Effect of Olive Cake and Cactus Cladodes Incorporation in Goat Kids’ Diet on the Rumen Microbial Community Profile and Meat Fatty Acid Composition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
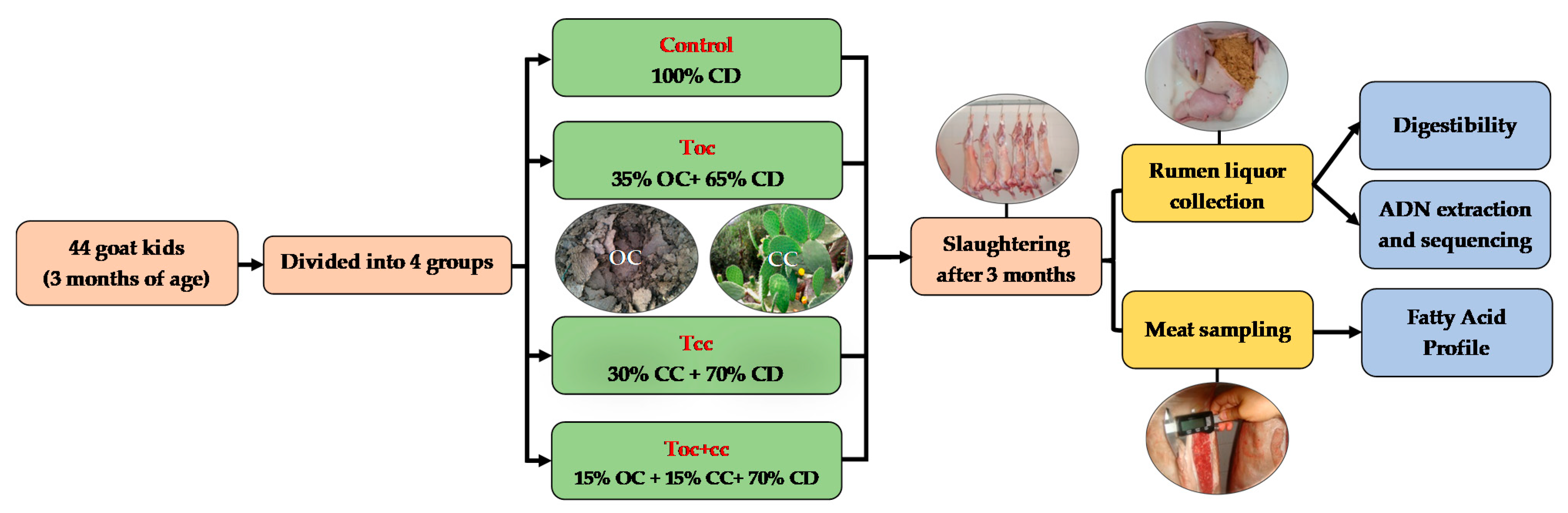
2.1. Animals and Diets
2.2. Slaughter and Sampling
2.3. Diets’ In Vitro Digestibility
2.4. Microbiota Analysis
2.4.1. DNA Extraction
2.4.2. 16S rRNA Gene Library Construction and Sequencing
2.5. Meat Fatty Acid
2.6. Data Analysis
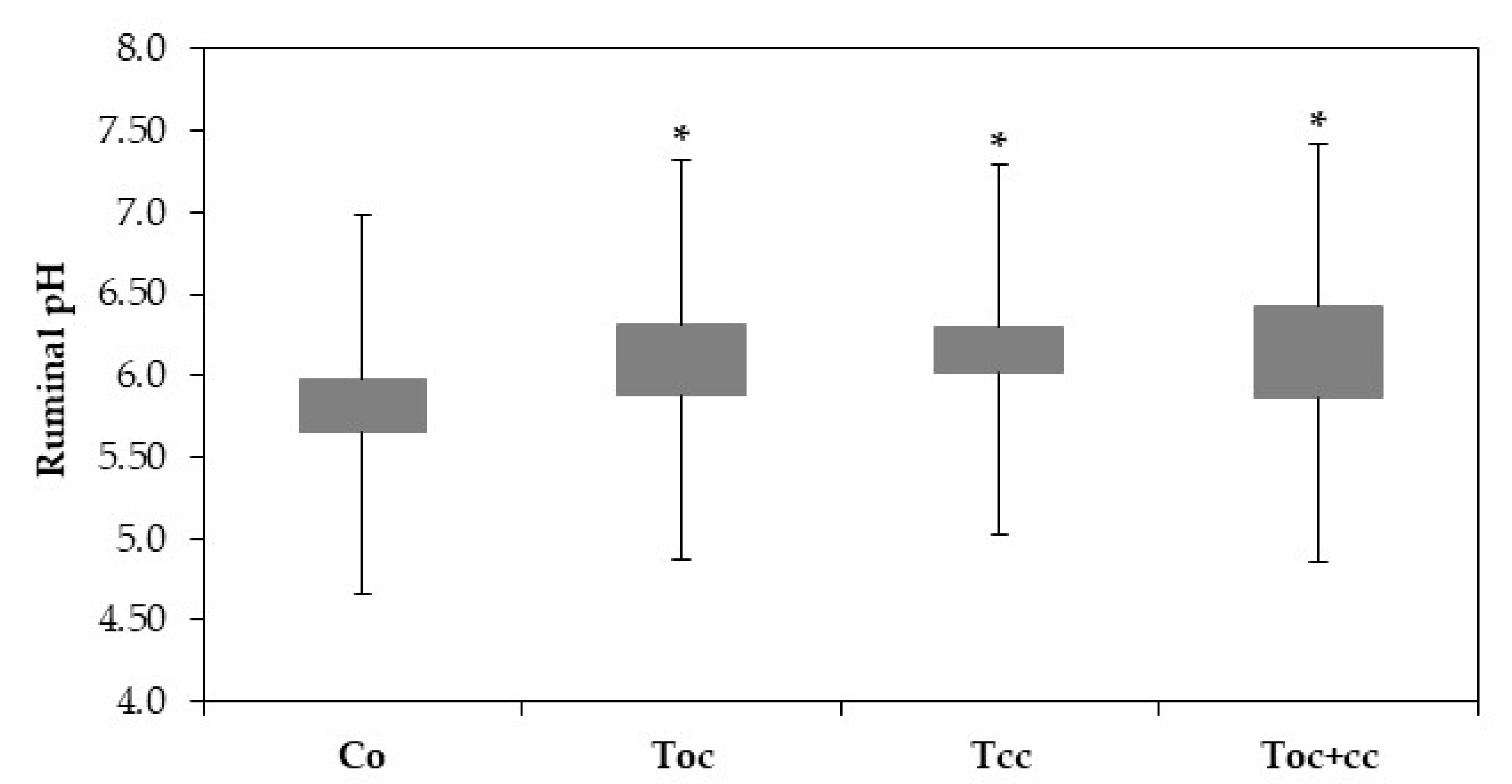
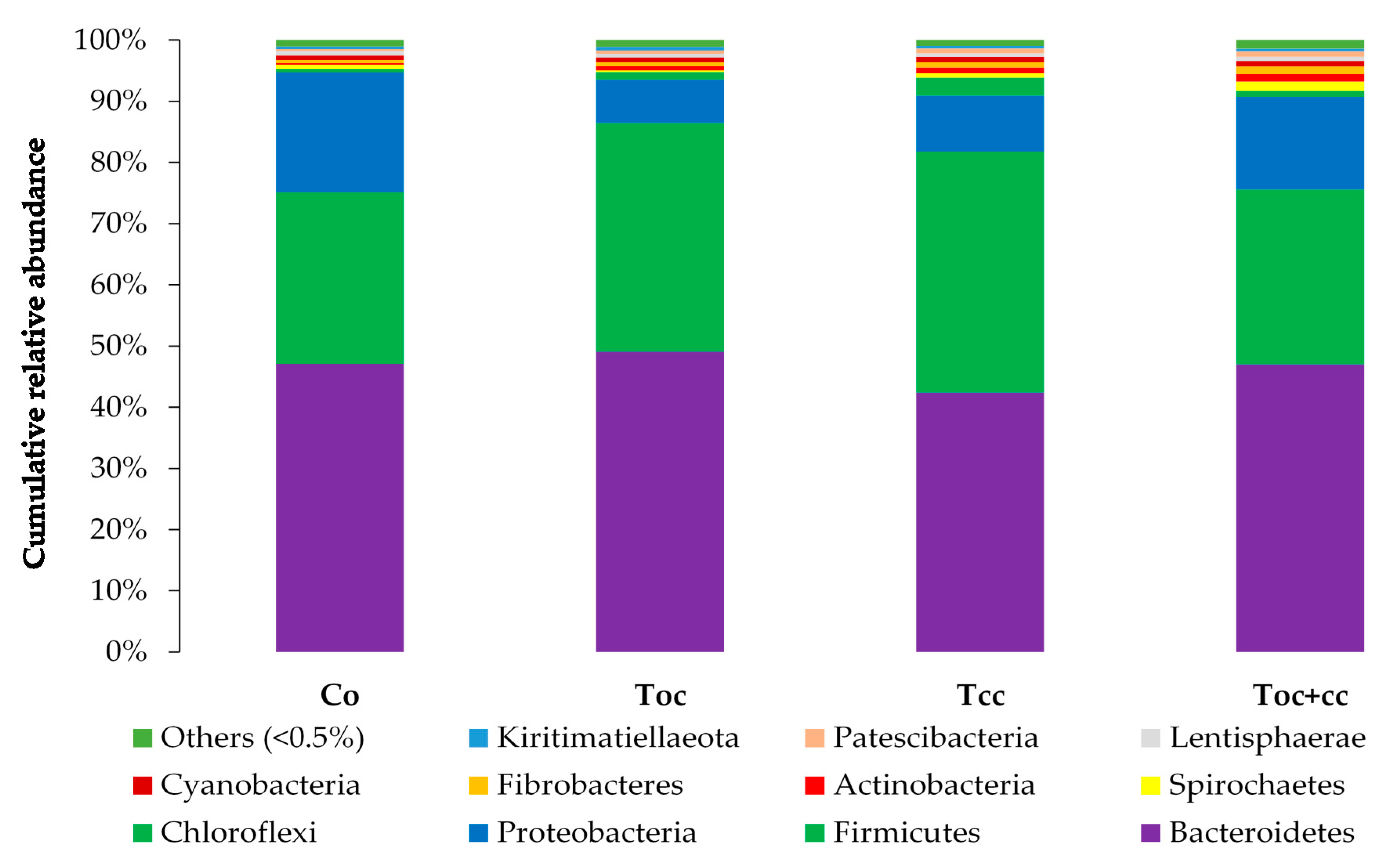
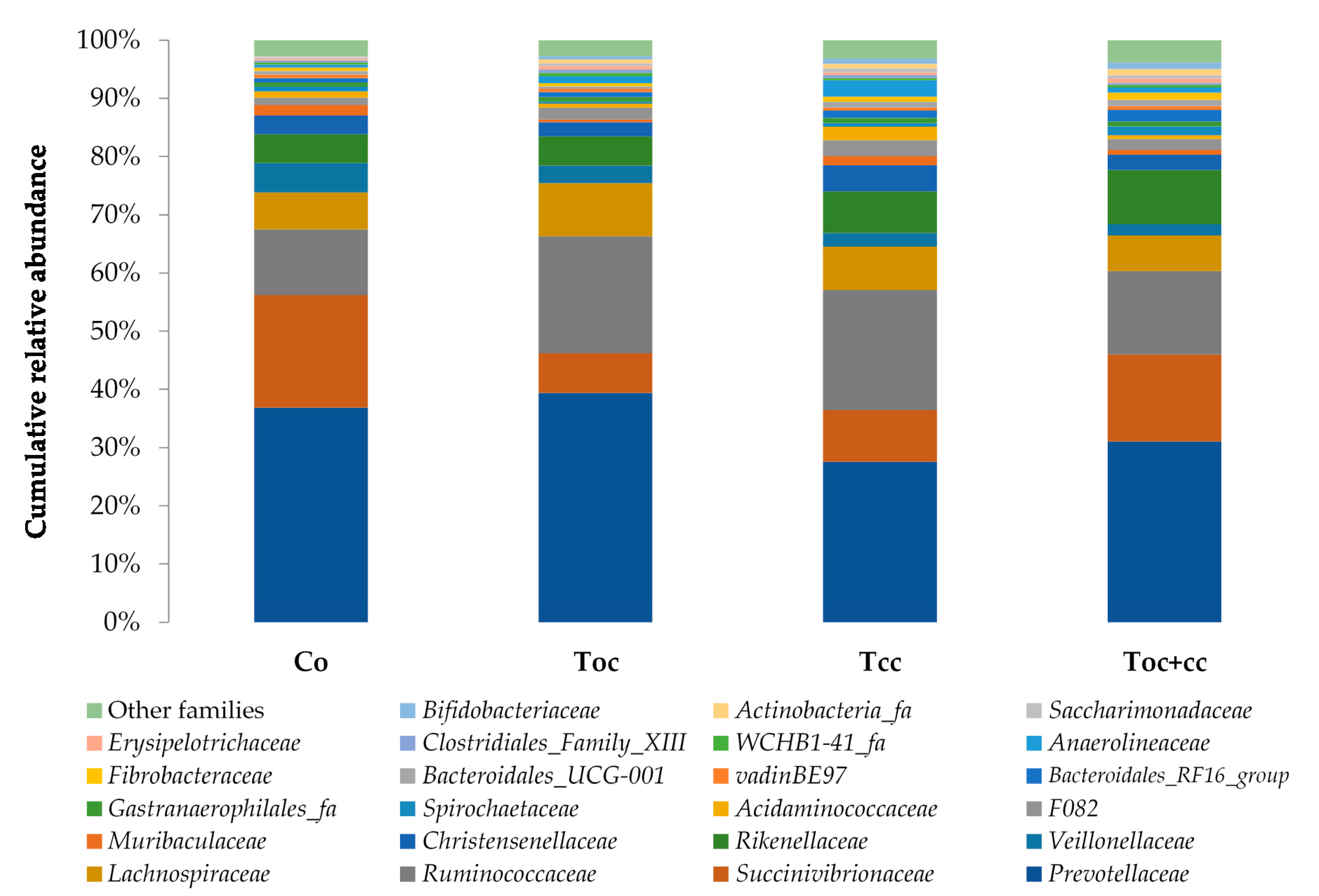
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chebli, Y.; Chentouf, M.; Ozer, P.; Hornick, J.L.; Cabaraux, J.F. Forest and silvopastoral cover changes and its drivers in northern Morocco. Appl. Geogr. 2018, 101, 23–35. [Google Scholar] [CrossRef]
- Webb, E.C.; Casey, N.H.; Simela, L. Goat meat quality. Small Rumin. Res. 2005, 60, 153–166. [Google Scholar] [CrossRef]
- Wallace, J.R.; Sasson, G.; Garnsworthy, P.C.; Tapio, I.; Gregson, E.; Bani, P.; Huhtanen, P.; Bayat, A.R.; Strozzi, F.; Biscarini, F.; et al. A heritable subset of the core rumen microbiome dictates dairy cow productivity and emissions. Sci. Adv. 2019, 5, eaav8391. [Google Scholar] [CrossRef] [Green Version]
- Chebli, Y.; El Otmani, S.; Chentouf, M.; Hornick, J.L.; Cabaraux, J.F. Temporal variations in chemical composition, in vitro digestibility, and metabolizable energy of plant species browsed by goats in Southern Mediterranean forest rangeland. Animals 2021, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; El Otmani, S.; Elame, F.; Moula, N.; Chentouf, M.; Hornick, J.L.; Cabaraux, J.F. Silvopastoral system in Morocco: Focus on their importance, strategic functions, and recent changes in the Mediterranean side. Sustainability 2021, 13, 10744. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The effects of dietary consumption of plants secondary compounds on small ruminants’ products quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Mele, M.; Serra, A.; Pauselli, M.; Luciano, G.; Lanza, M.; Pennisi, P.; Conte, G.; Taticchi, A.; Esposto, S.; Morbidini, L. The use of stoned olive cake and rolled linseed in the diet of intensively reared lambs: Effect on the intramuscular fatty-acid composition. Animal 2014, 8, 152–162. [Google Scholar] [CrossRef] [PubMed]
