Prognostic and Functional Significant of Heat Shock Proteins (HSPs) in Breast Cancer Unveiled by Multi-Omics Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression Analysis of HSP Members Using UALCAN and Oncomine
2.2. Genetic Alterations and Epigenetic Regulation of HSP Members Using cBioPortal and UALCAN
2.3. Evaluation of the Prognostic Value of HSP Members Using KM Plotter Database and Human Protein Atlas
2.4. Relationship between HSP Members and Clinical-Pathological Parameters Using Bc-GenExMiner and GOBO
2.5. Analysis of HSPs Co-Expressed Genes and Pathways Enrichment Analysis
2.6. Interactome Construction Using STRING Database
2.7. HSP-Mediated Regulatory Network of miRNAs-mRNAs
2.8. Identification of HSP Proteins and Isoforms in Breast Cancer Tissues
3. Results
3.1. Gene Expression Analysis of HSP Family Members between Normal and Cancer Tissues
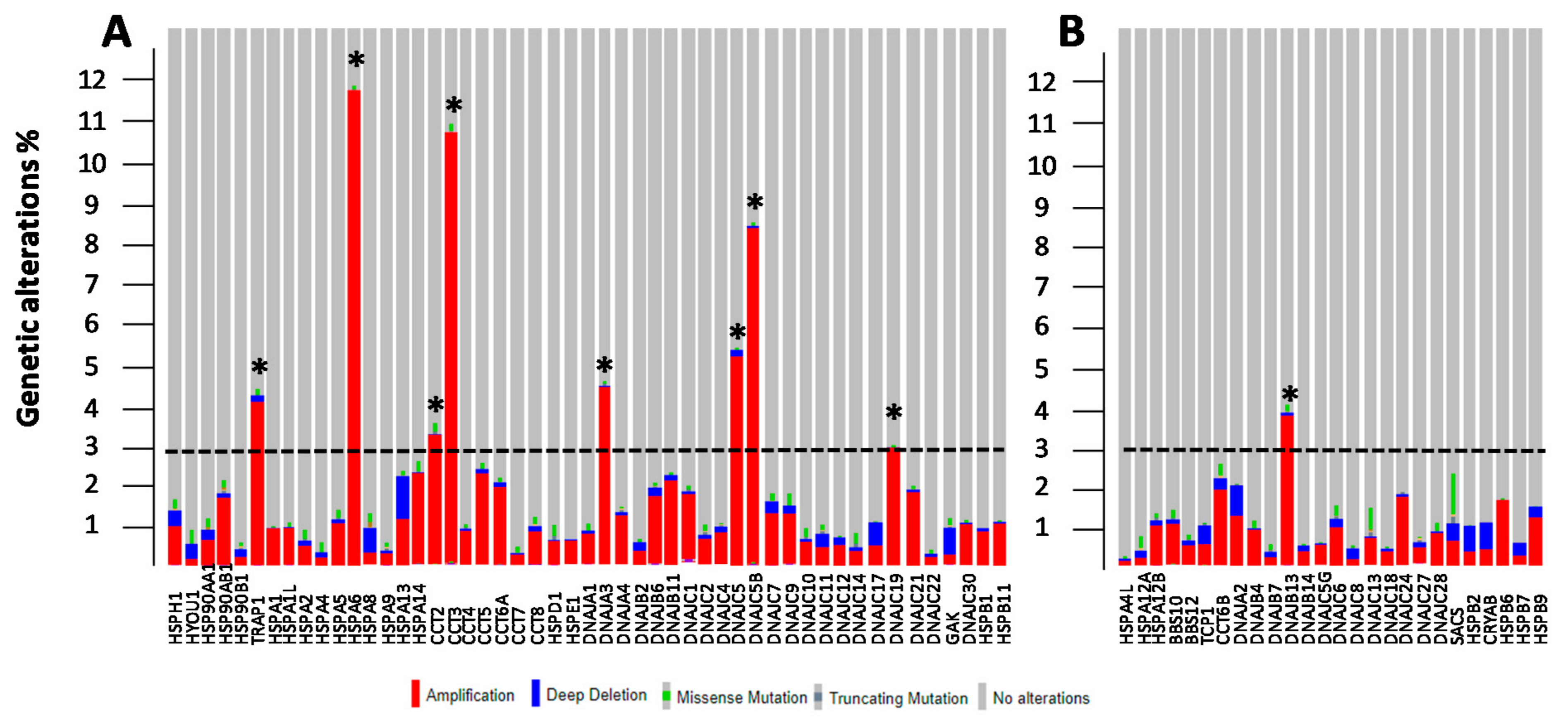
3.2. Genetic and Epigenetic Alterations of HSP Members
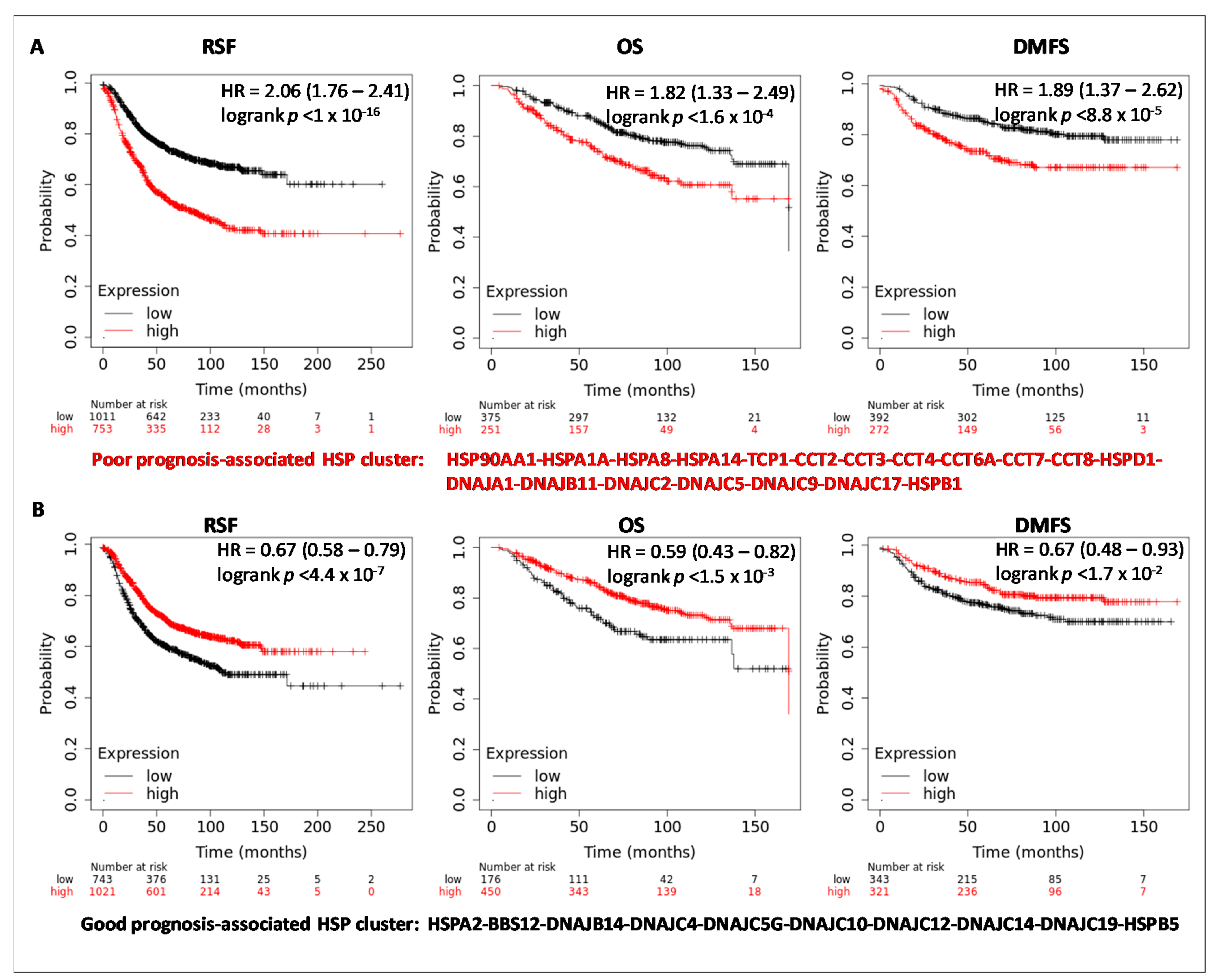
3.3. HSPs Expression and Clinical Outcome
3.4. Relationship between HSP Members and Clinical-Pathological Parameters
3.5. HSPs-Gene Co-Expression Networks
3.6. Regulatory Network Analysis of HSPs-miRNA-mRNA
3.7. Proteomics Expression of HSP Members and Prognostic Significance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Byler, S.; Goldgar, S.; Heerboth, S.; Leary, M.; Housman, G.; Moulton, K.; Sarkar, S. Genetic and epigenetic aspects of breast cancer progression and therapy. Anticancer Res. 2014, 34, 1071–1077. [Google Scholar]
- Testa, U.; Castelli, G.; Pelosi, E. Breast Cancer: A molecularly heterogenous disease needing subtype-specific treatments. Med. Sci. 2020, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivenbark, A.G.; O’Connor, S.M.; Coleman, W.B. Molecular and cellular heterogeneity in breast cancer: Challenges for personalized medicine. Am. J. Pathol. 2013, 183, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Gong, G.; Kwon, M.J.; Han, J.; Lee, H.J.; Lee, S.K.; Lee, J.E.; Lee, S.H.; Park, S.; Choi, J.S.; Cho, S.Y.; et al. A new molecular prognostic score for predicting the risk of distant metastasis in patients with HR+/HER2- early breast cancer. Sci. Rep. 2017, 7, 45554. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.W. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012, 21, 50–57. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.H.; Yeh, C.T. Functional compartmentalization of HSP60-survivin interaction between mitochondria and cytosol in cancer cells. Cells 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabani, M.; Martineau, C.N. Multiple hsp70 isoforms in the eukaryotic cytosol: Mere redundancy or functional specificity? Curr. Genom. 2008, 9, 338–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephanou, A.; Latchman, D.S. Transcriptional modulation of heat-shock protein gene expression. Biochem. Res. Int. 2011, 2011, 238601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef]
- Hoter, A.; Rizk, S.; Naim, H.Y. The multiple roles and therapeutic potential of molecular chaperones in prostate cancer. Cancers 2019, 11, 1194. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat shock proteins: Agents of cancer development and therapeutic targets in anti-cancer therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, D.; Grantham, J.; Zhu, X.O.; Wei Lin, J.; van Oosterum, M.; Taylor, R.; Edwards, M. The role of heat shock proteins in mammalian differentiation and development. Environ. Med. Annu. Rep. Res. Inst. Environ. Med. Nagoya Univ. 1999, 43, 79–87. [Google Scholar]
- Voellmy, R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit. Rev. Eukaryot. Gene Expr. 1994, 4, 357–401. [Google Scholar]
- Joly, A.L.; Wettstein, G.; Mignot, G.; Ghiringhelli, F.; Garrido, C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J. Innate Immun. 2010, 2, 238–247. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Lanczky, A.; Nagy, A.; Bottai, G.; Munkacsy, G.; Szabo, A.; Santarpia, L.; Gyorffy, B. miRpower: A web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat. 2016, 160, 439–446. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef] [Green Version]
- Jezequel, P.; Campone, M.; Gouraud, W.; Guerin-Charbonnel, C.; Leux, C.; Ricolleau, G.; Campion, L. bc-GenExMiner: An easy-to-use online platform for gene prognostic analyses in breast cancer. Breast Cancer Res. Treat. 2012, 131, 765–775. [Google Scholar] [CrossRef]
