Sugarcane Ratooning Ability: Research Status, Shortcomings, and Prospects
Abstract
:Simple Summary
Abstract
1. Introduction
| Country Name | Ratoon Percentage (%) | Ratoon Age (Year) | References |
|---|---|---|---|
| America | 80–85 | 2–3 | [14] |
| Brazil | 80–90 | 4–5 | [14,15] |
| Australia | 80–85 | 2–3 | [14] |
| South Africa | 80–90 | 4–5 | [14,15] |
| China | 50–70 | 2–3 | [15] |
| India | >50 | 1–2 | [7,8,9,10] |
| World | 75 | 4–7 | [8,14,15] |
2. Definition of Sugarcane Ratooning Ability
3. Phenotypes of Ratooning Ability in Sugarcane
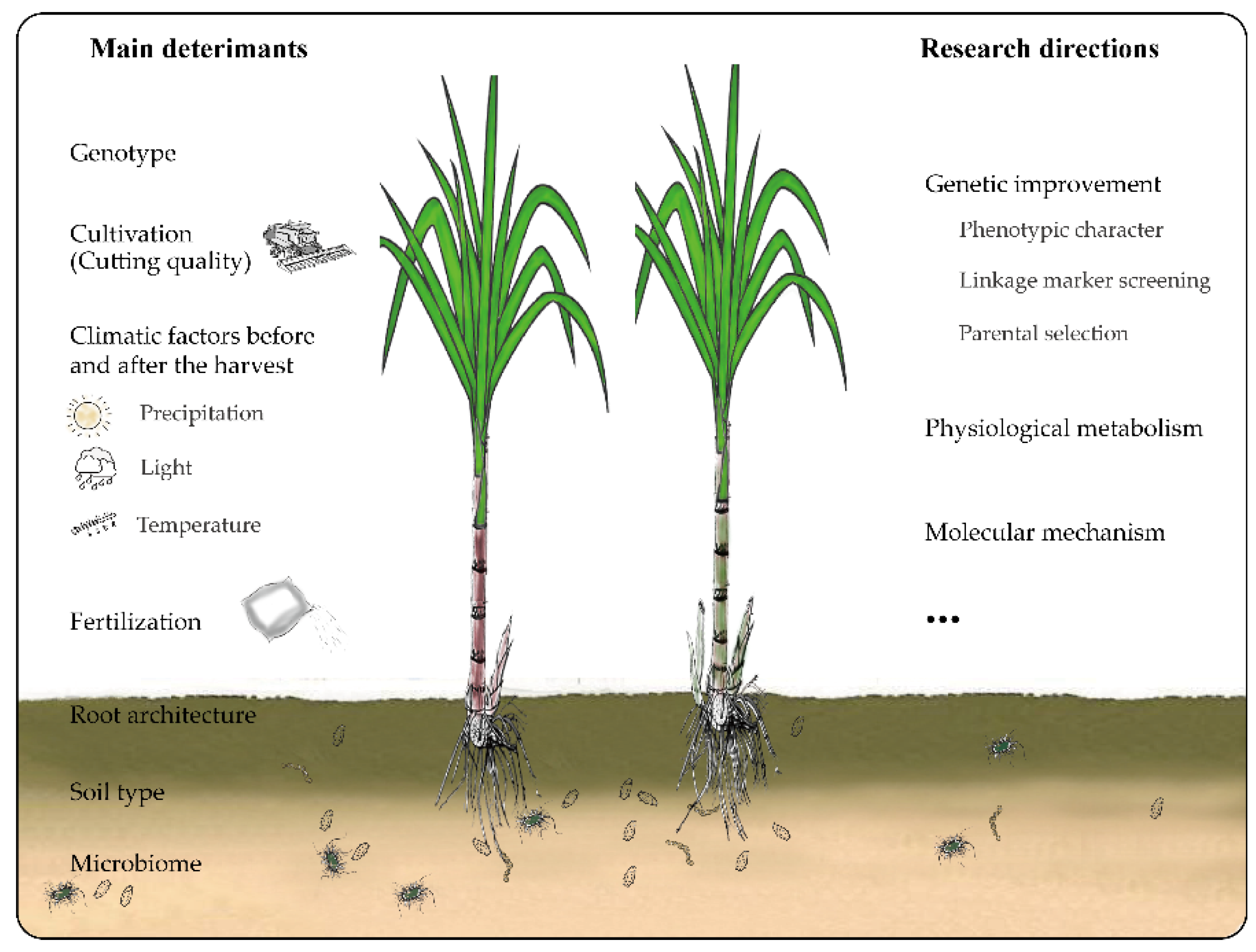
4. Main Factors Influencing Longevity and Productivity of Ratoon Sugarcane
5. Genetic Research on the Variation in Ratooning Ability between Different Sugarcane Genotypes
6. Mechanism Underlying the Variation in Sugarcane Ratooning Ability
7. Shortcomings of Existing Research
8. Research Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD-FAO. Agricultural Outlook 2020–2029. Available online: https://www.oecd-ilibrary.org/sites/3736a600-en/index.html?itemId=/content/component/3736a600-en (accessed on 4 September 2021).
