Pomacea canaliculata Ampullar Proteome: A Nematode-Based Bio-Pesticide Induces Changes in Metabolic and Stress-Related Pathways
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Snail Maintenance and Treatment
2.2. Transmission Electron Microscopy (TEM)
2.3. Protein Extraction and Preparation
2.4. LC-MS/MS
2.5. Data Analysis
2.6. Bioinformatic Analysis
3. Results
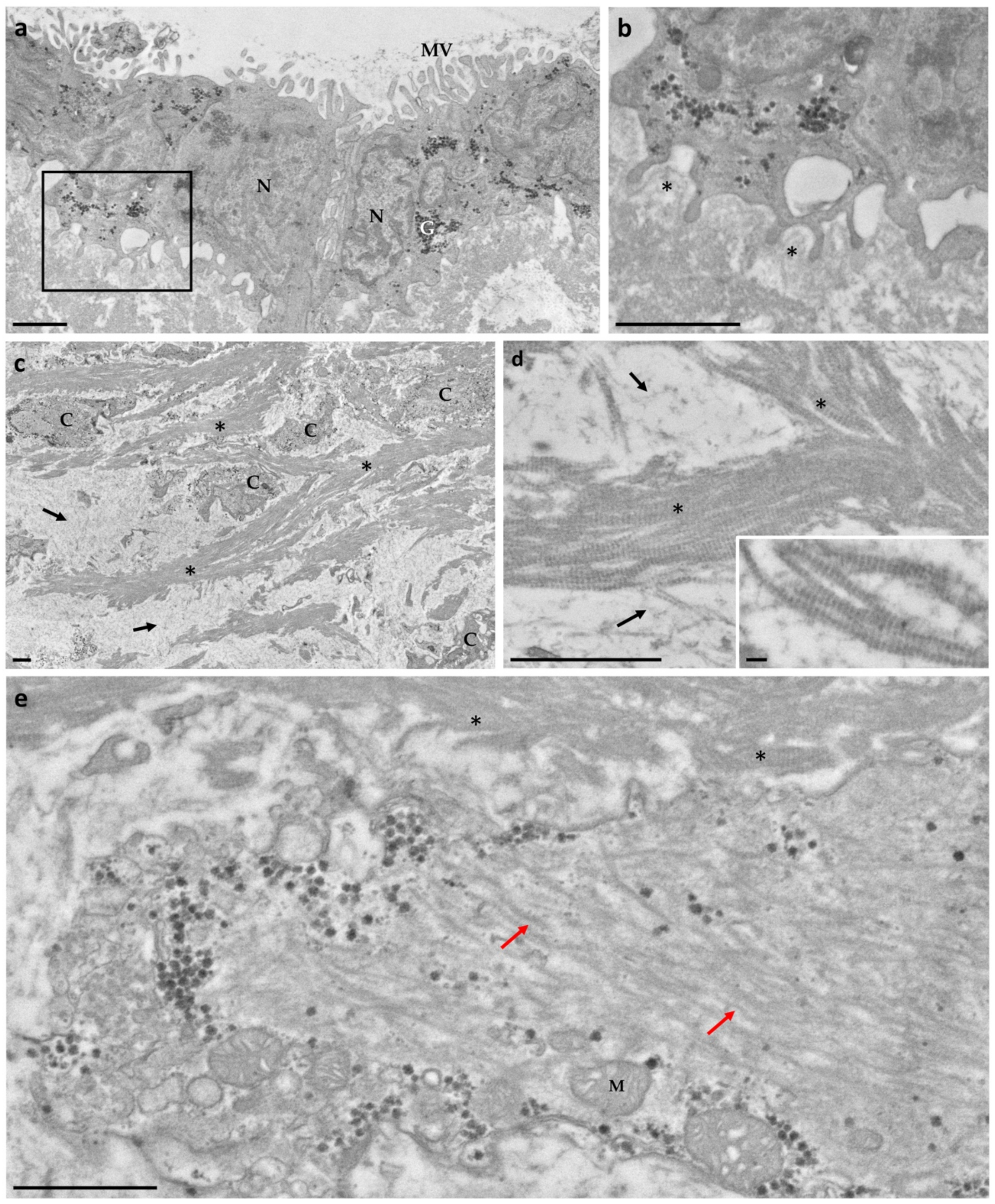
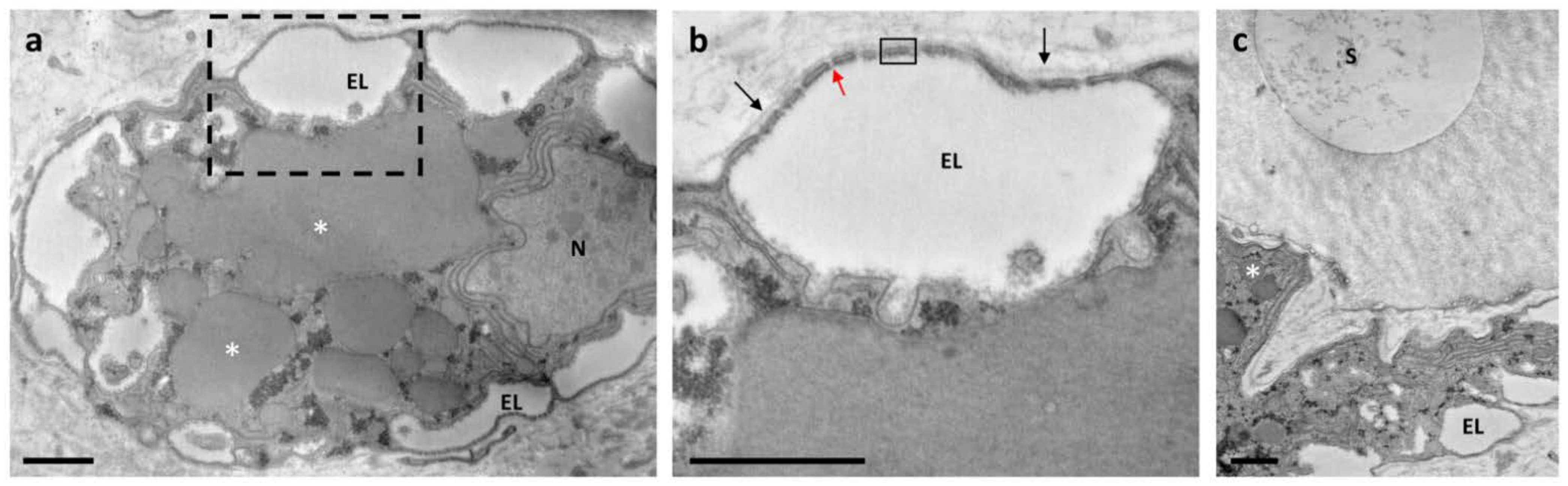
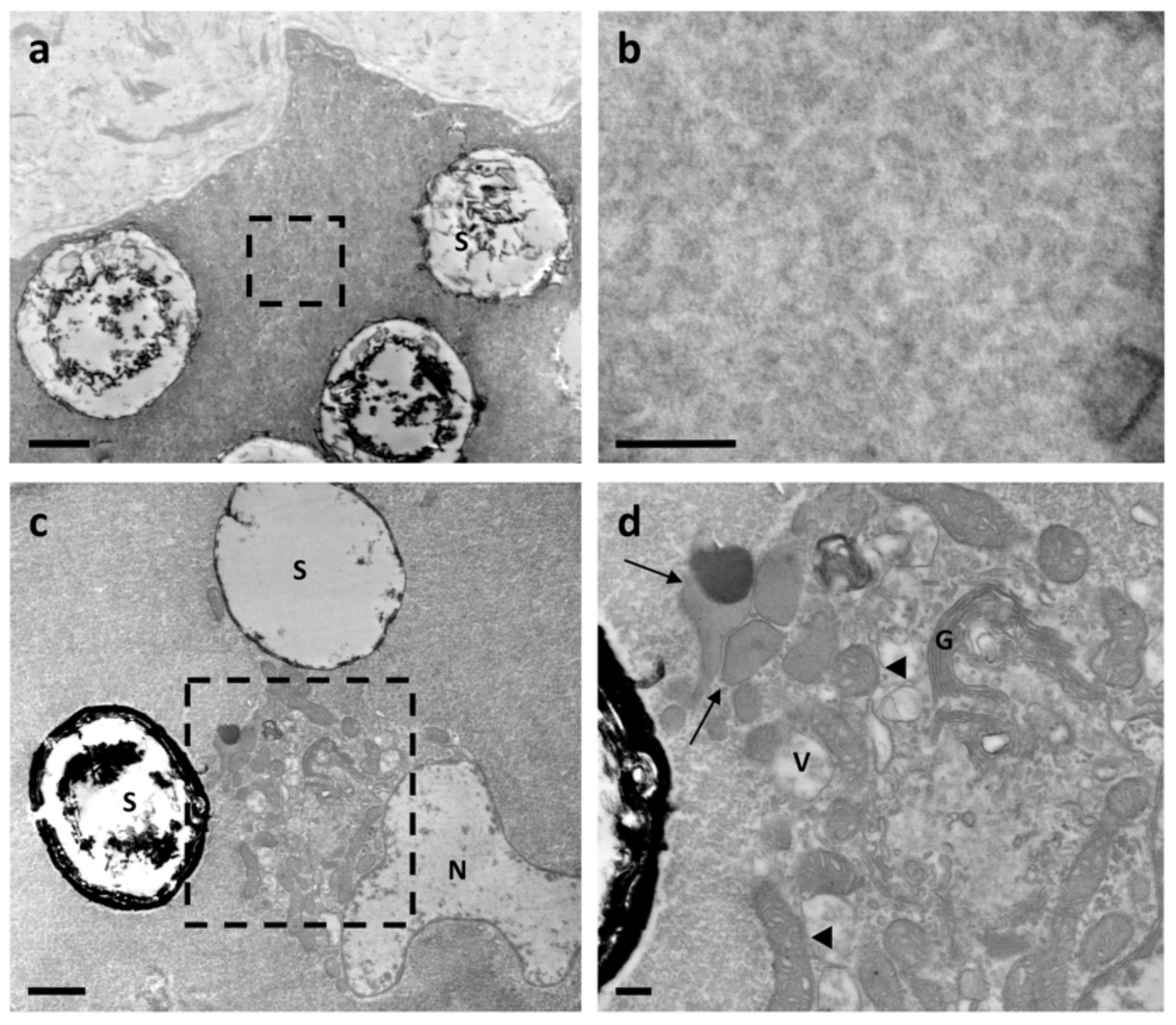
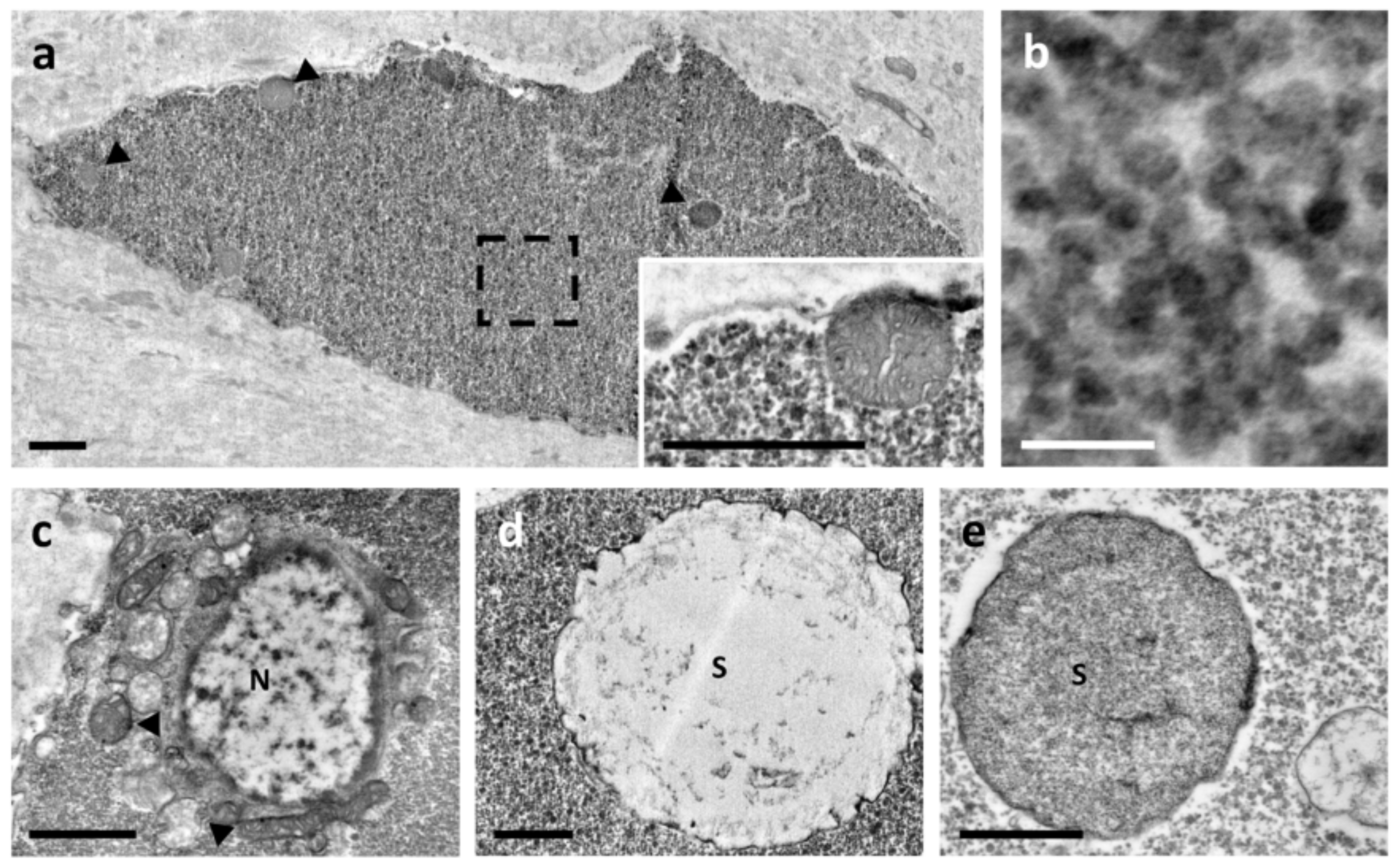
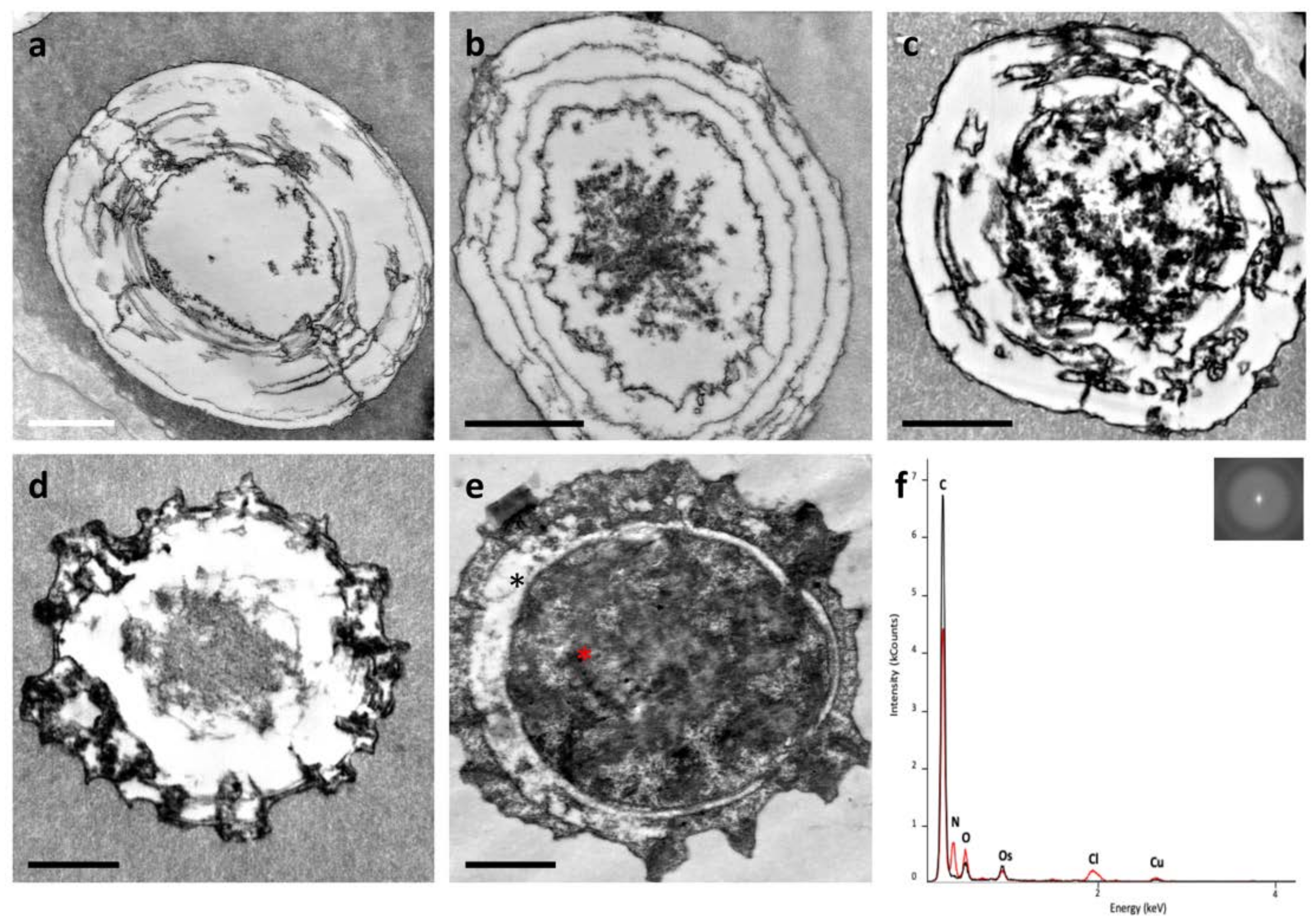
3.1. Ultrastructure Analysis Revealed a Variety of Cells in Ampullae
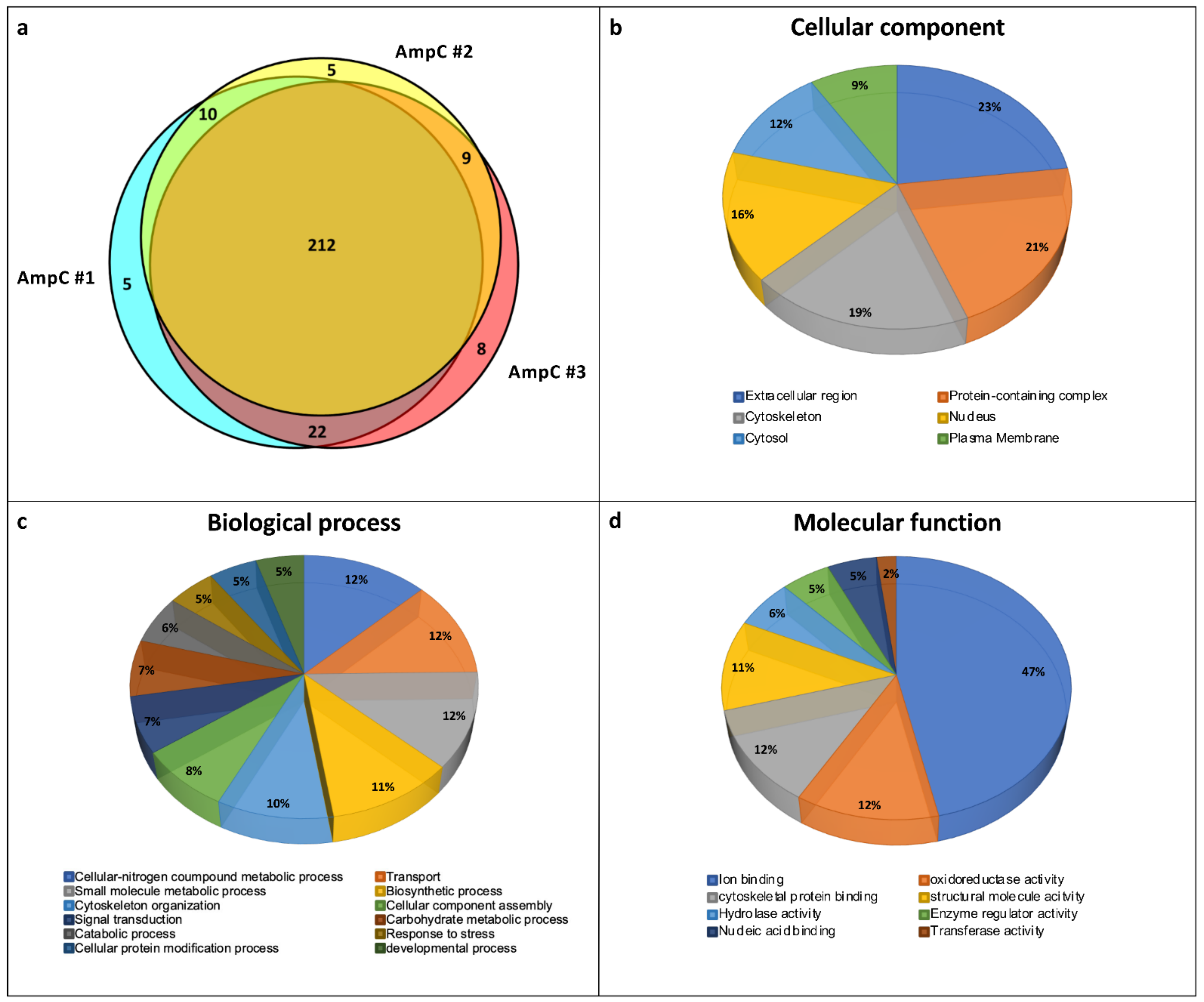
3.2. Protein Profile of Control Ampulla
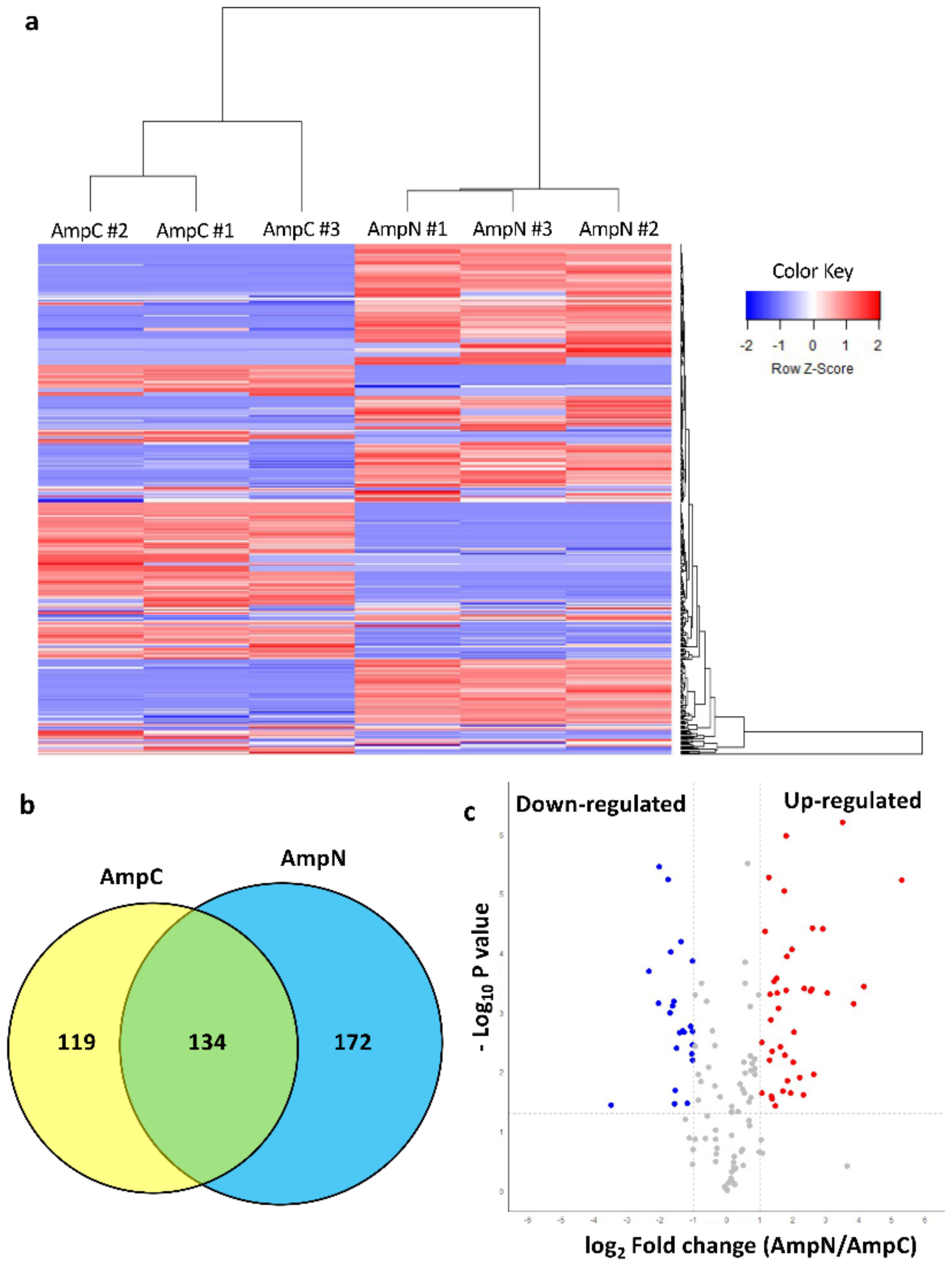
3.3. Nematode Exposure Determined a Rapid Change in the Protein Profile of Ampullae
3.4. Nematode Exposure Induced an Up-Regulation of the Antioxidant Defence
3.5. Nematode Exposure Induced a Stress Response
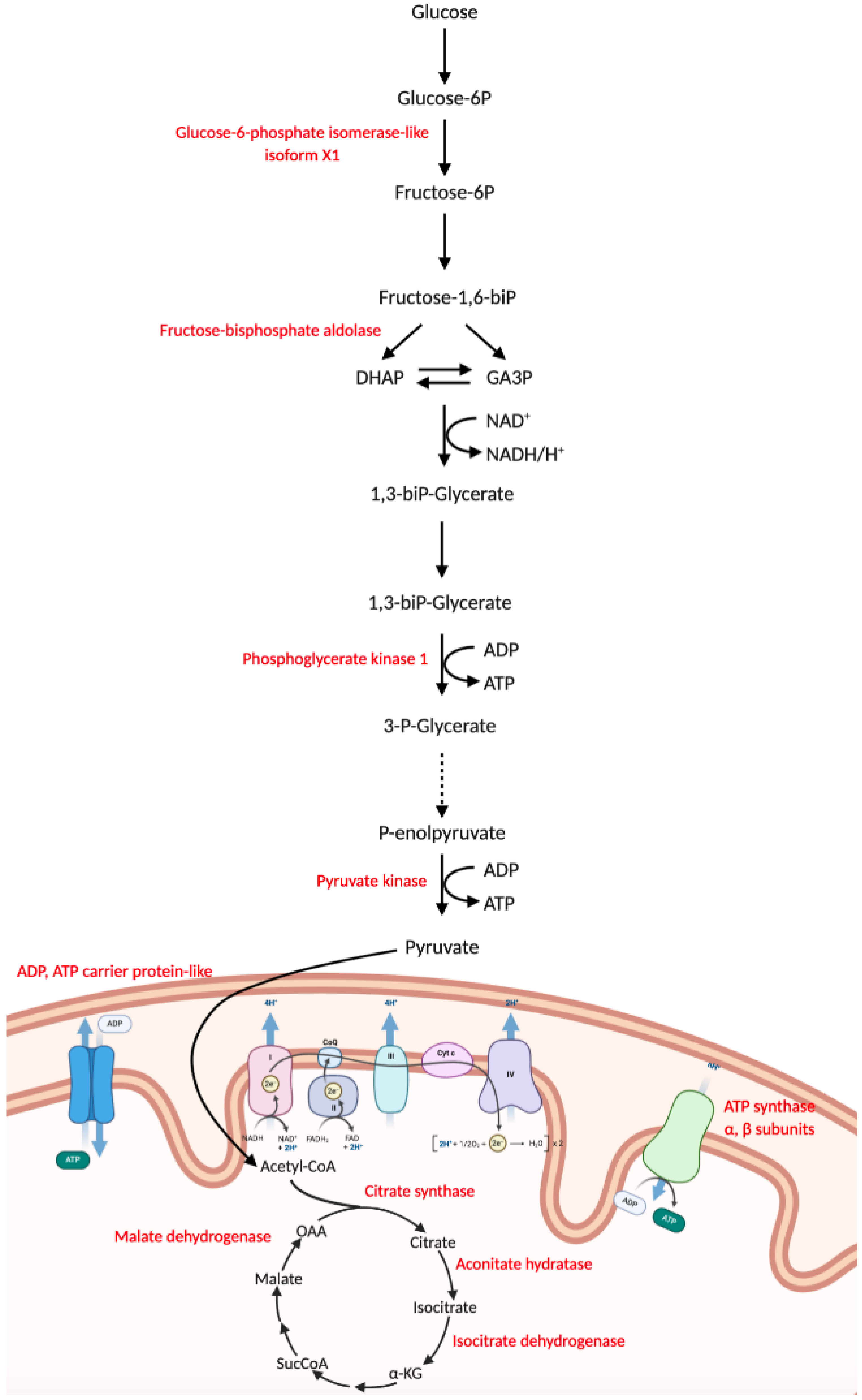
3.6. Nematode Exposure Induced Changes in Energy Metabolism
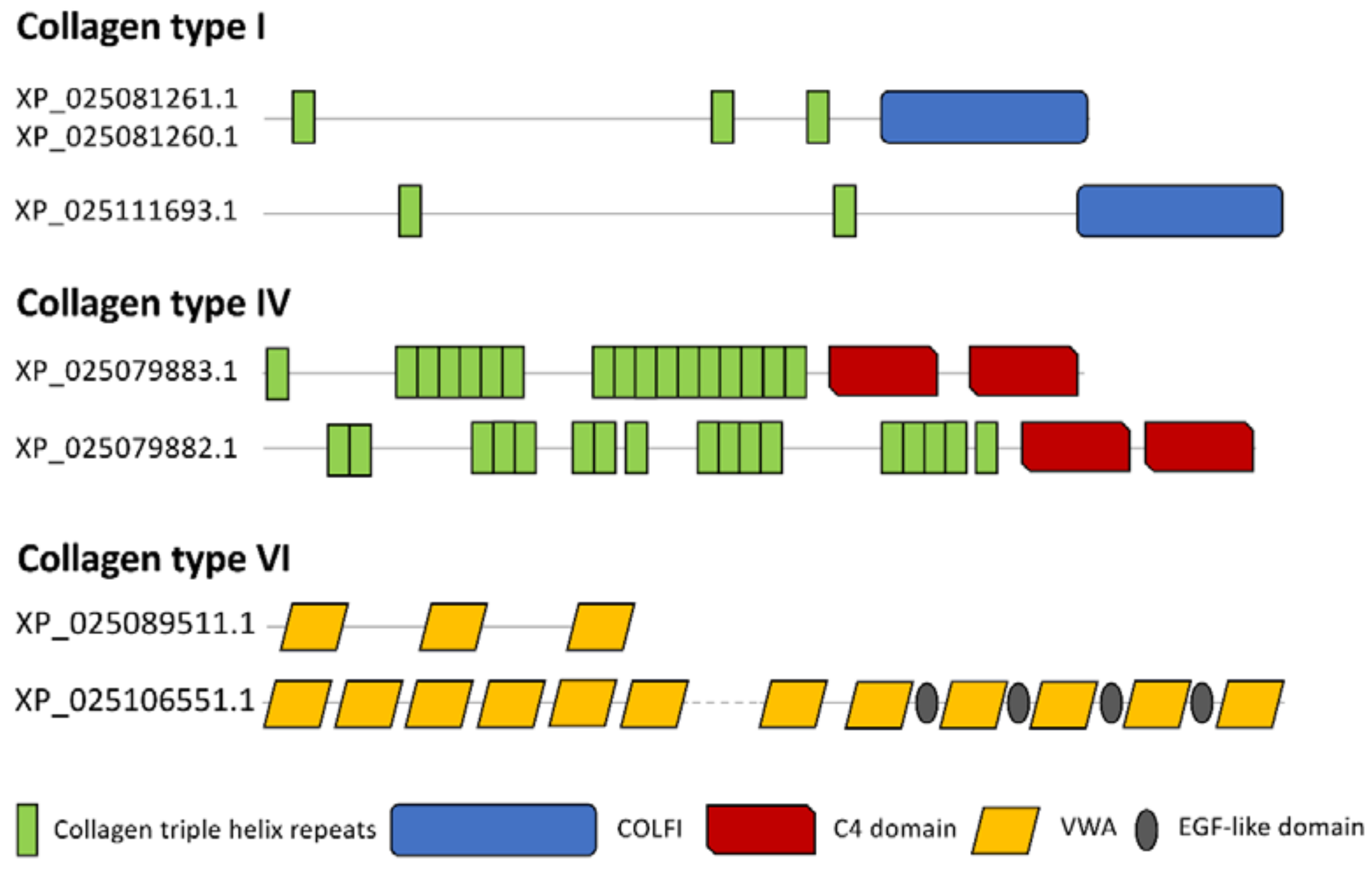
3.7. Nematode Exposure Influenced Cytoskeletal Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carver, S.; Mills, J.N.; Parmenter, C.A.; Parmenter, R.R.; Richardson, K.S.; Harris, R.L.; Douglass, R.J.; Kuenzi, A.J.; Luis, A.D. Toward a Mechanistic Understanding of Environmentally Forced Zoonotic Disease Emergence: Sin Nombre Hantavirus. Bioscience 2015, 65, 651–666. [Google Scholar] [CrossRef] [Green Version]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The Spread of Invasive Species and Infectious Disease as Drivers of Ecosystem Change. Front. Ecol. Environ. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Coustau, C.; Gourbal, B.; Duval, D.; Yoshino, T.P.; Adema, C.M.; Mitta, G. Advances in Gastropod Immunity from the Study of the Interaction between the Snail Biomphalaria glabrata and Its Parasites: A Review of Research Progress over the Last Decade. Fish. Shellfish Immunol. 2015, 46, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Tascedda, F.; Malagoli, D.; Accorsi, A.; Rigillo, G.; Blom, J.M.C.; Ottaviani, E. Molluscs as Models for Translational Medicine. Med. Sci. Monit. Basic Res. 2015, 21, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Malagoli, D. Going beyond a Static Picture: The Apple Snail Pomacea canaliculata Can Tell Us the Life History of Molluscan Hemocytes. Invertebr. Surviv. J. 2018, 15, 61–65. [Google Scholar]
- Yang, Q.-Q.; Yu, X.-P. A New Species of Apple Snail in the Genus Pomacea (Gastropoda: Caenogastropoda: Ampullariidae). Zool. Stud. 2019, 58, e13. [Google Scholar] [CrossRef] [PubMed]
- Dumidae, A.; Janthu, P.; Subkrasae, C.; Polseela, R.; Mangkit, B.; Thanwisai, A.; Vitta, A. Population Genetics Analysis of a Pomacea Snail (Gastropoda: Ampullariidae) in Thailand and Its Low Infection by Angiostrongylus cantonensis. Zool. Stud. 2021, 60, 31. [Google Scholar] [CrossRef]
- Wu, J.Y.; Wu, Y.T.; Li, M.C.; Chiu, Y.W.; Liu, M.Y.; Liu, L.L. Reproduction and Juvenile Growth of the Invasive Apple Snails Pomacea canaliculata and P. scalaris (Gastropoda: Ampullariidae) in Taiwan. Zool. Stud. 2011, 50, 61–68. [Google Scholar]
- Liang, K.; Zhang, J.; Fang, L.; Zhao, B.; Luo, M.; Parajuli, P.; Ouyang, Y. The Biological Control of Pomacea canaliculata Population by Rice-Duck Mutualism in Paddy Fields. Biocontrol Sci. Technol. 2013, 23, 674–690. [Google Scholar] [CrossRef]
- Yam, R.S.W.; Fan, Y.-T.; Wang, T.-T. Importance of Macrophyte Quality in Determining Life-History Traits of the Apple Snails Pomacea canaliculata: Implications for Bottom-Up Management of an Invasive Herbivorous Pest in Constructed Wetlands. Int. J. Environ. Res. Public Health 2016, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Montanari, A.; Bergamini, G.; Ferrari, A.; Ferri, A.; Nasi, M.; Simonini, R.; Malagoli, D. The Immune Response of the Invasive Golden Apple Snail to a Nematode-Based Molluscicide Involves Different Organs. Biology 2020, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Glen, D.M.; George, S.K. The Rhabditid Nematode Phasmarhabditis hermaphrodita as a Potential Biological Control Agent for Slugs. Biocontrol. Sci. Technol. 1993, 3, 503–511. [Google Scholar] [CrossRef]
