Regulation of Milk Protein Synthesis by Free and Peptide-Bound Amino Acids in Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Determination of Limiting AAs
3. Free and Peptide-Bound AAs Supply
3.1. EAAs Promote MP Synthesis
3.2. Met
3.3. Lys
3.4. BCAAs
3.5. AA Balance
3.6. PBAAs Are Involved in MP Synthesis
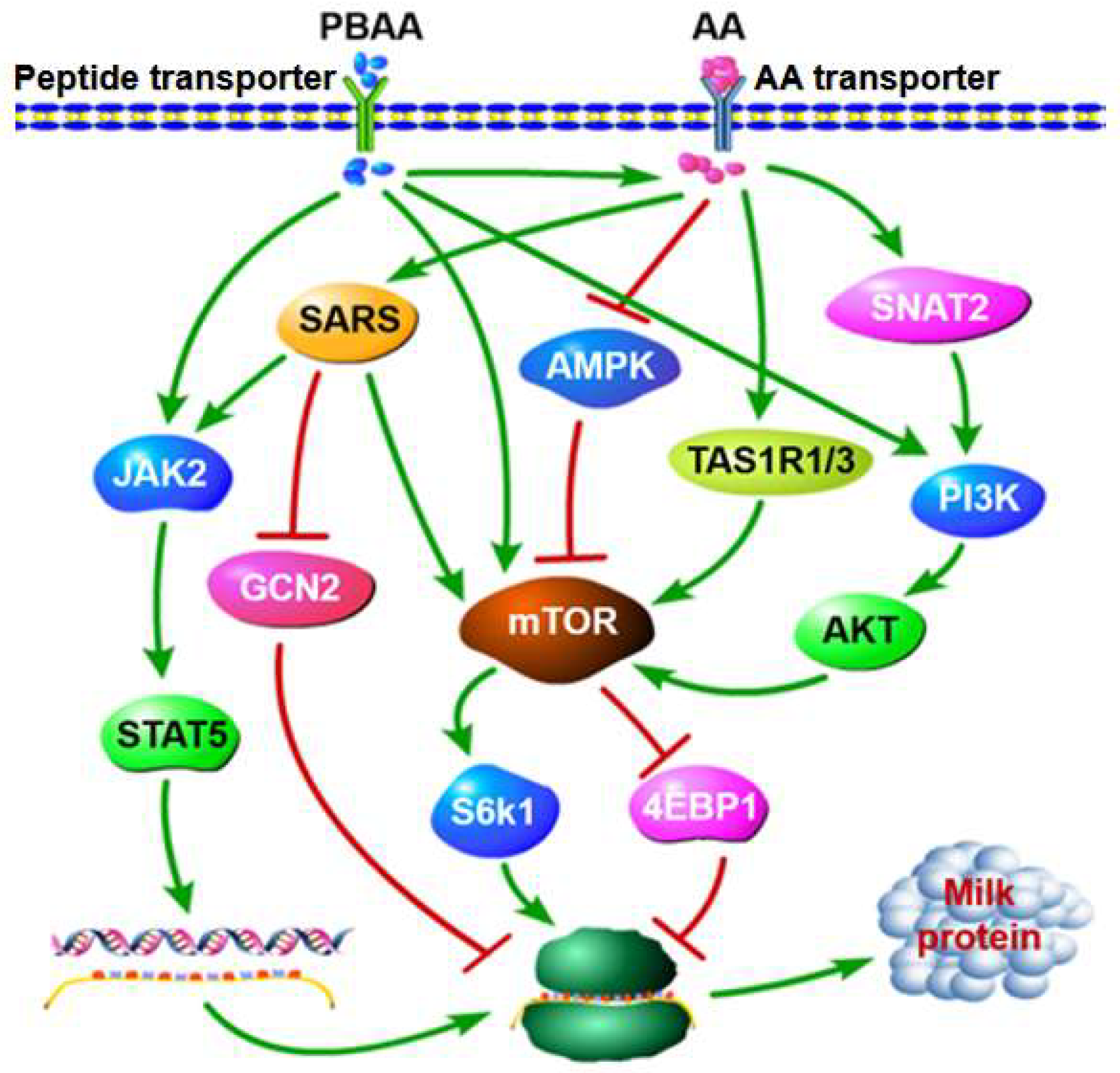
4. Mechanism of FAA and PBAA Promotion of MP Synthesis
4.1. Mechanism of the Promotion of MP Synthesis by FAA
4.1.1. AA Transport Systems
4.1.2. Role of AAs in Regulation of MP Synthesis
 inhibition.