- Ringner, M.; Fredlund, E.; Hakkinen, J.; Borg, A.; Staaf, J. GOBO: Gene expression-based outcome for breast cancer online. PLoS ONE 2011, 6, e17911. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Chisanga, D.; Alessandro, R.; Ang, C.S.; Askenase, P.; Batagov, A.O.; Benito-Martin, A.; Camussi, G.; Clayton, A.; et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J. Extracell. Vesicles 2017, 6, 1321455. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Cancemi, P.; Buttacavoli, M.; Roz, E.; Feo, S. Expression of alpha-enolase (ENO1), myc promoter-binding protein-1 (MBP-1) and matrix metalloproteinases (MMP-2 and MMP-9) reflect the nature and aggressiveness of breast tumors. Int. J. Mol. Sci. 2019, 20, 3952. [Google Scholar] [CrossRef] [Green Version]
- Di Cara, G.; Marabeti, M.R.; Musso, R.; Riili, I.; Cancemi, P.; Pucci Minafra, I. New Insights into the occurrence of matrix metalloproteases -2 and -9 in a cohort of breast cancer patients and proteomic correlations. Cells 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancemi, P.; Buttacavoli, M.; Di Cara, G.; Albanese, N.N.; Bivona, S.; Pucci-Minafra, I.; Feo, S. A multiomics analysis of S100 protein family in breast cancer. Oncotarget 2018, 9, 29064–29081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci-Minafra, I.; Di Cara, G.; Musso, R.; Cancemi, P.; Albanese, N.N.; Roz, E.; Minafra, S. Retrospective proteomic screening of 100 breast cancer tissues. Proteomes 2017, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Cancemi, P.; Di Cara, G.; Albanese, N.N.; Costantini, F.; Marabeti, M.R.; Musso, R.; Riili, I.; Lupo, C.; Roz, E.; Pucci-Minafra, I. Differential occurrence of S100A7 in breast cancer tissues: A proteomic-based investigation. Proteom. Clin. Appl. 2012, 6, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Cancemi, P.; Di Cara, G.; Albanese, N.N.; Costantini, F.; Marabeti, M.R.; Musso, R.; Lupo, C.; Roz, E.; Pucci-Minafra, I. Large-scale proteomic identification of S100 proteins in breast cancer tissues. BMC Cancer 2010, 10, 476. [Google Scholar] [CrossRef] [Green Version]
- Buttacavoli, M.; Albanese, N.N.; Roz, E.; Pucci-Minafra, I.; Feo, S.; Cancemi, P. Proteomic profiling of colon cancer tissues: Discovery of new candidate biomarkers. Int. J. Mol. Sci. 2020, 21, 3096. [Google Scholar] [CrossRef]
- Coppola, A.; Cancemi, P.; Tomasello, L.; Guarnotta, V.; Pitrone, M.; Failla, V.; Cillino, S.; Feo, S.; Pizzolanti, G.; Giordano, C. Anti-inflammatory action of heterogeneous nuclear ribonucleoprotein A2/B1 in patients with autoimmune endocrine disorders. J. Clin. Med. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiCara, G.; Marengo, G.; Albanese, N.N.; Marabeti, M.R.; Musso, R.; Cancemi, P.; Pucci-Minafra, I. Proteomic profiling of Trastuzumab (Herceptin(R))-sensitive and -resistant SKBR-3 breast cancer cells. Anticancer Res. 2013, 33, 489–503. [Google Scholar]
- Musso, R.; Di Cara, G.; Albanese, N.N.; Marabeti, M.R.; Cancemi, P.; Martini, D.; Orsini, E.; Giordano, C.; Pucci-Minafra, I. Differential proteomic and phenotypic behaviour of papillary and anaplastic thyroid cell lines. J. Proteom. 2013, 90, 115–125. [Google Scholar] [CrossRef]
- Coppola, A.; Tomasello, L.; Pizzolanti, G.; Pucci-Minafra, I.; Albanese, N.; Di Cara, G.; Cancemi, P.; Pitrone, M.; Bommarito, A.; Carissimi, E.; et al. In vitro phenotypic, genomic and proteomic characterization of a cytokine-resistant murine beta-TC3 cell line. PLoS ONE 2012, 7, e32109. [Google Scholar] [CrossRef] [Green Version]
- Minafra, L.; Di Cara, G.; Albanese, N.N.; Cancemi, P. Proteomic differentiation pattern in the U937 cell line. Leuk. Res. 2011, 35, 226–236. [Google Scholar] [CrossRef]
- Cancemi, P.; Albanese, N.N.; DiCara, G.; Marabeti, M.R.; Costantini, F.; Minafra, S.; Pucci-Minafra, I. Multiple changes induced by fibroblasts on breast cancer cells. Connect. Tissue Res. 2010, 51, 88–104. [Google Scholar] [CrossRef] [Green Version]
- Pucci-Minafra, I.; Albanese, N.N.; Di Cara, G.; Minafra, L.; Marabeti, M.R.; Cancemi, P. Breast cancer cells exhibit selective modulation induced by different collagen substrates. Connect. Tissue Res. 2008, 49, 252–256. [Google Scholar] [CrossRef]
