Comparison of the Acute Effects of Hold-Relax and Static Stretching among Older Adults
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
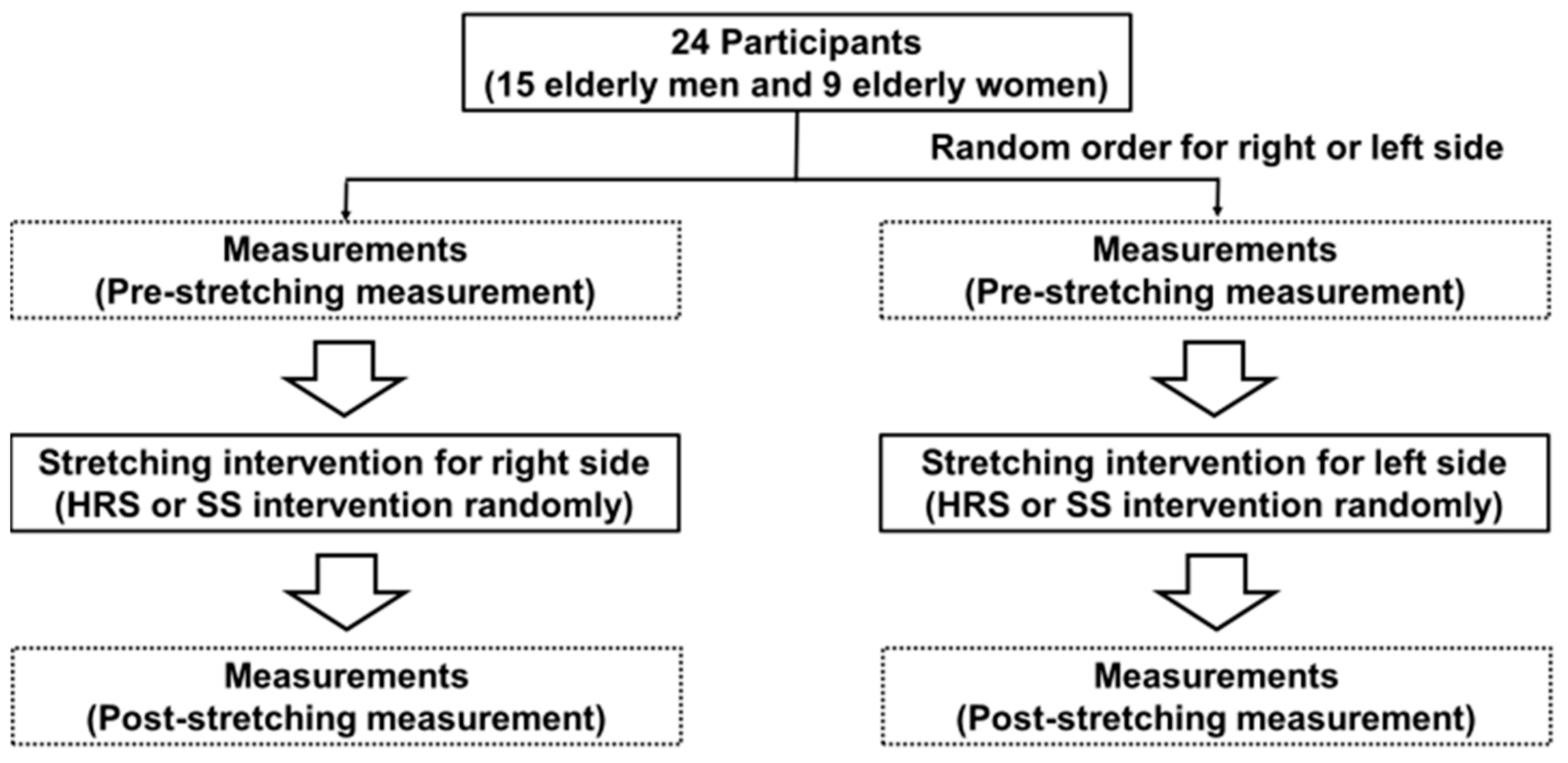
2.1. Experimental Protocol
2.2. Subjects
2.3. Assessment of DF Angle and Passive Torque
2.4. Assessment of Medial Gastrocnemius Stiffness
2.5. HRS and SS Techniques
2.6. Statistical Analysis
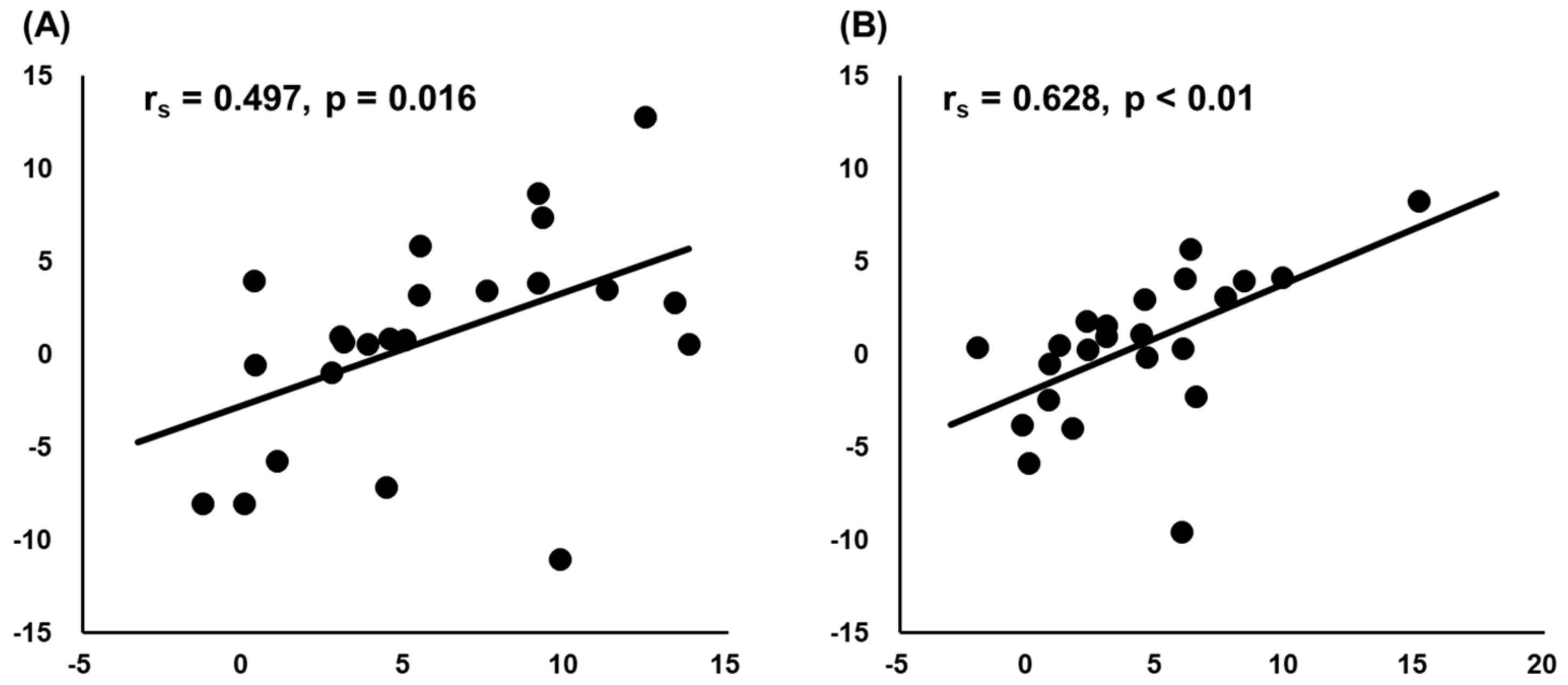
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajdosik, R.L.; Linden, D.W.V.; Williams, A.K. Influence of age on concentric isokinetic torque and passive extensibility variables of the calf muscles of women. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Gajdosik, R.L.; Linden, D.W.V.; McNair, P.J.; Riggin, T.J.; Albertson, J.S.; Mattick, D.J.; Wegley, J.C. Viscoelastic properties of short calf muscle-tendon units of older women: Effects of slow and fast passive dorsiflexion stretches in vivo. Eur. J. Appl. Physiol. 2005, 95, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, H.; Mita, K.; Watakabet, M.; Alkataki, K.; Suzuki, N.; Okuwa, T.; Yabe, K. Age-related changes in the interactive mobility of the hip and knee joints: A geometrical analysis. Gait. Posture 2002, 15, 236–243. [Google Scholar] [CrossRef]
- Holland, G.J.; Tanaka, K.; Shigematsu, R.; Nakagaichi, M. Flexibility and physical functions of older adults: A review. J. Aging Phys. Act. 2002, 10, 169–206. [Google Scholar] [CrossRef]
- Campbell, A.J.; Borrie, M.J.; Spears, G.F. Risk factors for falls in a community-based prospective study of people 70 years and older. J. Gerontol. 1989, 44, M112–M117. [Google Scholar] [CrossRef]
- Johnson, E.G.; Bradley, B.D.; Witkowski, K.R.; McKee, R.Y.; Telesmanic, C.L.; Chavez, A.S.; Kennedy, K.L.; Zimmerman, G.J. Effect of a static calf muscle-tendon unit stretching program on ankle dorsiflexion range of motion of older women. J. Geriatr. Phys. Ther. 2007, 30, 49–52. [Google Scholar] [CrossRef]
