High-Risk Human Papillomavirus and Epstein–Barr Virus Coinfection: A Potential Role in Head and Neck Carcinogenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Human Papillomavirus and Epstein–Barr Virus Tropism
3. Epidemiology of HPV/EBV Coinfection in HNSCCs
3.1. Oral Cavity
3.2. Nasopharynx
3.3. Oropharynx
3.4. HPV and EBV Infection in Other HNSCCs
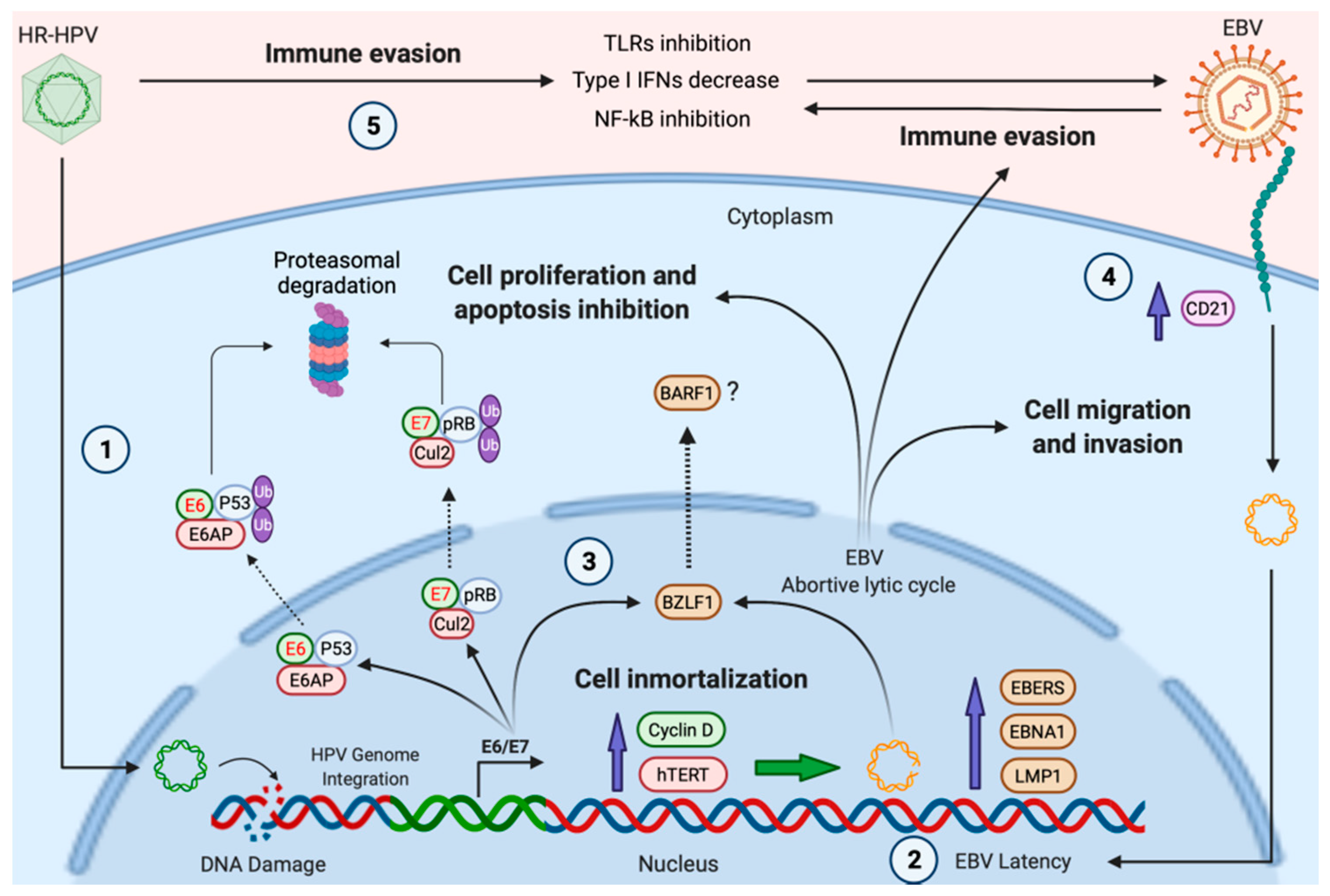
4. Possible Mechanisms of HR-HPV/EBV Cooperation
4.1. HPV Facilitates EBV Entry in Epithelial Cells
4.2. HPV Infection Could Promote EBV Latency Establishment and Lytic Cycle Activation in Head and Neck Epithelial Cells
4.3. Immune Evasion Orchestrated by HPV Facilitates the EBV Second Infection
4.3.1. Modulation of Toll-Like Receptors (TLRs)
4.3.2. Regulation of IRF Signaling and IFNs Production
4.3.3. Interference with Other Innate Effector Molecules
4.3.4. Disruption of NF-κB Signaling Pathway
4.3.5. Downregulation of Major Histocompatibility Complex (MHC) by HPV Oncoproteins
4.4. Other Mechanisms Involved in EBV/HPV-Associated HNCs
4.4.1. Cooperation between HR-HPV E6 and EBV LMP1
4.4.2. Gene Promoter Methylation Promoted by HR-HPV/EBV Infection
4.4.3. Reduced Activity of Detoxifying Enzymes Promoted by HR-HPV/EBV Infection
4.4.4. Gene Polymorphisms and HR-HPV/EBV Coinfection
5. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.S.; Siddiqui, S.A.; Perween, R. Epidemiological profile of head and neck cancer patients in Western Uttar Pradesh and analysis of distributions of risk factors in relation to site of tumor. J. Cancer Res. Ther. 2017, 13, 430–435. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Chien, Y.C.; Chen, J.Y.; Liu, M.Y.; Yang, H.I.; Hsu, M.M.; Chen, C.J.; Yang, C.S. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 2001, 345, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Batur, P. Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines. Clevel. Clin. J. Med. 2019, 86, 173–178. [Google Scholar] [CrossRef]
- Drop, B.; Strycharz-Dudziak, M.; Kliszczewska, E.; Polz-Dacewicz, M. Coinfection with Epstein-Barr Virus (EBV), Human Papilloma Virus (HPV) and Polyoma BK Virus (BKPyV) in Laryngeal, Oropharyngeal and Oral Cavity Cancer. Int. J. Mol. Sci. 2017, 18, 2752. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Ekshyyan, O.; Moore-Medlin, T.; Rong, X.; Nathan, S.; Gu, X.; Abreo, F.; Rosenthal, E.L.; Shi, M.; Guidry, J.T.; et al. Association between human papilloma virus/Epstein-Barr virus coinfection and oral carcinogenesis. J. Oral Pathol. Med. 2015, 44, 28–36. [Google Scholar] [CrossRef]
- Laantri, N.; Attaleb, M.; Kandil, M.; Naji, F.; Mouttaki, T.; Dardari, R.; Belghmi, K.; Benchakroun, N.; El Mzibri, M.; Khyatti, M. Human papillomavirus detection in moroccan patients with nasopharyngeal carcinoma. Infect. Agents Cancer 2011, 6, 3. [Google Scholar] [CrossRef]
- Gupta, I.; Ghabreau, L.; Al-Thawadi, H.; Yasmeen, A.; Vranic, S.; Al Moustafa, A.E.; Malki, M.I. Co-incidence of Human Papillomaviruses and Epstein-Barr Virus Is Associated With High to Intermediate Tumor Grade in Human Head and Neck Cancer in Syria. Front. Oncol. 2020, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawadi, H.; Gupta, I.; Jabeen, A.; Skenderi, F.; Aboulkassim, T.; Yasmeen, A.; Malki, M.I.; Batist, G.; Vranic, S.; Al Moustafa, A.E. Co-presence of human papillomaviruses and Epstein-Barr virus is linked with advanced tumor stage: A tissue microarray study in head and neck cancer patients. Cancer Cell Int. 2020, 20, 361. [Google Scholar] [CrossRef]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef] [PubMed]
- Möhl, B.S.; Chen, J.; Sathiyamoorthy, K.; Jardetzky, T.S.; Longnecker, R. Structural and Mechanistic Insights into the Tropism of Epstein-Barr Virus. Mol. Cells 2016, 39, 286–291. [Google Scholar] [CrossRef]
- McBride, A.A. Human papillomaviruses: Diversity, infection and host interactions. Nat. Rev. Microbiol. 2021, 1–14. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Surviladze, Z.; Sterkand, R.T.; Ozbun, M.A. Interaction of human papillomavirus type 16 particles with heparan sulfate and syndecan-1 molecules in the keratinocyte extracellular matrix plays an active role in infection. J. Gen. Virol. 2015, 96, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Ozbun, M.A.; Campos, S.K. The long and winding road: Human papillomavirus entry and subcellular trafficking. Curr. Opin. Virol. 2021, 50, 76–86. [Google Scholar] [CrossRef]
- Werner, J.; Decarlo, C.A.; Escott, N.; Zehbe, I.; Ulanova, M. Expression of integrins and Toll-like receptors in cervical cancer: Effect of infectious agents. Innate Immun. 2011, 18, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.; Filippova, M.; Filippov, V.; Bashkirova, S.; Zhang, G.; Reeves, M.E.; Duerksen-Hughes, P. Overexpression of HPV16 E6* Alters β-Integrin and Mitochondrial Dysfunction Pathways in Cervical Cancer Cells. Cancer Genom. Proteom. 2016, 13, 259–273. [Google Scholar]
- Eckhardt, M.; Zhang, W.; Gross, A.M.; Von Dollen, J.; Johnson, J.R.; Franks-Skiba, K.E.; Swaney, D.L.; Johnson, T.L.; Jang, G.M.; Shah, P.S.; et al. Multiple Routes to Oncogenesis Are Promoted by the Human Papillomavirus-Host Protein Network. Cancer Discov. 2018, 8, 1474–1489. [Google Scholar] [CrossRef]
- Romero-Medina, M.C.; Venuti, A.; Melita, G.; Robitaille, A.; Ceraolo, M.G.; Pacini, L.; Sirand, C.; Viarisio, D.; Taverniti, V.; Gupta, P.; et al. Human papillomavirus type 38 alters wild-type p53 activity to promote cell proliferation via the downregulation of integrin alpha 1 expression. PLoS Pathog. 2020, 16, e1008792. [Google Scholar] [CrossRef]
- Hutt-Fletcher, L.M. The Long and Complicated Relationship between Epstein-Barr Virus and Epithelial Cells. J. Virol. 2017, 91, e01677-16. [Google Scholar] [CrossRef]
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The interplay between Epstein-Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Temple, R.M.; Zhu, J.; Budgeon, L.; Christensen, N.D.; Meyers, C.; Sample, C.E. Efficient replication of Epstein-Barr virus in stratified epithelium in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 16544–16549. [Google Scholar] [CrossRef]
- Chen, J.; Sathiyamoorthy, K.; Zhang, X.; Schaller, S.; Perez White, B.E.; Jardetzky, T.S.; Longnecker, R. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat. Microbiol. 2018, 3, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Wang, H.B.; Zhang, A.; Chen, M.L.; Fang, Z.X.; Dong, X.D.; Li, S.B.; Du, Y.; Xiong, D.; et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat. Microbiol. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Qiu, Y.; Huang, D.; Zhang, S.; Xie, L.; Qi, L.; Yu, C.; Zhou, X.; Hu, G.; et al. Clinical significance of EphA2 expression in squamous-cell carcinoma of the head and neck. J. Cancer Res. Clin. Oncol. 2011, 137, 761–769. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lin, S.J.; Lin, K.M.; Chou, Y.C.; Lin, C.W.; Yu, S.C.; Chen, C.L.; Shen, T.L.; Chen, C.K.; Lu, J.; et al. Regulation of EBV LMP1-triggered EphA4 downregulation in EBV-associated B lymphoma and its impact on patients’ survival. Blood 2016, 128, 1578–1589. [Google Scholar] [CrossRef]
- Chesnokova, L.S.; Nishimura, S.L.; Hutt-Fletcher, L.M. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc. Natl. Acad. Sci. USA 2009, 106, 20464–20469. [Google Scholar] [CrossRef]