- Pucci-Minafra, I.; Cancemi, P.; Di Cara, G.; Minafra, L.; Feo, S.; Forlino, A.; Tira, M.E.; Tenni, R.; Martini, D.; Ruggeri, A.; et al. Decorin transfection induces proteomic and phenotypic modulation in breast cancer cells 8701-BC. Connect. Tissue Res. 2008, 49, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Pucci-Minafra, I.; Cancemi, P.; Albanese, N.N.; Di Cara, G.; Marabeti, M.R.; Marrazzo, A.; Minafra, S. New protein clustering of breast cancer tissue proteomics using actin content as a cellularity indicator. J. Proteome Res. 2008, 7, 1412–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, J.K.; Xiong, X.; Ren, X.; Yang, J.M.; Song, J. Heat shock proteins in cancer immunotherapy. J. Oncol. 2019, 2019, 3267207. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zhi, J.; Peng, X.; Zhong, X.; Xu, A. Clinical significance of HSP27 expression in colorectal cancer. Mol. Med. Rep. 2010, 3, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Fucarino, A.; Pitruzzella, A. Role of HSP60/HSP10 in Lung Cancer: Simple Biomarkers or Leading Actors? J. Oncol. 2020, 2020, 4701868. [Google Scholar] [CrossRef] [PubMed]
- Merendino, A.M.; Bucchieri, F.; Campanella, C.; Marciano, V.; Ribbene, A.; David, S.; Zummo, G.; Burgio, G.; Corona, D.F.; Conway de Macario, E.; et al. Hsp60 is actively secreted by human tumor cells. PLoS ONE 2010, 5, e9247. [Google Scholar] [CrossRef] [Green Version]
- Shan, N.; Zhou, W.; Zhang, S.; Zhang, Y. Identification of HSPA8 as a candidate biomarker for endometrial carcinoma by using iTRAQ-based proteomic analysis. Oncotargets Ther. 2016, 9, 2169–2179. [Google Scholar] [CrossRef] [Green Version]
- Seigneuric, R.; Mjahed, H.; Gobbo, J.; Joly, A.L.; Berthenet, K.; Shirley, S.; Garrido, C. Heat shock proteins as danger signals for cancer detection. Front. Oncol. 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoppino, F.C.M.; Guerrero-Gimenez, M.E.; Castro, G.N.; Ciocca, D.R. Comprehensive transcriptomic analysis of heat shock proteins in the molecular subtypes of human breast cancer. BMC Cancer 2018, 18, 700. [Google Scholar] [CrossRef] [Green Version]
- Klimczak, M.; Biecek, P.; Zylicz, A.; Zylicz, M. Heat shock proteins create a signature to predict the clinical outcome in breast cancer. Sci. Rep. 2019, 9, 7507. [Google Scholar] [CrossRef] [Green Version]
- Acun, T.; Senses, K.M. Downregulation of DNAJC10 (ERDJ5) is associated with poor survival in breast cancer. Breast Cancer 2020, 27, 483–489. [Google Scholar] [CrossRef]
- Zhang, Y.; Chou, S.D.; Murshid, A.; Prince, T.L.; Schreiner, S.; Stevenson, M.A.; Calderwood, S.K. The role of heat shock factors in stress-induced transcription. Methods Mol. Biol. 2011, 787, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, B.; Godek, K.M.; Compton, D. Aneuploidy. Curr. Biol. CB 2015, 25, R538–R542. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, N.; Storchova, Z. Causes and consequences of protein folding stress in aneuploid cells. Cell Cycle 2015, 14, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Tsai, H.J.; Gordon, M.R.; Li, R. Cellular stress associated with aneuploidy. Dev. Cell 2018, 44, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Showalter, A.E.; Martini, A.C.; Nierenberg, D.; Hosang, K.; Fahmi, N.A.; Gopalan, P.; Khaled, A.S.; Zhang, W.; Khaled, A.R. Investigating chaperonin-containing TCP-1 subunit 2 as an essential component of the chaperonin complex for tumorigenesis. Sci. Rep. 2020, 10, 798. [Google Scholar] [CrossRef]
- Lopez, T.; Dalton, K.; Frydman, J. The mechanism and function of group II chaperonins. J. Mol. Biol. 2015, 427, 2919–2930. [Google Scholar] [CrossRef] [Green Version]
- Roh, S.H.; Kasembeli, M.; Bakthavatsalam, D.; Chiu, W.; Tweardy, D.J. Contribution of the type II chaperonin, TRiC/CCT, to oncogenesis. Int. J. Mol. Sci. 2015, 16, 26706–26720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.; Shevde, L.A.; Samant, R.S. Multi-faceted role of HSP40 in cancer. Clin. Exp. Metastasis 2009, 26, 559–567. [Google Scholar] [CrossRef]
- Acun, T.; Doberstein, N.; Habermann, J.K.; Gemoll, T.; Thorns, C.; Oztas, E.; Ried, T. HLJ1 (DNAJB4) gene is a novel biomarker candidate in breast cancer. Omics J. Integr. Biol. 2017, 21, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal. Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieb, V.; Weigelt, K.; Scheinost, L.; Fischer, K.; Greither, T.; Marcou, M.; Theil, G.; Klocker, H.; Holzhausen, H.J.; Lai, X.; et al. Serum levels of miR-320 family members are associated with clinical parameters and diagnosis in prostate cancer patients. Oncotarget 2018, 9, 10402–10416. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Liu, X.; Li, M.; Zeng, Y.; Feng, Z.; Su, X.; Huang, Y.; Chen, M.; Yang, X. Potential tumor suppressing role of microRNA-545 in epithelial ovarian cancer. Oncol. Lett. 2018, 15, 6386–6392. [Google Scholar] [CrossRef] [Green Version]