- Gajdosik, R.L.; Linden, D.W.V.; McNair, P.J.; Williams, A.K.; Riggin, T.J. Effects of an eight-week stretching program on the passive-elastic properties and function of the calf muscles of older women. Clin. Biomech. 2005, 20, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ikezoe, T.; Nishishita, S.; Umehara, J.; Kimura, M.; Ichihashi, N. Acute effects of static stretching on the shear elastic moduli of the medial and lateral gastrocnemius muscles in young and elderly women. Musculoskelet. Sci. Pract. 2017, 32, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.R.; Jackson, K.; Franz, J.R.; Dicharry, J.; Evans, J.; Kerrigan, D.C. Effect of a supervised hip flexor stretching program on gait in frail elderly patients. PM&R 2011, 3, 330–335. [Google Scholar]
- Zotz, T.G.G.; Loureiro, A.P.C.; Valderramas, S.R.; Gomes, A.R.S. Stretching–An important strategy to prevent musculoskeletal aging: A systematic review and meta-analysis. Top. Geriatr. Rehabil. 2014, 30, 246–255. [Google Scholar] [CrossRef]
- Stathokostas, L.; Little, R.M.D.; Vandervoort, A.A.; Paterson, D.H. Flexibility training and functional ability in older adults: A systematic review. J. Aging Res. 2012, 2012, 306818. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Suga, T.; Takao, K.; Tanaka, T.; Misaki, J.; Miyake, Y.; Nagano, A.; Isaka, T. Potential Relationship between Passive Plantar Flexor Stiffness and Running Performance. Int. J. Sports Med. 2018, 39, 204–209. [Google Scholar] [CrossRef]
- Blazevich, A.J. Adaptations in the passive mechanical properties of skeletal muscle to altered patterns of use. J. Appl. Physiol. 2019, 126, 1483–1491. [Google Scholar] [CrossRef]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; Lebrasseur, N.K.; An, K.-N. Shear wave elastography of passive skeletal muscle stiffness: Influences of sex and age throughout adulthood. Clin. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Akagi, R.; Yamashita, Y.; Ueyasu, Y. Age-Related Differences in Muscle Shear Moduli in the Lower Extremity. Ultrasound. Med. Biol. 2015, 41, 2906–2912. [Google Scholar] [CrossRef]
- Hirata, K.; Yamadera, R.; Akagi, R. Can Static Stretching Reduce Stiffness of the Triceps Surae in Older Men? Med. Sci. Sports Exerc. 2020, 52, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Yamadera, R.; Akagi, R. Associations between Range of Motion and Tissue Stiffness in Young and Older People. Med. Sci. Sports Exerc. 2020, 52, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ikezoe, T.; Tokugawa, T.; Ichihashi, N. Acute Effects of Stretching on Passive Properties of Human Gastrocnemius Muscle-Tendon Unit: Analysis of Differences Between Hold-Relax and Static Stretching. J. Sport Rehabil. 2015, 24, 955–964. [Google Scholar] [CrossRef]