- El Otmani, S.; Chebli, Y.; Chentouf, M.; Hornick, J.L.; Cabaraux, J.F. Effects of olive cake and cactus cladodes as alternative feed resources on goat milk production and quality. Agriculture 2021, 11, 3. [Google Scholar] [CrossRef]
- El Otmani, S.; Chebli, Y.; Hornick, J.L.; Cabaraux, J.F.; Chentouf, M. Growth performance, carcass characteristics and meat quality of male goat kids supplemented by alternative feed resources: Olive cake and cactus cladodes. Anim. Feed Sci. Technol. 2021, 272, 114746. [Google Scholar] [CrossRef]
- Chentli, A.; Gillmann, L.; Bouazza, L.; Medjkal, S.; Limami, A.M.; Le Paven, M.C.M.; Bousseboua, H. Effects of secondary compounds from cactus and acacias trees on rumen microbial profile changes performed by Real-Time PCR. Int. J. Adv. Res. 2014, 2, 660–671. [Google Scholar]
- Banskalieva, V.; Sahlu, T.; Goetsch, A.L. Fatty acid composition of goat muscles and fat depots: A review. Small Rumin. Res. 2000, 37, 255–268. [Google Scholar] [CrossRef]
- Infascelli, L.; Tudisco, R.; Iommelli, P.; Capitanio, F. Milk Quality and animal welfare as a possible marketing lever for the economic development of rural areas in Southern Italy. Animals 2021, 11, 1059. [Google Scholar] [CrossRef]
- Luciano, G.; Pauselli, M.; Servili, M.; Mourvaki, E.; Serra, A.; Monahan, F.J.; Lanza, M.; Priolo, A.; Zinnai, A.; Mele, M. Dietary olive cake reduces the oxidation of lipids, including cholesterol, in lamb meat enriched in polyunsaturated fatty acids. Meat Sci. 2013, 93, 703–714. [Google Scholar] [CrossRef]
- Mannelli, F.; Cappucci, A.; Pini, F.; Pastorelli, R.; Decorosi, F.; Giovannetti, L.; Mele, M.; Minieri, S.; Conte, G.; Pauselli, M.; et al. Effect of different types of olive oil pomace dietary supplementation on the rumen microbial community profile in Comisana ewes. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Pallara, G.; Buccioni, A.; Pastorelli, R.; Minieri, S.; Mele, M.; Rapaccini, S.; Messini, A.; Pauselli, M.; Servili, M.; Giovannetti, L.; et al. Effect of stoned olive pomace on rumen microbial communities and polyunsaturated fatty acid biohydrogenation: An in vitro study. BMC Vet. Res. 2014, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Tudisco, R.; Chiofalo, B.; Addi, L.; Lo Presti, V.; Rao, R.; Calabrò, S.; Musco, N.; Grossi, M.; Cutrignelli, M.I.; Mastellone, V.; et al. Effect of hydrogenated palm oil dietary supplementation on milk yield and composition, fatty acids profile and Stearoyl-CoA desaturase expression in goat milk. Small Rumin. Res. 2015, 132, 72–78. [Google Scholar] [CrossRef]
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim. Feed Sci. Technol. 2008, 147, 223–246. [Google Scholar] [CrossRef]
- Arco-Pérez, A.; Ramos-Morales, E.; Yáñez-Ruiz, D.R.; Abecia, L.; Martín-García, A.I. Nutritive evaluation and milk quality of including of tomato or olive by-products silages with sunflower oil in the diet of dairy goats. Anim. Feed Sci. Technol. 2017, 232, 57–70. [Google Scholar] [CrossRef]
- El Otmani, S.; Chentouf, M.; Hornick, J.L.; Cabaraux, J.F. Chemical composition and in vitro digestibility of alternative feed resources for ruminants in Mediterranean climates: Olive cake and cactus cladodes. J. Agric. Sci. 2019, 157, 260–271. [Google Scholar] [CrossRef]
- Mahouachi, M.; Atti, N.; Hajji, H. Use of spineless cactus (Opuntia ficus indica f. inermis) for dairy goats and growing kids: Impacts on milk production, kid’s growth, and meat quality. Sci. World J. 2012, 2012, 321567. [Google Scholar] [CrossRef] [Green Version]
- Kotsampasi, B.; Bampidis, V.A.; Tsiaousi, A.; Christodoulou, C.; Petrotos, K.; Amvrosiadis, I.; Fragioudakis, N.; Christodoulou, V. Effects of dietary partly destoned exhausted olive cake supplementation on performance, carcass characteristics and meat quality of growing lambs. Small Rumin. Res. 2017, 156, 33–41. [Google Scholar] [CrossRef]
- Atti, N.; Maamouri, O.; Hajji, H.; Mahouachi, M. Utilisation du cactus inerme comme aliment de base pour la chèvre en lactation: Impacts sur la production laitière et la croissance des chevreaux. Livest. Res. Rural Dev. 2010, 22, 7. [Google Scholar]