- Surapaneni, S.K.; Bhat, Z.R.; Tikoo, K. MicroRNA-941 regulates the proliferation of breast cancer cells by altering histone H3 Ser 10 phosphorylation. Sci. Rep. 2020, 10, 17954. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Wang, F.; Chapin, W.; Huang, R.S. Identification of microRNAs as breast cancer prognosis markers through the cancer genome atlas. PLoS ONE 2016, 11, e0168284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokumaru, Y.; Asaoka, M.; Oshi, M.; Katsuta, E.; Yan, L.; Narayanan, S.; Sugito, N.; Matsuhashi, N.; Futamura, M.; Akao, Y.; et al. High expression of microRNA-143 is associated with favorable tumor immune microenvironment and better survival in estrogen receptor positive breast cancer. Int. J. Mol. Sci. 2020, 21, 3213. [Google Scholar] [CrossRef] [PubMed]
- Imam, J.S.; Buddavarapu, K.; Lee-Chang, J.S.; Ganapathy, S.; Camosy, C.; Chen, Y.; Rao, M.K. MicroRNA-185 suppresses tumor growth and progression by targeting the Six1 oncogene in human cancers. Oncogene 2010, 29, 4971–4979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahiri, A.; Leivonen, S.K.; Luders, T.; Steinfeld, I.; Ragle Aure, M.; Geisler, J.; Makela, R.; Nord, S.; Riis, M.L.; Yakhini, Z.; et al. Deregulation of cancer-related miRNAs is a common event in both benign and malignant human breast tumors. Carcinogenesis 2014, 35, 76–85. [Google Scholar] [CrossRef]
- Huang, S.; Chen, Y.; Wu, W.; Ouyang, N.; Chen, J.; Li, H.; Liu, X.; Su, F.; Lin, L.; Yao, Y. miR-150 promotes human breast cancer growth and malignant behavior by targeting the pro-apoptotic purinergic P2X7 receptor. PLoS ONE 2013, 8, e80707. [Google Scholar] [CrossRef]
- Cloutier, P.; Coulombe, B. Regulation of molecular chaperones through post-translational modifications: Decrypting the chaperone code. Biochim. Biophys. Acta 2013, 1829, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.K.; Kam, H.; Kim, K.Y.; Park, S.I.; Lee, Y.S. Targeting heat shock protein 27 in cancer: A druggable target for cancer treatment? Cancers 2019, 11, 1195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wong, L.L.; Koay, E.S. Phosphorylation of Ser78 of Hsp27 correlated with HER-2/neu status and lymph node positivity in breast cancer. Mol. Cancer 2007, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Burns, T.F. Targeting heat shock proteins in cancer: A promising therapeutic approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevtsov, M.; Balogi, Z.; Khachatryan, W.; Gao, H.; Vigh, L.; Multhoff, G. Membrane-associated heat shock proteins in oncology: From basic research to new theranostic targets. Cells 2020, 9, 1263. [Google Scholar] [CrossRef]
- Santos, T.G.; Martins, V.R.; Hajj, G.N.M. Unconventional secretion of heat shock proteins in cancer. Int. J. Mol. Sci. 2017, 18, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappa, F.; Farina, F.; Zummo, G.; David, S.; Campanella, C.; Carini, F.; Tomasello, G.; Damiani, P.; Cappello, F.; Macario-Conway, D.E.M.; et al. HSP-molecular chaperones in cancer biogenesis and tumor therapy: An overview. Anticancer Res. 2012, 32, 5139–5150. [Google Scholar] [PubMed]





| HSP Family | Gene Name | Chromosomic Localization | Affimetrix ID | Protein Name | Uniprot Access (Abbreviated Name) | Molecular Weigth (Da) |
|---|---|---|---|---|---|---|
| HSP110 (HSPH) | HSPH1 | 13q12.3 | 235573_at | Heat shock 105 kDa/110 kDa protein 1 | Q92598 (HS105) | 96,865 |
| HYOU1 | 11q23.3 | 200825_s_at | Hypoxia up-regulated protein 1 | Q9Y4L1 (HYOU1) | 111,335 | |
| HSP90 (HSPC) | HSP90AA1 | 14q32.31 | 214328_s_at | Heat shock protein HSP 90-alpha | P07900 (HS90A) | 84,660 |
| HSP90AB1 | 6Q21,1 | 200064_at | Heat shock protein HSP 90-beta | P08238 (HS90B) | 83,264 | |
| HSP90B1 | 12q23.3 | 200599_s_at | Endoplasmin | P14625 (ENPL) | 92,469 | |
| TRAP1 | 16P13.3 | 201391_at | Heat shock protein 75 kDa, mitochondrial | Q12931 (TRAP1) | 80,110 | |
| HSP70 (HSPA) | HSPA1A | 6p21.33 | 200799_at | Heat shock 70 kDa protein 1A | P0DMV8 (HS71A) | 70,052 |
| HSPA1B | 6p21.33 | 202581_at | Heat shock 70 kDa protein 1B | P0DMV9 (HS71B) | 70,052 | |
| HSPA1L | 6p21.33 | 210189_at | Heat shock 70 kDa protein 1-like | P34931 (HS71L) | 70,375 | |
| HSPA2 | 14q23.3 | 211538_s_at | Heat shock-related 70 kDa protein 2 | P54652 (HSP72) | 70,021 | |
| HSPA4 | 5q31.1 | 208815_x_at | Heat shock 70 kDa protein 4 | P34932 (HSP74) | 94,331 | |
| HSPA4L | 4q28 | 205543_at | Heat shock 70 kDa protein 4L | O95757 (HS74L) | 94,512 | |