- Chesnokova, L.S.; Hutt-Fletcher, L.M. Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J. Virol. 2011, 85, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, E.M.; Wildner, G.P.; Kruse-Boitschenko, U.; Hoffmeister, B.; Goodman, S.L.; Raguse, J.D. Immunohistochemical analysis of integrins αvβ3, αvβ5 and α5β1, and their ligands, fibrinogen, fibronectin, osteopontin and vitronectin, in frozen sections of human oral head and neck squamous cell carcinomas. Exp. Ther. Med. 2011, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Kan, X.; Li, Y.; Liu, M.; Lu, J.G. Elevated expression of integrin αv and β5 subunit in laryngeal squamous-cell carcinoma associated with lymphatic metastasis and angiogenesis. Pathol. Res. Pract. 2013, 209, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.R.; Chang, Y.; Chen, Y.L.; Hsieh, S.H.; Hsu, K.F.; Wang, C.F.; Tsai, S.T.; Jin, Y.T. Cyclic alphavbeta6-targeting peptide selected from biopanning with clinical potential for head and neck squamous cell carcinoma. Head Neck 2010, 32, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Yachie, A. Cell type specific infection of Epstein-Barr virus (EBV) in EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Crit. Rev. Oncol. Hematol. 2002, 44, 283–294. [Google Scholar] [CrossRef]
- Horvath, C.A.; Boulet, G.A.; Renoux, V.M.; Delvenne, P.O.; Bogers, J.P. Mechanisms of cell entry by human papillomaviruses: An overview. Virol. J. 2010, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Longnecker, R. Epithelial cell infection by Epstein-Barr virus. FEMS Microbiol. Rev. 2019, 43, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, P.; Gottschalk, E.Y.; Meneses, P.I. HPV entry into cells. Mutat. Res. Rev. Mutat. Res. 2017, 772, 13–22. [Google Scholar] [CrossRef]
- Speight, P.M.; Farthing, P.M. The pathology of oral cancer. Br. Dent. J. 2018, 225, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Krüger, M.; Pabst, A.M.; Walter, C.; Sagheb, K.; Günther, C.; Blatt, S.; Weise, K.; Al-Nawas, B.; Ziebart, T. The prevalence of human papilloma virus (HPV) infections in oral squamous cell carcinomas: A retrospective analysis of 88 patients and literature overview. J. Craniomaxillofac. Surg. 2014, 42, 1506–1514. [Google Scholar] [CrossRef]
- Petito, G.; Carneiro, M.A.; Santos, S.H.; Silva, A.M.; Alencar, R.C.; Gontijo, A.P.; Saddi, V.A. Human papillomavirus in oral cavity and oropharynx carcinomas in the central region of Brazil. Braz. J. Otorhinolaryngol. 2017, 83, 38–44. [Google Scholar] [CrossRef]
- Chakrobarty, B.; Roy, J.G.; Majumdar, S.; Uppala, D. Relationship among tobacco habits, human papilloma virus (HPV) infection, p53 polymorphism/mutation and the risk of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2014, 18, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Termine, N.; Panzarella, V.; Falaschini, S.; Russo, A.; Matranga, D.; Lo Muzio, L.; Campisi, G. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: A meta-analysis (1988–2007). Ann. Oncol. 2008, 19, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ling, Y.; Dong, C.; Zhou, X.; Wang, F. The relationship between oral squamous cell carcinoma and human papillomavirus: A meta-analysis of a Chinese population (1994–2011). PLoS ONE 2012, 7, e36294. [Google Scholar] [CrossRef]
- Melo, B.A.C.; Vilar, L.G.; de Oliveira, N.R.; de Lima, P.O.; Pinheiro, M.B.; Domingueti, C.P.; Pereira, M.C. Human papillomavirus infection and oral squamous cell carcinoma—A systematic review. Braz. J. Otorhinolaryngol. 2021, 87, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Rojas-Alcayaga, G.; Pennacchiotti, G.; Carrillo, D.; Muñoz, J.P.; Peña, N.; Montes, R.; Lobos, N.; Aguayo, F. Human papillomavirus infection in oral squamous cell carcinomas from Chilean patients. Exp. Mol. Pathol. 2015, 99, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Pandya, S.; Singh, M.; Mehrotra, R. Identification of high-risk human papillomavirus-16 and -18 infections by multiplex PCR and their expression in oral submucous fibrosis and oal squamous cell carcinoma. Head Neck Oncol. 2013, 5, 1–10. [Google Scholar]
- Yen, C.Y.; Lu, M.C.; Tzeng, C.C.; Huang, J.Y.; Chang, H.W.; Chen, R.S.; Liu, S.Y.; Liu, S.T.; Shieh, B.; Li, C. Detection of EBV infection and gene expression in oral cancer from patients in Taiwan by microarray analysis. J. Biomed. Biotechnol. 2009, 2009, 904589. [Google Scholar] [CrossRef]
- Acharya, S.; Ekalaksananan, T.; Vatanasapt, P.; Loyha, K.; Phusingha, P.; Promthet, S.; Kongyingyoes, B.; Pientong, C. Association of Epstein-Barr virus infection with oral squamous cell carcinoma in a case-control study. J. Oral Pathol. Med. 2014, 44, 252–257. [Google Scholar] [CrossRef]
- Heawchaiyaphum, C.; Iizasa, H.; Ekalaksananan, T.; Burassakarn, A.; Kiyono, T.; Kanehiro, Y.; Yoshiyama, H.; Pientong, C. Epstein-Barr Virus Infection of Oral Squamous Cells. Microorganisms 2020, 8, 419. [Google Scholar] [CrossRef]
- Kikuchi, K.; Noguchi, Y.; de Rivera, M.W.G.-N.; Hoshino, M.; Sakashita, H.; Yamada, T.; Inoue, H.; Miyazaki, Y.; Nozaki, T.; González-López, B.S.; et al. Detection of Epstein-Barr virus genome and latent infection gene expression in normal epithelia, epithelial dysplasia, and squamous cell carcinoma of the oral cavity. Tumor Biol. 2016, 37, 3389–3404. [Google Scholar] [CrossRef]
- Rahman, R.; Poomsawat, S.; Juengsomjit, R.; Buajeeb, W. Overexpression of Epstein-Barr virus-encoded latent membrane protein-1 (LMP-1) in oral squamous cell carcinoma. BMC Oral Health 2019, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Nong, X.; Zhang, M.; Wang, M. Epstein-Barr virus infection and oral squamous cell carcinoma risk: A meta-analysis. PLoS ONE 2017, 12, e0186860. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.A.P.; Teodoro, I.P.P.; de Galiza, L.E.; Filho, P.H.B.M.; Marques, F.M.; Pinheiro Junior, R.F.F.; Macedo, G.E.C.; Facundo, H.T.; da Silva, C.G.L.; Lima, M.V.A. Association between Epstein-Barr Virus and Oral Carcinoma: A Systematic Review with Meta-Analysis. Crit. Rev. Oncog. 2019, 24, 349–368. [Google Scholar] [CrossRef]
- Jalouli, J.; Jalouli, M.M.; Sapkota, D.; Ibrahim, S.O.; Larsson, P.A.; Sand, L. Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res. 2012, 32, 571–580. [Google Scholar] [PubMed]
- Polz-Gruszka, D.; Morshed, K.; Jarzyński, A.; Polz-Dacewicz, M. Prevalence of Polyoma BK Virus (BKPyV), Epstein-Barr Virus (EBV) and Human Papilloma Virus (HPV) in Oropharyngeal Cancer. Pol. J. Microbiol. 2015, 64, 323–328. [Google Scholar] [CrossRef]
- Vanshika, S.; Preeti, A.; Sumaira, Q.; Vijay, K.; Shikha, T.; Shivanjali, R.; Shankar, S.U.; Mati, G.M. Incidence OF HPV and EBV in oral cancer and their clinico-pathological correlation—A pilot study of 108 cases. J. Oral Biol. Craniofac. Res. 2021, 11, 180–184. [Google Scholar] [CrossRef]
- Broccolo, F.; Ciccarese, G.; Rossi, A.; Anselmi, L.; Drago, F.; Toniolo, A. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in keratinizing versus non- keratinizing squamous cell carcinoma of the oropharynx. Infect. Agents Cancer 2018, 13, 32. [Google Scholar] [CrossRef]
- Naqvi, S.U.; Khan, S.; Ahmed, A.; Lail, A.; Gul, S.; Ahmed, S. Prevalence of EBV, CMV, and HPV in oral squamous cell carcinoma patients in the Pakistani population. J. Med. Virol. 2020, 92, 3880–3883. [Google Scholar] [CrossRef]
- Tang, L.L.; Chen, W.Q.; Xue, W.Q.; He, Y.Q.; Zheng, R.S.; Zeng, Y.X.; Jia, W.H. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016, 374, 22–30. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, W.; Jin, M.; Zhang, J.; Li, S.; Tong, F.; Zhou, Y. Differential expression of EBV proteins LMP1 and BHFR1 in EBV-associated gastric and nasopharyngeal cancer tissues. Mol. Med. Rep. 2016, 13, 4151–4158. [Google Scholar] [CrossRef]
