Changes Occurring on the Activity of Salivary Alpha-Amylase Proteoforms in Two Naturalistic Situations Using a Spectrophotometric Assay
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Measurement of sAA in the sample using a colorimetric commercial kit (Alpha-Amylase, Beckman Coulter Inc., Fullerton, CA, USA) following the International Medicine (IFCC) method [19] that was previously validated for TsAA [20]. It uses 2-chloro-4-nitrophenyl-α-D-maltotrioside (CNPG3) as an enzyme substrate that directly reacts with sAA, producing 2-chloro-4-nitrophenol (CNP). The resulting absorbance increase per minute is directly related to sAA activity in the sample. This gave the value of TsAA.
- Depletion of the sample by the lectin ConA, which provided a fast glycoprotein depletion of the sample. For this step, a fixed volume of sample was mixed in a 1:16 ratio with ConA (Cocanavalin A-Sepharose® 4B, Sigma-Aldrich, St. Louis, MO, USA) and incubated at 4 °C for 15 min. Then, the supernatant was removed and analyzed for sAA activity corresponding to the non-glycosylated proteoform.
- The difference between the sAA measured in step 1 (TsAA activity) and the sAA measured in step 2 (NGsAA activity) was considered as GsAA activity. In all cases, sAA activity was expressed as IU/mL [21].
- Imprecision. Three saliva samples with different TsAA activity (A = 400.62 IU/mL; B = 183.58 IU/mL; C = 171.18 IU/mL) underwent the procedure three times. The within-run coefficient of variation (CV) was then calculated as the percentage of the standard deviation (SD) of the replicates, divided by the mean.
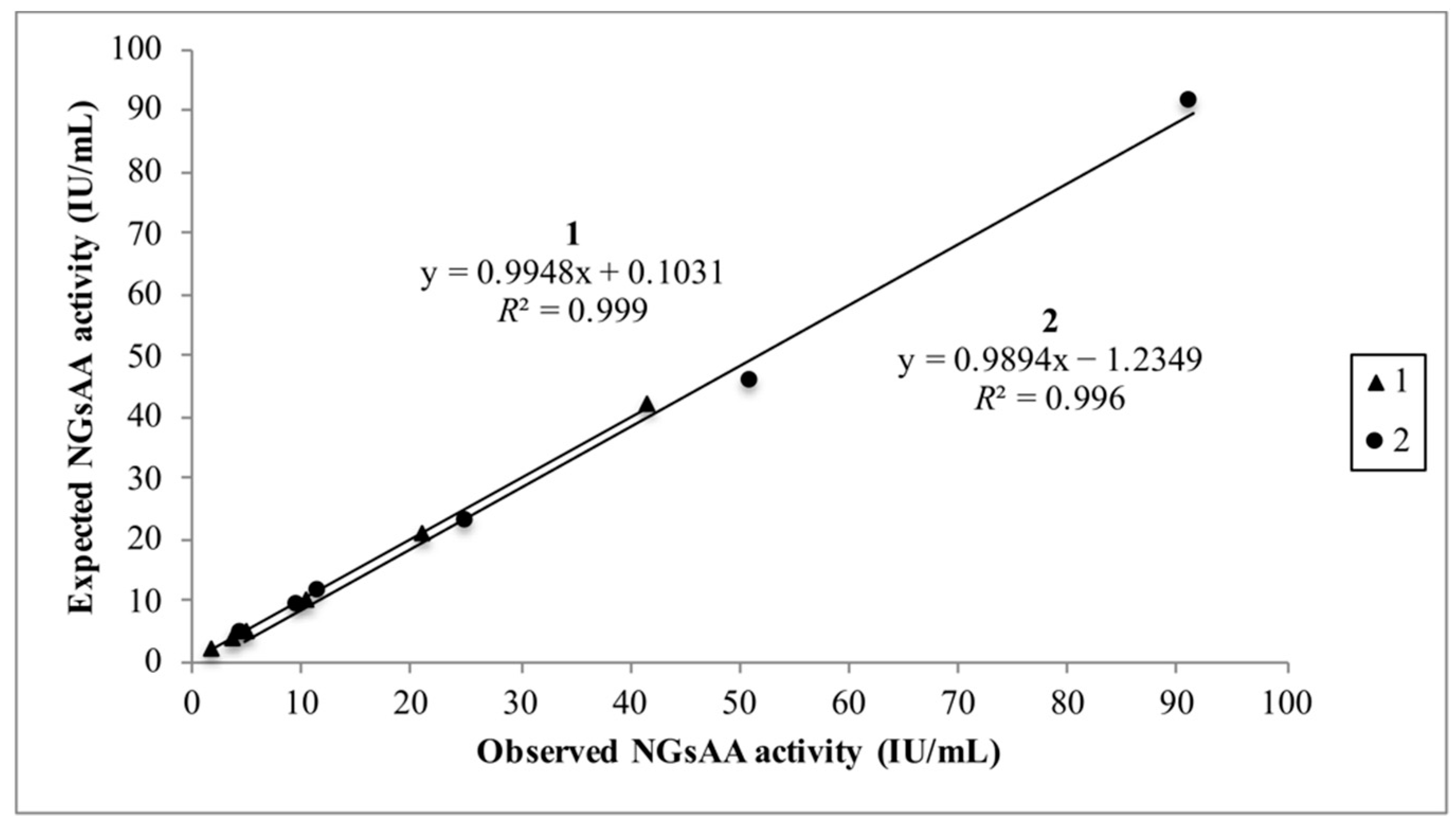
- Accuracy. Two samples with different NGsAA activities were serially diluted (1/2, 1/4, 1/8, 1/10, 1/20) with deionized water. Then, linear regression between the observed and the expected results was performed, and the slope, y-intercept, and R2 were calculated.
