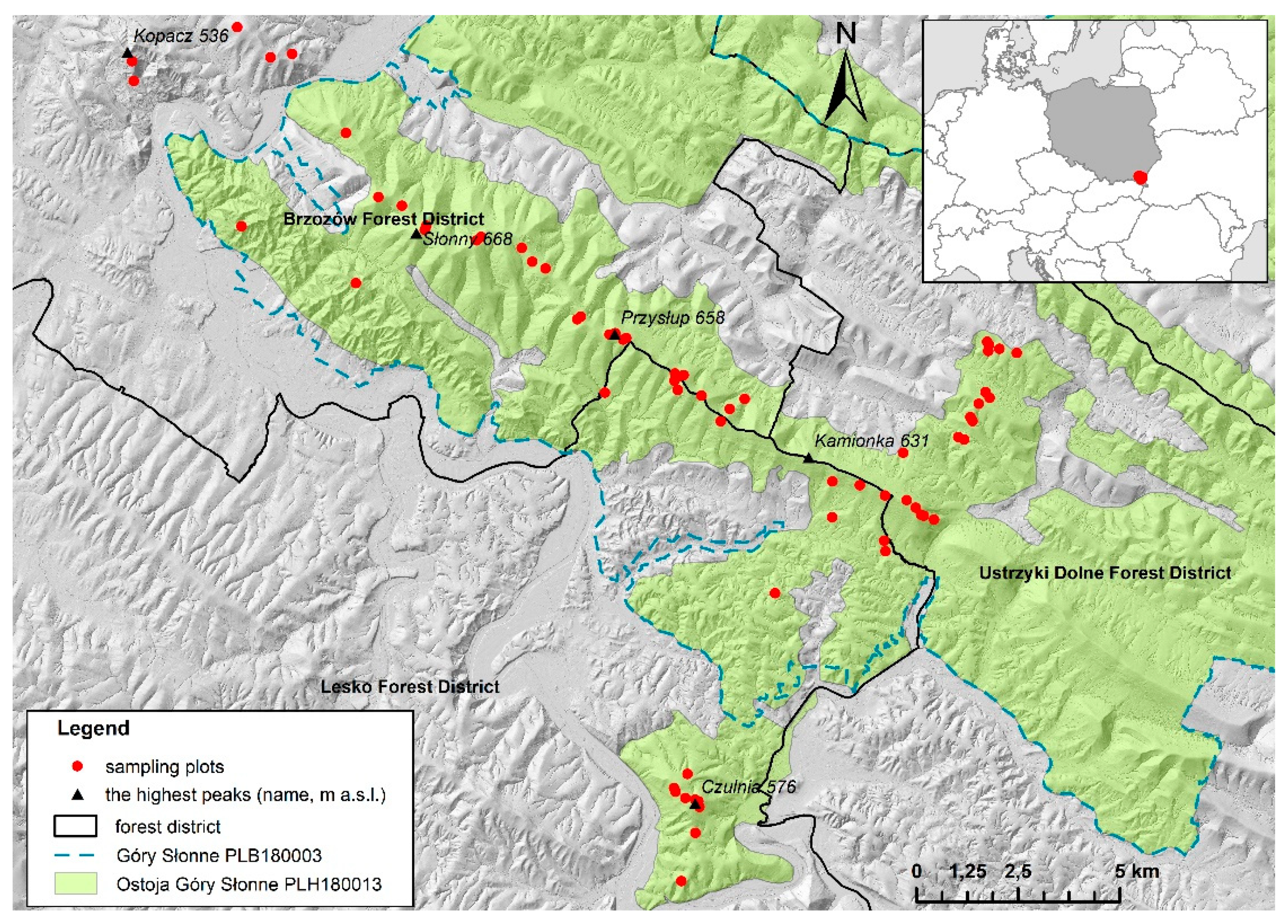
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
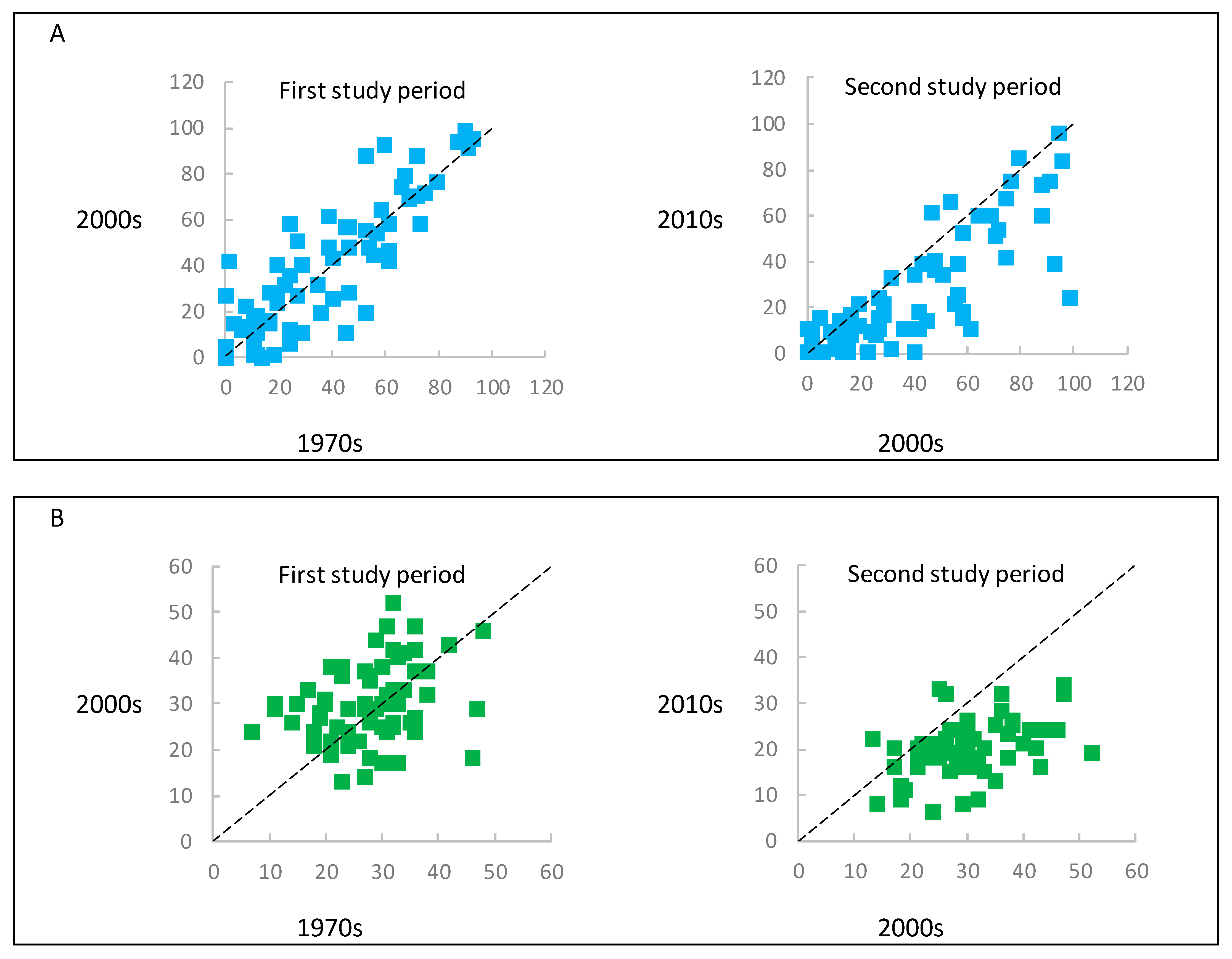
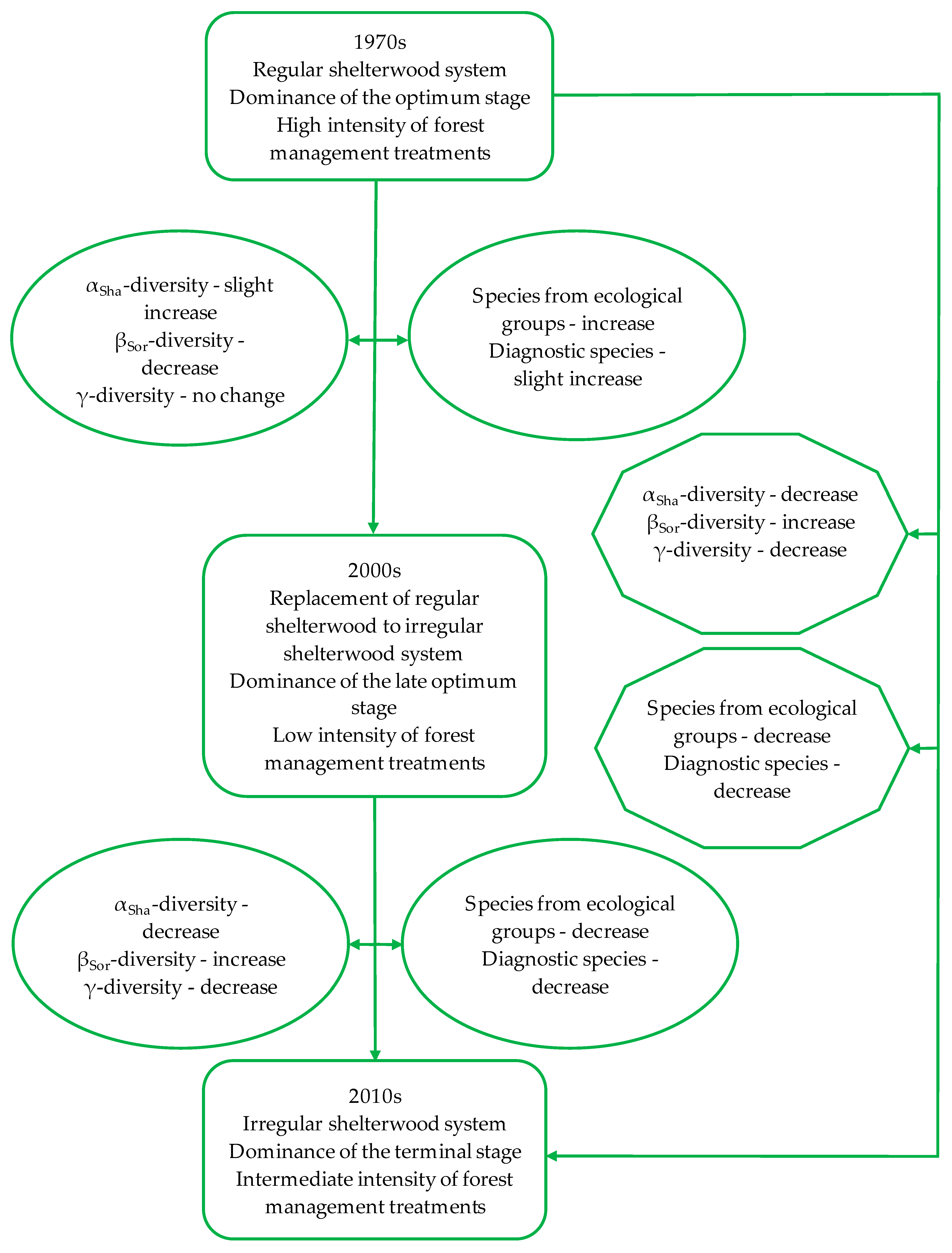
3. Results
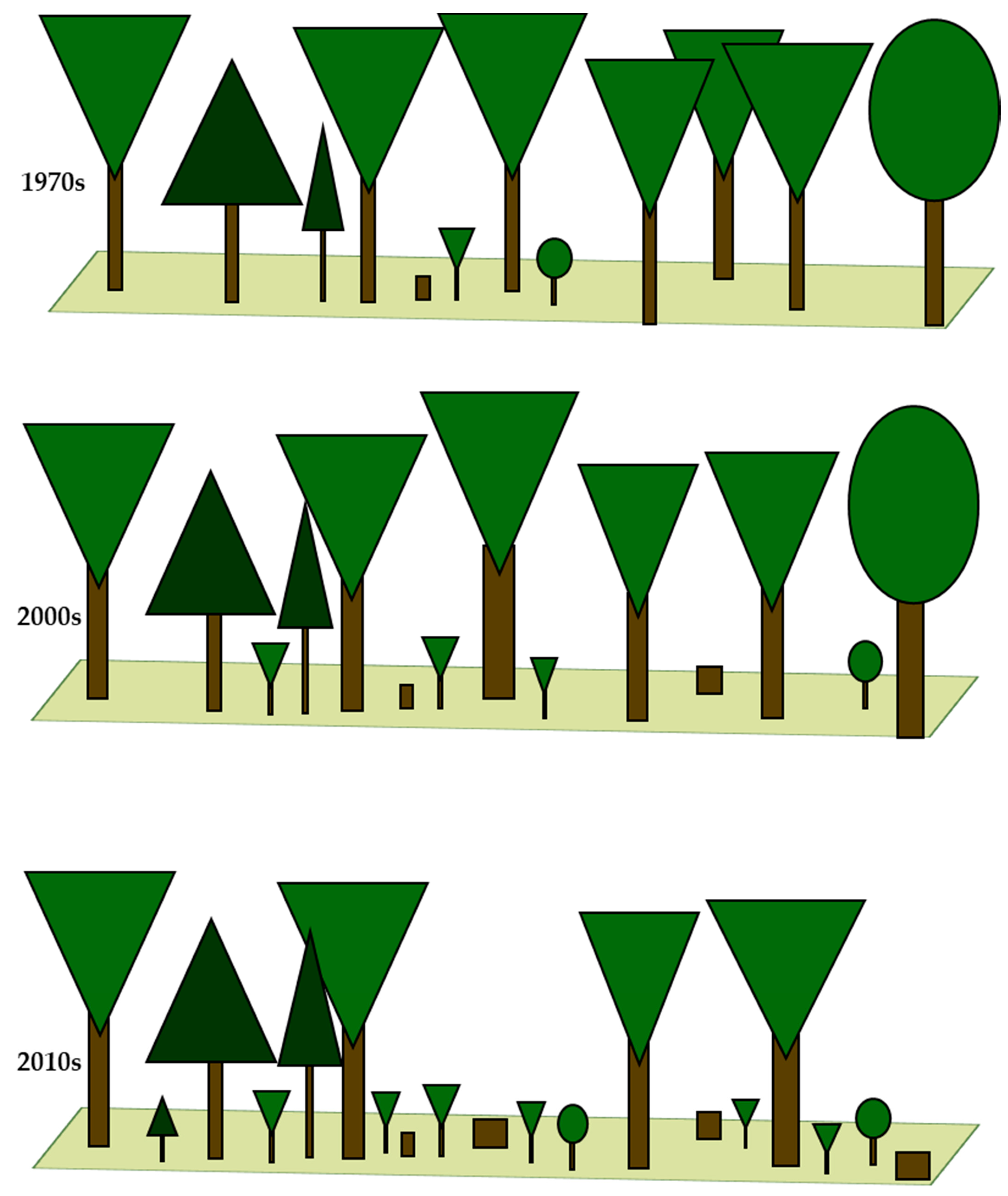
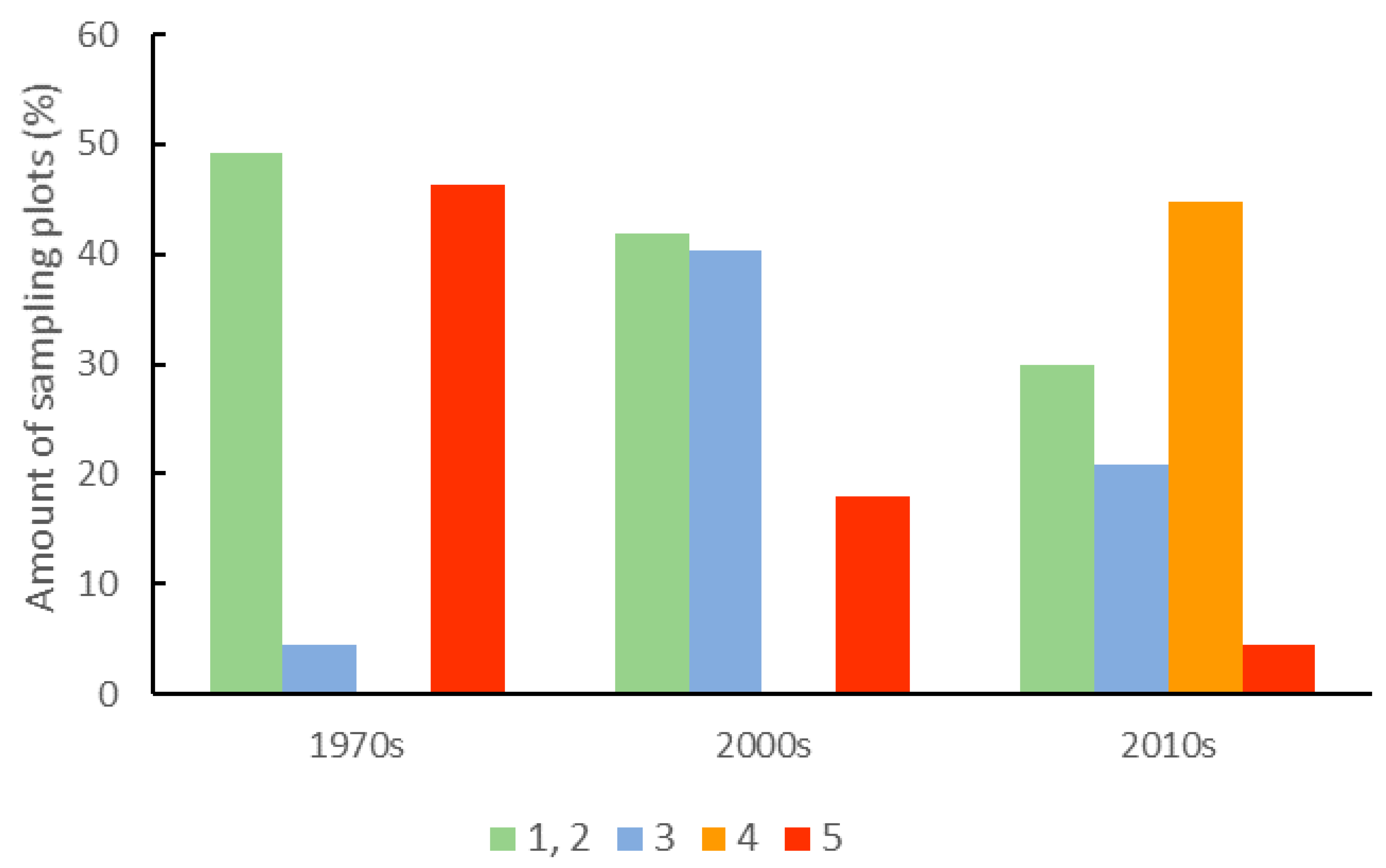
3.1. Dynamics of Change in the Forest Structure and Intensity of Forest Management
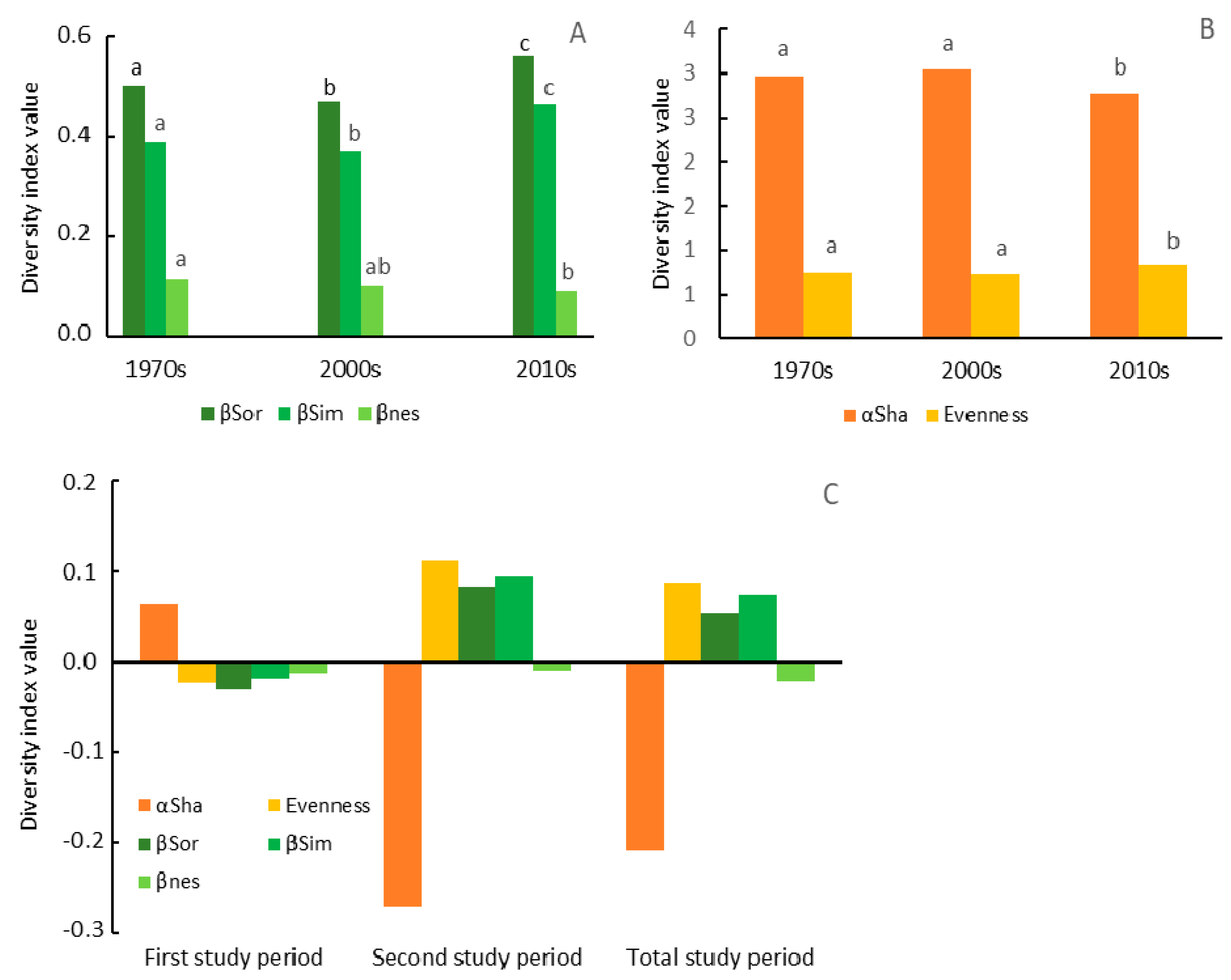
3.2. Dynamics of Change in the Herbaceous Plant Diversity Metrics
3.3. Impact of Changes in the Forest Structure, as Well as Intensity of Forest Management on the Herbaceous Plant Diversity Metrics
4. Discussion
4.1. Dynamics of Change in the Herbaceous Plant Diversity Metrics
4.2. Impact of Changes in Forest Structure as Well Intensity of Forest Management on Herbaceous Plant Diversity Metrics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Rackham, O. Ancient woodlands: Modern threats. New Phytol. 2008, 180, 571–586. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Perring, M.P.; Bernhardt-Römermann, M.; Baeten, L.; Midolo, G.; Blondeel, H.; Depauw, L.; Landuyt, D.; Maes, S.L.; De Lombaerde, E.; Carón, M.M.; et al. Global environmental change effects on plant community composition trajectories depend upon management legacies. Glob. Chang. Biol. 2018, 24, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Bürgi, M.; Li, L.; Kizos, T. Exploring links between culture and biodiversity: Studying land use intensity from the plot to the landscape level. Biodivers. Conserv. 2015, 24, 3285–3303. [Google Scholar] [CrossRef]