- Tan, L.; Grewal, P.S. Pathogenicity of Moraxella Osloensis, a Bacterium Associated with the Nematode Phasmarhabditis hermaphrodita, to the Slug Deroceras reticulatum. Appl. Environ. Microbiol. 2001, 67, 5010–5016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rae, R.G.; Tourna, M.; Wilson, M.J. The Slug Parasitic Nematode Phasmarhabditis hermaphrodita Associates with Complex and Variable Bacterial Assemblages That Do Not Affect Its Virulence. J. Invertebr. Pathol. 2010, 104, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, A.; Bucci, L.; de Eguileor, M.; Ottaviani, E.; Malagoli, D. Comparative Analysis of Circulating Hemocytes of the Freshwater Snail Pomacea canaliculata. Fish Shellfish Immunol. 2013, 34, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, A.; Ottaviani, E.; Malagoli, D. Effects of Repeated Hemolymph Withdrawals on the Hemocyte Populations and Hematopoiesis in Pomacea canaliculata. Fish Shellfish Immunol. 2014, 38, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Lofaro, F.D.; Accorsi, A.; Ross, E.; Malagoli, D. Toward the Molecular Deciphering of P Pomacea canaliculata Immunity: First Proteomic Analysis of Circulating Hemocytes. Proteomics 2019, 19, e1800314. [Google Scholar] [CrossRef]
- Cueto, J.A.; Rodriguez, C.; Vega, I.A.; Castro-Vazquez, A. Immune Defenses of the Invasive Apple Snail Pomacea canaliculata (Caenogastropoda, Ampullariidae): Phagocytic Hemocytes in the Circulation and the Kidney. PLoS ONE 2015, 10, e0123964. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Assessment of the Kidney and Lung as Immune Barriers and Hematopoietic Sites in the Invasive Apple Snail Pomacea canaliculata. PeerJ 2018, 6, e5789. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Simon, V.; Conget, P.; Vega, I.A. Both Quiescent and Proliferating Cells Circulate in the Blood of the Invasive Apple Snail Pomacea canaliculata. Fish Shellfish Immunol. 2020, 107, 95–103. [Google Scholar] [CrossRef]
- Accorsi, A.; Benatti, S.; Ross, E.; Nasi, M.; Malagoli, D. A Prokineticin-like Protein Responds to Immune Challenges in the Gastropod Pest Pomacea canaliculata. Dev. Comp. Immunol. 2017, 72, 37–43. [Google Scholar] [CrossRef]
- Cueto, J.A.; Vega, I.A.; Castro-Vazquez, A. Multicellular Spheroid Formation and Evolutionary Conserved Behaviors of Apple Snail Hemocytes in Culture. Fish Shellfish Immunol. 2013, 34, 443–453. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ren, Y.; Wang, H.; Li, S.; Jiang, F.; Yin, L.; Qiao, X.; Zhang, G.; Qian, W.; et al. The Genome of the Golden Apple Snail Pomacea canaliculata Provides Insight into Stress Tolerance and Invasive Adaptation. Gigascience 2018, 7, giy101. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, M.; Wang, H.; Zhang, H.; Zhang, X.; Thiyagarajan, V.; Qian, P.Y.; Qiu, J.W. De Novo Assembly of the Transcriptome of an Invasive Snail and Its Multiple Ecological Applications. Mol. Ecol. Resour. 2012, 12, 1133–1144. [Google Scholar] [CrossRef]
- Sun, J.; Mu, H.; Ip, J.C.H.; Li, R.; Xu, T.; Accorsi, A.; Sánchez Alvarado, A.; Ross, E.; Lan, Y.; Sun, Y.; et al. Signatures of Divergence, Invasiveness, and Terrestrialization Revealed by Four Apple Snail Genomes. Mol. Biol. Evol. 2019, 36, 1507–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ip, J.C.H.; Mu, H.; Zhang, Y.; Sun, J.; Heras, H.; Chu, K.H.; Qiu, J.-W. Understanding the Transition from Water to Land: Insights from Multi-Omic Analyses of the Perivitelline Fluid of Apple Snail Eggs. J. Proteom. 2019, 194, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Sun, J.; Heras, H.; Chu, K.H.; Qiu, J.-W. Dataset for the Proteomic and Transcriptomic Analyses of Perivitelline Fluid Proteins in Pomacea Snail Eggs. Data Brief 2017, 15, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.-J.; Park, Y.-S. Key Determinants of Freshwater Gastropod Diversity and Distribution: The Implications for Conservation and Management. Water 2020, 12, 1908. [Google Scholar] [CrossRef]
- Heras, H.; Frassa, M.V.; Fernández, P.E.; Galosi, C.M.; Gimeno, E.J.; Dreon, M.S. First Egg Protein with a Neurotoxic Effect on Mice. Toxicon 2008, 52, 481–488. [Google Scholar] [CrossRef]