- Akagi, R.; Takahashi, H. Acute effect of static stretching on hardness of the gastrocnemius muscle. Med. Sci. Sports Exerc. 2013, 45, 1348–1354. [Google Scholar] [CrossRef]
- Nakamura, M.; Sato, S.; Hiraizumi, K.; Kiyono, R.; Fukaya, T.; Nishishita, S. Effects of static stretching programs performed at different volume-equated weekly frequencies on passive properties of muscle-tendon unit. J. Biomech. 2020, 2020, 109670. [Google Scholar] [CrossRef]
- Sato, S.; Kiyono, R.; Takahashi, N.; Yoshida, T.; Takeuchi, K.; Nakamura, M. The acute and prolonged effects of 20-s static stretching on muscle strength and shear elastic modulus. PLoS ONE 2020, 15, e0228583. [Google Scholar] [CrossRef]
- Mizuno, T.; Matsumoto, M.; Umemura, Y. Viscoelasticity of the muscle-tendon unit is returned more rapidly than range of motion after stretching. Scand. J. Med. Sci. Sports 2013, 23, 23–30. [Google Scholar] [CrossRef]
- Weppler, C.H.; Magnusson, S.P. Increasing muscle extensibility: A matter of increasing length or modifying sensation? Phys. Ther. 2010, 90, 438–449. [Google Scholar] [CrossRef]
- Gajdosik, R.L.; Allred, J.D.; Gabbert, H.L.; Sonsteng, B.A. A stretching program increases the dynamic passive length and passive resistive properties of the calf muscle-tendon unit of unconditioned younger women. Eur. J. Appl. Physiol. 2007, 99, 449–454. [Google Scholar] [CrossRef]
- Morse, C.I.; Degens, H.; Seynnes, O.R.; Maganaris, C.N.; Jones, D.A. The acute effect of stretching on the passive stiffness of the human gastrocnemius muscle tendon unit. J. Physiol. 2008, 586, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ikezoe, T.; Takeno, Y.; Ichihashi, N. Effects of a 4-week static stretch training program on passive stiffness of human gastrocnemius muscle-tendon unit in vivo. Eur. J. Appl. Physiol. 