- Franklin, J.; Serra-Diaz, J.; Syphard, A.; Regan, H. Global change and terrestrial plant community dynamics. Proc. Nat. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Naaf, T.; Wulf, M. Habitat specialists and generalists drive homogenization and differentiation of temperate forest plant communities at the regional scale. Biodivers. Conserv. 2010, 143, 848–855. [Google Scholar] [CrossRef]
- Kopecký, M.; Hédl, R.; Szabó, P. Non-random extinctions dominate plant community changes in abandoned coppices. J. Appl. Ecol. 2013, 50, 79–87. [Google Scholar] [CrossRef]
- Dieler, J.; Uhl, E.; Biber, P.; Müller, J.; Rötzer, T.; Pretzsch, H. Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zone of Europe. J. For. Res. 2017, 136, 739–766. [Google Scholar] [CrossRef]
- Hilmers, T.; Friess, N.; Bässler, C.; Heurich, M.; Brandl, R.; Pretzsch, H.; Seidl, R.; Müller, J. Biodiversity along temperate forest succession. J. Appl. Ecol. 2018, 5, 2756–2766. [Google Scholar] [CrossRef]
- Lelli, C.; Bruun, H.H.; Chiaruccia, A.; Donatia, D.; Frascarolia, F.; Fritz, O.; Goldberg, I.; Nascimbene, J.; Tøttrup, A.; Rahbek, C.; et al. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Durak, T.; Bugno-Pogoda, A.; Durak, R. Application of forest inventories to assess the forest developmental stages on plots dedicated to long-term vegetation studies. For. Ecol. Manag. 2021, 489, 119041. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S.; et al. The impact of even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. J. Appl. Ecol. 2018, 55, 267–278. [Google Scholar] [CrossRef]
- Decocq, G.; Aubert, M.; Dupont, F.; Alard, D.; Saguez, R.; Wattez-Franger, A.; de Foucault, B.; Delelis-Dusollier, A.; Bardat, J. Plant diversity in a managed temperate deciduous forest: Understorey response to two silvicultural systems. J. Appl. Ecol. 2004, 41, 1065–1079. [Google Scholar] [CrossRef]
- Ciancio, O.; Corona, P.; Lamonaca, A.; Portoghesi, L.; Travaglini, D. Conversion of clearcut beech coppices into high forests with continuous cover: A case study in central Italy. For. Ecol. Manag. 2006, 224, 235–240. [Google Scholar] [CrossRef]
- Puettmann, K.J.; Wilson, S.M.; Baker, S.C.; Donoso, P.J.; Drössler, L.; Amente, G.; Harvey, B.D.; Knoke, T.; Lu, Y.; Nocentini, S.; et al. Silvicultural alternatives to conventional even-aged forest management—what limits global adoption? For. Ecosyst. 2015, 2, 1–16. [Google Scholar] [CrossRef]