inhibition.4.2. Mechanism of PBAAs Promoting MP Synthesis
4.2.1. PBAA Transport Systems
4.2.2. Role of PBAAs in MP Synthesis
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitabatake, N.; Kinekawa, Y.I. Digestibility of bovine milk whey protein and β-lactoglobulin in vitro and in vivo. J. Agric. Food Chem. 1998, 46, 4917–4923. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Etzel, M.R. Manufacture and use of dairy fractions. J. Nutr. 2004, 134, 996S–1002S. [Google Scholar] [CrossRef] [PubMed]
- Clare, D.A.; Swaisgood, H.E. Bioactive milk peptides: A prospectus. J. Dairy Sci. 2000, 83, 1187–1195. [Google Scholar] [CrossRef]
- Meyer, B.; Florian, B.; Gregor, V.; Monika, P. Distribution of protein oxidation products in the proteome of thermally processed milk. J. Agric. Food Chem. 2012, 60, 7306–7311. [Google Scholar] [CrossRef] [PubMed]
- Pizzano, R.; Carla, M.; Adalgisa, N.M.; Francesco, A. Occurrence of major whey proteins in the pH 4.6 insoluble protein fraction from UHT-treated milk. J. Agric. Food Chem. 2012, 60, 8044–8050. [Google Scholar] [CrossRef] [Green Version]
- Laporte, M.F.; Paquin, P. Near-infrared analysis of fat; protein; and casein in cow’s milk. J. Agric. Food Chem. 1999, 47, 2600–2605. [Google Scholar] [CrossRef]
- Li, Z.H.; Liu, Y.; Jin, X.L.; Lo, L.J.; Liu, J.X. Expression profiles of microRNAs from lactating and non-lactating bovine mammary glands and identification of miRNA related to lactation. BMC Genom. 2012, 13, 731–745. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.Z.; Shi, K.; Wu, X.H.; Xue, M.Y.; Wei, Z.H.; Liu, J.X.; Liu, H.Y. Lactation-related metabolic mechanism investigated based on mammary gland metabolomics and 4 biofluids’ metabolomics relationships in dairy cows. BMC Genom. 2017, 18, 936–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.T.; Wang, Q.J.; Zou, Y.X.; White, R.R.; Liu, J.X.; Liu, H.Y. Short communication: Comparative proteomic analysis of the lactating and nonlactating bovine mammary gland. J. Dairy Sci. 2017, 100, 5928–5935. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.T.; Zou, Y.X.; White, R.R.; Liu, J.X.; Liu, H.Y. Transcriptomic profiles of the bovine mammary gland during lactation and the dry period. Funct. Integr. Genom. 2018, 18, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.T.; Chen, Q.; Wang, Q.J.; White, R.R.; Liu, J.X.; Liu, H.Y. Complementary transcriptomic and proteomic analyses reveal regulatory mechanisms of milk protein production in dairy cows consuming different forages. Sci. Rep. 2017, 7, 44234–44247. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.T.; Wang, Q.J.; Zhao, F.Q.; Liu, J.X.; Liu, H.Y. Understanding the regulatory mechanisms of milk production using integrative transcriptomic and proteomic analyses: Improving inefficient utilization of crop byproducts as forage in dairy industry. BMC Genom. 2018, 19, 403–420. [Google Scholar] [CrossRef]
- Curtis, R.V.; Kim, J.J.M.; Doelman, J.; Cant, J.P. Maintenance of plasma branched-chain amino acid concentrations during glucose infusion directs essential amino acids to extra-mammary tissues in lactating dairy cows. J. Dairy Sci. 2018, 101, 4542–4553. [Google Scholar] [CrossRef]
- Omphalius, C.; Lemosquet, S.; Ouellet, D.R.; Bahloul, L.; Lapierre, H. Postruminal infusions of amino acids or glucose affect metabolisms of splanchnic; mammary; and other peripheral tissues and drive amino acid use in dairy cows. J. Dairy Sci. 2020, 103, 2233–2254. [Google Scholar] [CrossRef]