- Song, L.; Wang, X.; Yang, Z.; Lv, Z.; Wu, Z. Angiostrongylus cantonensis in the Vector Snails Pomacea canaliculata and Achatina Fulica in China: A Meta-Analysis. Parasitol. Res. 2016, 115, 913–923. [Google Scholar] [CrossRef]
- Accorsi, A.; Ross, E.; Ottaviani, E.; Sánchez Alvarado, A. Pomacea canaliculata: A New Model System for Studying Development and Regeneration of Complex Eyes. J. Histochem 2017, 61, 11. [Google Scholar]
- Accorsi, A.; Ross, E.; McClain, M.; McKinney, S.; Alvarado, A.S. Complete Regeneration of a Camera-Type Eye in the Research Organism Pomacea canaliculata. FASEB J. 2018, 32, 232.4. [Google Scholar] [CrossRef]
- Bever, M.M.; Borgens, R.B. Electrical Responses to Amputation of the Eye in the Mystery Snail. J. Exp. Zool. 1988, 245, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, L.L.; Yang, S.; Zhang, J.E.; Zhao, N.Q.; Wu, H.; He, Z.; Yan, T.M.; Guo, J. Regeneration of Excised Shell by the Invasive Apple Snail Pomacea canaliculata. Mar. Freshw. Behav. Physiol. 2017, 50, 17–29. [Google Scholar] [CrossRef]
- Bergamini, G.; Ahmad, M.; Cocchi, M.; Malagoli, D. A New Protocol of Computer-Assisted Image Analysis Highlights the Presence of Hemocytes in the Regenerating Cephalic Tentacles of Adult Pomacea canaliculata. Int. J. Mol. Sci. 2021, 22, 5023. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, E.; Accorsi, A.; Rigillo, G.; Malagoli, D.; Blom, J.M.C.; Tascedda, F. Epigenetic Modification in Neurons of the Mollusc Pomacea canaliculata after Immune Challenge. Brain Res. 2013, 1537, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.B. The Functional Anatomy of the Mantle Cavity, Kidney and Blood System of Some Pilid Gastropods (Prosobranchia). Proc. Zool. Soc. Lond. 1965, 146, 70–94. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Koch, E.; Vega, I.A.; Gamarra-Luques, C.; Castro-Vazquez, A. Urate Cells and Tissues in the South American Apple Snail Pomacea canaliculata. J. Molluscan Stud. 2008, 74, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Giraud-Billoud, M.; Castro-Vazquez, A.; Campoy-Diaz, A.D.; Giuffrida, P.M.; Vega, I.A. Tolerance to Hypometabolism and Arousal Induced by Hibernation in the Apple Snail Pomacea canaliculata (Caenogastropoda, Ampullariidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 224, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Rivera-Ingraham, G.A.; Moreira, D.C.; Burmester, T.; Castro-Vazquez, A.; Carvajalino-Fernández, J.M.; Dafre, A.; Niu, C.; Tremblay, N.; Paital, B.; et al. Twenty Years of the ‘Preparation for Oxidative Stress’ (POS) Theory: Ecophysiological Advantages and Molecular Strategies. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2019, 234, 36–49. [Google Scholar] [CrossRef]
- Boraldi, F.; Lofaro, F.D.; Losi, L.; Quaglino, D. Dermal Alterations in Clinically Unaffected Skin of Pseudoxanthoma Elasticum Patients. J. Clin. Med. 2021, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Boraldi, F.; Moscarelli, P.; Lofaro, F.D.; Sabia, C.; Quaglino, D. The Mineralization Process of Insoluble Elastin Fibrillar Structures: Ionic Environment vs Degradation. Int. J. Biol. Macromol. 2020, 149, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially Modified Protein Abundance Index (EmPAI) for Estimation of Absolute Protein Amount in Proteomics by the Number of Sequenced Peptides per Protein. Mol. Cell Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lofaro, F.; Boraldi, F.; Garcia-Fermandez, M.; Estrella, L.; Valdivielso, P.; Quaglino, D. Relationship between Mitochondrial Structure and Bioenergetics in Pseudoxanthoma Elasticum Dermal Fibroblasts. Front. Cell Dev. Biol. 2020, 8, 610266. [Google Scholar] [CrossRef]
- De Souza, G.A.; Fortuin, S.; Aguilar, D.; Pando, R.H.; McEvoy, C.R.E.; van Helden, P.D.; Koehler, C.J.; Thiede, B.; Warren, R.M.; Wiker, H.G. Using a Label-Free Proteomics Method to Identify Differentially Abundant Proteins in Closely Related Hypo- and Hypervirulent Clinical Mycobacterium tuberculosis Beijing Isolates. Mol. Cell Proteom. 2010, 9, 2414–2423. [Google Scholar] [CrossRef] [Green Version]