- Atti, N.; Mahouachi, M.; Rouissi, H. The effect of spineless cactus (Opuntia ficus-indica f. inermis) supplementation on growth, carcass, meat quality and fatty acid composition of male goat kids. Meat Sci. 2006, 73, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Abidi, S.; Ben Salem, H.; Vasta, V.; Priolo, A. Supplementation with barley or spineless cactus (Opuntia ficus indica f. inermis) cladodes on digestion, growth and intramuscular fatty acid composition in sheep and goats receiving oaten hay. Small Rumin. Res. 2009, 87, 9–16. [Google Scholar] [CrossRef]
- Chiofalo, B.; Liotta, L.; Zumbo, A.; Chiofalo, V. Administration of olive cake for ewe feeding: Effect on milk yield and composition. Small Rumin. Res. 2004, 55, 169–176. [Google Scholar] [CrossRef]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian Australas. J. Anim. Sci. 2013, 26, 971–980. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Vera, R.R.; Aguilar, C.; Lira, R.; Peña, I.; Fernández, J. Feeding olive cake to ewes improves fatty acid profile of milk and cheese. Anim. Feed Sci. Technol. 2013, 184, 94–99. [Google Scholar] [CrossRef]
- Dusart, C. La Digestion Ruminale: Mise en Place d’un Modèle d’étude In Vitro à Long Terme en Cultures Batch. Ph.D. Thesis, Toulouse University, Toulouse, France, 2014. [Google Scholar]
- Jones, G.A.; McAllister, T.A.; Cheng, K.J.; Muir, A.D. Effect of sainfoin (Onobrychris viciifolia Scop.) condensed tannins on growth and proteolysis by 4 strains of rumen bacteria. Appl. Environ. Microbiol. 1994, 60, 1374–1378. [Google Scholar] [CrossRef] [Green Version]
- Mateos, I.; Saro, C.; Carro, M.D.; Ranilla, M.J. Using by-Products in Dairy Sheep Diets: Effects on Ruminal Bacterial Communities. In XVIII Jornadas Sobre Producción Animal, Zaragoza, Spain, 7 and 8 May 2019; Asociación Interprofesional para el Desarrollo Agrario: Zaragoza, Spain, 2019; Available online: http://www.aida-itea.org/aida-itea/files/jornadas/2019/Comunicaciones/2019_NyA_60.pdf (accessed on 10 September 2020).