| HSPA5 | 9q33.3 | 230031_at | Endoplasmic reticulum chaperone BiP | P11021 (BIP) | 72,333 | |
| HSPA6 | 1q23.3 | 117_at | Heat shock 70 kDa protein 6 | P17066 (HSP76) | 71,028 | |
| HSPA7 | 1q23.3 | - | Putative heat shock 70 kDa protein 7 | P48741 (HSP77) | 40,244 | |
| HSPA8 | 11q24.1 | 221891_x_at | Heat shock cognate 71 kDa protein | P11142 (HSP7C) | 70,898 | |
| HSPA9 | 5q31.1 | 200690_at | Stress-70 protein, mitochondrial | P38646 (GRP75) | 73,680 | |
| HSPA12A | 10q25.3 | 214434_at | Heat shock 70 kDa protein 12A | O43301 (HS12A) | 74,978 | |
| HSPA12B | 20p13 | 229172_at | Heat shock 70 kDa protein 12B | Q96MM6 (HS12B) | 75,688 | |
| HSPA13 | 21q11.2 | 202557_at | Heat shock 70 kDa protein 13 | P48723 (HSP13) | 51,927 | |
| HSPA14 | 10p13 | 226887_at | Heat shock 70 kDa protein 14 | Q0VDF9 (HSP7E) | 54,794 | |
| HSP60 (Chaperonins) | BBS10 | 12q21.2 | 219487_at | Bardet-Biedl syndrome 10 protein | Q8TAM1 (BBS10) | 80,838 |
| BBS12 | 4q27 | 229603_at | Bardet-Biedl syndrome 12 protein | Q6ZW61 (BBS12) | 79,085 | |
| TCP1 | 6q25.3 | 222010_at | T-complex protein 1 subunit alpha | P17987 (TCPA) | 60,344 | |
| CCT2 | 12q15 | 201947_s_at | T-complex protein 1 subunit beta | P78371 (TCPB) | 57,488 | |
| CCT3 | 1q23 | 200910_at | T-complex protein 1 subunit gamma | P49368 (TCPG) | 60,534 | |
| CCT4 | 2p15 | 200877_at | T-complex protein 1 subunit delta | P50991 (TCPD) | 57,924 | |
| CCT5 | 5p15.2 | 229068_at | T-complex protein 1 subunit epsilon | P48643 (TCPE) | 59,671 | |
| CCT6A | 7p11.2 | 201326_at | T-complex protein 1 subunit zeta | P40227 (TCPZ) | 58,024 | |
| CCT6B | 17q12 | 206587_at | T-complex protein 1 subunit zeta-2 | Q92526 (TCPW) | 57,821 | |
| CCT7 | 2p13.2 | 200812_at | T-complex protein 1 subunit eta | Q99832 (TCPH) | 59,367 | |
| CCT8 | 21q22.11 | 200873_s_at | T-complex protein 1 subunit theta | P50990 (TCPQ) | 59,621 | |
| HSPD1 | 2q33.1 | 200807_s_at | 60 kDa heat shock protein, mitochondrial | P10809 (CH60) | 61,055 | |
| HSPE1 | 2q33.1 | 205133_s_at | 10 kDa heat shock protein, mitochondrial | P61604 (CH10) | 10,932 | |
| MKKS | 20p12 | 218138_at | McKusick-Kaufman/Bardet-Biedl syndromes putative chaperonin | Q9NPJ1 (MKKS) | 62,342 | |
| HSP40 (DNAJ) | DNAJA1 | 9p21.1 | 200881_s_at | DnaJ homolog subfamily A member 1 | P31689 (DNJA1) | 44,868 |
| DNAJA2 | 16q12.1 | 226994_at | DnaJ homolog subfamily A member 2 | O60884 (DNJA2) | 45,746 | |
| DNAJA3 | 16p13.3 | 205963_s_at | DnaJ homolog subfamily A member 3, mitochondrial | Q96EY1 (DNJA3) | 52,489 | |
| DNAJA4 | 15q25.1 | 225061_at | DnaJ homolog subfamily A member 4 | Q8WW22 (DNJA4) | 44,798 | |
| DNAJB1 | 19p13.2 | 200666_s_at | DnaJ homolog subfamily B member 1 | P25685 (DNJB1) | 38,044 | |
| DNAJB2 | 2q35 | 202500_at | DnaJ homolog subfamily B member 2 | P25686 (DNJB2) | 35,580 | |
| DNAJB3 | 2q37.1 | - | DnaJ homolog subfamily B member 3 | Q8WWF6 (DNJB3) | 16,559 | |
| DNAJB4 | 1p31.1 | 203810_at | DnaJ homolog subfamily B member 4 | Q9UDY4 (DNJB4) | 37,807 | |
| DNAJB5 | 9p13.1 | 212817_at | DnaJ homolog subfamily B member 5 | O75953 (DNJB5) | 39,133 | |
| DNAJB6 | 7q36.3 | 209015_s_at | DnaJ homolog subfamily B member 6 | O75190 (DNJB6) | 36,087 | |
| DNAJB7 | 22q13.2 | 1552675_at | DnaJ homolog subfamily B member 7 | Q7Z6W7 (DNJB7) | 35,434 | |
| DNAJB8 | 3q21.3 | 237284_at | DnaJ homolog subfamily B member 8 | Q8NHS0 (DNJB8) | 25,686 | |
| DNAJB9 | 7q31.1 | 202843_at | DnaJ homolog subfamily B member 9 | Q9UBS3 (DNJB9) | 25,518 | |
| DNAJB11 | 3q27.3 | 223054_at | DnaJ homolog subfamily B member 11 | Q9UBS4 (DJB11) | 40,514 | |
| DNAJB12 | 10q22.1 | 202866_at | DnaJ homolog subfamily B member 12 | Q9NXW2 (DJB12) | 41,860 | |
| DNAJB13 | 11q13.4 | 230936_at | DnaJ homolog subfamily B member 13 | P59910 (DJB13) | 36,118 | |
| DNAJB14 | 4q23 | 226399_at | DnaJ homolog subfamily B member 14 | Q8TBM8 (DJB14) | 42,516 | |
| DNAJC1 | 10p12.31 | 218409_at | DnaJ homolog subfamily C member 1 | Q96KC8 (DNJC1) | 63,883 | |
| DNAJC2 | 7q22.1 | 213097_s_at | DnaJ homolog subfamily C member 2 | Q99543 (DNJC2) | 71,996 | |
| DNAJC3 | 13q32.1 | 225284_at | DnaJ homolog subfamily C member 3 | Q13217 (DNJC3) | 57,580 | |
| DNAJC4 | 11q13.1 | 228622_s_at | DnaJ homolog subfamily C member 4 | Q9NNZ3 (DNJC4) | 27,593 | |
| DNAJC5 | 20q13.33 | 224611_s_at | DnaJ homolog subfamily C member 5 | Q9H3Z4 (DNJC5) | 22,149 | |
| DNAJC5B | 8q13.1 | 232798_at | DnaJ homolog subfamily C member 5B | Q9UF47 (DNJ5B) | 22,496 | |