- Huang, X.; Yoder, P.S.; Teixeira, I.A.M.A.; Hanigan, M.D. Assessing amino acid uptake and metabolism in mammary glands of lactating dairy cows intravenously infused with methionine; lysine; and histidine or with leucine and isoleucine. J. Dairy Sci. 2021, 104, 3032–3051. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Rulquin, H.; Pisulewski, P.M.; Vérité, R.; Guinard, J. Milk production and composition as a function of postruminal lysine and methionine supply: A nutrient-response approach. Livest. Prod. Sci. 1993, 37, 69–90. [Google Scholar] [CrossRef]
- Schwab, C.G. Rumen-protected amino acids for dairy cattle: Progress towards determining lysine and methionine requirements. Anim. Feed Sci. Technol. 1996, 59, 87–101. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.Y.; Wang, Y.M.; Yang, Z.Q.; Liu, J.X.; Wu, Y.M.; Yan, T.; Ye, H.W. Effects of dietary supplementation of methionine and lysine on milk production and nitrogen utilization in dairy cows. J. Dairy Sci. 2010, 93, 3661–3670. [Google Scholar] [CrossRef]
- Morris, D.L.; Kononoff, P.J. Effects of rumen-protected lysine and histidine on milk production and energy and nitrogen utilization in diets containing hydrolyzed feather meal fed to lactating Jersey cows. J. Dairy Sci. 2020, 103, 7110–7123. [Google Scholar] [CrossRef]
- Paz, H.A.; Kononoff, P.J. Lactation responses and amino acid utilization of dairy cows fed low-fat distillers dried grains with solubles with or without rumen-protected lysine supplementation. J. Dairy Sci. 2014, 97, 6519–6530. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, Z.; Wang, L.; Saremi, B.; Helmbrecht, A.; Wang, Z.; Loor, J.J. Increasing the availability of threonine; isoleucine; valine; and leucine relative to lysine while maintaining an ideal ratio of lysine: Methionine alters mammary cellular metabolites, mammalian target of rapamycin signaling, and gene transcription. J. Dairy Sci. 2018, 101, 5502–5514. [Google Scholar] [CrossRef] [Green Version]
- Lean, I.J.; de Ondarza, M.B.; Sniffen, C.J.; Santos, J.E.; Griswold, P.K.E. Meta-analysis to predict the effects of metabolizable amino acids on dairy cattle performance. J. Dairy Sci. 2018, 101, 340–364. [Google Scholar] [CrossRef]
- Arriola Apelo, S.I.; Singer, L.M.; Ray, W.K.; Helm, R.F.; Lin, X.Y. Casein synthesis is independently and additively related to individual essential amino acid supply. J. Dairy Sci. 2014, 97, 2998–3005. [Google Scholar] [CrossRef] [Green Version]
- Yoder, P.S.; Huang, X.; Teixeira, I.A.; Cant, J.P.; Hanigan, M.D. Effects of jugular infused methionine, lysine, and histidine as a group or leucine and isoleucine as a group on production and metabolism in lactating dairy cows. J. Dairy Sci. 2020, 103, 2387–2404. [Google Scholar] [CrossRef] [Green Version]
- Arriola Apelo, S.I.; Knapp, J.R.; Hanigan, M.D. Invited review: Current representation and future trends of predicting amino acid utilization in the lactating dairy cow. J. Dairy Sci. 2014, 97, 4000–4017. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.N.; Rulquin, H.; Andrade, A.; Faverdin, P.; Peyraud, J.L.; Lemosquet, S. Milk protein synthesis in response to the provision of an “ideal” amino acid profile at 2 levels of metabolizable protein supply in dairy cows. J. Dairy Sci. 2012, 95, 5876–5887. [Google Scholar] [CrossRef]