3. Results
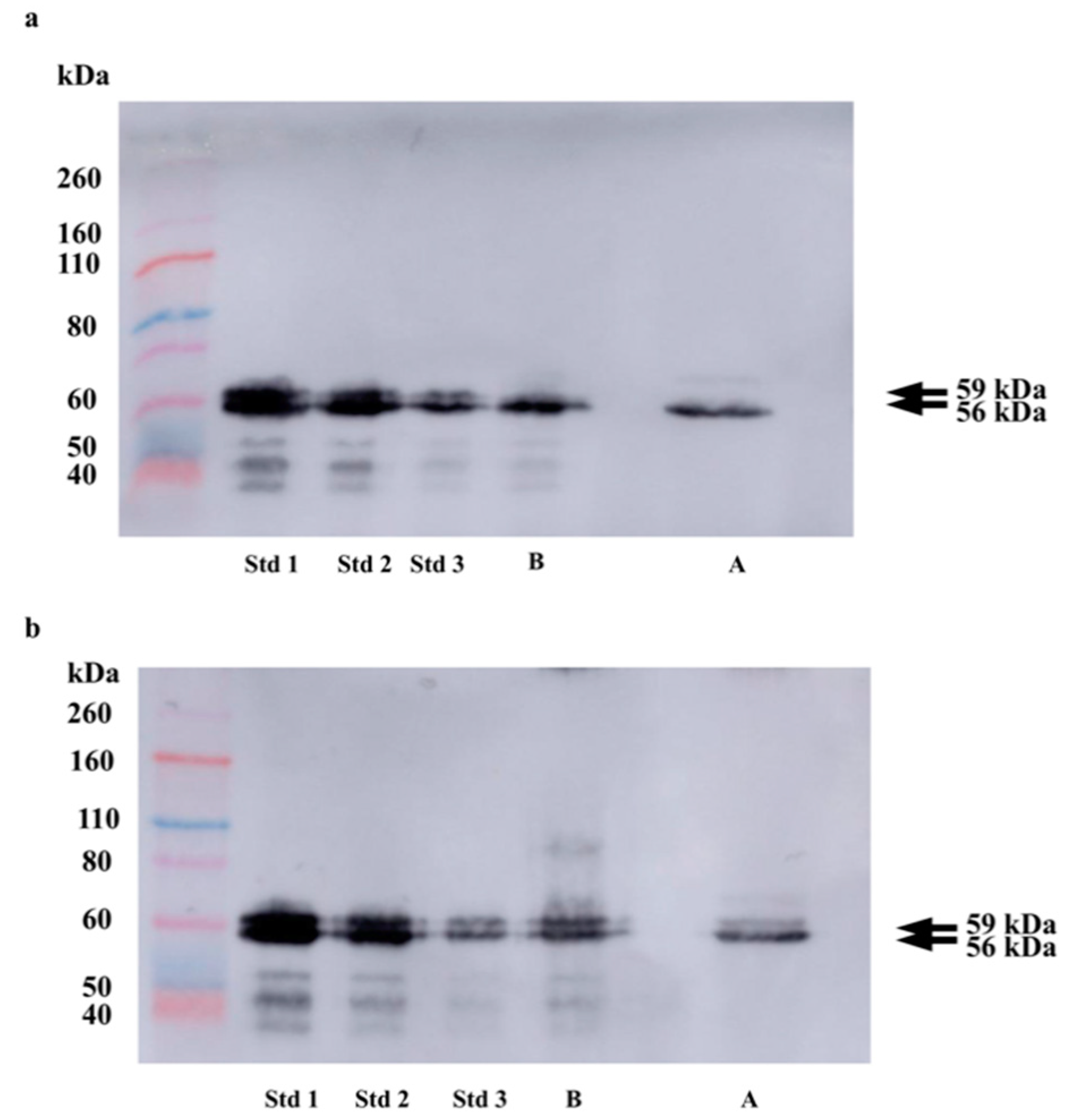
3.1. Validation of the Glycoprotein Depletion Procedure
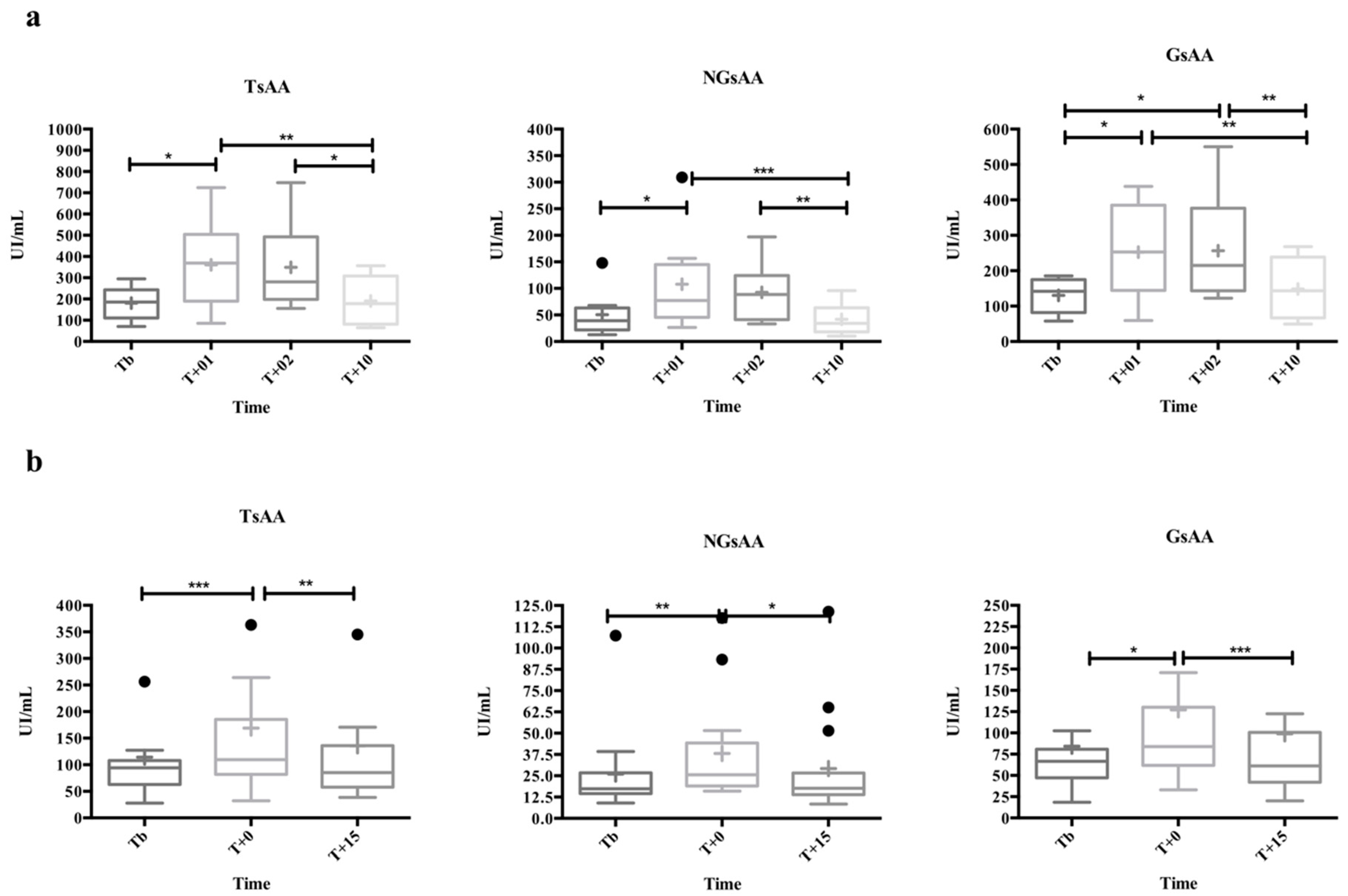
3.2. Activity Results in the Stress Models
3.2.1. Physical Effort
3.2.2. Psychological Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Y.; Chen, X.; Sato, T.; Rankin, S.A.; Tsuji, R.F.; Ge, Y. Purification and High-Resolution Top-Down Mass Spectrometric Characterization of Human Salivary alpha-Amylase. Anal. Chem. 2012, 84, 3339–3346. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef]
- Strahler, J.; Skoluda, N.; Kappert, M.B.; Nater, U.M. Simultaneous measurement of salivary cortisol and alpha-amylase: Application and recommendations. Neurosci. Biobehav. Rev. 2017, 83, 657–677. [Google Scholar] [CrossRef]
- Rohleder, N.; Nater, U.M. Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology 2009, 34, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Esler, M. How to assess sympathetic activity in humans. J. Hypertens. 1999, 17, 719–734. [Google Scholar] [CrossRef]
- Merritt, A.D.; Lovrien, E.W.; Rivas, M.L.; Conneally, P.M. Human amylase loci. Genetic linkage with the Duffy blood group locus and assignment to linkage group I. Am. J. Hum. Genet. 1973, 25, 523–538. [Google Scholar] [PubMed]
- Hirtz, C.; Chevalier, F.; Centeno, D.; Rofidal, V.; Egea, J.C.; Rossignol, M.; Sommerer, N.; De Périère, D.D. MS characterization of multiple forms of alpha-amylase in human saliva. Proteomics 2005, 5, 4597–4607. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Vialaret, J.; de Périère, D.D.; Escribano, D.; Lehmann, S.; Tecles, F.; Cerón, J.J.; Hirtz, C. Variation of human salivary alpha-amylase proteoforms in three stimulation models. Clin. Oral Investig. 2019, 24, 475–486. [Google Scholar] [CrossRef]