| DNAJC5G | 2p23.3 | 1552450_a_at | DnaJ homolog subfamily C member 5G | Q8N7S2 (DNJ5G) | 21,433 | |
| DNAJC6 | 1p31.3 | 204721_s_at | Putative tyrosine-protein phosphatase auxilin | O75061 (AUXI) | 99,997 | |
| DNAJC7 | 17q21.2 | 202416_at | DnaJ homolog subfamily C member 7 | Q99615 (DNJC7) | 56,441 | |
| DNAJC8 | 1p35.3 | 212490_at | DnaJ homolog subfamily C member 8 | O75937 (DNJC8) | 29,842 | |
| DNAJC9 | 10q22.2 | 213088_s_at | DnaJ homolog subfamily C member 9 | Q8WXX5 (DNJC9) | 29,910 | |
| DNAJC10 | 2q32.1 | 229588_at | DnaJ homolog subfamily C member 10 | Q8IXB1 (DJC10) | 91,080 | |
| DNAJC11 | 1p36.31 | 215792_s_at | DnaJ homolog subfamily C member 11 | Q9NVH1 (DJC11) | 63,278 | |
| DNAJC12 | 10q21.3 | 223722_at | DnaJ homolog subfamily C member 12 | Q9UKB3 (DJC12) | 23,415 | |
| DNAJC13 | 3q22.1 | 212467_at | DnaJ homolog subfamily C member 13 | O75165 (DJC13) | 254,415 | |
| DNAJC14 | 12q13.2 | 223420_at | DnaJ homolog subfamily C member 14 | Q6Y2X3 (DJC14) | 78,569 | |
| DNAJC15 | 13q14.11 | 227808_at | DnaJ homolog subfamily C member 15 | Q9Y5T4 (DJC15) | 16,383 | |
| DNAJC16 | 1p36.21 | 212911_at | DnaJ homolog subfamily C member 16 | Q9Y2G8 (DJC16) | 90,591 | |
| DNAJC17 | 15q15.1 | 219861_at | DnaJ homolog subfamily C member 17 | Q9NVM6 (DJC17) | 34,687 | |
| DNAJC18 | 5q31.2 | 238115_at | DnaJ homolog subfamily C member 18 | Q9H819 (DJC18) | 41,551 | |
| DNAJC19 | 3q26.33 | 225358_at | Mitochondrial import inner membrane translocase subunit TIM14 | Q96DA6 (TIM14) | 12,499 | |
| HSCB | 22q12.1 | 223647_x_at | Iron-sulfur cluster co-chaperone protein HscB | Q8IWL3 (HSC20) | 27,422 | |
| DNAJC21 | 5p13.1 | 230893_at | DnaJ homolog subfamily C member 21 | Q5F1R6 (DJC21) | 62,028 | |
| DNAJC22 | 12q13.12 | 220441_at | DnaJ homolog subfamily C member 22 | Q8N4W6 (DJC22) | 38,086 | |
| SEC63 | 6q21 | 229969_at | Translocation protein SEC63 homolog | Q9UGP8 (SEC63) | 87,997 | |
| DNAJC24 | 11p13 | 213853_at | DnaJ homolog subfamily C member 24 | Q6P3W2 (DJC24) | 17,139 | |
| DNAJC25 | 9q31.3 | 226859_at | DnaJ homolog subfamily C member 25 | Q9H1X3 (DJC25) | 42,404 | |
| GAK | 4p16.3 | 40225_at | Cyclin-G-associated kinase | O14976 (GAK) | 143,191 | |
| DNAJC27 | 2p23.3 | 227859_at | DnaJ homolog subfamily C member 27 | Q9NZQ0 (DJC27) | 30,855 | |
| DNAJC28 | 21q22.11 | 220372_at | DnaJ homolog subfamily C member 28 | Q9NX36 (DJC28) | 45,806 | |
| SACS | 13q12.12 | 213262_at | Sacsin | Q9NZJ4 (SACS) | 521,126 | |
| DNAJC30 | 7q11.23 | 223367_at | DnaJ homolog subfamily C member 30, mitochondrial | Q96LL9 (DJC30) | 25,961 | |
| HSP20 (HSPB) | HSPB1 | 7q11.23 | 201841_s_at | Heat shock protein beta-1 | P04792 (HSPB1) | 22,783 |
| HSPB2 | 11q23.1 | 205824_at | Heat shock protein beta-2 | Q16082 (HSPB2) | 20,233 | |
| HSPB3 | 5q11.2 | 206375_s_at | Heat shock protein beta-3 | Q12988 (HSPB3) | 16,966 | |
| CRYAA | 21q22.3 | 210199_at | Alpha-crystallin A chain | P02489 (CRYAA) | 19,909 | |
| CRYAB | 11q22.3 | 209283_at | Alpha-crystallin B chain | P02511 (CRYAB) | 20,159 | |
| HSPB6 | 19q13.12 | 226304_at | Heat shock protein beta-6 | O14558 (HSPB6) | 17,136 | |
| HSPB7 | 1p36.13 | 218934_s_at | Heat shock protein beta-7 | Q9UBY9 (HSPB7) | 18,611 | |
| HSPB8 | 12q24.23 | 221667_s_at | Heat shock protein beta-8 | Q9UJY1 (HSPB8) | 21,604 | |
| HSPB9 | 17q21.2 | 230510_at | Heat shock protein beta-9 | Q9BQS6 (HSPB9) | 17,486 | |
| OFD1 | 8q22.3 | 203569_s_at | Oral-facial-digital syndrome 1 protein | O75665 (OFD1) | 116,671 | |
| HSPB11 | 1p32 | 215691_x_at | Intraflagellar transport protein 25 homolog | Q9Y547 (IFT25) | 16,297 |
| List of HSPs Up-Regulated in BC | UALCAN Gene Expression between Normal and Cancer Tissues (p Value) | List of HSPs Up-Regulated in BC | UALCAN Gene Expression between Normal and Cancer Tissues (p Value) | List of HSPs Down-Regulated in BC | UALCAN Gene Expression between Normal and Cancer Tissues (p Value) |
|---|---|---|---|---|---|
| HSPH1 | 1.62 × 10−12 | HSPB1 | 1.62 × 10−12 | HSPA4L | 1.63 × 10−5 |
| HYOU1 | <1 × 10−12 | HSPB11 | 1.62 × 10−12 | HSPA12A | 2.24 × 10−12 |
| HSP90AA1 | 1.62 × 10−12 | DNAJA1 | <1 × 10−12 | HSPA12B | 1.62 × 10−12 |
| HSP90AB1 | 1.62 × 10−12 | DNAJA3 | <1 × 10−12 | BBS10 | 1.1 × 10−16 |
| HSP90B1 | 1.62 × 10−12 | DNAJA4 | 1.62 × 10−12 | BBS12 | 1.62 × 10−12 |
| TRAP1 | 1.62 × 10−12 | DNAJB2 | 1.7 × 10−12 | TCP1 | 3.19 × 10−9 |
| HSPA1A | 5.62 × 10−4 | DNAJB6 | 5.59 × 10−10 | CCT6B | 4.61 × 10−10 |
| HSPA1L | 8.43 × 10−5 | DNAJB11 | 1.62 × 10−12 | HSPB2 | <1 × 10−12 |