- Li, Y.R.; Song, X.P.; Wu, J.M.; Li, C.N.; Liang, Q.; Liu, X.H.; Wang, W.Z.; Tan, H.W.; Yang, L.T. Sugar industry and improved sugarcane farming technologies in China. Sugar Tech 2016, 18, 603–611. [Google Scholar] [CrossRef]
- Gascho, G.J.; Ruelke, O.C.; West, S.H. Residual effect of germination temperature in sugarcane. Crop Sci. 1973, 13, 274–276. [Google Scholar] [CrossRef]
- Pissolato, M.D.; Cruz, L.P.; Silveira, N.M.; Machado, E.C.; Ribeiro, R.V. Sugarcane regrowth is dependent on root system size: An approach using young plants grown in nutrient solution. Bragantia 2021, 80, e4321. [Google Scholar] [CrossRef]
- Sehtiya, H.L.; Dendsay, J.P.S. Sugarcane ratooning ability I. study on morphology of stubbles. Indian Sugar 1992, 42, 751–754. [Google Scholar]
- Hunsigi, G.; Krishna, K.R. Science of Field Crops; Oxford & IBH Publishing Co., Pvt. Ltd.: New Delhi, India, 1998; pp. 328–352. [Google Scholar]
- Singh, H.; Rathore, A.K.; Tamrakar, S.K. Agro-techniques for ratoon management in sugarcane. Indian Sugar 2015, 65, 32–34. [Google Scholar]
- Gomathi, R.; Rao, P.N.G.; Rakkiyappan, P.; Sundara, B.P.; Shiyamala, S. Physiological studies on ratoon ability of sugarcane varieties under tropical Indian condition. Am. J. Plant Sci. 2013, 4, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Chumphu, S.; Jongrungklang, N.; Songsri, P. Association of physiological responses and root distribution patterns of ratooning ability and yield of the second ratoon cane in sugarcane elite clones. Agronomy 2019, 9, 200. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Rai, R.K.; Suman, A.; Srivastava, T.K.; Singh, K.P.; Arya, N.; Yadav, R.L. Soil-root interface changes in sugarcane plant and ratoon crops under subtropical conditions: Implications for dry-matter accumulation. Commun. Soil Sci. Plant Anal. 2015, 46, 454–475. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.C.; Yadav, S.P.; Srivastav, M.L.; Singh, I.S.; Sharma, B.L. Effect of cultural operations and fertilizer application in ratoon for enhancing the sugarcane ratoon cane productivity. Agrica 2017, 6, 62–65. [Google Scholar] [CrossRef]
- Bashir, S.; Hassan, M.; Fiaz, N.; Khan, Z.; Ali, Z. Ratooning potential of different promising sugarcane genotypes at varying harvesting dates. J. Agric. Biol. Sci. 2013, 8, 437–440. [Google Scholar]
- Aslam, M.; Rauf, H.A.; Ahmad, N. Ratooning potential of different sugarcane clones under Southern Punjab conditions. Pak. Sugar J. 2020, 45, 21–26. [Google Scholar]
- Li, Y.R. Modern Sugarcane Cultivation; China Agriculture Press: Beijing, China, 2010; pp. 313–333. [Google Scholar]
- Hu, S.F. Discussion on characteristic of growth and high-yield cultivation techniques of ratoon cane. Hortic. Seed 2015, 48, 19–20. [Google Scholar]
- Osman, A.M.; Kumar, G.V.; Natarajan, U.S.; Babu, C. Investigations on sugar cane families for ratooning ability and iron chlorosis tolerance under field conditions. Indian Sugar 2006, 56, 25–30. [Google Scholar]
- Milligan, S.B.; Gravois, K.A.; Martin, F.A. Inheritance of sugarcane ratooning ability and the relationship of younger crop traits to older crop traits. Crop Sci. 1996, 36, 45–50. [Google Scholar] [CrossRef]