- Arike, L.; Peil, L. Spectral Counting Label-Free Proteomics. In Shotgun Proteomics: Methods and Protocols; Martins-de-Souza, D., Ed.; Springer: New York, NY, USA, 2014; pp. 213–222. ISBN 978-1-4939-0685-7. [Google Scholar]
- Kokkinopoulou, M.; Güler, M.A.; Lieb, B.; Barbeck, M.; Ghanaati, S.; Markl, J. 3D-Ultrastructure, Functions and Stress Responses of Gastropod (Biomphalaria glabrata) Rhogocytes. PLoS ONE 2014, 9, e101078. [Google Scholar] [CrossRef] [Green Version]
- Kokkinopoulou, M.; Spiecker, L.; Messerschmidt, C.; Barbeck, M.; Ghanaati, S.; Landfester, K.; Markl, J. On the Ultrastructure and Function of Rhogocytes from the Pond Snail Lymnaea stagnalis. PLoS ONE 2015, 10, e0141195. [Google Scholar] [CrossRef] [Green Version]
- Simkiss, K.; Mason, A.Z. Metal Ions: Metabolic and Toxic Effects. In Biology of Mollusca; Wilbur, K.M., Ed.; Academic Press: New York, NY, USA, 1983; pp. 101–164. ISBN 978-0-12-751402-4. [Google Scholar]
- Vega, I.; Giraud-Billoud, M.; Koch, E.; Gamarra-Luques, C.; Castro-Vazquez, A. Uric Acid Accumulation within Intracellular Corpuscles of the Midgut Gland in Pomacea canalaculata (Caenogastropoda, Ampullariidae). Veliger 2007, 48, 276–283. [Google Scholar]
- Gaudet, P.; Dessimoz, C. Gene Ontology: Pitfalls, Biases, and Remedies. In The Gene Ontology Handbook; Dessimoz, C., Škunca, N., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 189–205. ISBN 978-1-4939-3743-1. [Google Scholar]
- Song, X.; Cai, L.; Li, Y.; Zhu, J.; Jin, P.; Chen, L.; Ma, F. Identification and Characterization of Transforming Growth Factor β Induced Gene (TGFBIG) from Branchiostoma belcheri: Insights into Evolution of TGFBI Family. Genomics 2014, 103, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, C.; Vandenbulcke, F.; Bocquet, B.; Tasiemski, A.; Desmons, A.; Verstraete, M.; Salzet, M.; Cocquerelle, C. Cathepsin L and Cystatin B Gene Expression Discriminates Immune Cœlomic Cells in the Leech Theromyzon tessulatum. Dev. Comp. Immunol. 2008, 32, 795–807. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.; Goetz, G.; White, S.; Goetz, F. Analysis of Genes Isolated from Plated Hemocytes of the Pacific Oyster, Crassostreas Gigas. Mar. Biotechnol. 2009, 11, 24–44. [Google Scholar] [CrossRef]
- Gnatyshyna, L.; Falfushynska, H.; Stoliar, O.; Dallinger, R. Preliminary Study of Multiple Stress Response Reactions in the Pond Snail Lymnaea stagnalis Exposed to Trace Metals and a Thiocarbamate Fungicide at Environmentally Relevant Concentrations. Arch. Environ. Contam. Toxicol. 2020, 79, 89–100. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Shamsuri, R.B.; Ubaidillah, A.M. Evaluation of Sodium Fluoride Toxicity in Schistosoma Infected Snails: Assessment of Antioxidants, Antiapoptotic, Hypoprotein and Hypocholesterol Activities. J. Parasit. Dis. 2016, 40, 1451–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieb, B.; Altenhein, B.; Markl, J.; Vincent, A.; van Olden, E.; van Holde, K.E.; Miller, K.I. Structures of Two Molluscan Hemocyanin Genes: Significance for Gene Evolution. Proc. Natl. Acad. Sci. USA 2001, 98, 4546–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhang, H.; Cheng, D.; Tan, K.; Ye, T.; Ma, H.; Li, S.; Zheng, H. Differential Responses of a Pi-Class Glutathione S-Transferase (CnGSTp) Expression and Antioxidant Status between Golden and Brown Noble Scallops under Pathogenic Stress. Fish Shellfish Immunol. 2020, 105, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanina, A.V.; Cherkasov, A.S.; Sokolova, I.M. Effects of Cadmium on Cellular Protein and Glutathione Synthesis and Expression of Stress Proteins in Eastern Oysters, Crassostrea virginica Gmelin. J. Exp. Biol. 2008, 211, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Yarmola, E.G.; Bubb, M.R. Profilin: Emerging Concepts and Lingering Misconceptions. Trends Biochem. Sci. 2006, 31, 197–205. [Google Scholar] [CrossRef]
- Silacci, P.; Mazzolai, L.; Gauci, C.; Stergiopulos, N.; Yin, H.L.; Hayoz, D. Gelsolin Superfamily Proteins: Key Regulators of Cellular Functions. Cell Mol. Life Sci. 2004, 61, 2614–2623. [Google Scholar] [CrossRef] [Green Version]