- Belenguer, Á.; Frutos, P.; Bernard, L.; Hervás, G.; Chilliard, Y.; Toral, P.G. Comparación de la fermentación y la comunidad bacteriana del rumen en vacas y cabras alimentadas con la misma dieta. In Proceedings of the AIDA (2013) XV Jornadas Sobre Producción Animal, Zaragoza, Spain, 14–15 May 2013; Asociación Interprofesional para el Desarrollo Agrario: Zaragoza, Spain, 2013; Tomo II, pp. 860–862. [Google Scholar]
- Brooker, J.D.; O’Donovan, L.A.; Skene, I.; Clarke, K.; Blackall, L.; Muslera, P. Streptococcus caprinus sp. Nov., a tannin-resistant ruminal bacterium from feral goats. Lett. Appl. Microbiol. 1994, 18, 313–318. [Google Scholar] [CrossRef]
- Hilal, B.; El Otmani, S.; Chentouf, M.; Boujenane, L. Morphological characterization of the local goat population “Beni Arrous”. Options Méditerranéennes 2014, 437, 433–437. [Google Scholar]
- El Otmani, S.; Hilal, B.; Chentouf, M. Milk production and composition of ‘Beni Arousse’ North Moroccan local goat. Options Méditerranéennes 2013, 108, 457–461. [Google Scholar]
- Palmieri, A.D.; de Carvalho, G.G.P.; Tosto, M.S.L.; Leite, V.M.; Santos, S.A.; Borja, M.S.; Azevêdo, J.A.G.; Júnior, J.E.d.F.; Leite, L.C.; Ayres, M.C.C.; et al. Nutritional and productive performance of goats kids fed diets with detoxified castor meal. Anim. Feed Sci. Technol. 2016, 216, 81–92. [Google Scholar] [CrossRef]
- Mabjeesh, S.J.; Cohen, M.; Arieli, A. In vitro methods for measuring the dry matter digestibility of ruminant feedstuffs: Comparison of methods and inoculum source. J. Dairy Sci. 2000, 83, 2289–2294. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyrinck, A.M.; Etxeberria, U.; Taminiau, B.; Daube, G.; Van Hul, M.; Everard, A.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol. Nutr. Food Res. 2016, 61, 1500899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open-source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The silva ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Mioč, B.; Pavić, V.; Vnučec, I.; Prpić, Z.; Kostelić, A.; Sušić, V. Effect of olive cake on daily gain, carcass characteristics and chemical composition of lamb meat. Czech J. Anim. Sci. 2007, 52, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International. Fatty Acids in Oils and Fats, Preparation of Methyl Esters Boron Trifluoride Method, 15th ed.; AOAC Official Method 969.33; AOAC International: Washington, DC, USA, 1990. [Google Scholar]
- Sauvant, D.; Meschy, F.; Mertens, D. Les composantes de l’acidose ruminale et les effets acidogènes des rations. Prod. Anim. 1999, 12, 49–60. [Google Scholar] [CrossRef]
- Giger-Reverdin, S.; Sauvant, D. Meta-analysis of the acidogenicity of ingredients. J. Anim. Sci. 2001, 79 (Suppl. 1), 79. [Google Scholar]
- Iqbal, M.W.; Zhang, Q.; Yang, Y.; Zou, C.; Li, L.; Liang, X.; Wei, S.; Lin, B. Ruminal fermentation and microbial community differently influenced by four typical subtropical forages in vitro. Anim. Nutr. 2018, 4, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, K.; Wang, Z.; Bai, X.; Peng, Q.; Jin, L. Bacterial community diversity associated with different utilization efficiencies of nitrogen in the gastrointestinal tract of goats. Front. Microbiol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yao, S.; Chen, Y.; Gao, S.; Yang, Y.; Deng, J.; Ren, Z.; Shen, L.; Cui, H.; Hu, Y.; et al. Use of antimicrobial peptides as a feed additive for juvenile goats. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census Collaborators; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Bajzer, M.; Seeley, R.J. Physiology: Obesity and gut flora. Nature 2006, 444, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, H.; Yu, Z.; Niu, W.; Qiu, Q.; Su, H.; Cao, B. Effects of the gender differences in cattle rumen fermentation on anaerobic fermentation of wheat straw. J. Clean. Prod. 2018, 205, 845–853. [Google Scholar] [CrossRef]
- Biscarini, F.; Palazzo, F.; Castellani, F.; Masetti, G.; Grotta, L.; Cichelli, A.; Martino, G. Rumen microbiome in dairy calves fed copper and grape-pomace dietary supplementations: Composition and predicted functional profile. PLoS ONE 2018, 13, e0205670. [Google Scholar] [CrossRef]
- Cappucci, A.; Alves, S.P.; Bessa, R.J.B.; Buccioni, A.; Mannelli, F.; Pauselli, M.; Viti, C.; Pastorelli, R.; Roscini, V.; Serra, A.; et al. Effect of increasing amounts of olive crude phenolic concentrate in the diet of dairy ewes on rumen liquor and milk fatty acid composition. J. Dairy Sci. 2018, 101, 4992–5005. [Google Scholar] [CrossRef]
- Belenguer, A.; Ben Bati, M.; Hervás, G.; Toral, P.G.; Yáñez-Ruiz, D.R.; Frutos, P. Impact of oxalic acid on rumen function and bacterial community in sheep. Animal 2013, 7, 940–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grilli, D.J.; Fliegerova, K.; Kopecný, J.; Lama, S.P.; Egea, V.; Sohaefer, N.; Pereyra, C.; Soledad Ruiz, M.; Sosa, M.A.; Arenas, G.N.; et al. Analysis of the rumen bacterial diversity of goats during shift from forage to concentrate diet. Anaerobe 2016, 42, 17–26. [Google Scholar] [CrossRef]
- Lin, H.; An, Y.; Hao, F.; Wang, Y.; Tang, H. Correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Narayanan, S.K.; Stewart, G.C.; Chengappa, M.M. Fusobacterium necrophorum infections in animals: Pathogenesis and pathogenic mechanisms. Anaerobe 2005, 11, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; DeLong, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes: Gammaproteobacteria, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–768. [Google Scholar] [CrossRef]
- Killer, J.; Kopečný, J.; Mrázek, J.; Havlík, J.; Koppová, I.; Benada, O.; Rada, V.; Kofroňová, O. Bombiscardovia coagulans gen. nov., sp. nov., a new member of the family Bifidobacteriaceae isolated from the digestive tract of bumblebees. Syst. Appl. Microbiol. 2010, 33, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, H.; Zheng, M.; Wang, K. Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Bioresour. Technol. 2015, 191, 53–58. [Google Scholar] [CrossRef]
- Combes, S.; Ikken, S.; Gidenne, T.; Balmisse, E.; Aymard, P.; Gabinaud, B.; Segura, M.; Barilly, C.; Travel, A. Influence de l’ingestion précoce ou du ratio protéine amidon sur les performances de croissance et le microbiote caecal. Journ. Rech. Cunicole 2017, 17, 87–90. [Google Scholar]
- Zened, A. Particularités du Microbiote et son Activité Lors de la Déviation de la Biohydrogénation Ruminale de L’acide Linoléique de la Voie Trans-11 à la Voie Trans-10. Ph.D. Thesis, Toulouse University, Toulouse, France, 2011. Available online: http://ethesis.inp-toulouse.fr/archive/00001711/ (accessed on 3 August 2020).