- Shaw, M.E.A. An index to measure sugar cane ratoon performance. Sugar Azucar 1989, 84, 22–23. [Google Scholar]
- Abu-Ellail, F.F.B.; El-Azez, Y.M.A.; Bassiony, N.A. Assessment of ratooning ability and genetic variability of promising sugarcane varieties under middle Egypt conditions. Electron. J. Plant Breed. 2019, 10, 143–154. [Google Scholar] [CrossRef]
- Olaoye, G. Estimate of ratooning ability in sugar cane under conditions of low available soil moisture in a savanna ecology of Nigeria. Sugar Cane Inter. 2001, 3, 8–13. [Google Scholar]
- Ding, X.E.; Lin, P.P.; Yu, F.; Deng, Z.H. Research progress of sugarcane rationing ability. Sugar Crops China 2020, 42, 12–18. [Google Scholar]
- Silva, V.S.G.; Oliveira, M.W.; Silva, A.C.; Silva, A.F.; Galvão, E.R.; Santana, M. Agro-industrial quality of plant cane, first and second ratoon in sugarcane varieties. Aust. J. Crop Sci. 2017, 11, 1216–1220. [Google Scholar] [CrossRef]
- Chou, K.Y. The bud and the root as fundamental factors in successful stubble cane production. Zuowu Xuebao 1963, 2, 437–450. [Google Scholar]
- Tripathi, B.K.; Gill, S.S.; Misa, G.P.; Lai, S. Screening of sugarcane (Saccharum spp. hybrids) genotypes for ratooning ability. Indian Sugar 1982, 32, 577–580. [Google Scholar]
- Gravois, K.A.; Milligan, S.B.; Martin, F.A. Indirect selection for increased sucrose yield in early sugarcane testing stages. Field Crops Res. 1991, 26, 67–73. [Google Scholar] [CrossRef]
- Chapman, L.S.; Ferraris, R.; Ludlow, M.M. Ratooning ability of cane varieties: Variation in yield and yield components. In Proceedings of the 14th Conference of the Australian Society of Sugar Cane Technologist, Mackay, Australia, 28 April–1 May 1992; Volume 14, pp. 130–138. [Google Scholar]
- Rafiq, M.; Chattha, A.A.; Mian, M.R. Ratooning potential of different sugarcane genotypes under Faisalabad conditions. J. Agric. Res. 2006, 44, 269–275. [Google Scholar]
- Qin, W.; Wu, C.W.; Zhao, J.; Yao, L.; Chen, X.K.; Yang, K.; Zeng, Q.Q. Research on ratoon ability of sugarcane II. Relationship between the impact factors and sugarcane ratoon. Sugar Crops China 2017, 39, 1–3. [Google Scholar]
- Qin, W.; Wu, C.W.; Zhao, J.; Zhao, P.F.; Yang, K.; Chen, X.K.; Yao, L.; Zeng, Q.Q. Research on ratoon ability of sugarcane I. Relationship between ratooning ability and morphological characteristics of ratoon stools. Southwest China J. Agric. Sci. 2017, 30, 989–993. [Google Scholar]
- Chapman, L.S. Constraints to production in ratoon crops. In Proceedings of the 10th Conference of the Australian Society of Sugar Cane Technologist, Cairns, Australia, 27–30 April 1998; Volume 10, pp. 189–192. [Google Scholar]
- Ferraris, R.; Chapman, L.S.; Ludlow, M.M. Ratooning ability of cane varieties: Interception of light and efficiency of light use. In Proceedings of the 15th Conference of the Australian Society of Sugar Cane Technologist, Cairns, Australia, 27–30 April 1993; Volume 15, pp. 316–322. [Google Scholar]
- Su, G.D.; Wu, B.Q. Review on biological characteristics of ratoon sugarcane. Sugarcane Cane Sugar 1980, 12, 10–13. [Google Scholar]