| HSPA2 | 1.69 × 10−12 | DNAJC1 | 1.11 × 10−16 | CRYAB | 1.62 × 10−12 |
| HSPA4 | <1 × 10−12 | DNAJC2 | 1.62 × 10−12 | HSPB6 | <1 × 10−12 |
| HSPA5 | 1.62 × 10−12 | DNAJC4 | 3.87 × 10−7 | HSPB7 | 3.76 × 10−11 |
| HSPA6 | 2.87 × 10−7 | DNAJC5 | <1 × 10−12 | HSPB9 | 3.89 × 10−3 |
| HSPA8 | 1.62 × 10−12 | DNAJC5B | 1.05 × 10−9 | DNAJA2 | 4.36 × 10−5 |
| HSPA9 | <1 × 10−12 | DNAJC7 | 1.62 × 10−12 | DNAJB4 | 1.62 × 10−12 |
| HSPA13 | 1.62 × 10−12 | DNAJC9 | <1 × 10−12 | DNAJB7 | 2.01 × 10−5 |
| HSPA14 | <1 × 10−12 | DNAJC10 | 1.11 × 10−16 | DNAJB13 | 2.13 × 10−6 |
| CCT2 | 1.62 × 10−12 | DNAJC11 | 2.97 × 10−9 | DNAJB14 | 1.62 × 10−3 |
| CCT3 | <1 × 10−12 | DNAJC12 | 1.62 × 10−12 | DNAJC5G | 1.21 × 10−4 |
| CCT4 | 1.98 × 10−4 | DNAJC14 | 1.62 × 10−12 | DNAJC6 | 1.64 × 10−12 |
| CCT5 | <1 × 10−12 | DNAJC17 | 8.1 × 10−5 | DNAJC8 | 3.4 × 10−8 |
| CCT6A | 1.11 × 10−16 | DNAJC19 | <1 × 10−12 | DNAJC13 | 3.5 × 10−3 |
| CCT7 | <1 × 10−12 | DNAJC21 | 1.64 × 10−13 | DNAJC18 | <1 × 10−12 |
| CCT8 | 1.62 × 10−12 | DNAJC22 | 1.62 × 10−12 | DNAJC24 | 7.52 × 10−5 |
| HSPD1 | 1.62 × 10−12 | GAK | 1.62 × 10−12 | DNAJC27 | 1.62 × 10−12 |
| HSPE1 | <1 × 10−12 | DNAJC30 | 1.27 × 10−7 | DNAJC28 | 8.74 × 10−12 |
| SACS | <1 × 10−12 |
| Gene | RFS | OS | DMFS | |||
|---|---|---|---|---|---|---|
| Used Cut-Off | Logrank P | Used Cut-Off | Logrank P | Used Cut-Off | Logrank P | |
| HSP90AA1 | 20,364 | <1 × 10−16 | 23,504 | 5.7 × 10−7 | 20,054 | 3.8 × 10−4 |
| HSPA1A | 9077 | 9.4 × 10−5 | 13,346 | 2.5 × 10−3 | 9089 | 8 × 10−4 |
| HSPA1B | 2046 | 8.3 × 10−4 | 1254 | 9.2 × 10−4 | 1829 | 6.8 × 10−3 |
| HSPA8 | 14,679 | <1 × 10−16 | 14,679 | 5.5 × 10−3 | 14,018 | 1.7 × 10−2 |
| HSPA14 | 490 | 1.7 × 10−5 | 477 | 1.7 × 10−4 | 490 | 2.2 × 10−3 |
| TCP1 | 601 | 7.4 × 10−11 | 676 | 1.5 × 10−7 | 562 | 1.2 × 10−4 |
| CCT2 | 5366 | <1 × 10−16 | 5370 | 2.7 × 10−8 | 4520 | 2 × 10−7 |
| CCT3 | 4554 | 2.9 × 10−15 | 4619 | 1.2 × 10−2 | 4735 | 1.9 × 10−3 |
| CCT4 | 5934 | 1.10 × 10−11 | 5587 | 2.9 × 10−2 | 6778 | 3.32 × 10−2 |
| CCT6A | 1676 | <1 × 10−16 | 1667 | 2 × 10−8 | 1678 | 7.6 × 10−6 |
| CCT7 | 2498 | <1 × 10−16 | 2394 | 5 × 10−4 | 2466 | 1.4 × 10−3 |
| CCT8 | 5676 | <1 × 10−16 | 4599 | 5.5 × 10−5 | 3613 | 1.1 × 10−4 |
| HSPD1 | 11,874 | <1 × 10−16 | 11,640 | 7.7 × 10−7 | 10,253 | 5.6 × 10−6 |
| DNAJA1 | 5070 | 2 × 10−14 | 4099 | 2.7 × 10−4 | 4939 | 1 × 10−4 |
| DNAJB1 | 2284 | 8.7 × 10−4 | 2312 | 4 × 10−2 | 2369 | 1.1 × 10−2 |
| DNAJB11 | 1771 | 1.3 × 10−9 | 2300 | 4.20 × 10−2 | 1910 | 2.5 × 10−4 |
| DNAJC2 | 1153 | 2.5 × 10−16 | 781 | 1.7 × 10−3 | 1049 | 9.1 × 10−4 |
| DNAJC5 | 768 | 3.9 × 10−2 | 748 | 1.8 × 10−3 | 591 | 4.8 × 10−2 |
| DNAJC9 | 1494 | <1 × 10−16 | 1296 | 1.5 × 10−7 | 1328 | 1.3 × 10−6 |
| DNAJC17 | 128 | 1.6 × 10−2 | 128 | 2 × 10−2 | 163 | 3.6 × 10−3 |
| HSPB1 | 9981 | 2.4 × 10−12 | 5940 | 3.5 × 10−4 | 7584 | 1.3 × 10−7 |
| HSPA2 | 536 | 2.2 × 10−10 | 624 | 5.8 × 10−5 | 563 | 1.7 × 10−6 |
| BBS12 | 186 | 1 × 10−10 | 185 | 3.8 × 10−4 | 187 | 4.7 × 10−3 |
| DNAJB8 | 26 | 4 × 10−5 | 23 | 3.7 × 10−2 | 24 | 7.4 × 10−3 |
| DNAJB12 | 644 | 6.4 × 10−10 | 644 | 1.5 × 10−2 | 879 | 2.5 × 10−2 |
| DNAJB14 | 757 | 5.5 × 10−8 | 1147 | 1.2 × 10−4 | 1164 | 2.7 × 10−2 |
| DNAJC4 | 487 | 3.3 × 10−10 | 489 | 9 × 10−3 | 445 | 2.9 × 10−3 |
| DNAJC5G | 54 | 1.3 × 10−8 | 27 | 2.3 × 10−3 | 54 | 1.5 × 10−2 |
| DNAJC10 | 171 | 5.3 × 10−12 | 202 | 9.6 × 10−3 | 187 | 4.3 × 10−2 |
| DNAJC12 | 92 | 1.4 × 10−7 | 127 | 5.3 × 10−4 | 92 | 1.8 × 10−5 |
| DNAJC14 | 433 | 3.8 × 10−10 | 393 | 5.2 × 10−3 | 529 | 1.5 × 10−2 |
| DNAJC16 | 402 | 8.3 × 10−11 | 361 | 5.8 × 10−9 | 402 | 4.7 × 10−8 |
| DNAJC19 | 562 | 1.4 × 10−10 | 578 | 1.6 × 10−7 | 578 | 3.1 × 10−7 |
| CRYAB | 575 | 1.7 × 10−2 | 522 | 3.8 × 10−3 | 848 | 3.2 × 10−2 |
| Gene Name | ER+/PR+ | ER-/PR- | HER+ | HER- | N+ | N- | G1 | G2 | G3 |
|---|---|---|---|---|---|---|---|---|---|
| HSPA1A | <0.0001 *** | - | - | 0.0315 * | - | - | - | ||
| HSPA8 | <0.0001 *** | 0.0022 ** | 0.0002 *** | - | - | - | |||
| HSPA14 | <0.0001 *** | <0.0001 *** | 0.0134 ** | <0.0001 *** | |||||
| HSPB1 | <0.0001 *** | - | - | <0.0001 *** | <0.0001 *** | ||||
| HSP90AA1 | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | |||||
| TCP1 | <0.0001 *** | <0.0001 *** | 0.0175 ** | <0.0001 *** | |||||
| CCT2 | - | - | 0.0066 ** | 0.0003 *** | <0.0001 *** | ||||
| CCT3 | <0.0001 *** | 0.0001 *** | 0.0025 ** | <0.0001 *** | |||||
| CCT4 | <0.0001 *** | - | - | - | - | <0.0001 *** | |||
| CCT6A | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | |||||