- Koyama, I.; Komine, S.; Yakushijin, M.; Hokari, S. Glycosylated salivary a-amylases are capable of maltotriose hydrolysis and glucose formation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 126, 553–560. [Google Scholar] [CrossRef]
- Timón, R.; Olcina, G.; Camacho-Cardeñosa, M.; Camacho-Cardenosa, A.; Martinez-Guardado, I.; Marcos-Serrano, M. 48-h recovery of biochemical parameters and physical performance after two modalities of CrossFit workouts. Biol. Sport 2019, 36, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Falgenbaum, A.; McFarland, J.; Herman, R.; Naclerlo, F.; Ratamess, N.; Kang, J.; Myer, G. Reliability of the One Repetition-Maximum Power Clean Test in Adolescent Athletes. J. Strength Cond. Res. 2012, 26, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Petrakova, L.; Doering, B.K.; Vits, S.; Engler, H.; Rief, W.; Schedlowski, M.; Grigoleit, J.S. Psychosocial stress increases salivary alpha-Amylase activity independently from plasma noradrenaline levels. PLoS ONE 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Guillén Riquelme, A. Validación de la adaptación española del State-Trait Anxiety Inventory en diferentes muestras españolas. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2014. [Google Scholar]
- Bieling, P.J.; Antony, M.M.; Swinson, R.P. The state-trait anxiety inventory, trait version: Structure and content re-examined. Behav. Res. Ther. 1998, 36, 777–788. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kammerer, M.; O’Reilly, R.; Taylor, A.; Glover, V. Salivary alpha-amylase stability, diurnal profile and lack of response to the cold hand test in young women. Stress. Int. J. Biol. Stress 2009, 12, 549–554. [Google Scholar] [CrossRef] [PubMed]