- Bashir, S.; Fiaz, N.; Ghaar, A.; Khalid, F. Ratooning ability of sugarcane genotypes at different harvesting dates. Inter. Sugar J. 2012, 114, 273–276. [Google Scholar]
- Hassan, M.U.; Fiaz, N.; Mudassir, M.A.; Yasin, M. Exploring the ratooning potential of sugarcane (Saccharum officinarum L.) genotypes under varying harvesting times of plant crop. Pak. J. Agric. Res. 2017, 30, 303–309. [Google Scholar] [CrossRef]
- Milligan, S.B.; Gravois, K.A.; Bischoff, K.P.; Martin, F.A. Crop effects on genetic relationships among sugarcane traits. Crop Sci. 1990, 30, 927–931. [Google Scholar] [CrossRef]
- Qin, W.; Wu, C.W.; Yao, L.; Chen, X.K.; Zhao, P.F.; Zeng, Q.Q. Relationship between ratoon ability and the change of endogenous hormone in sugarcane at sprouting stage. Acta Bot. Boreali-Occident. Sin. 2014, 34, 143–149. [Google Scholar]
- Ramburana, S.; Wettergreena, T.; Berrya, S.D.; Shongweb, B. Genetic, environmental and management contributions to ratoon decline in sugarcane. Field Crops Res. 2013, 146, 105–112. [Google Scholar] [CrossRef]
- Ricaud, R.; Arceneaux, A. Some factors affecting ratoon cane yield and longevity in Louisiana. In Proceedings of the 8th Conference of the Australian Society of Sugar Cane Technologist, Townsville, Australia, 28 April–1 May 1986; Volume 19, pp. 18–24. [Google Scholar]
- Bhale, V.M.G. Effect of growth regulators and cultural treatment on productivity of ratoon cane. Indian Sugar 1994, 44, 645–651. [Google Scholar]
- Hogarth, D.M.; Berding, N. Breeding for a better industry: Conventional breeding. Sugarcane Inter. 2006, 24, 26–31. [Google Scholar]
- Qin, W.; Wu, C.W.; Yang, K.; Zhao, P.F.; Zhao, J.; Liu, J.Y.; Yao, L.; Zhai, F.G.; Xia, H.M. Breeding potential evaluation on ratooning ability of sugarcane progeny from ROC varieties. Sugar Crops China 2015, 37, 5–7. [Google Scholar]
- Yang, R.Z. The preliminary study on cold-resistance and rationing ability of sugarcane. Sugarcane Cane Sugar 1996, 6, 13–17. [Google Scholar]
- Li, Q.W.; Deng, H.H.; Zhou, Y.H. Coefficients of relationship among cultivars and ancestors and their relationships with varietal performance. Sugarcane 1997, 4, 1–5. [Google Scholar]
- Jackson, P. Genetic relationships between attributes in sugarcane clones closely related to Saccharum spontaneum. Euphytica 1994, 79, 101–108. [Google Scholar] [CrossRef]
- Burnera, D.M.; Haleb, A.L.; Viatorc, R.P.; Belesky, D.P.; Houx, J.H.; Ashworth, A.J.; Fritschi, F.B. Ratoon cold tolerance of Pennisetum, Erianthus, and Saccharum bioenergy feedstocks. Ind. Crops Prod. 2017, 109, 327–334. [Google Scholar] [CrossRef]
- Huang, Y.X.; Zhang, B.Q.; Zhou, S.; Yang, C.F.; Gao, Y.J.; Duan, W.X.; Li, Y.R.; Zhang, G.M. Genetic variation and correlation analysis of characters in BC1 progeny of intergeneric hybrid (Erianthus arundinaceus × Saccharum spontaneum). J. China Agric. Univ. 2018, 7, 19–25. [Google Scholar]
- Duan, W.X.; Huang, Y.X.; Zhou, S.; Zhang, B.Q.; Gao, Y.J.; Yang, C.F.; Liu, X.H.; Zhang, G.M. Preliminary identification and evaluation of smut resistance in sugarcane × Narenga porphyrocoma (Hance) Bor hybrid F1. Southwest China J. Agric. Sci. 2017, 30, 1560–1564. [Google Scholar]