- Pan, J.J.; Ng, W.T.; Zong, J.F.; Chan, L.L.; O’Sullivan, B.; Lin, S.J.; Sze, H.C.; Chen, Y.B.; Choi, H.C.; Guo, Q.J.; et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016, 122, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.L.; Chen, Y.P.; Mao, Y.P.; Wang, Z.X.; Guo, R.; Chen, L.; Tian, L.; Lin, A.H.; Li, L.; Sun, Y.; et al. Validation of the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma From Endemic Areas in the Intensity-Modulated Radiotherapy Era. J. Natl. Compr. Cancer Netw. 2017, 15, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Okekpa, S.I.; Mydin, R.B.S.M.N.; Mangantig, E.; Azmi, N.S.A.; Zahari, S.N.S.; Kaur, G.; Musa, Y. Nasopharyngeal Carcinoma (NPC) Risk Factors: A Systematic Review and Meta-Analysis of the Association with Lifestyle, Diets, Socioeconomic and Sociodemographic in Asian Region. Asian Pac. J. Cancer Prev. 2019, 20, 3505–3514. [Google Scholar] [CrossRef] [PubMed]
- Bei, J.X.; Zuo, X.Y.; Liu, W.S.; Guo, Y.M.; Zeng, Y.X. Genetic susceptibility to the endemic form of NPC. Chin. Clin. Oncol. 2016, 5, 15. [Google Scholar] [CrossRef]
- Fan, H.C.; Chen, C.Y.; Hsu, Y.C.; Chou, R.H.; Teng, C.J.; Chiu, C.H.; Hsu, C.Y.; Muo, C.H.; Chang, M.Y.; Chang, K.H. Increased risk of incident nasopharyngeal carcinoma with exposure to air pollution. PLoS ONE 2018, 13, e0204568. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Yu, D.; Liu, Y.; Xue, K.; Zhao, X. Role of Epstein-Barr Virus in the Development of Nasopharyngeal Carcinoma. Open Med. 2017, 12, 171–176. [Google Scholar] [CrossRef]
- Fan, C.; Tang, Y.; Wang, J.; Xiong, F.; Guo, C.; Wang, Y.; Xiang, B.; Zhou, M.; Li, X.; Wu, X.; et al. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J. Cancer 2018, 9, 2852–2864. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef]
- Asante, D.B.; Asmah, R.H.; Adjei, A.A.; Kyei, F.; Simpong, D.L.; Brown, C.A.; Gyasi, R.K. Detection of Human Papillomavirus Genotypes and Epstein-Barr Virus in Nasopharyngeal Carcinomas at the Korle-Bu Teaching Hospital, Ghana. Sci. World J. 2017, 2017, 2721367. [Google Scholar] [CrossRef]
- Breda, E.; Catarino, R.J.; Azevedo, I.; Lobão, M.; Monteiro, E.; Medeiros, R. Epstein-Barr virus detection in nasopharyngeal carcinoma: Implications in a low-risk area. Braz. J. Otorhinolaryngol. 2010, 76, 310–315. [Google Scholar] [CrossRef]
- Dogan, S.; Hedberg, M.L.; Ferris, R.L.; Rath, T.J.; Assaad, A.M.; Chiosea, S.I. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck 2014, 36, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Svajdler, M.; Kaspirkova, J.; Mezencev, R.; Laco, J.; Torday, T.; Dubinsky, P.; Straka, L.; Ondic, O.; Michal, M.; Skalova, A. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a non-endemic eastern european population. Neoplasma 2016, 63, 107–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruuskanen, M.; Irjala, H.; Minn, H.; Vahlberg, T.; Randen-Brady, R.; Hagström, J.; Syrjänen, S.; Leivo, I. Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: A nationwide study in Finland. Head Neck 2019, 41, 349–357. [Google Scholar] [CrossRef]
- Rumayor Piña, A.; Dos Santos, H.T.; Carlos, R.; Altemani, A.; de Almeida, O.P. Epstein-Barr Virus in Nasopharyngeal Carcinoma of Guatemalan and Brazilian Patients. Int. J. Surg. Pathol. 2017, 25, 304–309. [Google Scholar] [CrossRef]
- Tatlı Doğan, H.; Kılıçarslan, A.; Doğan, M.; Süngü, N.; Güler Tezel, G.; Güler, G. Retrospective analysis of oncogenic human papilloma virus and Epstein-Barr virus prevalence in Turkish nasopharyngeal cancer patients. Pathol. Res. Pract. 2016, 212, 1021–1026. [Google Scholar] [CrossRef]
- Tham, T.; Machado, R.; Russo, D.P.; Herman, S.W.; Teegala, S.; Costantino, P. Viral markers in nasopharyngeal carcinoma: A systematic review and meta-analysis on the detection of p16INK4a, human papillomavirus (HPV), and Ebstein-Barr virus (EBV). Am. J. Otolaryngol. 2021, 42, 102762. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, C.; Song, Y.; Li, Q.; Huang, J.; Ma, X. Epstein-Barr virus DNA level as a novel prognostic factor in nasopharyngeal carcinoma: A meta-analysis. Medicine 2016, 95, e5130. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, L.; Zhang, Y.; Guo, R.; Li, W.F.; Mao, Y.P.; Tan, L.L.; Sun, Y.; Zhang, F.; Liu, L.Z.; et al. Survival analysis of patients with advanced-stage nasopharyngeal carcinoma according to the Epstein-Barr virus status. Oncotarget 2016, 7, 24208–24216. [Google Scholar] [CrossRef]
- Liu, T.B.; Zheng, Z.H.; Pan, J.; Pan, L.L.; Chen, L.H. Prognostic role of plasma Epstein-Barr virus DNA load for nasopharyngeal carcinoma: A meta-analysis. Clin. Investig. Med. 2017, 40, E1–E12. [Google Scholar] [CrossRef]
- Du, Y.Y.; Luo, D.H.; Sun, X.S.; Tang, L.Q.; Mai, H.Q.; Chen, Q.Y.; Zhong, J.H.; Mai, D.M.; Zhang, W.R.; Chen, W.H.; et al. Combining pretreatment plasma Epstein-Barr virus DNA level and cervical node necrosis improves prognostic stratification in patients with nasopharyngeal carcinoma: A cohort study. Cancer Med. 2019, 8, 6841–6852. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.S.; Forslund, O.; Andersson, F.C.; Lindstedt, M.; Greiff, L. Intralesional EBV-DNA load as marker of prognosis for nasopharyngeal cancer. Sci. Rep. 2019, 9, 15432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Zeng, S.; Hu, X. LMP1 expression is positively associated with metastasis of nasopharyngeal carcinoma: Evidence from a meta-analysis. J. Clin. Pathol. 2012, 65, 41–45. [Google Scholar] [CrossRef]
- Hu, C.; Wei, W.; Chen, X.; Woodman, C.B.; Yao, Y.; Nicholls, J.M.; Joab, I.; Sihota, S.K.; Shao, J.Y.; Derkaoui, K.D.; et al. A global view of the oncogenic landscape in nasopharyngeal carcinoma: An integrated analysis at the genetic and expression levels. PLoS ONE 2012, 7, e41055. [Google Scholar] [CrossRef]
- Luo, F.-F.; Li, Z.-Y.; Huang, S.-N.; Chen, G.; Xie, T.-T.; LI, Y.-Q.; Xing, W.-W.; Li, W.-Y.; Lu, Y.-K.; Ding, H. Prevalence of human papillomavirus in patients with nasopharyngeal carcinoma: A meta-analysis. Int. J. Clin. Exp. Med. 2017, 10, 9837–9847. [Google Scholar]
- Stenmark, M.H.; McHugh, J.B.; Schipper, M.; Walline, H.M.; Komarck, C.; Feng, F.Y.; Worden, F.P.; Wolf, G.T.; Chepeha, D.B.; Prince, M.E.; et al. Nonendemic HPV-positive nasopharyngeal carcinoma: Association with poor prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 580–588. [Google Scholar] [CrossRef]
- Tung, Y.C.; Lin, K.H.; Chu, P.Y.; Hsu, C.C.; Kuo, W.R. Detection of human papilloma virus and Epstein-Barr virus DNA in nasopharyngeal carcinoma by polymerase chain reaction. Kaohsiung J. Med. Sci. 1999, 15, 256–262. [Google Scholar]
- Mirzamani, N.; Salehian, P.; Farhadi, M.; Tehran, E.A. Detection of EBV and HPV in nasopharyngeal carcinoma by in situ hybridization. Exp. Mol. Pathol. 2006, 81, 231–234. [Google Scholar] [CrossRef]
- Jiang, W.; Chamberlain, P.D.; Garden, A.S.; Kim, B.Y.; Ma, D.; Lo, E.J.; Bell, D.; Gunn, G.B.; Fuller, C.D.; Rosenthal, D.I.; et al. Prognostic value of p16 expression in Epstein-Barr virus-positive nasopharyngeal carcinomas. Head Neck 2016, 38, E1459–E1466. [Google Scholar] [CrossRef]
- Simon, J.; Schroeder, L.; Ingarfield, K.; Diehl, S.; Werner, J.; Brenner, N.; Liu, Z.; Pawlita, M.; Pring, M.; Butt, J.; et al. Epstein-Barr virus and human papillomavirus serum antibodies define the viral status of nasopharyngeal carcinoma in a low endemic country. Int. J. Cancer 2020, 147, 461–471. [Google Scholar] [CrossRef]
- Osborne, R.F.; Brown, J.J. Carcinoma of the oral pharynx: An analysis of subsite treatment heterogeneity. Surg. Oncol. Clin. N. Am. 2004, 13, 71–80. [Google Scholar] [CrossRef]
- Pan, C.; Issaeva, N.; Yarbrough, W.G. HPV-driven oropharyngeal cancer: Current knowledge of molecular biology and mechanisms of carcinogenesis. Cancers Head Neck 2018, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, A.W.; Galloway, T.J.; Bhayani, M.K.; Liu, J.C. Effect of HPV Status on Survival of Oropharynx Cancer with Distant Metastasis. Otolaryngol. Head Neck Surg. 2020, 163, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Wendt, M.; Hammarstedt-Nordenvall, L.; Zupancic, M.; Friesland, S.; Landin, D.; Munck-Wikland, E.; Dalianis, T.; Näsman, A.; Marklund, L. Long-Term Survival and Recurrence in Oropharyngeal Squamous Cell Carcinoma in Relation to Subsites, HPV, and p16-Status. Cancers 2021, 13, 2553. [Google Scholar] [CrossRef] [PubMed]