- Revenu, C.; Ubelmann, F.; Hurbain, I.; El-Marjou, F.; Dingli, F.; Loew, D.; Delacour, D.; Gilet, J.; Brot-Laroche, E.; Rivero, F.; et al. A New Role for the Architecture of Microvillar Actin Bundles in Apical Retention of Membrane Proteins. Mol. Biol. Cell 2012, 23, 324–336. [Google Scholar] [CrossRef]
- Langhorst, M.F.; Solis, G.P.; Hannbeck, S.; Plattner, H.; Stuermer, C.A.O. Linking Membrane Microdomains to the Cytoskeleton: Regulation of the Lateral Mobility of Reggie-1/Flotillin-2 by Interaction with Actin. FEBS Lett. 2007, 581, 4697–4703. [Google Scholar] [CrossRef] [Green Version]
- Neumann-Giesen, C.; Fernow, I.; Amaddii, M.; Tikkanen, R. Role of EGF-Induced Tyrosine Phosphorylation of Reggie-1/Flotillin-2 in Cell Spreading and Signaling to the Actin Cytoskeleton. J. Cell Sci. 2007, 120, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Colombatti, A.; Bonaldo, P.; Doliana, R. Type A Modules: Interacting Domains Found in Several Non-Fibrillar Collagens and in Other Extracellular Matrix Proteins. Matrix 1993, 13, 297–306. [Google Scholar] [CrossRef]
- Boer, H.H.; Sminia, T. Sieve Structure of Slit Diaphragms of Podocytes and Pore Cells of Gastropod Molluscs. Cell Tissue Res. 1976, 170, 221–229. [Google Scholar] [CrossRef]
- Sminia, T.; Boer, H.H. Hemocyanin Production in Pore Cells of the Freshwater Snail Lymnaea stagnalis. Z. Zellforsch. Mikrosk. Anat. 1973, 145, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Weavers, H.; Prieto-Sánchez, S.; Grawe, F.; Garcia-López, A.; Artero, R.; Wilsch-Bräuninger, M.; Ruiz-Gómez, M.; Skaer, H.; Denholm, B. The Insect Nephrocyte Is a Podocyte-like Cell with a Filtration Slit Diaphragm. Nature 2009, 457, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Morphological Grounds for the Obligate Aerial Respiration of an Aquatic Snail: Functional and Evolutionary Perspectives. PeerJ 2021, 9, e10763. [Google Scholar] [CrossRef]
- Dallinger, R.; Chabicovsky, M.; Hödl, E.; Prem, C.; Hunziker, P.; Manzl, C. Copper in Helix pomatia (Gastropoda) Is Regulated by One Single Cell Type: Differently Responsive Metal Pools in Rhogocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1185–R1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haszprunar, G. The Molluscan Rhogocyte (Pore-Cell, Blasenzelle, Cellule Nucale), and Its Significance for Ideas on Nephridial Evolution. J. Molluscan Stud. 1996, 62, 185–211. [Google Scholar] [CrossRef]
- Marigómez, I.; Soto, M.; Cajaraville, M.P.; Angulo, E.; Giamberini, L. Cellular and Subcellular Distribution of Metals in Molluscs. Microsc. Res. Tech. 2002, 56, 358–392. [Google Scholar] [CrossRef] [PubMed]
- Nott, J.A.; Bebianno, M.J.; Langston, W.J.; Ryan, K.P. Cadmium in the Gastropod Littorina littorea. J. Mar. Biol. Assoc. United Kingd. 1993, 73, 655–665. [Google Scholar] [CrossRef]
- Beuerlein, K.; Löhr, S.; Westermann, B.; Ruth, P.; Schipp, R. Components of the Cellular Defense and Detoxification System of the Common Cuttlefish Sepia officinalis (Mollusca, Cephalopoda). Tissue Cell 2002, 34, 390–396. [Google Scholar] [CrossRef]
- Skelding, J.M.; Newell, P.F. On the Functions of the Pore Cells in the Connective Tissue of Terrestrial Pulmonate Molluscs. Cell Tissue Res. 1975, 156, 381–390. [Google Scholar] [CrossRef]
- Albrecht, U.; Keller, H.; Gebauer, W.; Markl, J. Rhogocytes (Pore Cells) as the Site of Hemocyanin Biosynthesis in the Marine Gastropod Haliotis tuberculata. Cell Tissue Res. 2001, 304, 455–462. [Google Scholar] [CrossRef]
- Martin, A.M.; Martin, G.G.; Butler, R.; Goffredi, S.K. Synthesis of Keyhole Limpet Hemocyanin by the Rhogocytes of Megathura crenulata. Invertebr. Biol. 2011, 130, 302–312. [Google Scholar] [CrossRef]
- Sairi, F.; Valtchev, P.; Gomes, V.G.; Dehghani, F. Distribution and Characterization of Rhogocyte Cell Types in the Mantle Tissue of Haliotis laevigata. Mar. Biotechnol. 2015, 17, 168–179. [Google Scholar] [CrossRef]
- Sminia, T. Structure and Function of Blood and Connective Tissue Cells of the Fresh Water Pulmonate Lymnaea stagnalis Studied by Electron Microscopy and Enzyme Histochemistry. Z. Zellforsch. Mikrosk. Anat. 1972, 130, 497–526. [Google Scholar] [CrossRef]
- Sminia, T.; Vlugh-van Dallen, J.E. Hemocyanin Synthesis in Pore Cells of the Terrestrial Snail Helix aspersa. Cell Tissue Res. 1977, 183, 299–301. [Google Scholar] [CrossRef]