- DeCaro, J.A. Methodological Considerations in the Use of Salivary α-Amylase as a Stress Marker in Field Research. Am. J. Hum. Biol. 2008, 20, 617–619. [Google Scholar] [CrossRef]
- Barranco, T.; Rubio, C.P.; Tvarijonaviciute, A.; Rubio, M.; Damia, E.; Lamy, E.; Cugat, R.; Cerón, J.J.; Tecles, F.; Escribano, D. Changes of salivary biomarkers under different storage conditions: Effects of temperature and length of storage. Biochem. Med. 2019, 29, 94–111. [Google Scholar] [CrossRef]
- Van der Heiden, C.; Bais, R.; Gerhardt, W.; Lorentz, K.; Rosalki, S. IFCC methods for measurement of catalytic concentration of enzymes—Part 9. IFCC method for alpha-amylase {[}1,4-alpha-D-glucan 4-glucanohydrolase, EC 3.2.1.1]. Clin. Chim. Acta 1999, 281, S5–S39. [Google Scholar]
- Tecles, F.; Fuentes-Rubio, M.; Tvarijonaviciute, A.; Martínez-Subiela, S.; Fatjó, J.; Cerón, J.J. Assessment of stress associated with an oral public speech in veterinary students by salivary biomarkers. J. Vet. Med. Educ. 2014, 41, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the way of reporting alpha- Amylase values in saliva in different naturalistic situations: A pilot study. PLoS ONE 2017, 12, e0180100. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef] [PubMed]
- Clegg, R.M.; Loontiens, F.G.; Landschoot, A.V.; Jovin, T.M. Binding Kinetics of Methyl α-D-Mannopyranoside to Concanavalin A: Temperature-Jump Relaxation Study with 4-Methylumbelliferyl α-D-Mannopyranoside as a Fluorescence Indicator Ligand. Biochemistry 1981, 20, 4687–4692. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N.; Schlotz, W.; Ehlert, U.; Kirschbaum, C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 2007, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G*Power: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003; ISBN 0618124055. [Google Scholar]