2012, 112, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ikezoe, T.; Takeno, Y.; Ichihashi, N. Acute and prolonged effect of static stretching on the passive stiffness of the human gastrocnemius muscle tendon unit in vivo. J. Orthop. Res. 2011, 29, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Balle, S.S.; Magnusson, S.P.; McHugh, M.P. Effects of contract-relax vs static stretching on stretch-induced strength loss and length-tension relationship. Scand. J. Med. Sci. Sports 2015, 25, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Konrad, A.; Stafilidis, S.; Tilp, M. Effects of acute static, ballistic, and PNF stretching exercise on the muscle and tendon tissue properties. Scand. J. Med. Sci. Sports 2017, 27, 1070–1080. [Google Scholar] [CrossRef]
- Puentedura, E.J.; Huijbregts, P.A.; Celeste, S.; Edwards, D.; In, A.; Landers, M.R.; Fernandez-de-las-Penas, C. Immediate effects of quantified hamstring stretching: Hold-relax proprioceptive neuromuscular facilitation versus static stretching. Phys. Ther. Sport 2011, 12, 122–126. [Google Scholar] [CrossRef]
- Condon, M.S.; Hutton, R.S. Soleus muscle electromyographic activity and ankle dorsiflexion range of motion during four stretching procedures. Phys. Ther. 1987, 67, 24–30. [Google Scholar] [CrossRef]
- Chow, T.P.; Ng, G.Y. Active, passive and proprioceptive neuromuscular facilitation stretching are comparable in improving the knee flexion range in people with total knee replacement: A randomized controlled trial. Clin. Rehabil. 2010, 24, 911–918. [Google Scholar] [CrossRef]
- González-Ravé, J.M.; Sanchez-Gomez, A.; Santos-Garcia, D.J. Efficacy of two different stretch training programs (passive vs. proprioceptive neuromuscular facilitation) on shoulder and hip range of motion in older people. J. Strength. Cond. Res. 2012, 26, 1045–1051. [Google Scholar]
- Mitchell, U.H.; Myrer, J.W.; Hopkins, J.T.; Hunter, I.; Feland, J.B.; Hilton, S.C. Acute stretch perception alteration contributes to the success of the PNF “contract-relax” stretch. J. Sport Rehabil. 2007, 16, 85–92. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Simonsen, E.B.; Aagaard, P.; Dyhre-Poulsen, P.; McHugh, M.P.; Kjaer, M. Mechanical and physical responses to stretching with and without preisometric contraction in human skeletal muscle. Arch. Phys. Med. Rehabil. 1996, 77, 373–378. [Google Scholar] [CrossRef]