- O’Hara, K.L. What is close-to-nature silviculture in a changing world? Forestry 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Decocq, G.; Aubert, M.; Dupont, F.; Bardat, J.; Wattez-Franger, A.; Saguez, R.; de Foucault, B.; Alard, D.; Delelis-Dusollier, A. Silviculture-driven vegetation change in a European temperate deciduous forest. Ann. For. Sci. 2005, 62, 313–323. [Google Scholar] [CrossRef]
- Baeten, L.; Bauwens, B.; De Schrijver, A.; De Keersmaeker, L.; Van Calster, H.; Vandekerkhove, K.; Roelandt, B.; Beeckman, H.; Verheyen, K. Herb layer changes (1954–2000) related to the conversion of coppice-withstandards forest and soil acidification. Appl. Veg. Sci. 2009, 12, 187–197. [Google Scholar] [CrossRef]
- Müllerová, J.; Hédl, R.; Szabó, P. Coppice abandonment and its implications for species diversity in forest vegetation. For. Ecol. Manag. 2015, 343, 88–100. [Google Scholar] [CrossRef]
- Durak, T.; Holeksa, J. Biotic homogenisation and differentiation along a habitat gradient resulting from the ageing of manager beech stands. For. Ecol. Manag. 2015, 351, 47–56. [Google Scholar] [CrossRef]
- FOREST EUROPE 2020: State of Europe’s Forests 2020. Available online: https://foresteurope.org/wp-content/uploads/2016/08/SoEF_2020.pdf (accessed on 13 April 2021).
- Bakker, J.P.; Olff, H.; Willems, J.H.; Zobel, M. Why do we need permanent plots in the study of long-term vegetation dynamics? J. Veg. Sci. 1996, 7, 147–156. [Google Scholar] [CrossRef]