- Alegria-Schaffer, A.; Lodge, A.; Vattem, K. Performing and Optimizing Western Blots with an Emphasis on Chemiluminescent Detection. In Methods in Enzymology. Guide to Protein Purification, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Elsevier Publishing: London, UK, 2009; pp. 573–599. [Google Scholar]
- Belen’KY, D.M.; Mikhajlov, V.I.; Rosenfeld, E.L. Carbohydrate Content of Acid α-Glucoside (y-Amylase) from Human Liver. Clin. Chim. Acta 1979, 93, 365–370. [Google Scholar] [CrossRef]
- Kato, S.; Ishibashi, M.; Tatsuda, D.; Tokunaga, H.; Tokunaga, M. Efficient expression, purification and characterization of mouse salivary amylase secreted from methylotrophic yeast, Pichia pastoris. Yeast 2001, 18, 643–655. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.; Chen, L.; Zhang, M.; Lin, J.; Zhang, J.; Chen, W. Age Differences of Salivary Alpha-Amylase Levels of Basal and Acute Responses to Citric Acid Stimulation Between Chinese Children and Adults. Front. Physiol. 2015, 6, 1–11. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.C.; Peterson, S.E.; Loring, J.F. Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res. 2014, 24, 143–160. [Google Scholar] [CrossRef]
| Variable | Mean ± SD 1 |
|---|---|
| Age (years) | 33.1 ± 5.19 |
| Bodyweight (kg) | 82.1 ± 7.59 |
| Height (m) | 1.8 ± 0.07 |
| Body Mass Index | 25.0 ± 2.29 |
| 1RM Power Clean (kg) | 85.0 ± 11.02 |
| TSST | CrossFit WODs | |||
|---|---|---|---|---|
| T + 0 | T + 01 | |||
| 59 kDa | 56 kDa | 59 kDa | 56 kDa | |
| Before sample depletion | 0.51 | 0.61 | 0.57 | 0.62 |
| After sample depletion | 0.10 | 0.67 | 0.17 | 0.56 |
| % of difference | 80.4 | 9.8 | 70.2 | 9.7 |