- Wang, L.; Li, P.; Tang, Z.; Yan, X.; Feng, B. Structural modulation of the gut microbiota and the relationship with body weight: Compared evaluation of liraglutide and saxagliptin treatment. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Cook, A.R.; Riley, P.W.; Murdoch, H.; Evans, P.N.; McDonald, I.R. Howardella ureilytica gen. nov., sp. nov., a Gram-positive, coccoid-shaped bacterium from a sheep rumen. Int. J. Syst. Evol. Microbiol. 2007, 57, 2940–2945. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Chen, W.J.; Adeolu, M.; Chai, Y. Molecular signatures for the class Coriobacteriia and its different clades; proposal for division of the class Coriobacteriia into the emended order Coriobacteriales, containing the emended family Coriobacteriaceae and Atopobiaceae fam. nov., and Eggerthe. Int. J. Syst. Evol. Microbiol. 2013, 63, 3379–3397. [Google Scholar] [CrossRef] [Green Version]
- Prins, R.A.; Lankhorst, A.; Van der Meer, P.; Van Nevel, C.J. Some characteristics of Anaerovibrio lipolytica, a rumen lipolytic organism. Antonie Van Leeuwenhoek 1975, 41, 1–11. [Google Scholar] [CrossRef]
- Singh, K.M.; Pandya, P.R.; Tripathi, A.K.; Patel, G.R.; Parnerkar, S.; Kothari, R.K.; Joshi, C.G. Study of rumen metagenome community using qPCR under different diets. Meta Gene 2014, 2, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, T.C. Lipid metabolism in the rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Pantoja, J.; Firkins, J.L.; Eastridge, M.L.; Hull, B.L. Effects of fat saturation and source of fibre on site of nutrient digestion and milk production by lactating dairy cows. J. Dairy Sci. 1994, 77, 2341–2356. [Google Scholar] [CrossRef]
- Mkhize, N.R.; Scogings, P.F.; Nsahlai, I.V.; Dziba, L.E. Diet selection of goats depends on season: Roles of plant physical and chemical traits. Afr. J. Range Forage Sci. 2014, 31, 209–214. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Sawanon, S.; Koike, S.; Kobayashi, Y. Evidence for the possible involvement of Selenomonas ruminantium in rumen fiber digestion. FEMS Microbiol. Lett. 2011, 325, 170–179. [Google Scholar] [CrossRef]
- Hocquette, J.; Picard, B.; Doreau, M.; Bauchart, D.; Micol, D. La viande des ruminants. De nouvelles approches pour améliorer et maîtriser la qualité. Viandes Prod. Carnés 2009, 24, 7–18. [Google Scholar]
- Guo, B.; Li, D.; Zhou, B.; Jiang, Y.; Bai, H.; Zhang, Y.; Xu, Q.; Zhao, W.; Chen, G. Comparative characterization of bacterial communities in geese consuming of different proportions of ryegrass. PLoS ONE 2019, 14, e0223445. [Google Scholar] [CrossRef] [PubMed]
- Alsaker, K.V.; Paredes, C.; Papoutsakis, E.T. Metabolite stress and tolerance in the production of biofuels and chemicals: Gene-expression-based systems analysis of butanol, butyrate, and acetate stresses in the anaerobe Clostridium acetobutylicum. Biotechnol. Bioeng. 2010, 105, 1131–1147. [Google Scholar] [CrossRef] [PubMed]
- Lerma-Reyes, I.; Mendoza-Martínez, G.D.; Rojo-Rubio, R.; Mejia, M.; García-Lopez, J.C.; Lee-Rangel, H.A. Influence of supplemental canola or soybean oil on milk yield, fatty acid profile and postpartum weight changes in grazing dairy goats. Asian Australas. J. Anim. Sci. 2018, 31, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.; Aguilar, C.; Lira, R.; Toro, P.; Barrales, L.; Peña, I.; Squella, F.; Pérez, P.; Quenaya, J.; Yutronic, H.; et al. Feeding dry olive cake modifies subcutaneous fat composition in lambs, noting cake resistance to degradation and peroxidation. Chil. J. Agric. Res. 2009, 69, 548–559. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
| Item | Diet 1 | |||
|---|---|---|---|---|
| Co | TOC | TCC | TOC+CC | |
| Diet ingredients (on DM basis)2 | ||||
| Oat hay (g/kg TD) | 508 | 427 | 466 | 456 |
| Barley (g/kg CD) | 390 | 0 | 0 | 0 |
| Olive cake (g/kg CD) | 0 | 350 | 0 | 150 |
| Cactus cladodes (g/kg CD) | 0 | 0 | 300 | 150 |
| Faba beans (g/kg CD) | 590 | 630 | 680 | 680 |
| Vitamin-mineral supplement (g/kg CD) | 20 | 20 | 20 | 20 |