- Liu, X.H. Inheritance, DNA Methylation of the Hybrid Progeny of S. officinarum L. and Narenga porphypocoma (Hance) Bor and Drought-Tolerant Gene Mining. Ph.D. Thesis, Guangxi University, Nanning, China, 2018. [Google Scholar]
- Duan, W.X.; Zhang, B.Q.; Zhou, S.; Huang, Y.X.; Wang, Z.P.; Gao, Y.J.; Yang, C.F.; Lin, S.H.; Zhang, G.M. Identification and preliminary evaluation of smut resistance in BC1 hybrids derived from Saccharum L.× Narenga porphyrocoma (Hance) Bor. J. China Agric. Univ. 2018, 23, 29–37. [Google Scholar]
- Huang, Y.X.; Duan, W.X.; Zhang, B.Q.; Yang, C.F.; Gao, Y.J.; Zhou, S.; Zhang, G.M.; Li, X. Evaluation of ratoon characteristic of 138 exotic sugarcane germplasm. J. Yunnan Agric. Univ. 2019, 34, 564–570. [Google Scholar]
- Deng, Z.H.; Lin, Y.Q.; Lin, H.M.; Chen, R.K. The selection effect of early stage rationing in sugarcane breeding. J. Fujian Agric. For. Univ. 2002, 31, 137–141. [Google Scholar]
- Zhou, M.M.; Shoko, M.D. Simultaneous selection for yield and ratooning ability in sugarcane genotypes using analysis of covariance. S. Afr. J. Plant Soil 2012, 29, 93–100. [Google Scholar] [CrossRef]
- Rowan, F.S.; Murilo, M.P.; Tammy, L.S. Photosynthesis in sugarcane. In Sugarcane: Physiology, Biochemistry, and Functional Biology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 121–154. [Google Scholar]
- Faralli, M.; Lawson, T. Natural genetic variation in photosynthesis: An untapped resource to increase crop yield potential? Plant J. 2020, 3, 518–528. [Google Scholar] [CrossRef]
- Ye, Y.P. Studies on the Physiological and Biochemical Mechanism on Ethephon Promoting Effective Tillering in Sugarcane. Ph.D. Thesis, Guangxi University, Nanning, China, 2006. [Google Scholar]
- Qiu, L.H.; Fan, Y.G.; Luo, H.M.; Huang, X.; Chen, R.F.; Yang, R.Z.; Wu, J.M.; Li, Y.R. Advances of regulation study on tillering formation and stem forming from available tillers in sugarcane (Saccharum officinarum). J. Plant Physiol. 2018, 54, 192–202. [Google Scholar]
- Pribil, M.; Hermann, F.; Dun, G.; Karno, P.; Ngo, C.; O’Neill, S.; Wang, L.; Bonnett, G.D.; Chandler, P.M.; Beveridge, C.A.; et al. Altering sugarcane shoot architecture through genetic engineering: Prospects for increasing cane and sugar yield. In Proceedings of the 29th Conference of the Australian Society of Sugar Cane Technologist, Cairns, Australia, 8–11 May 2007; Volume 29, pp. 251–257. [Google Scholar]
- Wang, J.; Wu, B.; Lu, K.; Wei, Q.; Qian, J.; Chen, Y.; Fang, Z. The amino acid permease OsAAP5 regulates tiller number and grain yield in rice. Plant Physiol. 2019, 180, 1031–1045. [Google Scholar] [CrossRef]
- Magarey, R.C. Microbiological aspects of sugarcane yield decline. Aust. J. Agric. Res. 1996, 47, 307–322. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Magarey, R.C.; Stirling, G.R.; Blair, B.L.; Bell, M.J.; Garside, A.L. Management practices to improve soil health and reduce the effects of detrimental soil biota associated with yield decline of sugarcane in Queensland. Soil Till. Res. 2003, 72, 125–137. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Blair, B.L.; Magarey, R.C.; Stirling, G.R.; Bell, M.J.; Garside, A.L. Effect of rotation breaks and organic matter amendments on the capacity of soils to develop biological suppression towards soil organisms associated with yield decline of sugarcane. Appl. Soil Ecol. 2005, 28, 271–282. [Google Scholar] [CrossRef]