- De Lombaerde, E.; Verheyen, K.; Perring, M.P.; Bernhardt-Römermann, M.; Van Calster, H.; Brunet, J.; Chudomelová, M.; Decocq, G.; Diekmann, M.; Durak, T.; et al. Responses of competitive understorey species to spatial environmental gradients inaccurately explain temporal changes. Basic Appl. Ecol. 2018, 30, 52–64. [Google Scholar] [CrossRef]
- Van Calster, H.; Baeten, L.; De Schrijver, A.; De Keersmaeker, L.; Rogister, J.E.; Verheyen, K.; Hermy, M. Management driven changes (1967–2005) in soil acidity and the understorey plant community following conversion of a coppice-with-standards forest. For. Ecol. Manag. 2007, 241, 258–271. [Google Scholar] [CrossRef]
- Durak, T.; Durak, R. Vegetation changes in meso-and eutrophic submontane oak–hornbeam forests under long-term high forest management. For. Ecol. Manag. 2015, 354, 206–214. [Google Scholar] [CrossRef]
- Heinrichs, S.; Schmidt, W. Biotic homogenization of herb layer composition between two contrasting beech forest communities on limestone over 50 years. Appl. Veg. Sci. 2017, 20, 271–281. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjaltén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity differences between managed and unmanaged forests: Meta analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Burivalova, Z.; Koh, L.P.; Hellweg, S. Impact of forest management on species richness: Global meta-analysis and economic trade-offs. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Olden, J.D.; Rooney, T.P. On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 2006, 15, 113–120. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1970, 21, 213–251. [Google Scholar] [CrossRef]
- Loreau, M. Are communities saturated? On the relationship between α, β, and ϒ diversity. Ecol. Lett. 2000, 3, 73–76. [Google Scholar] [CrossRef]
- Melo, A.S.; Fernando, T.; Rancel, L.V.B.; Diniz-Filho, J.A.F. Environmental drivers of beta-diversity patterns in New-World birds and mammals. Ecography 2009, 32, 226–236. [Google Scholar] [CrossRef]
- Allouche, O.; Kalyuzhny, M.; Moreno-Rueda, G.; Pizarro, M.; Kadmon, R. Area-heterogeneity tradeoff and the diversity of ecological communities. Proc. Nat. Acad. Sci. USA 2012, 109, 17495–17500. [Google Scholar] [CrossRef]
- Dzwonko, Z. Forest communities of the Gory Słonne Range (Polish Eastern Carpathians). Fragm. Flor. Geobot. 1977, 23, 161–200. [Google Scholar]
- Skiba, S.; Drewnik, M. Soil map of the polish carpathian mountains. Rocz. Bieszcz. 2003, 11, 15–20. [Google Scholar]
- Meteorological Data. Averages and Monthly Totals. Available online: https://meteomodel.pl/dane/srednie-miesieczne/?imgwid=349220690&par=tm&max_empty=2 (accessed on 16 March 2021).