- Tucker, H.A.; Malacco, V.M.R.; Hanigan, M.D.; Donkin, S.S. Postruminal protein supply upregulates hepatic lysine oxidation and ornithine transcarbamoylase in lactating dairy cattle. J. Dairy Sci. 2021, 104, 4251–4259. [Google Scholar] [CrossRef]
- Lemosquet, S.; Raggio, G.; Lobley, G.E.; Rulquin, H.; Guinard-Flament, J.; Lapierre, H. Whole-body glucose metabolism and mammary energetic nutrient metabolism in lactating dairy cows receiving digestive infusions of casein and propionic acid. J. Dairy Sci. 2009, 92, 6068–6082. [Google Scholar] [CrossRef]
- Nichols, K.; Bannink, A.; Doelman, J.; Dijkstra, J. Mammary gland metabolite utilization in response to exogenous glucose or long-chain fatty acids at low and high metabolizable protein levels. J. Dairy Sci. 2019, 102, 7150–7167. [Google Scholar] [CrossRef]
- Qi, H.; Meng, C.; Jin, X.; Li, X.; Li, P.; Gao, X. Methionine promotes milk protein and fat synthesis and cell proliferation via the SNAT2-PI3K signaling pathway in bovine mammary epithelial cells. J. Agric. Food Chem. 2018, 66, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.T.; White, R.R.; Liu, J.X.; Liu, H.Y. Seryl-tRNA synthetase-mediated essential amino acids regulate β-casein synthesis via cell proliferation and mTOR signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2018, 101, 10456–10468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Vailati-Riboni, M.; Trevisi, E.; Drackley, J.K.; Luchini, D.N.; Loor, J.J. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 2016, 99, 8716–8732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giallongo, F.; Harper, M.T.; Oh, J.; Lopes, J.C.; Lapierre, H.; Patton, R.A.; Parys, C.; Shinzato, I.; Hristov, A.N. Effects of rumen-protected methionine, lysine, and histidine on lactation performance of dairy cows. J. Dairy Sci. 2016, 99, 4437–4452. [Google Scholar] [CrossRef] [Green Version]
- Batistel, F.; Arroyo, J.M.; Bellingeri, A.; Wang, L.; Saremi, B.; Parys, C.; Trevisi, E.; Cardoso, F.C.; Loor, J.J. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cow. J. Dairy Sci. 2017, 100, 7455–7467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junior, V.C.; Lopes, F.; Schwab, C.G.; Toledo, M.Z.; Collao-Saenz, E.A. Effects of rumen-protected methionine supplementation on the performance of high production dairy cows in the tropics. PLoS ONE 2021, 16, e0243953. [Google Scholar] [CrossRef]
- Park, J.K.; Yeo, J.; Bae, G.; Kim, E.J.; Kim, C. Effects of supplementing limiting amino acids on milk production in dairy cows consuming a corn grain and soybean meal-based diet. J. Anim. Sci. Technol. 2020, 62, 485–494. [Google Scholar] [CrossRef]
- Gao, H.N.; Zhao, S.G.; Zheng, N.; Zhang, Y.D.; Wang, S.S.; Zhou, X.Q.; Wang, J.Q. Combination of histidine; lysine; methionine; and leucine promotes β-casein synthesis via the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2017, 100, 7696–7709. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Peng, J.; Loor, J.J. Methionine and valine activate the mammalian target of rapamycin complex 1 pathway through heterodimeric amino acid taste receptor (TAS1R1/TAS1R3) and intracellular Ca2+ in bovine mammary epithelial cells. J. Dairy Sci. 2018, 101, 11354–11363. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Giallongo, F.; Hristov, A.N.; Lapierre, H.; Cassidy, T.W.; Heyler, K.S.; Varga, G.A.; Parys, C. Effect of dietary protein level and rumen-protected amino acid supplementation on amino acid utilization for milk protein in lactating dairy cows. J. Dairy Sci. 2015, 98, 1885–1902. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.B.D.; Whitehouse, N.L.; Aragona, K.M.; Schwab, C.S.; Reis, S.F.; Brito, A.F. Production and nitrogen utilization in lactating dairy cows fed ground field peas with or without ruminally protected lysine and methionine. J. Dairy Sci. 2017, 100, 6239–6255. [Google Scholar] [CrossRef]