- Lin, W.X.; Wu, L.K.; Lin, S.; Zhang, A.J.; Zhou, M.M.; Lin, R.; Wang, H.B.; Chen, J.; Zhang, Z.X.; Lin, R.Y. Metaproteomic analysis of ratoon sugarcane rhizospheric soil. BMC Microbiol. 2013, 13, 135. [Google Scholar] [CrossRef] [Green Version]
- Ramburanab, S.; Nxumaloa, N. Regional, seasonal, cultivar and crop-year effects on sugarcane responses to residue mulching. Field Crops Res. 2017, 210, 136–146. [Google Scholar] [CrossRef]
- Singh, N.; Joshi, M.C.; Siddique, A. Influence of ethophon and agroseb on sprouting and cane yield of sugarcane ratoon. Indian Sugar 2009, 58, 11. [Google Scholar]
- Shukla, S.K.; Yadav, R.L.; Suman, A.; Singh, P.N. Improving rhizospheric environment and sugarcane ratoon yield through bioagents amended farm yard manure in udic ustochrept soil. Soil Till. Res. 2008, 99, 158–168. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Magarey, R.C.; Stirling, G.R.; Holt, J.A.; Brown, J.D. Rotation-induced changes in soil biological properties and their effect on yield decline in sugarcane. In Proceedings of the 21st Conference of the Australian Society of Sugar Cane Technologist, Townsville, Australia, 27–30 April 1999; Volume 21, pp. 79–86. [Google Scholar]
- Gao, X.; Wu, Z.; Liu, R.; Wu, J.; Zeng, Q.; Qi, Y. Rhizosphere bacterial community characteristics over different years of sugarcane ratooning in consecutive monoculture. BioMed Res. Inter. 2019, 2019, 4943150. [Google Scholar] [CrossRef] [PubMed]
- Nong, Z.M.; Shi, G.Y.; Zeng, Q.; Ye, X.L.; Qin, H.D.; Hu, C.J. Diversity of culturable rhizosphere bacteria of ratoon sugarcane grown in latosolic red soil condition. Southwest China J. Agric. Sci. 2019, 32, 1079–1086. [Google Scholar]
- Wu, H.L.; Wang, X.H.; He, L.L.; Li, F.S. Analysis and comparison of chromosome karyotype of ten sugarcane varieties. Chin. Agric. Sci. Bull. 2013, 29, 72–78. [Google Scholar]
- D’Hont, A.; Grivet, L.; Feldmann, P.; Rao, P.S.; Berding, N.; Glauszmann, J.C. Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Mol. Gen. Genet. 1996, 250, 404–413. [Google Scholar] [CrossRef]
- Piperidis, G.; Piperidis, N.; D’Hont, A. Molecular cytogenetic investigation of chromosome composition and transmission in sugarcane. Mol. Gen. Genet. 2010, 284, 65–73. [Google Scholar] [CrossRef]
- Irvine, J.E. Saccharum species as horticultural classes. Theor. Appl. Genet. 1999, 98, 186–194. [Google Scholar] [CrossRef]
- Papini-Terzi, F.S.; Rocha, F.R.; Vencio, R.Z.N.; Felix, J.M.; Branco, D.S.; Waclawovsky, A.J.; del Bem, L.E.V.; Lembke, C.G.; Costa, M.D.L.; Nishiyama, M.Y., Jr.; et al. Sugarcane genes associated with sucrose content. BMC Genom. 2009, 10, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, N.; Siraree, A.; Yadav, S.; Kumar, S.; Singh, J.; Pandey, D.; Singh, R. Marker-trait association study for sucrose and yield contributing traits in sugarcane (Saccharum spp. hybrid). Euphytica 2015, 205, 185–201. [Google Scholar] [CrossRef]