- Mazul, A.L.; Rodriguez-Ormaza, N.; Taylor, J.M.; Desai, D.D.; Brennan, P.; Anantharaman, D.; Gheit, T.; Tommasino, M.; Abedi-Ardekani, B.; Olshan, A.F.; et al. Prognostic significance of non-HPV16 genotypes in oropharyngeal squamous cell carcinoma. Oral Oncol. 2016, 61, 98–103. [Google Scholar] [CrossRef]
- Smith, E.M.; Rubenstein, L.M.; Haugen, T.H.; Hamsikova, E.; Turek, L.P. Tobacco and alcohol use increases the risk of both HPV-associated and HPV-independent head and neck cancers. Cancer Causes Control. 2010, 21, 1369–1378. [Google Scholar] [CrossRef]
- Stjernstrøm, K.D.; Jensen, J.S.; Jakobsen, K.K.; Grønhøj, C.; von Buchwald, C. Current status of human papillomavirus positivity in oropharyngeal squamous cell carcinoma in Europe: A systematic review. Acta Oto-Laryngol. 2019, 139, 1112–1116. [Google Scholar] [CrossRef]
- Mariz, B.A.L.A.; Kowalski, L.P.; William, W.N.; de Castro, G.; Chaves, A.L.F.; Santos, M.; de Oliveira, T.B.; Araújo, A.L.D.; Normando, A.G.C.; Ribeiro, A.C.P.; et al. Global prevalence of human papillomavirus-driven oropharyngeal squamous cell carcinoma following the ASCO guidelines: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 156, 103116. [Google Scholar] [CrossRef]
- Haeggblom, L.; Ramqvist, T.; Tommasino, M.; Dalianis, T.; Näsman, A. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Donà, M.G.; Spriano, G.; Pichi, B.; Rollo, F.; Laquintana, V.; Covello, R.; Pellini, R.; Giuliani, M.; Pescarmona, E.; Benevolo, M. Human papillomavirus infection and p16 overexpression in oropharyngeal squamous cell carcinoma: A case series from 2010 to 2014. Future Microbiol. 2015, 10, 1283–1291. [Google Scholar] [CrossRef]
- Anantharaman, D.; Abedi-Ardekani, B.; Beachler, D.C.; Gheit, T.; Olshan, A.F.; Wisniewski, K.; Wunsch-Filho, V.; Toporcov, T.N.; Tajara, E.H.; Levi, J.E.; et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int. J. Cancer 2017, 140, 1968–1975. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; de Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.; Kruk-Zagajewska, A.; Wal, M.; Jopek, A.; Wierzbicka, M.; Kuch, A. Epstein-Barr virus and human papillomavirus infections and oropharyngeal squamous cell carcinomas. Clin. Exp. Med. 2002, 2, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Tyan, Y.S.; Liu, S.T.; Ong, W.R.; Chen, M.L.; Shu, C.H.; Chang, Y.S. Detection of Epstein-Barr virus and human papillomavirus in head and neck tumors. J. Clin. Microbiol. 1993, 31, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Guillen, J.M.; Palacios-Saucedo, G.C.; Rivera-Morales, L.G.; Alonzo-Morado, M.V.; Burciaga-Bernal, S.B.; Montufar-Martinez, M.; Ortiz-Lopez, R.; Gonzalez-Villasana, V.; Martinez-Torres, A.C.; Serna-Hernandez, J.C.; et al. Infection and coinfection by human papillomavirus, Epstein-Barr virus and Merkel cell polyomavirus in patients with squamous cell carcinoma of the larynx: A retrospective study. PeerJ 2018, 6, e5834. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Johnstone, B.M. Human papillomavirus as a risk factor for oral squamous cell carcinoma: A meta-analysis, 1982–1997. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 622–635. [Google Scholar] [CrossRef]
- Dayyani, F.; Etzel, C.J.; Liu, M.; Ho, C.H.; Lippman, S.M.; Tsao, A.S. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010, 2, 15. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef]
- Shaikh, M.H.; McMillan, N.A.; Johnson, N.W. HPV-associated head and neck cancers in the Asia Pacific: A critical literature review & meta-analysis. Cancer Epidemiol. 2015, 39, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, L.; Li, H.; Gao, J.; Yang, Y.; Zhou, F.; Gao, C.; Li, M.; Jin, Q. Human papillomavirus infection and laryngeal cancer risk: A systematic review and meta-analysis. J. Infect. Dis. 2013, 207, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Altekin, I.; Taş, A.; Yalcin, O.; Guven, S.G.; Aslan, Z.; Adali, M.K.; Karasalihoğlu, A.R. Frequency of Epstein-Barr virus and human papilloma virus in patients with nasopharyngeal carcinoma. Eur. Arch. Otorhinolaryngol. 2020, 277, 2041–2047. [Google Scholar] [CrossRef]
- Deng, Z.; Uehara, T.; Maeda, H.; Hasegawa, M.; Matayoshi, S.; Kiyuna, A.; Agena, S.; Pan, X.; Zhang, C.; Yamashita, Y.; et al. Epstein-Barr virus and human papillomavirus infections and genotype distribution in head and neck cancers. PLoS ONE 2014, 9, e113702. [Google Scholar] [CrossRef]
- Hutt-Fletcher, L.M. Epstein-Barr virus entry. J. Virol. 2007, 81, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Gu, X.; Nathan, C.O.; Hutt-Fletcher, L. Laser-capture microdissection of oropharyngeal epithelium indicates restriction of Epstein-Barr virus receptor/CD21 mRNA to tonsil epithelial cells. J. Oral Pathol. Med. 2008, 37, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Gu, X.; Moore-Medlin, T.N.; Nathan, C.A.; Hutt-Fletcher, L.M. Oral dysplasia and squamous cell carcinoma: Correlation between increased expression of CD21, Epstein-Barr virus and CK19. Oral Oncol. 2012, 48, 836–841. [Google Scholar] [CrossRef][Green Version]
- Li, Q.X.; Young, L.S.; Niedobitek, G.; Dawson, C.W.; Birkenbach, M.; Wang, F.; Rickinson, A.B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 1992, 356, 347–350. [Google Scholar] [CrossRef]
- Knox, P.G.; Li, Q.X.; Rickinson, A.B.; Young, L.S. In vitro production of stable Epstein-Barr virus-positive epithelial cell clones which resemble the virus:cell interaction observed in nasopharyngeal carcinoma. Virology 1996, 215, 40–50. [Google Scholar] [CrossRef][Green Version]
- Brooks, L.; Yao, Q.Y.; Rickinson, A.B.; Young, L.S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: Coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 1992, 66, 2689–2697. [Google Scholar] [CrossRef]
- Murono, S.; Yoshizaki, T.; Park, C.S.; Furukawa, M. Association of Epstein-Barr virus infection with p53 protein accumulation but not bcl-2 protein in nasopharyngeal carcinoma. Histopathology 1999, 34, 432–438. [Google Scholar] [CrossRef]
- Fingeroth, J.D.; Diamond, M.E.; Sage, D.R.; Hayman, J.; Yates, J.L. CD21-Dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 1999, 73, 2115–2125. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, W.; Mei, X.; Hu, H. Transcriptomic Analyses Reveal Gene Expression Profiles and Networks in Nasopharyngeal Carcinoma. Biomed Res. Int. 2021, 2021, 8890176. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, Z.; He, Y.; He, Z.; Ban, Z.; Zhu, Y.; Ding, L.; Yang, C.; Jeong, J.H.; Yuan, W.; et al. EphA2 promotes tumorigenicity of cervical cancer by up-regulating CDK6. J. Cell. Mol. Med. 2021, 25, 2967–2975. [Google Scholar] [CrossRef]
- Fuentes-González, A.M.; Muñoz-Bello, J.O.; Manzo-Merino, J.; Contreras-Paredes, A.; Pedroza-Torres, A.; Fernández-Retana, J.; Pérez-Plasencia, C.; Lizano, M. Intratype variants of the E2 protein from human papillomavirus type 18 induce different gene expression profiles associated with apoptosis and cell proliferation. Arch. Virol. 2019, 164, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Ganguly, N. Transcriptomic analyses of genes differentially expressed by high-risk and low-risk human papilloma virus E6 oncoproteins. Virusdisease 2015, 26, 105–116. [Google Scholar] [CrossRef]