| CCT7 | <0.0001 *** | - | - | <0.0001 *** | <0.0001 *** | ||||
| CCT8 | <0.0001 *** | - | - | - | - | <0.0001 *** | |||
| HSPD1 | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | |||||
| DNAJA1 | <0.0001 *** | 0.0091 ** | <0.0001 *** | <0.0001 *** | |||||
| DNAJB11 | <0.0001 *** | <0.0001 *** | - | - | - | - | - | ||
| DNAJC2 | <0.0001 *** | - | - | 0.0002 *** | <0.0001 *** | ||||
| DNAJC5 | <0.0001 *** | <0.0001 *** | 0.0050 ** | - | - | - | |||
| DNAJC9 | <0.0001 *** | 0.0042 ** | 0.0213 * | <0.0001 *** | |||||
| DNAJC17 | <0.0001 *** | - | - | - | - | <0.0001 *** | |||
| HSPA2 | <0.0001 *** | <0.0001 *** | - | - | <0.0001 *** | ||||
| BBS12 | <0.0001 *** | <0.0001 *** | 0.0404 * | - | - | - | |||
| DNAJB14 | <0.0001 *** | - | - | - | - | <0.0001 *** | |||
| DNAJC4 | 0.01 * | 0.0057 ** | <0.0001 *** | <0.0001 *** | |||||
| DNAJC5G | - | - | - | - | - | - | - | - | - |
| DNAJC10 | - | - | <0.0001 *** | - | - | 0.00011 *** | |||
| DNAJC12 | <0.0001 *** | <0.0001 *** | - | - | <0.0001 *** | ||||
| DNAJC14 | <0.0001 *** | 0.0003 *** | - | - | - | - | - | ||
| DNAJC19 | <0.0001 *** | <0.0001 *** | - | - | - | - | - | ||
| CRYAB | <0.0001 *** | <0.0001 *** | - | - | - | - | - |
| HSPs Co-Expressed miRNAs Up-Regulated in Poor Prognosis | HSPs Co-Expressed miRNAs Up-Regulated in Good Prognosis | ||
|---|---|---|---|
| hsa-mir-320b-2 | 7.13 × 10−7 | hsa-mir-23a | - |
| hsa-mir-545 | 4.38 × 10−12 | hsa-mir-143 | 1.62 × 10−12 |
| hsa-mir-941-2 | - | hsa-mir-185 | 1.42 × 10−4 |
| hsa-mir-651 | <1 × 10−12 | hsa-mir-196b | 5.07 × 10−2 |
| hsa-mir-383 | 5.48 × 10−7 | hsa-mir-145 | <1 × 10−12 |
| hsa-mir-1914 | - | hsa-mir-150 | 4.4 × 10−11 |
| hsa-mir-570 | 5.56 × 10−5 | hsa-mir-1228 | 3.04 × 10−2 |
| hsa-mir-647 | - | hsa-mir-1910 | 4.10 × 10−5 |
| hsa-mir-1227 | - | hsa-mir-450a-1 | - |
| hsa-mir-593 | - | hsa-mir-548i-1 | - |
| hsa-mir-300 | - | hsa-mir-1247 | 1.24 × 10−14 |
| hsa-mir-643 | 9.17 × 10−11 | hsa-mir-411 | 2.00 × 10−5 |
| hsa-mir-626 | - | hsa-mir-1299 | - |
| hsa-mir-1289-1 | - | hsa-mir-370 | 4.12 × 10−5 |
| hsa-mir-544a | - | ||
| HSPs-miRNAs network associated with poor prognosis | hsa-mir-1200 | - | |
| hsa-mir-3654 | 8.15 × 10−9 | ||
| hsa-mir-16-5p | - | hsa-mir-770 | 6.09 × 10−05 |
| hsa-mir-23b-3p | - | hsa-mir-555 | - |
| hsa-mir-15a-5p | 3.88 × 10−15 | hsa-mir-604 | - |
| hsa-mir-34a-5p | - | ||
| hsa-mir-1-3p | - | HSPs-miRNAs network associated with good prognosis | |
| hsa-mir-103a-3p | <1 × 10−12 | ||
| hsa-mir-148a-3p | 6.5 × 10−13 | hsa-mir-16-5p | - |
| hsa-mir-424-5p | - | hsa-mir-155-5p | 1.62 × 10−12 |
| hsa-mir-107 | 2.4 × 10−11 | hsa-mir-146a-5p | 5.77 × 10−05 |
| hsa-mir-155-5p | 1.62 × 10−12 | hsa-mir-124-3p | - |
| hsa-mir-497-5p | <1 × 10−12 | hsa-mir-1-3p | - |
| hsa-mir-181a-5p | 8.31 × 10−03 | hsa-mir-182-5p | 1.62 × 10−12 |
| hsa-mir-21-3p | <1 × 10−12 | hsa-mir-191-5p | <1 × 10−12 |
| hsa-mir-15b-3p | 6.96 × 10−10 | hsa-mir-17-5p | <1 × 10−12 |
| hsa-mir-186-5p | 0.00446 | hsa-mir-23b-3p | - |
| hsa-mir-195-5p | <1 × 10−12 | hsa-mir-34a-5p | - |
| hsa-mir-141-5p | 1.62 × 10−12 | hsa-mir-15a-5p | 3.88 × 10−15 |
| hsa-mir-29a-3p | 1.96 × 10−12 | hsa-mir-20a-5p | 1.1 × 10−11 |
| hsa-mir-197-3p | - | hsa-mir-195-5p | <1 × 10−12 |
| hsa-mir-302c-3p | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buttacavoli, M.; Di Cara, G.; D’Amico, C.; Geraci, F.; Pucci-Minafra, I.; Feo, S.; Cancemi, P. Prognostic and Functional Significant of Heat Shock Proteins (HSPs) in Breast Cancer Unveiled by Multi-Omics Approaches. Biology 2021, 10, 247. https://doi.org/10.3390/biology10030247
Buttacavoli M, Di Cara G, D’Amico C, Geraci F, Pucci-Minafra I, Feo S, Cancemi P. Prognostic and Functional Significant of Heat Shock Proteins (HSPs) in Breast Cancer Unveiled by Multi-Omics Approaches. Biology. 2021; 10(3):247. https://doi.org/10.3390/biology10030247
Chicago/Turabian StyleButtacavoli, Miriam, Gianluca Di Cara, Cesare D’Amico, Fabiana Geraci, Ida Pucci-Minafra, Salvatore Feo, and Patrizia Cancemi. 2021. "Prognostic and Functional Significant of Heat Shock Proteins (HSPs) in Breast Cancer Unveiled by Multi-Omics Approaches" Biology 10, no. 3: 247. https://doi.org/10.3390/biology10030247
APA StyleButtacavoli, M., Di Cara, G., D’Amico, C., Geraci, F., Pucci-Minafra, I., Feo, S., & Cancemi, P. (2021). Prognostic and Functional Significant of Heat Shock Proteins (HSPs) in Breast Cancer Unveiled by Multi-Omics Approaches. Biology, 10(3), 247. https://doi.org/10.3390/biology10030247