- Doelman, J.; Kim, J.J.M.; Carson, M.; Metcalf, J.A.; Cant, J.P. Branched-chain amino acid and lysine deficiencies exert different effects on mammary translational regulation. J. Dairy Sci. 2015, 98, 7846–7855. [Google Scholar] [CrossRef] [Green Version]
- Lobos, N.E.; Wattiaux, M.A.; Broderick, G.A. Effect of rumen-protected lysine supplementation of diets based on corn protein fed to lactating dairy cows. J. Dairy Sci. 2021, 104, 6620–6632. [Google Scholar] [CrossRef]
- Haque, M.N.; Rulquin, H.; Lemosquet, S. Milk protein responses in dairy cows to changes in postruminal supplies of arginine, isoleucine, and valine. J. Dairy Sci. 2013, 96, 420–430. [Google Scholar] [CrossRef]
- Hultquist, K.M.; Casper, D.P. Effects of feeding rumen-degradable valine on milk production in late-lactating dairy cows. J. Dairy Sci. 2016, 99, 1201–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giallongo, F.; Hristov, A.N.; Oh, J.; Frederick, T.; Weeks, H.; Werner, J.; Lapierre, H.; Patton, R.A. Effects of slow-release urea and rumen-protected methionine and histidine on performance of dairy cows. J. Dairy Sci. 2015, 98, 3292–3308. [Google Scholar] [CrossRef]
- Giallongo, F.; Harper, M.T.; Oh, J.; Parys, C.; Shinzato, I.; Hristov, A.N. Histidine deficiency has a negative effect on lactational performance of dairy cows. J. Dairy Sci. 2017, 100, 2784–2800. [Google Scholar] [CrossRef]
- McLain, K.A.; Morris, D.L.; Kononoff, P.J. Effect of feeding hydrolyzed feather meal and rumen-protected lysine on milk protein and energy utilization in late-lactation Jersey cows. J. Dairy Sci. 2021, 104, 8708–8720. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.B.D.; Moura, D.C.; Whitehouse, N.L.; Brito, A.F. Production and nitrogen metabolism in lactating dairy cows fed finely ground field pea plus soybean meal or canola meal with or without rumen-protected methionine supplementation. J. Dairy Sci. 2020, 103, 3161–3176. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, N.R.; Sylvester, J.T. Effects of 2-hydroxy-4-(methylthio) butanoic acid (HMB) and its isopropyl ester on milk production and composition by Holstein cows. J. Dairy Sci. 2005, 88, 2487–2497. [Google Scholar] [CrossRef] [Green Version]
- Graulet, B.; Richard, C.; Robert, J.C. Methionine availability in plasma of dairy cows supplemented with methionine hydroxy analog isopropyl ester. J. Dairy Sci. 2005, 88, 3640–3649. [Google Scholar] [CrossRef] [Green Version]
- Rulquin, H.; Graulet, B.; Delaby, L.; Robert, J.C. Effect of different forms of methionine on lactational performance of dairy cows. J. Dairy Sci. 2006, 89, 4387–4394. [Google Scholar] [CrossRef]