- Verma, D.; Church, T.M.; Swaminathan, S. Epstein-Barr Virus Lytic Replication Induces ACE2 Expression and Enhances SARS-CoV-2 Pseudotyped Virus Entry in Epithelial Cells. J. Virol. 2021, 95, e0019221. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Deng, W.; Yip, Y.L.; Zeng, M.S.; Lo, K.W.; Tsao, S.W. Epstein-Barr virus infection and persistence in nasopharyngeal epithelial cells. Chin. J. Cancer 2014, 33, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Sixbey, J.W.; Vesterinen, E.H.; Nedrud, J.G.; Raab-Traub, N.; Walton, L.A.; Pagano, J.S. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature 1983, 306, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Frangou, P.; Middeldorp, J.; Niedobitek, G. Epstein-Barr virus replication in tongue epithelial cells. J. Gen. Virol. 2002, 83, 2995–2998. [Google Scholar] [CrossRef]
- Vincent-Bugnas, S.; Vitale, S.; Mouline, C.C.; Khaali, W.; Charbit, Y.; Mahler, P.; Prêcheur, I.; Hofman, P.; Maryanski, J.L.; Doglio, A. EBV infection is common in gingival epithelial cells of the periodontium and worsens during chronic periodontitis. PLoS ONE 2013, 8, e80336. [Google Scholar] [CrossRef]
- Rheu, G.B.; Ji, S.; Ryu, J.J.; Lee, J.B.; Shin, C.; Lee, J.Y.; Huh, J.B.; Shin, S.W. Risk assessment for clinical attachment loss of periodontal tissue in Korean adults. J. Adv. Prosthodont. 2011, 3, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Tsang, C.M.; Zhang, G.; Seto, E.; Takada, K.; Deng, W.; Yip, Y.L.; Man, C.; Hau, P.M.; Chen, H.; Cao, Y.; et al. Epstein-Barr virus infection in immortalized nasopharyngeal epithelial cells: Regulation of infection and phenotypic characterization. Int. J. Cancer 2010, 127, 1570–1583. [Google Scholar] [CrossRef]
- Tsang, C.M.; Yip, Y.L.; Lo, K.W.; Deng, W.; To, K.F.; Hau, P.M.; Lau, V.M.; Takada, K.; Lui, V.W.; Lung, M.L.; et al. Cyclin D1 overexpression supports stable EBV infection in nasopharyngeal epithelial cells. Proc. Natl. Acad. Sci. USA 2012, 109, E3473–E3482. [Google Scholar] [CrossRef]
- Yip, Y.L.; Tsang, C.M.; Deng, W.; Cheung, P.Y.; Jin, Y.; Cheung, A.L.; Lung, M.L.; Tsao, S.W. Expression of Epstein-Barr virus-encoded LMP1 and hTERT extends the life span and immortalizes primary cultures of nasopharyngeal epithelial cells. J. Med. Virol. 2010, 82, 1711–1723. [Google Scholar] [CrossRef]
- Raghunandan, B.N.; Sanjai, K.; Kumaraswamy, J.; Papaiah, L.; Pandey, B.; Jyothi, B.M. Expression of human telomerase reverse transcriptase protein in oral epithelial dysplasia and oral squamous cell carcinoma: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2016, 20, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Moharil, R.B.; Khandekar, S.; Dive, A.; Bodhade, A. Cyclin D1 in oral premalignant lesions and oral squamous cell carcinoma: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2020, 24, 397. [Google Scholar] [CrossRef] [PubMed]
- de la Cour, C.D.; Sperling, C.D.; Belmonte, F.; Syrjänen, S.; Verdoodt, F.; Kjaer, S.K. Prevalence of human papillomavirus in oral epithelial dysplasia: Systematic review and meta-analysis. Head Neck 2020, 42, 2975–2984. [Google Scholar] [CrossRef]
- Sarkar, S.; Alam, N.; Chakraborty, J.; Biswas, J.; Mandal, S.S.; Roychoudhury, S.; Panda, C.K. Human papilloma virus (HPV) infection leads to the development of head and neck lesions but offers better prognosis in malignant Indian patients. Med. Microbiol. Immunol. 2017, 206, 267–276. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Guidry, J.T.; Myers, J.E.; Bienkowska-Haba, M.; Songock, W.K.; Ma, X.; Shi, M.; Nathan, C.O.; Bodily, J.M.; Sapp, M.J.; Scott, R.S. Inhibition of Epstein-Barr Virus Replication in Human Papillomavirus-Immortalized Keratinocytes. J. Virol. 2019, 93, e01216-18. [Google Scholar] [CrossRef]
- Nawandar, D.M.; Wang, A.; Makielski, K.; Lee, D.; Ma, S.; Barlow, E.; Reusch, J.; Jiang, R.; Wille, C.K.; Greenspan, D.; et al. Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells. PLoS Pathog. 2015, 11, e1005195. [Google Scholar] [CrossRef]
- Liang, C.L.; Chen, J.L.; Hsu, Y.P.; Ou, J.T.; Chang, Y.S. Epstein-Barr virus BZLF1 gene is activated by transforming growth factor-beta through cooperativity of Smads and c-Jun/c-Fos proteins. J. Biol. Chem. 2002, 277, 23345–23357. [Google Scholar] [CrossRef] [PubMed]
- Iempridee, T.; Das, S.; Xu, I.; Mertz, J.E. Transforming growth factor beta-induced reactivation of Epstein-Barr virus involves multiple Smad-binding elements cooperatively activating expression of the latent-lytic switch BZLF1 gene. J. Virol. 2011, 85, 7836–7848. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Chavez, J.; Hernandez-Pando, R.; Lambert, P.F.; Gariglio, P. Down-regulation of transforming growth factor-beta type II receptor (TGF-betaRII) protein and mRNA expression in cervical cancer. Mol. Cancer 2008, 7, 3. [Google Scholar] [CrossRef]
- French, D.; Belleudi, F.; Mauro, M.V.; Mazzetta, F.; Raffa, S.; Fabiano, V.; Frega, A.; Torrisi, M.R. Expression of HPV16 E5 down-modulates the TGFbeta signaling pathway. Mol. Cancer 2013, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Makielski, K.R.; Lee, D.; Lorenz, L.D.; Nawandar, D.M.; Chiu, Y.F.; Kenney, S.C.; Lambert, P.F. Human papillomavirus promotes Epstein-Barr virus maintenance and lytic reactivation in immortalized oral keratinocytes. Virology 2016, 495, 52–62. [Google Scholar] [CrossRef]
- Szostek, S.; Zawilinska, B.; Kopec, J.; Kosz-Vnenchak, M. Herpesviruses as possible cofactors in HPV-16-related oncogenesis. Acta Biochim. Pol. 2009, 56, 337–342. [Google Scholar] [CrossRef]
- Kahla, S.; Oueslati, S.; Achour, M.; Kochbati, L.; Chanoufi, M.B.; Maalej, M.; Oueslati, R. Correlation between ebv co-infection and HPV16 genome integrity in Tunisian cervical cancer patients. Braz. J. Microbiol. 2012, 43, 744–753. [Google Scholar] [CrossRef]
- Ressing, M.E.; van Gent, M.; Gram, A.M.; Hooykaas, M.J.; Piersma, S.J.; Wiertz, E.J. Immune Evasion by Epstein-Barr Virus. Curr. Top. Microbiol. Immunol. 2015, 391, 355–381. [Google Scholar] [CrossRef]
- Lo Cigno, I.; Calati, F.; Albertini, S.; Gariglio, M. Subversion of Host Innate Immunity by Human Papillomavirus Oncoproteins. Pathogens 2020, 9, 292. [Google Scholar] [CrossRef]
- Gaudreault, E.; Fiola, S.; Olivier, M.; Gosselin, J. Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J. Virol. 2007, 81, 8016–8024. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, D.; Zhou, L.; Samanta, M.; Matsumoto, M.; Ebihara, T.; Seya, T.; Imai, S.; Fujieda, M.; Kawa, K.; Takada, K. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J. Exp. Med. 2009, 206, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.E.; Ma, Y.; Farhat, S.; Moscicki, A.B. Expression of nucleic acid-sensing Toll-like receptors predicts HPV16 clearance associated with an E6-directed cell-mediated response. Int. J. Cancer 2015, 136, 2402–2408. [Google Scholar] [CrossRef] [PubMed]
- Daud, I.I.; Scott, M.E.; Ma, Y.; Shiboski, S.; Farhat, S.; Moscicki, A.B. Association between toll-like receptor expression and human papillomavirus type 16 persistence. Int. J. Cancer 2011, 128, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Jouhi, L.; Mohamed, H.; Mäkitie, A.; Remes, S.M.; Haglund, C.; Atula, T.; Hagström, J. Toll-like receptor 5 and 7 expression may impact prognosis of HPV-positive oropharyngeal squamous cell carcinoma patients. Cancer Immunol. Immunother. 2017, 66, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Hasan, U.A.; Bates, E.; Takeshita, F.; Biliato, A.; Accardi, R.; Bouvard, V.; Mansour, M.; Vincent, I.; Gissmann, L.; Iftner, T.; et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 2007, 178, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Hasan, U.A.; Zannetti, C.; Parroche, P.; Goutagny, N.; Malfroy, M.; Roblot, G.; Carreira, C.; Hussain, I.; Müller, M.; Taylor-Papadimitriou, J.; et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J. Exp. Med. 2013, 210, 1369–1387. [Google Scholar] [CrossRef]