- Wang, H.B. Genetic Map Construction and QTL Mapping of Main Agronomic Traits in Sugarcane. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. [Google Scholar]
- Ukoskit, K.; Posudsavang, G.; Pongsiripat, N.; Chatwachirawong, P.; Klomsa-ard, P.; Poomipant, P.; Tragoonrung, S. Detection and validation of EST-SSR markers associated with sugar-related traits in sugarcane using linkage and association mapping. Genomics 2019, 111, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aljanabi, S.M.; Parmessur, Y.; Kross, H.; Dhayan, S.; Saumtally, S.; Ramdoyal, K.; Autrey, L.J.C.; Dookun-Saumtally, A. Identification of a major quantitative trait locus (QTL) for yellow spot (Mycovellosiella koepkei) disease resistance in sugarcane. Mol. Breed. 2007, 19, 1–14. [Google Scholar] [CrossRef]
- Daugrois, J.H.; Grivet, L.; Roques, D.; Hoarau, J.Y.; Lombard, H.; Glaszmann, J.C.; D’Hont, A. A putative major gene for rust resistance linked with a RFLP marker in sugarcane cultivar ‘R570’. Theor. Appl. Genet. 1996, 92, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Asnaghi, C.; Roques, D.; Ruffel, S.; Kaye, C.; Hoarau, J.Y.; Lismart, H.T.; Girard, J.C.; Raboin, L.M.; Risterucci, A.M.; Grivet, L.; et al. Targeted mapping of a sugarcane rust resistance gene (Bru1) using bulked segregant analysis and AFLP markers. Theor. Appl. Genet. 2004, 108, 759–764. [Google Scholar] [CrossRef]
- Le Cunff, L.; Garsmeur, O.; Raboin, L.M.; Pauquet, J.; Telismart, H.; Selvi, A.; Grivet, L.; Philippe, R.; Begum, D.; Deu, M.; et al. Diploid/polyploid syntenic shuttle mapping and haplotype-specific chromosome walking towards a rust resistance gene (Bru1) in highly polyploid sugarcane (2n~12x~115). Genetics 2008, 180, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Parco, A.S.; Avellaneda, M.C.; Hale, A.H.; Hoy, J.W.; Kimbeng, C.A.; Pontif, M.J.; Gravois, K.A.; Baisakh, N. Frequency and distribution of the brown rust resistance gene Bru1 and implications for the Louisiana sugarcane breeding programme. Plant Breed. 2014, 133, 654–659. [Google Scholar] [CrossRef]
- Wang, H.B.; Chen, P.H.; Yang, Y.Q.; D’Hont, A.; Lu, Y.H. Molecular insights into the origin of the brown rust resistance gene Bru1 among Saccharum species. Theor. Appl. Genet. 2017, 130, 2431–2443. [Google Scholar] [CrossRef]
- Li, Z.; Su, Y.C.; Yu, Q.; Chen, Y.; Gao, S.W.; Que, Y.X.; Xu, L.P. Molecular insights into brown rust resistance and potential epidemic based on Bru1 gene. Euphytica 2018, 214, 18978. [Google Scholar] [CrossRef]
- Zhang, J.S.; Nagai, C.; Yu, Q.Y.; Pan, Y.B.; Ayala-Silva, T.; Schnell, R.J.; Comstock, J.C.; Arumuganathan, A.K.; Ming, R. Genome size variation in three Saccharum species. Euphytica 2012, 185, 511–519. [Google Scholar] [CrossRef]
- Yu, X.H.; Wang, X.H.; Yang, Q.H. Genetic diversity and phylogenetic relationship of Saccharum spontaneum L. with different ploidy levels based on SRAP markers. Sugar Tech 2019, 21, 802–814. [Google Scholar] [CrossRef]
- Riaño-Pachón, D.M.; Mattiello, L. Draft genome sequencing of the sugarcane hybrid SP80-3280. F1000Research 2017, 6, 861. [Google Scholar] [CrossRef] [PubMed]