- Maddigan, M.E.; Peach, A.A.; Behm, D.G. A comparison of assisted and unassisted proprioceptive neuromuscular facilitation techniques and static stretching. J. Strength Cond. Res. 2012, 26, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, E.J.; Ryan, E.D.; Thompson, B.J.; McHugh, M.P.; Conchola, E.C. The influence of age on the viscoelastic stretch response. J. Strength. Cond. Res. 2014, 28, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.D.; Husbands-Beasley, J.; Blazevich, A.J. Effects of Contract-Relax, Static Stretching, and Isometric Contractions on Muscle-Tendon Mechanics. Med. Sci. Sports Exerc. 2015, 47, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
| SS | HRS | |||
|---|---|---|---|---|
| PRE | POST | PRE | POST | |
| DF ROM (°) | 30.9 ± 10.0 | 35.3 ± 10.8 ** | 31.7 ± 10.6 | 37.2 ± 11.1 **,# |
| Passive torque at DF ROM (Nm) | 27.1 ± 14.1 | 27.7 ± 12.4 | 28.3 ± 12.0 | 29.7 ± 10.5 |
| Muscle stiffness (Nm/cm) | 17.3 ± 14.1 | 8.4 ± 6.3 ** | 17.3 ± 11.4 | 14.6 ± 11.8 § |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Sato, S.; Kiyono, R.; Yahata, K.; Yoshida, R.; Fukaya, T.; Konrad, A. Comparison of the Acute Effects of Hold-Relax and Static Stretching among Older Adults. Biology 2021, 10, 126. https://doi.org/10.3390/biology10020126
Nakamura M, Sato S, Kiyono R, Yahata K, Yoshida R, Fukaya T, Konrad A. Comparison of the Acute Effects of Hold-Relax and Static Stretching among Older Adults. Biology. 2021; 10(2):126. https://doi.org/10.3390/biology10020126
Chicago/Turabian StyleNakamura, Masatoshi, Shigeru Sato, Ryosuke Kiyono, Kaoru Yahata, Riku Yoshida, Taizan Fukaya, and Andreas Konrad. 2021. "Comparison of the Acute Effects of Hold-Relax and Static Stretching among Older Adults" Biology 10, no. 2: 126. https://doi.org/10.3390/biology10020126
APA StyleNakamura, M., Sato, S., Kiyono, R., Yahata, K., Yoshida, R., Fukaya, T., & Konrad, A. (2021). Comparison of the Acute Effects of Hold-Relax and Static Stretching among Older Adults. Biology, 10(2), 126. https://doi.org/10.3390/biology10020126