- van Gent, M.; Gram, A.M.; Boer, I.G.J.; Geerdink, R.J.; Lindenbergh, M.F.S.; Lebbink, R.J.; Wiertz, E.J.; Ressing, M.E. Silencing the shutoff protein of Epstein-Barr virus in productively infected B cells points to (innate) targets for immune evasion. J. Gen. Virol. 2015, 96, 858–865. [Google Scholar] [CrossRef]
- van Gent, M.; Griffin, B.D.; Berkhoff, E.G.; van Leeuwen, D.; Boer, I.G.; Buisson, M.; Hartgers, F.C.; Burmeister, W.P.; Wiertz, E.J.; Ressing, M.E. EBV lytic-phase protein BGLF5 contributes to TLR9 downregulation during productive infection. J. Immunol. 2011, 186, 1694–1702. [Google Scholar] [CrossRef]
- van Gent, M.; Braem, S.G.; de Jong, A.; Delagic, N.; Peeters, J.G.; Boer, I.G.; Moynagh, P.N.; Kremmer, E.; Wiertz, E.J.; Ovaa, H.; et al. Epstein-Barr virus large tegument protein BPLF1 contributes to innate immune evasion through interference with toll-like receptor signaling. PLoS Pathog. 2014, 10, e1003960. [Google Scholar] [CrossRef] [PubMed]
- Fathallah, I.; Parroche, P.; Gruffat, H.; Zannetti, C.; Johansson, H.; Yue, J.; Manet, E.; Tommasino, M.; Sylla, B.S.; Hasan, U.A. EBV latent membrane protein 1 is a negative regulator of TLR9. J. Immunol. 2010, 185, 6439–6447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hang, X.; Xie, L. Toll-like receptor 3 (TLR3) functions as a pivotal target in latent membrane protein 1 (LMP1)-mediated nasopharyngeal carcinoma cell proliferation. Int. J. Clin. Exp. Pathol. 2020, 13, 153–162. [Google Scholar]
- Ruuskanen, M.; Leivo, I.; Minn, H.; Vahlberg, T.; Haglund, C.; Hagström, J.; Irjala, H. Expression of toll-like receptors in non-endemic nasopharyngeal carcinoma. BMC Cancer 2019, 19, 624. [Google Scholar] [CrossRef] [PubMed]
- Fiola, S.; Gosselin, D.; Takada, K.; Gosselin, J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J. Immunol. 2010, 185, 3620–3631. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.E.; Roman, R.M.; Rudenga, B.J.; Holers, V.M.; Craft, J.E. Epstein-Barr virus promotes interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum. 2010, 62, 1693–1701. [Google Scholar] [CrossRef]
- Beima-Sofie, K.; Wamalwa, D.; Maleche-Obimbo, E.; Lingappa, J.R.; Mackelprang, R.; Gantt, S.; John-Stewart, G.; Casper, C.; Slyker, J.A. Toll-like receptor 9 polymorphism is associated with increased Epstein-Barr virus and Cytomegalovirus acquisition in HIV-exposed infants. AIDS 2018, 32, 267–270. [Google Scholar] [CrossRef]
- Jabłońska, A.; Studzińska, M.; Szenborn, L.; Wiśniewska-Ligier, M.; Karlikowska-Skwarnik, M.; Gęsicki, T.; Paradowska, E. TLR4 896A/G and TLR9 1174G/A polymorphisms are associated with the risk of infectious mononucleosis. Sci. Rep. 2020, 10, 13154. [Google Scholar] [CrossRef]
- Chiang, H.S.; Liu, H.M. The Molecular Basis of Viral Inhibition of IRF- and STAT-Dependent Immune Responses. Front. Immunol. 2018, 9, 3086. [Google Scholar] [CrossRef]
- Ronco, L.V.; Karpova, A.Y.; Vidal, M.; Howley, P.M. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998, 12, 2061–2072. [Google Scholar] [CrossRef]
- Nees, M.; Geoghegan, J.M.; Hyman, T.; Frank, S.; Miller, L.; Woodworth, C.D. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J. Virol. 2001, 75, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- Raikhy, G.; Woodby, B.L.; Scott, M.L.; Shin, G.; Myers, J.E.; Scott, R.S.; Bodily, J.M. Suppression of Stromal Interferon Signaling by Human Papillomavirus 16. J. Virol. 2019, 93, e00458-19. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Hurst, J.; Voges, M.; Krauss, P.; Münch, P.; Iftner, T.; Stubenrauch, F. High-risk human papillomaviruses repress constitutive kappa interferon transcription via E6 to prevent pathogen recognition receptor and antiviral-gene expression. J. Virol. 2011, 85, 11372–11380. [Google Scholar] [CrossRef]
- DeCarlo, C.A.; Severini, A.; Edler, L.; Escott, N.G.; Lambert, P.F.; Ulanova, M.; Zehbe, I. IFN-κ, a novel type I IFN, is undetectable in HPV-positive human cervical keratinocytes. Lab. Investig. 2010, 90, 1482–1491. [Google Scholar] [CrossRef][Green Version]
- Rincon-Orozco, B.; Halec, G.; Rosenberger, S.; Muschik, D.; Nindl, I.; Bachmann, A.; Ritter, T.M.; Dondog, B.; Ly, R.; Bosch, F.X.; et al. Epigenetic silencing of interferon-kappa in human papillomavirus type 16-positive cells. Cancer Res. 2009, 69, 8718–8725. [Google Scholar] [CrossRef]
- Sunthamala, N.; Thierry, F.; Teissier, S.; Pientong, C.; Kongyingyoes, B.; Tangsiriwatthana, T.; Sangkomkamhang, U.; Ekalaksananan, T. E2 proteins of high risk human papillomaviruses down-modulate STING and IFN-κ transcription in keratinocytes. PLoS ONE 2014, 9, e91473. [Google Scholar] [CrossRef]
- Hahn, A.M.; Huye, L.E.; Ning, S.; Webster-Cyriaque, J.; Pagano, J.S. Interferon regulatory factor 7 is negatively regulated by the Epstein-Barr virus immediate-early gene, BZLF-1. J. Virol. 2005, 79, 10040–10052. [Google Scholar] [CrossRef]
- Bentz, G.L.; Liu, R.; Hahn, A.M.; Shackelford, J.; Pagano, J.S. Epstein-Barr virus BRLF1 inhibits transcription of IRF3 and IRF7 and suppresses induction of interferon-beta. Virology 2010, 402, 121–128. [Google Scholar] [CrossRef]
- Morrison, T.E.; Mauser, A.; Wong, A.; Ting, J.P.; Kenney, S.C. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity 2001, 15, 787–799. [Google Scholar] [CrossRef]
- Wu, L.; Fossum, E.; Joo, C.H.; Inn, K.S.; Shin, Y.C.; Johannsen, E.; Hutt-Fletcher, L.M.; Hass, J.; Jung, J.U. Epstein-Barr virus LF2: An antagonist to type I interferon. J. Virol. 2009, 83, 1140–1146. [Google Scholar] [CrossRef]
- Wang, J.T.; Doong, S.L.; Teng, S.C.; Lee, C.P.; Tsai, C.H.; Chen, M.R. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J. Virol. 2009, 83, 1856–1869. [Google Scholar] [CrossRef]
- Hooykaas, M.J.G.; van Gent, M.; Soppe, J.A.; Kruse, E.; Boer, I.G.J.; van Leenen, D.; Groot Koerkamp, M.J.A.; Holstege, F.C.P.; Ressing, M.E.; Wiertz, E.J.H.J.; et al. EBV MicroRNA BART16 Suppresses Type I IFN Signaling. J. Immunol. 2017, 198, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qin, Z.; Wang, J.; Zheng, X.; Lu, J.; Zhang, X.; Wei, L.; Peng, Q.; Zheng, Y.; Ou, C.; et al. Epstein-Barr Virus miR-BART6-3p Inhibits the RIG-I Pathway. J. Innate Immun. 2017, 9, 574–586. [Google Scholar] [CrossRef]
- Berti, F.C.B.; Pereira, A.P.L.; Cebinelli, G.C.M.; Trugilo, K.P.; Brajão de Oliveira, K. The role of interleukin 10 in human papilloma virus infection and progression to cervical carcinoma. Cytokine Growth Factor Rev. 2017, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petrini, C.G.; Bastos, L.B.; Duarte, G.; Dos Santos Melli, P.P.; Alves-Filho, J.C.; Quintana, S.M. Downregulation of IL-2 and IL-23 in Cervical Biopsies of Cervical Intraepithelial Lesions: A Cross-Sectional Study. Acta Cytol. 2020, 64, 442–451. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kang, J.W.; Cho, M.; Cho, C.W.; Lee, S.; Choe, Y.K.; Kim, Y.; Choi, I.; Park, S.N.; Kim, S.; et al. Down modulation of IL-18 expression by human papillomavirus type 16 E6 oncogene via binding to IL-18. FEBS Lett. 2001, 501, 139–145. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Matamoros, J.A.; da Silva, M.I.F.; de Moura, P.M.M.F.; Leitão, M.D.C.G.; Coimbra, E.C. Reduced Expression of IL-1β and IL-18 Proinflammatory Interleukins Increases the Risk of Developing Cervical Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 2715–2721. [Google Scholar] [CrossRef]
- Havard, L.; Delvenne, P.; Fraré, P.; Boniver, J.; Giannini, S.L. Differential production of cytokines and activation of NF-kappaB in HPV-transformed keratinocytes. Virology 2002, 298, 271–285. [Google Scholar] [CrossRef]
- Niiro, H.; Otsuka, T.; Abe, M.; Satoh, H.; Ogo, T.; Nakano, T.; Furukawa, Y.; Niho, Y. Epstein-Barr virus BCRF1 gene product (viral interleukin 10) inhibits superoxide anion production by human monocytes. Lymphokine Cytokine Res. 1992, 11, 209–214. [Google Scholar]