- Garsmeur, O.; Droc, G.; Antonise, R.; Grimwood, J.; Potier, B.; Aitken, K.; Jenkins, J.; Martin, G.; Charron, C.; Hervouet, C.; et al. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat. Commun. 2018, 9, 2638. [Google Scholar] [CrossRef] [PubMed]
- Raboin, L.M.; Oliveira, K.M.; Lecunff, L.; Telismart, H.; Roques, D.; Butterfield, M.; Hoarau, J.Y.; D‘Hont, A. Genetic mapping in sugarcane, a high polyploidy, using bi-parental progeny: Identification of a gene controlling stalk colour and a new rust resistance gene. Theor. Appl. Genet. 2006, 113, 1382–1391. [Google Scholar] [CrossRef]
- Aitken, K.S.; Farmer, A.; Berkman, P.; Muller, C.; Wei, X.; Demano, E.; Jackson, P.A.; Magwire, M.; Dietrich, B.; Kota, R. Generation of a 345K sugarcane SNP chip. In Proceedings of the 38th Conference of the Australian Society of Sugar Cane Technologist, Mackay, Australia, 27–29 April 2016; Volume 29, pp. 1165–1172. [Google Scholar]
- You, Q. Development of an Axiom Sugarcane100K SNP Array and Its Applications: Construction of Linkage Maps and Identification of SCYLV-Resistance QTLs. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. [Google Scholar]
- You, Q.; Yang, X.; Peng, Z.; Sariful, M.I.; Sushma, S.; Luo, Z.L.; Comstock, J.C.; Xu, L.P.; Wang, J.P. Development of an Axiom sugarcane100K SNP array for genetic map construction and QTL identification. Theor. Appl. Genet. 2019, 132, 2829–2845. [Google Scholar] [CrossRef]
- You, Q.; Sood, S.; Luo, Z.; Liu, H.; Sariful, M.I.; Zhang, M.; Wang, J. Identifying genomic regions controlling ratoon stunting disease resistance in sugarcane clonal F1 population. Crop J. 2020, 9, 1070–1078. [Google Scholar] [CrossRef]
- Yang, X.P.; Islam, M.S.; Sood, S.; Maya, S.; Hanson, E.A.; Comstock, J.; Wang, J.P. Identifying quantitative trait loci (QTLs) and developing diagnostic markers linked to orange rust resistance in sugarcane (Saccharum spp.). Front. Plant Sci. 2018, 9, 350. [Google Scholar] [CrossRef]
- Yang, X.P.; Todd, J.; Arundale, R.; Binder, J.B.; Luo, Z.L.; Islam, M.S.; Sood, S.; Wang, J.P. Identifying loci controlling fiber composition in polyploid sugarcane (Saccharum spp.) through genome-wide association study. Ind. Crops Prod. 2019, 130, 598–605. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Wang, Z.; Lu, G.; Zeng, R.; Que, Y. Sugarcane Ratooning Ability: Research Status, Shortcomings, and Prospects. Biology 2021, 10, 1052. https://doi.org/10.3390/biology10101052
Xu F, Wang Z, Lu G, Zeng R, Que Y. Sugarcane Ratooning Ability: Research Status, Shortcomings, and Prospects. Biology. 2021; 10(10):1052. https://doi.org/10.3390/biology10101052
Chicago/Turabian StyleXu, Fu, Zhoutao Wang, Guilong Lu, Rensen Zeng, and Youxiong Que. 2021. "Sugarcane Ratooning Ability: Research Status, Shortcomings, and Prospects" Biology 10, no. 10: 1052. https://doi.org/10.3390/biology10101052
APA StyleXu, F., Wang, Z., Lu, G., Zeng, R., & Que, Y. (2021). Sugarcane Ratooning Ability: Research Status, Shortcomings, and Prospects. Biology, 10(10), 1052. https://doi.org/10.3390/biology10101052