- Ma, Y.F.; Batistel, F.; Xu, T.L.; Han, L.Q.; Bucktrout, R.; Liang, Y.; Coleman, D.N.; Parys, C.; Loor, J.J. Phosphorylation of AKT serine/threonine kinase and abundance of milk protein synthesis gene networks in mammary tissue in response to supply of methionine in periparturient Holstein cows. J. Dairy Sci. 2018, 102, 4264–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Xia, F.; Hanigan, M.D.; Lin, X.Y.; Yan, Z.G.; White, R.R.; Hu, Z.Y.; Hou, Q.L.; Wang, Z.H. Short-term lactation and mammary metabolism responses in lactating goats to graded removal of methionine from an intravenously infused complete amino acid mixture. J. Dairy Sci. 2019, 102, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Socha, M.T.; Putnam, D.E.; Garthwaite, B.D.; Whitehouse, N.L.; Kierstead, N.A.; Schwab, C.G.; Ducharme, G.A.; Robert, J.C. Improving intestinal amino acid supply of pre- and postpartum dairy cows with rumen-protected methionine and lysine. J. Dairy Sci. 2005, 88, 1113–1126. [Google Scholar] [CrossRef]
- Armentano, L.E.; Swain, S.M.; Ducharme, G.A. Lactation responses to ruminally protected methionine and lysine at two amounts of ruminally available nitrogen. J. Dairy Sci. 1993, 76, 2963–2969. [Google Scholar] [CrossRef]
- BaileyPAS, H.R.; KaufmanPAS, J.D.; Estes, K.A.; ZimmermanPAS, C.A.; BartonPAS, B.A.; Ríus, A.G. Rumen-protected lysine supplementation increased milk production in dairy cows fed a lysine-deficient diet. Appl. Anim. Sci. 2019, 35, 482–490. [Google Scholar]
- Lin, X.J.; Li, S.S.; Zou, Y.X.; Zhao, F.Q.; Liu, J.X.; Liu, H.Y. Lysine stimulates protein synthesis by promoting the expression of ATB0;+ and activating the mTOR pathway in bovine mammary epithelial cells. J. Nutr. 2018, 148, 1426–1433. [Google Scholar] [CrossRef]
- Monirujjaman, M.; Ferdouse, A. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jenness, R. The Composition of Milk. Lactation: A Comprehensive Treatise; Larson, B.L., Smith, V.R., Eds.; Academic Press: New York, NY, USA, 1974; Volume III, pp. 3–107. [Google Scholar]
- Mepham, T.B. Amino acid utilization by lactating mammary gland. J. Dairy Sci. 1982, 65, 287–298. [Google Scholar] [CrossRef]
- Castro, J.J.; Arriola Apelo, S.I.; Appuhamy, J.A.D.R.N.; Hanigan, M.D. Development of a model describing regulation of casein synthesis by the mammalian target of rapamycin (mTOR) signaling pathway in response to insulin; amino acids; and acetate. J. Dairy Sci. 2016, 99, 6714–6736. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhou, Z.; Saremi, B.; Helmbrecht, A.; Wang, Z.; Loor, J.J. Varying the ratio of Lys: Met while maintaining the ratios of Thr: Phe, Lys: Thr, Lys: His, and Lys: Val alters mammary cellular metabolites; mammalian target of rapamycin signaling; and gene transcription. J. Dairy Sci. 2017, 101, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Ronquillo, M.G.; Faciola, A.P.; Nursoy, H.; Broderick, G.A. Effect of increasing dietary protein with constant lysine:methionine ratio on production and omasal flow of nonammonia nitrogen in lactating dairy cows. J. Dairy Sci. 2021, 104, 5319–5331. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.V.; Karges, K.; Rezamand, P.; Laarman, A.H.; Chibisa, G.E. Production performance and nitrogen metabolism in dairy cows fed supplemental blends of rumen undegradable protein and rumen-protected amino acids in low- compared with high-protein diets containing corn distillers grains. J. Dairy Sci. 2021, 104, 4134–4145. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Choung, J.J.; Chamberlain, D.G. Determination of the first-limiting amino acid for milk production in dairy cows consuming a diet of grass silage and a cereal-based supplement containing feather meal. J. Sci. Food Agric. 1999, 79, 1703–1708. [Google Scholar] [CrossRef]