- Cohen, J.I.; Lekstrom, K. Epstein-Barr virus BARF1 protein is dispensable for B-cell transformation and inhibits alpha interferon secretion from mononuclear cells. J. Virol. 1999, 73, 7627–7632. [Google Scholar] [CrossRef]
- Shim, A.H.; Chang, R.A.; Chen, X.; Longnecker, R.; He, X. Multipronged attenuation of macrophage-colony stimulating factor signaling by Epstein-Barr virus BARF1. Proc. Natl. Acad. Sci. USA 2012, 109, 12962–12967. [Google Scholar] [CrossRef] [PubMed]
- Wiech, T.; Nikolopoulos, E.; Lassman, S.; Heidt, T.; Schöpflin, A.; Sarbia, M.; Werner, M.; Shimizu, Y.; Sakka, E.; Ooka, T.; et al. Cyclin D1 expression is induced by viral BARF1 and is overexpressed in EBV-associated gastric cancer. Virchows Arch. 2008, 452, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Dotan, S.; Krelin, Y.; Song, X.; Elkabets, M.; Carmi, Y.; Rider, P.; Cohen, I.; Romzova, M.; Kaplanov, I.; et al. Unique Versus Redundant Functions of IL-1α and IL-1β in the Tumor Microenvironment. Front. Immunol. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.M.; Ivanov, N.S.; Barr, S.A.; Chen, Y.; Skalsky, R.L. An Epstein-Barr Virus MicroRNA Blocks Interleukin-1 (IL-1) Signaling by Targeting IL-1 Receptor 1. J. Virol. 2017, 91, e00530-17. [Google Scholar] [CrossRef] [PubMed]
- Niebler, M.; Qian, X.; Höfler, D.; Kogosov, V.; Kaewprag, J.; Kaufmann, A.M.; Ly, R.; Böhmer, G.; Zawatzky, R.; Rösl, F.; et al. Post-translational control of IL-1β via the human papillomavirus type 16 E6 oncoprotein: A novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathog. 2013, 9, e1003536. [Google Scholar] [CrossRef]
- Szabo, S.J.; Sullivan, B.M.; Peng, S.L.; Glimcher, L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003, 21, 713–758. [Google Scholar] [CrossRef]
- Haabeth, O.A.; Lorvik, K.B.; Yagita, H.; Bogen, B.; Corthay, A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology 2016, 5, e1039763. [Google Scholar] [CrossRef]
- Spitkovsky, D.; Hehner, S.P.; Hofmann, T.G.; Möller, A.; Schmitz, M.L. The human papillomavirus oncoprotein E7 attenuates NF-kappa B activation by targeting the Ikappa B kinase complex. J. Biol. Chem. 2002, 277, 25576–25582. [Google Scholar] [CrossRef]
- Havard, L.; Rahmouni, S.; Boniver, J.; Delvenne, P. High levels of p105 (NFKB1) and p100 (NFKB2) proteins in HPV16-transformed keratinocytes: Role of E6 and E7 oncoproteins. Virology 2005, 331, 357–366. [Google Scholar] [CrossRef]
- Patel, D.; Huang, S.M.; Baglia, L.A.; McCance, D.J. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999, 18, 5061–5072. [Google Scholar] [CrossRef]
- Huang, S.M.; McCance, D.J. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J. Virol. 2002, 76, 8710–8721. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.E.; Kenney, S.C. BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function. Virology 2004, 328, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.; Dawson, C.W.; Hu, C.; Shah, K.M.; Owen, T.J.; Date, K.L.; Maia, S.P.; Shao, J.; Arrand, J.R.; Young, L.S.; et al. Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-kappaB pathway in carcinoma cells by inhibiting IKK phosphorylation. Mol. Cancer 2010, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.S.; Wang, J.T.; Doong, S.L.; Lee, C.P.; Chang, C.W.; Tsai, C.H.; Yeh, S.W.; Hsieh, C.Y.; Chen, M.R. Epstein-Barr virus BGLF4 kinase downregulates NF-κB transactivation through phosphorylation of coactivator UXT. J. Virol. 2012, 86, 12176–12186. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Y.; Xu, Z.; Zou, X.; Wang, P.; Ou, X.; Li, Y.; Peng, T.; Chen, D.; Li, M.; et al. Epstein-Barr virus tegument protein BGLF2 inhibits NF-κB activity by preventing p65 Ser536 phosphorylation. FASEB J. 2019, 33, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, H.; Duan, Z.; Hu, D.; Li, M.; Liu, S.; Li, Z.; Deng, X.; Wang, Z.; Tang, M.; et al. LMP1-augmented kappa intron enhancer activity contributes to upregulation expression of Ig kappa light chain via NF-kappaB and AP-1 pathways in nasopharyngeal carcinoma cells. Mol. Cancer 2009, 8, 92. [Google Scholar] [CrossRef]
- Chang, M.S.; Kim, D.H.; Roh, J.K.; Middeldorp, J.M.; Kim, Y.S.; Kim, S.; Han, S.; Kim, C.W.; Lee, B.L.; Kim, W.H.; et al. Epstein-Barr virus-encoded BARF1 promotes proliferation of gastric carcinoma cells through regulation of NF-κB. J. Virol. 2013, 87, 10515–10523. [Google Scholar] [CrossRef]
- Karin, M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a000141. [Google Scholar] [CrossRef]
- Vandermark, E.R.; Deluca, K.A.; Gardner, C.R.; Marker, D.F.; Schreiner, C.N.; Strickland, D.A.; Wilton, K.M.; Mondal, S.; Woodworth, C.D. Human papillomavirus type 16 E6 and E 7 proteins alter NF-kB in cultured cervical epithelial cells and inhibition of NF-kB promotes cell growth and immortalization. Virology 2012, 425, 53–60. [Google Scholar] [CrossRef]
- de Freitas, A.C.; de Oliveira, T.H.A.; Barros, M.R.; Venuti, A. hrHPV E5 oncoprotein: Immune evasion and related immunotherapies. J. Exp. Clin. Cancer Res. 2017, 36, 71. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.S.; Graham, S.V.; Cortese, M.S.; Ashrafi, G.H.; Araibi, E.H.; Dornan, E.S.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 2010, 407, 137–142. [Google Scholar] [CrossRef]
- Georgopoulos, N.T.; Proffitt, J.L.; Blair, G.E. Transcriptional regulation of the major histocompatibility complex (MHC) class I heavy chain, TAP1 and LMP2 genes by the human papillomavirus (HPV) type 6b, 16 and 18 E7 oncoproteins. Oncogene 2000, 19, 4930–4935. [Google Scholar] [CrossRef] [PubMed]
- Cyprian, F.S.; Al-Farsi, H.F.; Vranic, S.; Akhtar, S.; Al Moustafa, A.E. Epstein-Barr Virus and Human Papillomaviruses Interactions and Their Roles in the Initiation of Epithelial-Mesenchymal Transition and Cancer Progression. Front. Oncol. 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.E.; Chen, D.; Ghabreau, L.; Akil, N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med. Hypotheses 2009, 73, 184–186. [Google Scholar] [CrossRef]
- Al-Thawadi, H.; Ghabreau, L.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Batist, G.; Al Moustafa, A.E. Co-Incidence of Epstein-Barr Virus and High-Risk Human Papillomaviruses in Cervical Cancer of Syrian Women. Front. Oncol. 2018, 8, 250. [Google Scholar] [CrossRef]
- Lo, A.K.; Dawson, C.W.; Lo, K.W.; Yu, Y.; Young, L.S. Upregulation of Id1 by Epstein-Barr virus-encoded LMP1 confers resistance to TGFbeta-mediated growth inhibition. Mol. Cancer 2010, 9, 155. [Google Scholar] [CrossRef]
- Lin, J.; Guan, Z.; Wang, C.; Feng, L.; Zheng, Y.; Caicedo, E.; Bearth, E.; Peng, J.R.; Gaffney, P.; Ondrey, F.G. Inhibitor of differentiation 1 contributes to head and neck squamous cell carcinoma survival via the NF-kappaB/survivin and phosphoinositide 3-kinase/Akt signaling pathways. Clin. Cancer Res. 2010, 16, 77–87. [Google Scholar] [CrossRef]
- Shimabuku, T.; Tamanaha, A.; Kitamura, B.; Tanabe, Y.; Tawata, N.; Ikehara, F.; Arakaki, K.; Kinjo, T. Dual expression of Epstein-Barr virus, latent membrane protein-1 and human papillomavirus-16 E6 transform primary mouse embryonic fibroblasts through NF-κB signaling. Int. J. Clin. Exp. Pathol. 2014, 7, 1920–1934. [Google Scholar]
- Uehara, K.; Tanabe, Y.; Hirota, S.; Higa, S.; Toyoda, Z.; Kurima, K.; Kina, S.; Nakasone, T.; Arasaki, A.; Kinjo, T. Co-expression of low-risk HPV E6/E7 and EBV LMP-1 leads to precancerous lesions by DNA damage. BMC Cancer 2021, 21, 688. [Google Scholar] [CrossRef]
- Al Moustafa, A.E.; Al-Antary, N.; Aboulkassim, T.; Akil, N.; Batist, G.; Yasmeen, A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum. Vaccin. Immunother. 2016, 12, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Jarzynski, A.; Boguszewska, A.; Kliszczewska, E.; Rolniak, L.; Dworzanska, A.; Polz-Dacewicz, M. TP53 promoter methylation in EBV and HPV associated oropharyngeal carcinoma—A pilot study. Curr. Issues Pharm. Med. Sci. 2017, 30, 119–122. [Google Scholar] [CrossRef]