- Jaworski, A.; Kołodziej, Z. Beech (Fagus sylvatica L.) forest of a selection structure in the Bieszczady Mountains (southeastern Poland). J. For. Sci. 2004, 50, 301–312. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde; Springer-Verlag: Wien, Austria; New York, NY, USA, 1964. [Google Scholar]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Rooney, T.P.; Wiegmann, S.M.; Rogers, D.A.; Waller, D.M. Biotic impoverishment and homogenization in unfragmented forest understory communities. Conserv. Biol. 2004, 18, 787–798. [Google Scholar] [CrossRef]
- Durak, T.; Durak, R.; Węgrzyn, E.; Leniowski, K. The impact of changes in species richness and species replacement on patterns of taxonomic homogenization in the Carpathian forest ecosystems. Forests 2015, 6, 4391–4402. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L. Toward a mechanistic understanding and prediction of biotic homogenization. Am. Nat. 2003, 162, 442–460. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von pflanzen in Mitteleuropa. Scr. Geobot. 1992, 18, 1–248. [Google Scholar]
- Horn, H.S. Measurement of overlap in comparative ecological studies. Am. Nat. 1966, 100, 419–424. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski. [A Guide for the Identification of Polish Plant Communities]; Wydawnictwo Naukowe PWN: Warszawa, Polska, 2001. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Karp, D.S.; Rominger, A.J.; Zook, J.; Ranganathan, J.; Ehrlich, P.R.; Daily, G.C. Intensive agriculture erodes b-diversity at large scales. Ecol. Lett. 2012, 15, 963–970. [Google Scholar] [CrossRef] [PubMed]
| Regular Shelterwood System | Irregular Shelterwood Systems | |
|---|---|---|
| Rotation age | 80–110 years | 110–130 years |
| Regeneration period | 10–20 years | 30–50 years |
| Regeneration processes | System in which, in order to provide a source of seed and/or protection for regeneration, the mature stand is removed in two or more overstory removal cuttings. The first of which is an establishment cutting to establish the regeneration from the seeds. After 2–5 years, to provide the best conditions for the growth of a new generation of trees partial mature trees, removals are started. After 10–20 years, all the mature trees are removed by a final cut. | In dense stands, foresters choose irregularly distributed plots where, every 3–6 years, they cut a small group of trees, forming small gaps. This cycle is repeated within the previously formed gaps, where another small group of trees are cut, thus expanding the gaps in the stands. Process of expanding the gaps continues throughout the regeneration cycle. |
| Stand structure | even-aged | uneven-aged |
| Test Score | Mean (±SE) Values | |||
|---|---|---|---|---|
| F, x Chi2 | 1970s | 2000s | 2010s | |
| Cover of tree layer (%) | x6.7 * | 87.3 (1.02) a | 84.0 (1.16) a | 77.6 (2.60) b |
| Cover of shrub layer (%) | x40.0 *** | 5.6 (0.63) a | 8.8 (1.45) a | 27.2 (2.77) b |
| Average tree height (m) | x11.1 ** | 30.3 (0.47) a | 31.0 (0.55) a | 27.1 (0.73) b |
| Average DBH (cm) | 6.3 ** | 37.9 (1.43) a | 49.0 (3.51) b | 40.1 (2.35) a |
| Tree layer species richness (No. of species) | x27.5 *** | 2.9 (0.15) a | 2.3 (0.11) b | 1.8 (0.08) c |
| Shrub layer species richness (No. of species) | 3.5 * | 1.7 (0.11) a | 2.3 (0.17) ab | 2.3 (0.15) b |
| Age of stands (year) | 74.0 *** | 85.3 (2.45) a | 96.3 (2.50) b | 113.0 (2.49) c |
| Intensity of forest management (ranks) | 5.3 ** | 6.5 (0.31) a | 5.4 (0.28) b | 5.7 (0.25) ab |
| Test Score | Mean (±SE) Values | |||
|---|---|---|---|---|
| F, xChi2 | 1970s | 2000s | 2010s | |