| Chemical composition of diet | ||||
| Dry matter (DM; g/kg CM) | 885 | 887 | 292 | 435 |
| Ash (g/kg DM) | 47 | 47 | 77 | 62 |
| Crude protein (CP; g/kg DM) | 140 | 155 | 150 | 154 |
| Ether extract (EE; g/kg DM) | 35 | 60 | 33 | 44 |
| Neutral detergent fiber (NDF; g/kg DM) | 446 | 480 | 456 | 467 |
| Acid detergent fiber (ADF; g/kg DM) | 273 | 328 | 276 | 300 |
| Metabolizable energy (ME; MJ/kg DM) | 12 | 10 | 11 | 11 |
| Forage unit for meat (FUMeat/kg DM) | 0.8 | 0.7 | 0.7 | 0.7 |
| Digestible proteins in the intestines (DPI; g/kg DM) | 75 | 63 | 69 | 67 |
| Diet | Rumen Liquor | n | IVDMD | IVOMD |
|---|---|---|---|---|
| Co | Co | 18 | 0.560 | 0.540 |
| TOC | 6 | 0.596 | 0.575 | |
| TCC | 6 | 0.627 | 0.611 | |
| TOC+CC | 6 | 0.585 | 0.564 | |
| SEM | 0.012 | 0.013 | ||
| p-value | 0.242 | 0.246 | ||
| Toc | Co | 10 | 0.459 b | 0.436 b |
| TOC | 20 | 0.510 a | 0.489 a | |
| SEM | 0.013 | 0.013 | ||
| p-value | 0.021 | 0.022 | ||
| Tcc | Co | 10 | 0.571 b | 0.531 b |
| TCC | 20 | 0.607 a | 0.571 a | |
| SEM | 0.008 | 0.009 | ||
| p-value | 0.032 | 0.027 | ||
| Toc+cc | Co | 10 | 0.509 b | 0.477 b |
| TOC+CC | 20 | 0.585 a | 0.555 a | |
| SEM | 0.009 | 0.009 | ||
| p-value | <0.001 | <0.001 |
| Iterm | Co | TOC | TCC | TOC+CC | p-Value | SEM |
|---|---|---|---|---|---|---|
| Genus | ||||||
| Good’s coverage estimator (%) | 99.78 | 99.79 | 99.79 | 99.77 | 0.581 | 0.006 |
| Observed genus | 115.11 | 115.60 | 119.19 | 115.91 | 0.956 | 3.051 |
| Chao1 | 134.10 | 135.65 | 138.70 | 138.75 | 0.964 | 3.625 |
| Inverse Simpson index | 7.00 | 6.62 | 9.12 | 9.04 | 0.479 | 0.707 |
| Simpson evenness index | 0.058 | 0.057 | 0.074 | 0.071 | 0.516 | 0.005 |
| Species | ||||||
| Good’s coverage estimator (%) | 93.20 | 94.45 | 93.61 | 94.42 | 0.481 | 0.315 |
| Observed species | 1352 | 1162 | 1358 | 1144 | 0.464 | 61.8 |
| Chao1 | 2353 | 1950 | 2269 | 1945 | 0.434 | 105.1 |
| Inverse Simpson index | 29.20 | 47.23 | 67.17 | 59.96 | 0.508 | 9.36 |
| Simpson evenness index | 0.021 | 0.034 | 0.047 | 0.043 | 0.393 | 0.0057 |
| Family | Genus | Co | Toc | Tcc | Toc+cc | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| n = 11 | n = 11 | n = 11 | n = 11 | ||||
| Defluviitaleaceae | Defluviitaleaceae_UCG_011 | 0.017 b | 0.027 ab | 0.037 a | 0.012 b | 0.005 | 0.015 |
| Lachnospiraceae | Butyrivibrio_2 | 0.204 | 0.377 | 0.159 | 0.225 | 0.031 | 0.082 |
| Howardella | 0.082 | 0.043 | 0.054 | 0.008 | 0.016 | 0.076 | |
| Lachnoclostridium_10 | 0.023 | 0.032 | 0.058 | 0.022 | 0.008 | 0.246 | |
| Lachnospiraceae_NK4B4_group | 0.000 b | 0.005 a | 0.001 b | 0.000 b | 0.0006 | 0.044 | |
| Marvinbryantia | 0.142 | 0.157 | 0.237 | 0.098 | 0.022 | 0.067 | |
| Fusobacteriaceae | Fusobacterium | 0.000 | 0.000 | 0.000 | 0.002 | 0.0003 | 0.052 |
| Bifidobacteriaceae | Aeriscardovia | 0.012 a | 0.000 b | 0.001 b | 0.000 b | 0.0016 | 0.044 |
| Atopobiaceae | Atopobiaceae_ge | 0.010 | 0.039 | 0.029 | 0.042 | 0.007 | 0.096 |
| Veillonellaceae | Anaerovibrio | 0.011 | 0.025 | 0.008 | 0.011 | 0.003 | 0.128 |
| Selenomonas_1 | 0.346 | 0.372 | 0.182 | 0.171 | 0.038 | 0.098 | |
| NED5E9_fa | NED5E9_ge | 0.000 | 0.000 | 0.002 | 0.007 | 0.001 | 0.057 |
| Ruminococcaceae | Ruminococcaceae_UCG-001 | 0.029 | 0.072 | 0.047 | 0.007 | 0.018 | 0.095 |
| Item | Co | TOC | TCC | TOC+CC | SEM | p-Value |
|---|---|---|---|---|---|---|
| N | 11 | 11 | 11 | 11 | ||
| C4:0 | 0.308 | 0.457 | 0.370 | 0.452 | 0.039 | 0.651 |
| C6:0 | 0.319 ab | 0.443 a | 0.207 b | 0.235 b | 0.026 | 0.011 |
| C8:0 | 0.121 | 0.448 | 0.379 | 0.330 | 0.044 | 0.102 |
| C10:0 | 0.385 | 0.120 | 0.254 | 0.135 | 0.044 | 0.194 |
| C11:0 | 0.246 b | 0.692 a | 0.222 b | 0.426 ab | 0.057 | 0.025 |
| C12:0 | 0.831 a | 0.193 b | 0.220 b | 0.216 b | 0.078 | 0.033 |
| C13:0 | 0.268 | 0.299 | 0.274 | 0.157 | 0.031 | 0.183 |
| C14:0 | 0.984 | 1.11 | 0.967 | 1.25 | 0.056 | 0.234 |
| C14:1 | 0.514 | 0.601 | 0.474 | 0.676 | 0.035 | 0.212 |
| C15:0 | 6.81 | 5.85 | 5.62 | 5.29 | 0.377 | 0.804 |
| C15:1 | 0.888 | 0.519 | 0.613 | 0.572 | 0.062 | 0.461 |