- Chiumiento, I.R.; Ituarte, S.; Sun, J.; Qiu, J.W.; Heras, H.; Dreon, M.S. Hemocyanin of the Caenogastropod Pomacea canaliculata Exhibits Evolutionary Differences among Gastropod Clades. PLoS ONE 2020, 15, e0228325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, R.; Cruz-Hofling, M.; Matsuura, M. Functional and Dissociation Properties and Structural Organization of the Hemocyanin of Ampullaria canaliculata (Gastropoda, Mollusca). Comp. Biochem. Physiol. 1993, 105, 725–730. [Google Scholar] [CrossRef]
- Duerr, D.F. Qualitative Analysis of the Uric Acid, Xanthine, and Guanine Content of Several Snails. Rep. Am. Malc. Un. 1966, 66–67. [Google Scholar]
- Duerr, F.G. The Uric Acid Content of Several Species of Prosobranch and Pulmonate Snails as Related to Nitrogen Excretion. Comp. Biochem. Physiol. 1967, 22, 333–340. [Google Scholar] [CrossRef]
- Sun, J.; Mu, H.; Zhang, H.; Chandramouli, K.H.; Qian, P.-Y.; Wong, C.K.C.; Qiu, J.-W. Understanding the Regulation of Estivation in a Freshwater Snail through ITRAQ-Based Comparative Proteomics. J. Proteome Res. 2013, 12, 5271–5280. [Google Scholar] [CrossRef]
- Mayne, R.; Brewton, R.G. New Members of the Collagen Superfamily. Curr. Opin. Cell Biol. 1993, 5, 883–890. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb Perspect. Biol. 2011, 3, a004678. [Google Scholar] [CrossRef] [Green Version]
- Aouacheria, A.; Geourjon, C.; Aghajari, N.; Navratil, V.; Deléage, G.; Lethias, C.; Exposito, J.-Y. Insights into Early Extracellular Matrix Evolution: Spongin Short Chain Collagen-Related Proteins Are Homologous to Basement Membrane Type IV Collagens and Form a Novel Family Widely Distributed in Invertebrates. Mol. Biol. Evol. 2006, 23, 2288–2302. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Wang, L.; Yang, J.; Zhang, H.; Wang, L.; Song, L. A Four-CRD C-Type Lectin from Chlamys Farreri Mediating Nonself-Recognition with Broader Spectrum and Opsonization. Dev. Comp. Immunol. 2013, 39, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Yang, J.; Zhang, H.; Huang, M.; Kong, P.; Zhou, Z.; Song, L. A Multi-CRD C-Type Lectin with Broad Recognition Spectrum and Cellular Adhesion from Argopecten irradians. Dev. Comp. Immunol. 2012, 36, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Yu, D.; Yang, B.; Chen, L.; Hayouka, Z.; Chen, X.; Gong, Y.; Dai, H.; Wang, L.; Zhao, Y.; et al. Molecular Characterization, Expression and Immune Functions of Two C-Type Lectin from Venerupis philippinarum. Fish Shellfish Immunol. 2020, 107, 260–268. [Google Scholar] [CrossRef]
- Jeffroy, F.; Brulle, F.; Paillard, C. Differential Expression of Genes Involved in Immunity and Biomineralization during Brown Ring Disease Development and Shell Repair in the Manila Clam, Ruditapes philippinarum. J. Invertebr. Pathol. 2013, 113, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, B.; Lun, C.M.; Adema, C.M. Comparative ORESTES-Sampling of Transcriptomes of Immune-Challenged Biomphalaria glabrata Snails. J. Invertebr. Pathol. 2008, 99, 192–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canesi, L.; Scarpato, A.; Betti, M.; Ciacci, C.; Pruzzo, C.; Gallo, G. Bacterial Killing by Mytilus Hemocyte Monolayers as a Model for Investigating the Signaling Pathways Involved in Mussel Immune Defence. Mar. Environ. Res. 2002, 54, 547–551. [Google Scholar] [CrossRef]
- Delaporte, M.; Soudant, P.; Moal, J.; Giudicelli, E.; Lambert, C.; Séguineau, C.; Samain, J.-F. Impact of 20:4n−6 Supplementation on the Fatty Acid Composition and Hemocyte Parameters of the Pacific Oyster Crassostrea gigas. Lipids 2006, 41, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Hermes-Lima, M.; Storey, J.M.; Storey, K.B. Antioxidant Defenses and Metabolic Depression. The Hypothesis of Preparation for Oxidative Stress in Land Snails. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 120, 437–448. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Pernier, J.; Shekhar, S.; Jegou, A.; Guichard, B.; Carlier, M.-F. Profilin Interaction with Actin Filament Barbed End Controls Dynamic Instability, Capping, Branching, and Motility. Dev. Cell 2016, 36, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Tomanek, L. Proteomics to Study Adaptations in Marine Organisms to Environmental Stress. J. Proteom. 