- McCormick, T.M.; Canedo, N.H.; Furtado, Y.L.; Silveira, F.A.; de Lima, R.J.; Rosman, A.D.; Almeida Filho, G.L.; Carvalho, M.a.G. Association between human papillomavirus and Epstein—Barr virus DNA and gene promoter methylation of RB1 and CDH1 in the cervical lesions: A transversal study. Diagn. Pathol. 2015, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Lattario, F.; Furtado, Y.L.; Fonseca, R.; Silveira, F.A.; do Val, I.C.; Almeida, G.; Carvalho, M.G. Analysis of human papillomavirus and Epstein-Barr virus infection and aberrant death-associated protein kinase methylation in high-grade squamous intraepithelial lesions. Int. J. Gynecol. Cancer 2008, 18, 785–789. [Google Scholar] [CrossRef]
- Singh, P.; Ravanan, P.; Talwar, P. Death Associated Protein Kinase 1 (DAPK1): A Regulator of Apoptosis and Autophagy. Front. Mol. Neurosci. 2016, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Strycharz-Dudziak, M.; Fołtyn, S.; Dworzański, J.; Kiełczykowska, M.; Malm, M.; Drop, B.; Polz-Dacewicz, M. Glutathione Peroxidase (GPx) and Superoxide Dismutase (SOD) in Oropharyngeal Cancer Associated with EBV and HPV Coinfection. Viruses 2020, 12, 1008. [Google Scholar] [CrossRef]
- Sharma, U.; Singhal, P.; Bandil, K.; Patle, R.; Kumar, A.; Neyaz, K.; Bose, S.; Kumar Dewan, A.; Mehrotra, R.; Sharma, V.; et al. Genetic variations of TLRs and their association with HPV/EBV, co-infection along with nicotine exposure in the development of premalignant/malignant lesions of the oral cavity in Indian population. Cancer Epidemiol. 2019, 61, 38–49. [Google Scholar] [CrossRef]
- Yang, Z.H.; Dai, Q.; Gu, Y.J.; Guo, Q.X.; Gong, L. Cytokine and chemokine modification by Toll-like receptor polymorphisms is associated with nasopharyngeal carcinoma. Cancer Sci. 2012, 103, 653–658. [Google Scholar] [CrossRef]
- Dai, Q.; Li, X.P.; Chai, L.; Long, H.A.; Yang, Z.H. Polymorphisms of Toll-like receptor 9 are associated with nasopharyngeal carcinoma susceptibility. Tumor Biol. 2014, 35, 3247–3253. [Google Scholar] [CrossRef]
- Aromseree, S.; Middeldorp, J.M.; Pientong, C.; van Eijndhoven, M.; Ramayanti, O.; Lougheed, S.M.; Pegtel, D.M.; Steenbergen, R.D.; Ekalaksananan, T. High Levels of EBV-Encoded RNA 1 (EBER1) Trigger Interferon and Inflammation-Related Genes in Keratinocytes Expressing HPV16 E6/E7. PLoS ONE 2017, 12, e0169290. [Google Scholar] [CrossRef]
| Biological Process | Human Papillomavirus | Epstein–Barr Virus | References |
|---|---|---|---|
| Route of entry | Direct epithelial contact, apical entry (microlesions) | Salivary transmission, apical, basolateral, or basal entry | [21,33] |
| Tropism | Epithelial cells (mucosal or cutaneous) | Epithelial cells (mucosal), B cells, T cells, NK cells | [33,34] |
| Entry mechanism | Endocytosis | Membrane fusion | [35,36] |
| Receptors | Entry receptor complex, HSPGs, integrins | EphA2 | [24,37] |
| Tumor Type | No. of Cases | HPV Pooled Prevalence (%) | OR (95% CI) | Comments | Refs |
|---|---|---|---|---|---|
| OSCC | 4680 | 46.5 | 37.6–55.5 | HPV infection was 4.7 times more likely to be detected in OSCC than in normal mucosa. | [106] |
| OSCC | 610 | 58.0 | 54.1–61.9 | HPV positivity increased the probability of OSCC development by 12.7 times. | [44] |
| OSCC HNSCC (other sites) | 3238 4852 | 38.1 34.5 | 30.0–46.2 28.4–40.6 | The pooled HPV prevalence was greater in OSCC (38.1%) than in other HNSCCs (24.1%). | [43] |
| NPC | 1748 | 21.0 | 1.69–13.45 | HPV prevalence was higher in cases outside of China than in cases from regions in China (23% vs. 19%; p < 0.001). | [85] |
| NPC | 2453 | 19.9 | 13.6–27.1 | HR-HPV infection was higher in WHO type I NPCs (39.9%) compared to WHO types II/III tumors (23.3%). | [77] |
| OPSCC | 6009 | 44.8 | 36.4–53.5 | HPV pooled prevalence was more increased in New Zealand (74.5%), Sweden (70.0%), and Denmark (61.7%) than in Brazil (11.1%), Germany (25.0%), and the Netherlands (30.3%). | [98] |
| OPSCC HNSCC (all sites) | 925 5681 | 41.0 21.9 | 38.0–44.0 21.0–23.0 | HR-HPV (any genotype) and HPV16 increased the probability of HNSCC development by 1.83 and 4.44 times, respectively. | [107] |
| OPSCC HNSCC (other sites) | 5396 13,972 | 47.7 21.8 | 42.9–52.5 18.9–25.1 | - | [108] |
| OSCC OPSCC LSCC HNSCC (all sites) | 2642 969 1435 5046 | 23.5 35.6 24.0 25.9 | 21.9–25.1 32.6–38.7 21.8–26.3 24.7–27.2 | HPV prevalence was significantly higher in OPSCC than OSCC or LSCC. | [109] |
| OSCC OPSCC LSCC/HPSCC HNSCC (all sites) | 5478 3946 2739 12,163 | 24.2 45.8 22.1 29.5 | 18.7–30.2 38.9–52.9 16.4–28.3 25.5–33.6 | HPV16 prevalence was more increased in OPSCC (40.6%) than in OSCC (14.9%) or LSCC (13.4%). | [102] |
| OSCC OPSCC LSCC HNSCC (all sites) | 3153 2768 856 6777 | 37.5 40.5 23.6 37.0 | 35.9–39.2 38.7–42.3 22.1–25.0 36.0–38.0 | The association of HPV with cancer development was increased for OPSCC (OR: 14.7) compared to OSCC (OR: 4.1) or LSCC (OR: 3.2). | [110] |
| LSCC | 2559 | 28.0 | 23.5–32.9 | HPV infection was significantly associated with the risk of LSCC development (OR: 5.4). | [111] |
| Tumor Type | Method | No. of Cases | EBV Positivity (%) | Comments | Refs |
|---|---|---|---|---|---|
| OSCC | EBV-chip hybridization | 57 | 82.5 | - | [48] |
| OSCC | PCR for BNLF1 | 91 | 45.1 | EBV was significantly associated with the probability of OSCC development (OR 3.76). | [49] |
| OSCC | EBER in situ hybridization | 165 | 41.2 | - | [50] |
| NPC | EBER in situ hybridization | 92 | 57.6 | - | [72] |
| NPC | EBER in situ hybridization | 62 | 85.5 | - | [73] |
| NPC | EBER in situ hybridization | 150 | 62.0 | Overall survival was increased in EBV-positive patients (p = 0.005). | [74] |
| NPC | EBER in situ hybridization | 19 | 84.2 | - | [75] |
| NPC | EBER in situ hybridization | 82 | 87.8 | EBV positivity was evidenced in 92.6% of non-keratinizing carcinoma. | [76] |
| NPC | EBER in situ hybridization | 56 | 73.2 | EBV infection was not associated with the histopathological type of NPC. | [112] |
| NPC | - | 2329 | 76.7 | Meta-analysis. EBV prevalence was increased in WHO Type II/III (83.2%) compared to WHO Type I cases (21.3%). | [77] |
| OPSCC | Nested PCR | 62 | 29.0 | - | [56] |
| OPSCC | PCR-ELISA | 28 | 85.7 | - | [103] |
| OSCC NPC OPSCC HPSCC LSCC | EBER in situ hybridization | 37 20 74 50 28 | 0 60.0 1.4 0 0 | EBER positivity was significantly higher in NPCs compared to non-NPC HNSCCs (p < 0.001). | [113] |
| HNSCC (OSCC, PSCC, and LSCC) | PCR for LMP1 | 98 | 69.4 | LMP1 protein was also expressed in PSCC (100%) followed by OSCC (76.0%) and LSCC (33.3%). | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, R.; Carrillo-Beltrán, D.; Corvalán, A.H.; Aguayo, F. High-Risk Human Papillomavirus and Epstein–Barr Virus Coinfection: A Potential Role in Head and Neck Carcinogenesis. Biology 2021, 10, 1232. https://doi.org/10.3390/biology10121232
Blanco R, Carrillo-Beltrán D, Corvalán AH, Aguayo F. High-Risk Human Papillomavirus and Epstein–Barr Virus Coinfection: A Potential Role in Head and Neck Carcinogenesis. Biology. 2021; 10(12):1232. https://doi.org/10.3390/biology10121232
Chicago/Turabian StyleBlanco, Rancés, Diego Carrillo-Beltrán, Alejandro H. Corvalán, and Francisco Aguayo. 2021. "High-Risk Human Papillomavirus and Epstein–Barr Virus Coinfection: A Potential Role in Head and Neck Carcinogenesis" Biology 10, no. 12: 1232. https://doi.org/10.3390/biology10121232
APA StyleBlanco, R., Carrillo-Beltrán, D., Corvalán, A. H., & Aguayo, F. (2021). High-Risk Human Papillomavirus and Epstein–Barr Virus Coinfection: A Potential Role in Head and Neck Carcinogenesis. Biology, 10(12), 1232. https://doi.org/10.3390/biology10121232