| C16:0 | 12.5 | 12.2 | 14.7 | 13.5 | 0.507 | 0.112 |
| C16:1 | 1.62 | 2.20 | 1.80 | 1.43 | 0.136 | 0.356 |
| C17:0 | 3.30 | 4.54 | 4.11 | 4.08 | 0.272 | 0.609 |
| C17:1 | 1.74 | 1.25 | 1.36 | 1.13 | 0.096 | 0.286 |
| C18:0 | 14.9 | 18.4 | 17.8 | 18.3 | 0.799 | 0.454 |
| 9t-C18:1 | 1.03 | 1.47 | 0.777 | 0.792 | 0.097 | 0.068 |
| C18:1n-9 | 25.5 | 22.1 | 24.9 | 25.0 | 1.071 | 0.656 |
| 6t-C18:2 | 0.497 | 0.443 | 0.418 | 0.275 | 0.039 | 0.270 |
| C18:2n-6 | 6.12 | 5.15 | 4.76 | 6.14 | 0.238 | 0.184 |
| C20:0 | 0.430 | 0.381 | 0.764 | 0.276 | 0.081 | 0.059 |
| C18:3n-6 | 0.407 | 0.337 | 0.944 | 0.337 | 0.110 | 0.080 |
| C20:1 | 0.375 | 0.383 | 1.03 | 0.449 | 0.149 | 0.305 |
| C18:3n-3 | 0.489 | 0.687 | 0.546 | 0.620 | 0.054 | 0.668 |
| C21:0 | 1.24 ab | 1.93 a | 0.501 b | 0.727 b | 0.162 | 0.007 |
| C20:2 | 0.547 | 0.777 | 0.433 | 0.508 | 0.082 | 0.615 |
| C22:0 | 1.90 | 1.24 | 1.36 | 1.54 | 0.092 | 0.267 |
| C20:3n-6 | 0.503 | 0.606 | 0.436 | 0.764 | 0.052 | 0.202 |
| C22:1n-9 | 0.362 | 0.364 | 0.662 | 0.522 | 0.077 | 0.485 |
| C20:3n-3 | 0.309 | 0.310 | 0.283 | 0.410 | 0.035 | 0.520 |
| C20:4n-6 | 1.02 | 0.526 | 1.05 | 0.593 | 0.114 | 0.253 |
| C23:0 | 4.89 | 6.16 | 5.68 | 5.35 | 0.248 | 0.306 |
| C22:2 | 0.401 c | 1.05 a | 0.410 bc | 0.969 ab | 0.089 | 0.015 |
| C24:0 | 1.18 | 1.82 | 1.17 | 1.12 | 0.141 | 0.350 |
| C20:5n-3 | 0.734 | 1.36 | 1.15 | 1.40 | 0.128 | 0.385 |
| C24:1 | 3.57 a | 1.26 b | 1.56 b | 1.36 b | 0.205 | 0.002 |
| C22:6n-3 | 2.86 | 2.25 | 1.80 | 2.65 | 0.144 | 0.071 |
| Summary | ||||||
| SCFA | 1.13 | 1.47 | 1.21 | 1.15 | 0.089 | 0.424 |
| MCFA | 2.84 | 2.90 | 2.16 | 2.73 | 0.330 | 0.560 |
| LCFA | 96.0 | 95.6 | 96.6 | 96.1 | 0.376 | 0.451 |
| SFA | 50.6 | 56.4 | 54.6 | 53.5 | 0.883 | 0.195 |
| MUFA | 35.2 | 29.8 | 32.5 | 31.4 | 0.866 | 0.520 |
| PUFA | 13.9 | 13.5 | 12.2 | 14.7 | 0.650 | 0.087 |
| DFA | 64.0 | 61.7 | 62.5 | 64.4 | 0.755 | 0.733 |
| n-3 | 4.39 | 4.61 | 3.78 | 5.08 | 0.237 | 0.059 |
| n-6 | 8.55 | 7.07 | 7.61 | 8.11 | 0.344 | 0.409 |
| n-9 | 26.9 | 23.9 | 26.3 | 26.3 | 0.943 | 0.244 |
| Ratio | ||||||
| UFA/SFA | 0.971 | 0.768 | 0.819 | 0.861 | 0.035 | 0.207 |
| n-6/n-3 | 1.94 | 1.53 | 2.01 | 1.60 | 0.134 | 0.421 |
| PUFA/SFA | 0.275 | 0.240 | 0.224 | 0.274 | 0.019 | 0.303 |
| MUFA/PUFA | 2.54 | 2.20 | 2.66 | 2.14 | 0.312 | 0.513 |
| Index | ||||||
| AI | 0.351 | 0.390 | 0.420 | 0.408 | 0.014 | 0.420 |
| TI | 0.796 | 0.966 | 1.05 | 0.931 | 0.060 | 0.619 |
| (C18:0+C18:1)/C16:0 | 3.32 | 3.43 | 2.95 | 3.26 | 0.086 | 0.058 |
| Δ9C14 | 0.343 | 0.350 | 0.329 | 0.351 | 0.016 | 0.922 |
| Δ9C16 | 0.115 | 0.152 | 0.109 | 0.095 | 0.013 | 0.148 |
| Δ9C18 | 0.641 | 0.561 | 0.591 | 0.584 | 0.013 | 0.599 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Otmani, S.; Chebli, Y.; Taminiau, B.; Chentouf, M.; Hornick, J.-L.; Cabaraux, J.-F. Effect of Olive Cake and Cactus Cladodes Incorporation in Goat Kids’ Diet on the Rumen Microbial Community Profile and Meat Fatty Acid Composition. Biology 2021, 10, 1237. https://doi.org/10.3390/biology10121237
El Otmani S, Chebli Y, Taminiau B, Chentouf M, Hornick J-L, Cabaraux J-F. Effect of Olive Cake and Cactus Cladodes Incorporation in Goat Kids’ Diet on the Rumen Microbial Community Profile and Meat Fatty Acid Composition. Biology. 2021; 10(12):1237. https://doi.org/10.3390/biology10121237
Chicago/Turabian StyleEl Otmani, Samira, Youssef Chebli, Bernard Taminiau, Mouad Chentouf, Jean-Luc Hornick, and Jean-François Cabaraux. 2021. "Effect of Olive Cake and Cactus Cladodes Incorporation in Goat Kids’ Diet on the Rumen Microbial Community Profile and Meat Fatty Acid Composition" Biology 10, no. 12: 1237. https://doi.org/10.3390/biology10121237
APA StyleEl Otmani, S., Chebli, Y., Taminiau, B., Chentouf, M., Hornick, J.-L., & Cabaraux, J.-F. (2021). Effect of Olive Cake and Cactus Cladodes Incorporation in Goat Kids’ Diet on the Rumen Microbial Community Profile and Meat Fatty Acid Composition. Biology, 10(12), 1237. https://doi.org/10.3390/biology10121237