2014, 105, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cheong, J.-H. Role of Mitochondria-Cytoskeleton Interactions in the Regulation of Mitochondrial Structure and Function in Cancer Stem Cells. Cells 2020, 9, 1691. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Tomanek, L. Environmental Proteomics of the Mussel Mytilus: Implications for Tolerance to Stress and Change in Limits of Biogeographic Ranges in Response to Climate Change. Integr. Comp. Biol. 2012, 52, 648–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winston, G.W.; Di Giulio, R.T. Prooxidant and Antioxidant Mechanisms in Aquatic Organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Suwannatrai, K.; Suwannatrai, A.; Tabsripair, P.; Welbat, J.U.; Tangkawattana, S.; Cantacessi, C.; Mulvenna, J.; Tesana, S.; Loukas, A.; Sotillo, J. Differential Protein Expression in the Hemolymph of Bithynia siamensis goniomphalos Infected with Opisthorchis viverrini. PLoS Negl. Trop. Dis. 2016, 10, e0005104. [Google Scholar] [CrossRef] [Green Version]

| Accession | Description | Accession | Description |
|---|---|---|---|
| XP_025103230.1 | calponin-1-like | XP_025082010.1 | myosin-2 essential light chain-like |
| XP_025110638.1 | LQP: myosin heavy chain, striated muscle-like | XP_025087122.1 | myotrophin-like |
| XP_025104157.1 | LQP: titin-like | XP_025086366.1 | paramyosin-like isoform X1 |
| XP_025084779.1 | LQP: twitchin-like | XP_025104008.1 | titin homolog isoform X3 |
| XP_025087541.1 | myomodulin neuropeptides 1-like | XP_025104057.1 | titin homolog isoform X1 |
| XP_025098567.1 | myophilin-like | XP_025104087.1 | titin-like isoform X1 |
| XP_025096983.1 | myophilin-like | XP_025098601.1 | troponin C-like isoform X1 |
| XP_025079300.1 | myosin essential light chain, striated adductor muscle-like | XP_025085471.1 | troponin I-like isoform X1 |
| XP_025095035.1 | myosin regulatory light chain LC-2, mantle muscle-like isoform X2 | XP_025086357.1 | troponin T, skeletal muscle-like isoform X3 |
| XP_025095034.1 | myosin regulatory light chain LC-2, mantle muscle-like isoform X1 |
| Accession | Description | p-Value | log2 Fold Change |
|---|---|---|---|
| XP_025110638.1 | LQP: myosin heavy chain, striated muscle-like | 5.89 × 10−6 | 5.289117 |
| XP_025103379.1 | alpha-actinin, sarcomeric-like isoform X1 | 0.000361 | 4.139306 |
| XP_025090809.1 | LQP: elongation factor 1-alpha-like | 0.000708 | 3.831499 |
| XP_025086366.1 | paramyosin-like isoform X1 | 6.29 × 10−7 | 3.498598 |
| XP_025104956.1 | LQP: spectrin beta chain-like | 0.000468 | 3.035385 |
| XP_025083839.1 | heat shock cognate 71 kDa protein | 3.9 × 10−5 | 2.888477 |
| XP_025098387.1 | catalase-like isoform X1 | 0.010952 | 2.622116 |
| XP_025104758.1 | malate dehydrogenase, cytoplasmic-like | 3.81 × 10−5 | 2.592439 |
| XP_025106720.1 | LQP: transketolase-like | 0.000397 | 2.571769 |
| XP_025085843.1 | dihydropyrimidinase-like isoform X1 | 0.000429 | 2.521828 |
| XP_025107274.1 | LQP: glutathione S-transferase Mu 2-like | 0.000396 | 2.341715 |
| XP_025085667.1 | lysosomal aspartic protease-like | 0.024059 | 2.320860 |
| XP_025078538.1 | voltage-dependent anion-selective channel protein 2-like | 0.012484 | 2.206812 |
| XP_025082706.1 | protein/nucleic acid deglycase DJ-1-like | 0.002122 | 2.024145 |
| XP_025092675.1 | 60S acidic ribosomal protein P2-like | 0.006832 | 2.005876 |
| XP_025113405.1 | glutathione S-transferase S1-like | 8.63 × 10−5 | 1.972678 |
| XP_025082558.1 | LQP: protein singed-like | 0.022403 | 1.927912 |
| XP_025110989.1 | xylose isomerase-like | 0.01412 | 1.833387 |
| XP_025089044.1 | malate dehydrogenase, mitochondrial-like isoform X1 | 0.000113 | 1.817043 |
| XP_025087809.1 | vinculin-like isoform X1 | 0.000419 | 1.800653 |
| XP_025108883.1 | peptidyl-prolyl cis-trans isomerase-like | 1.06 × 10−6 | 1.797534 |
| XP_025090294.1 | 14-3-3 protein beta/alpha-A-like | 0.005248 | 1.763492 |
| XP_025106211.1 | glutamate receptor 1-like | 8.88 × 10−6 | 1.742724 |
| XP_025112868.1 | uncharacterized protein LOC112575321 isoform X1 | 0.020812 | 1.695959 |