| Frequency of species occurrence | x23.2 *** | 16.0 (±1.8) a | 17.4 (±1.9) a | 11.5 (±1.5) b |
| Species richness (No. of species) | 42.5 *** | 27.9 (±1.0) a | 30.2 (±1.0) a | 20.1 (±0.7) b |
| Total abundance of species (%) | x26.5 *** | 86.2 (±4.5) a | 98.1 (±3.8) a | 128.7 (±6.8) b |
| Number of species with high or low habitat requirements | ||||
| LL | 56.2 *** | 8.9 (±0.3) a | 10.1 (±0.3) b | 6.2 (±0.3) c |
| LH | 2.7 | 2.4 (±0.2) a | 2.8 (±0.2) a | 2.7 (±0.2) a |
| TL | x27.1 *** | 2.1 (±0.2) a | 2.4 (±0.2) a | 1.4 (±0.1) b |
| TH | 2.3 | 1.6 (±0.2) a | 1.9 (±0.2) a | 1.6 (±0.2) a |
| FH | x32.6 *** | 2.7 (±0.2) a | 3.8 (±0.2) b | 1.8 (±0.2) c |
| RL | x1.8 | 0.2 (±0.1) a | 0.4 (±0.1) a | 0.3 (±0.1) a |
| RH | 25.2 *** | 10.4 (± 0.5) a | 10.5 (±0.5) a | 7.0 (±0.4) b |
| NL | 0.6 | 0.4 (±0.1) a | 0.4 (±0.1) a | 0.3 (±0.1) a |
| NH | 19.5 *** | 9.7 (±0.5) a | 10.7 (±0.6) a | 7.0 (±0.4) b |
| ∆ Cover of Tree Layer (%) | ∆ Cover of Shrub Layer (%) | ∆ Average Tree Height (m) | ∆ Average DBH (cm) | ∆ Tree Layer Species Richness (No. of Species) | ∆ Shrub Layer Species Richness (No. of Species) | ∆ Age of Stands (Year) | |
|---|---|---|---|---|---|---|---|
| First study period | |||||||
| ∆αSha | 0.08 | −0.21 | 0.06 | −0.03 | 0.19 | 0.04 | 0.05 |
| ∆Evenness | 0.12 | −0.01 | −0.05 | −0.2 | 0.1 | −0.15 | −0.05 |
| ∆LL | 0.21 | −0.17 | 0.05 | −0.18 | 0.1 | 0.03 | −0.09 |
| ∆TL | −0.07 | −0.19 | 0.04 | 0.07 | 0.02 | −0.04 | 0.07 |
| ∆FH | −0.13 | −0.17 | 0.06 | 0.12 | 0.18 | 0.02 | 0.17 |
| ∆RH | −0.03 | −0.16 | 0.08 | 0.04 | 0.04 | 0.09 | 0,00 |
| ∆NH | −0.06 | −0.15 | 0.01 | 0,00 | 0.09 | 0.12 | −0.04 |
| ∆Species richness | 0.02 | −0.19 | 0.05 | −0.02 | 0.15 | 0.11 | 0.01 |
| ∆βSor | 0,00 | 0.15 | −0.18 | −0.06 | −0.14 | −0.08 | −0.06 |
| ∆βSim | −0.02 | 0.07 | 0,00 | 0,00 | −0.02 | 0.04 | 0.04 |
| ∆βnes | 0.04 | 0.03 | −0.14 | 0.06 | −0.08 | −0.07 | −0.09 |
| Second study period | |||||||
| ∆αSha | −0.05 | −0.26 | 0.18 | −0.06 | 0.03 | 0.05 | −0.07 |
| ∆Evenness | −0.03 | −0.07 | −0.01 | −0.17 | −0.12 | −0.26 | −0.2 |
| ∆LL | 0.2 | −0.3 | −0.08 | −0.23 | 0.07 | −0.09 | 0,00 |
| ∆TL | −0.12 | −0.05 | 0.19 | 0.03 | 0.03 | 0.06 | −0.07 |
| ∆FH | −0.09 | −0.3 | 0.08 | −0.08 | 0.14 | 0.13 | −0.18 |
| ∆RH | 0.04 | −0.35 | 0.09 | −0.06 | 0.18 | 0.12 | −0.03 |
| ∆NH | 0.07 | −0.4 | 0.07 | −0.1 | 0.15 | 0.14 | −0.04 |
| ∆Species richness | 0.02 | −0.37 | 0.18 | −0.11 | 0.17 | 0.08 | −0.07 |
| ∆βSor | −0.08 | 0.04 | −0.25 | 0.02 | −0.26 | −0.13 | 0.05 |
| ∆βSim | −0.3 | 0.01 | −0.08 | 0.21 | −0.24 | 0.12 | 0.11 |
| ∆βnes | 0.27 | 0.04 | 0.02 | −0.1 | 0.05 | −0.24 | −0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugno-Pogoda, A.; Durak, R.; Durak, T. Impact of Forest Management on the Temporal Dynamics of Herbaceous Plant Diversity in the Carpathian Beech Forests over 40 Years. Biology 2021, 10, 406. https://doi.org/10.3390/biology10050406
Bugno-Pogoda A, Durak R, Durak T. Impact of Forest Management on the Temporal Dynamics of Herbaceous Plant Diversity in the Carpathian Beech Forests over 40 Years. Biology. 2021; 10(5):406. https://doi.org/10.3390/biology10050406
Chicago/Turabian StyleBugno-Pogoda, Anna, Roma Durak, and Tomasz Durak. 2021. "Impact of Forest Management on the Temporal Dynamics of Herbaceous Plant Diversity in the Carpathian Beech Forests over 40 Years" Biology 10, no. 5: 406. https://doi.org/10.3390/biology10050406
APA StyleBugno-Pogoda, A., Durak, R., & Durak, T. (2021). Impact of Forest Management on the Temporal Dynamics of Herbaceous Plant Diversity in the Carpathian Beech Forests over 40 Years. Biology, 10(5), 406. https://doi.org/10.3390/biology10050406