| Saliva Specimens | Mean (IU/mL) | SD (IU/mL) | CV (%) |
|---|---|---|---|
| A | 95.17 | 5.05 | 5.30 |
| B | 38.29 | 0.32 | 0.86 |
| C | 34.35 | 2.53 | 7.36 |
| Specimen 1 | Specimen 2 | Specimen 3 | ||||
|---|---|---|---|---|---|---|
| Activity Measurement | NGsAA + GsAA | Activity Measurement | NGsAA + GsAA | Activity Measurement | NGsAA + GsAA | |
| TsAA (IU/mL) | 368.5 | 133.0 | 39.9 | |||
| NGsAA (IU/mL) | 69.8 | 21.7 | 6.9 | |||
| GsAA (IU/mL) | 285.8 | 355.8 | 91.4 | 113.2 | 26.8 | 33.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Aguilar, M.D.; Mateo, S.V.; Tecles, F.; Hirtz, C.; Escribano, D.; Cerón, J.J. Changes Occurring on the Activity of Salivary Alpha-Amylase Proteoforms in Two Naturalistic Situations Using a Spectrophotometric Assay. Biology 2021, 10, 227. https://doi.org/10.3390/biology10030227
Contreras-Aguilar MD, Mateo SV, Tecles F, Hirtz C, Escribano D, Cerón JJ. Changes Occurring on the Activity of Salivary Alpha-Amylase Proteoforms in Two Naturalistic Situations Using a Spectrophotometric Assay. Biology. 2021; 10(3):227. https://doi.org/10.3390/biology10030227
Chicago/Turabian StyleContreras-Aguilar, María D., Sandra V. Mateo, Fernando Tecles, Christophe Hirtz, Damián Escribano, and Jose J. Cerón. 2021. "Changes Occurring on the Activity of Salivary Alpha-Amylase Proteoforms in Two Naturalistic Situations Using a Spectrophotometric Assay" Biology 10, no. 3: 227. https://doi.org/10.3390/biology10030227
APA StyleContreras-Aguilar, M. D., Mateo, S. V., Tecles, F., Hirtz, C., Escribano, D., & Cerón, J. J. (2021). Changes Occurring on the Activity of Salivary Alpha-Amylase Proteoforms in Two Naturalistic Situations Using a Spectrophotometric Assay. Biology, 10(3), 227. https://doi.org/10.3390/biology10030227