| XP_025093398.1 | actin-interacting protein 1-like | 0.003751 | 1.623441 |
| XP_025113955.1 | glutathione S-transferase 1-like | 0.000842 | 1.553176 |
| XP_025089524.1 | collagen alpha-6(VI) chain-like | 0.000468 | 1.516933 |
| XP_025099134.1 | LQP: arginine kinase-like | 0.000265 | 1.510341 |
| XP_025093079.1 | 14-3-3 protein epsilon-like isoform X1 | 0.037068 | 1.464123 |
| XP_025106444.1 | filamin-A-like isoform X1 | 0.000299 | 1.419625 |
| XP_025095112.1 | elongation factor 1-beta-like | 0.027983 | 1.365221 |
| XP_025094704.1 | protein disulphide-isomerase-like isoform X1 | 0.004507 | 1.359329 |
| XP_025078628.1 | LQP: neurofilament medium polypeptide-like | 0.025379 | 1.346143 |
| XP_025090849.1 | vegetative incompatibility protein HET-E-1-like | 0.001314 | 1.324563 |
| XP_025099800.1 | radixin-like | 0.000492 | 1.316749 |
| XP_025099490.1 | heat shock protein 70 B2-like | 0.006393 | 1.295692 |
| XP_025110201.1 | LQP: uncharacterized protein LOC112573811 | 5.36 × 10−6 | 1.269061 |
| XP_025093885.1 | LQP: uncharacterized protein LOC112563776 | 4.27 × 10−5 | 1.148421 |
| XP_025090599.1 | fructose-bisphosphate aldolase-like isoform X1 | 0.022681 | 1.050548 |
| XP_025106551.1 | collagen alpha-3(VI) chain-like isoform X18 | 0.00317 | 1.046923 |
| XP_025094101.1 | FK506-binding protein 2-like | 0.002086 | −1.02828 |
| XP_025078843.1 | glycogenin-1-like isoform X1 | 0.00642 | −1.03570 |
| XP_025100514.1 | kinesin-like protein K39 | 0.003484 | −1.03774 |
| XP_025104144.1 | mammalian ependymin-related protein 1-like | 0.000133 | −1.04629 |
| XP_025087031.1 | thymosin beta-like isoform X2 | 0.004896 | −1.05945 |
| XP_025080405.1 | calumenin-like isoform X2 | 0.001715 | −1.09339 |
| XP_025109789.1 | transforming growth factor-beta-induced protein ig-h3-like | 0.033214 | −1.18800 |
| XP_025083419.1 | small cardioactive peptides-like isoform X1 | 0.002106 | −1.29382 |
| XP_025096878.1 | uncharacterized protein LOC112565575 isoform X1 | 0.002002 | −1.32377 |
| XP_025111371.1 | LQP: 40S ribosomal protein S12-like | 6.42 × 10−5 | −1.38759 |
| XP_025098601.1 | troponin C-like isoform X1 | 0.002188 | −1.41944 |
| XP_025081260.1 | collagen alpha-1(I) chain-like | 0.003941 | −1.51975 |
| XP_025082853.1 | uncharacterized protein LOC112557300 | 0.020629 | −1.55671 |
| XP_025083634.1 | thioredoxin-1-like | 0.034374 | −1.58664 |
| XP_025114384.1 | PDZ and LIM domain protein 3-like isoform X1 | 0.000647 | −1.60308 |
| XP_025104157.1 | LQP: titin-like | 0.000772 | −1.64498 |
| XP_025085606.1 | uncharacterized protein LOC112559006 | 9.59 × 10−5 | −1.69730 |
| XP_025104799.1 | uncharacterized protein LOC112570529 | 0.001003 | −1.72428 |
| XP_025090775.1 | LQP: tensin-1-like | 5.75 × 10−6 | −1.77371 |
| XP_025087544.1 | small heat shock protein p36-like | 3.5 × 10−6 | −2.04098 |
| XP_025104087.1 | titin-like isoform X1 | 0.000689 | −2.06979 |
| XP_025082862.1 | PDZ and LIM domain protein 5-like | 0.000199 | −2.36176 |
| XP_025082010.1 | myosin-2 essential light chain-like | 0.035777 | −3.49412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boraldi, F.; Lofaro, F.D.; Bergamini, G.; Ferrari, A.; Malagoli, D. Pomacea canaliculata Ampullar Proteome: A Nematode-Based Bio-Pesticide Induces Changes in Metabolic and Stress-Related Pathways. Biology 2021, 10, 1049. https://doi.org/10.3390/biology10101049
Boraldi F, Lofaro FD, Bergamini G, Ferrari A, Malagoli D. Pomacea canaliculata Ampullar Proteome: A Nematode-Based Bio-Pesticide Induces Changes in Metabolic and Stress-Related Pathways. Biology. 2021; 10(10):1049. https://doi.org/10.3390/biology10101049
Chicago/Turabian StyleBoraldi, Federica, Francesco Demetrio Lofaro, Giulia Bergamini, Agnese Ferrari, and Davide Malagoli. 2021. "Pomacea canaliculata Ampullar Proteome: A Nematode-Based Bio-Pesticide Induces Changes in Metabolic and Stress-Related Pathways" Biology 10, no. 10: 1049. https://doi.org/10.3390/biology10101049
APA StyleBoraldi, F., Lofaro, F. D., Bergamini, G., Ferrari, A., & Malagoli, D. (2021). Pomacea canaliculata Ampullar Proteome: A Nematode-Based Bio-Pesticide Induces Changes in Metabolic and Stress-Related Pathways. Biology, 10(10), 1049. https://doi.org/10.3390/biology10101049