- Kim, C.H.; Choung, J.J.; Chamberlain, D.G. Variability in the ranking of the three most-limiting amino acids for milk protein production in dairy cows consuming grass silage and a cereal-based supplement containing feather meal. J. Sci. Food Agric. 2000, 80, 1386–1392. [Google Scholar] [CrossRef]
- Yeo, J.M.; Knight, C.H.; Chamberlain, D.G. Effects of changes in dietary amino acid balance on milk yield and mammary function in dairy cows. J. Dairy Sci. 2003, 86, 1436–1444. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.Q. Effect of Ratio of Amino Acid on Milk Performance and Nitrogen Utilization in Dairy Cow. Master’s Thesis, Zhejiang University, Hangzhou, China, 2009; pp. 1–3. [Google Scholar]
- Abbasi, I.H.R.; Abbasi, F.; El-Hack, M.E.A.; Abdel-Latif, M.A.; Soomro, R.N.; Hayat, K.; Mohamed, M.A.E.; Bodinga, B.M.; Yao, J.; Cao, Y. Critical analysis of excessive utilization of crude protein in ruminants ration: Impact on environmental ecosystem and opportunities of supplementation of limiting amino acids. Environ. Sci. Pollut. Res. 2018, 25, 181–190. [Google Scholar] [CrossRef]
- Weekes, T.L.; Luimes, P.H.; Cant, J.P. Responses to amino acid imbalances and deficiencies in lactating dairy cows. J. Dairy Sci. 2006, 89, 2177–2187. [Google Scholar] [CrossRef]
- Haque, M.N.; Guinard-Flament, J.; Lamberton, P.; Mustière, C.; Lemosquet, S. Changes in mammary metabolism in response to the provision of an ideal amino acid profile at 2 levels of metabolizable protein supply in dairy cows: Consequences on efficiency. J. Dairy Sci. 2015, 98, 3951–3968. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.J.; Wu, H.H.; Zhang, C.Q.; Liu, J.X.; Wu, Y.M. Amplification of alfa-s1-casein gene and its expression influenced by prolactin in cultured mammary tissue from lactating dairy cows. J. Agric. Biotech. 2005, 13, 629–634. [Google Scholar]
- Liu, H.Y.; Yang, J.Y.; Wu, H.H.; Wu, Y.M.; Liu, J.X. Effects of methionine and its ratio to lysine on expression of αs1 casein gene in cultured bovine mammary epithelial cells. J. Anim. Feed Sci. 2007, 16, 330–334. [Google Scholar] [CrossRef]
- Li, S.S.; Loor, J.J.; Liu, H.Y.; Liu, L.; Hosseini, A.; Zhao, W.S.; Liu, J.X. Optimal ratios of essential amino acids stimulate β-casein synthesis via activation of the mammalian target of rapamycin signaling pathway in MAC-T cells and bovine mammary tissue explants. J. Dairy Sci. 2017, 100, 6676–6688. [Google Scholar] [CrossRef]
- Bickerstaffe, R.; Annison, E.F.; Linzell, J.F. The metabolism of glucose; acetate; lipids and amino acids in lactating cows. J. Agric. Sci. 1974, 82, 71–85. [Google Scholar] [CrossRef]
- Backwell, F.R.C.; Bequette, B.J.; Wilson, D.; Metcalf, J.A.; Franklin, M.F.; Beever, D.E.; Lobley, G.E.; MacRae, J.C. Evidence for the utilization of peptides for milk protein synthesis in the lactating dairy goat in vivo. Am. J. Physiol. 1996, 271, R955–R960. [Google Scholar] [CrossRef]
- Tagari, H.; Webb, K., Jr.; Theurer, B.; Huber, T.; DeYoung, D.; Cuneo, P.; Santos, J.E.P.; Simas, J.; Sadik, M.; Alio, A.; et al. Mammary uptake; portal-drained visceral flux; and hepatic metabolism of free and peptide-bound amino acids in cows fed steam-flaked or dry-rolled sorghum grain diets. J. Dairy Sci. 2008, 91, 679–697. [Google Scholar] [CrossRef]
- Chen, G.; Sniffen, C.J.; Russell, J.B. Concentration and estimated flow of peptides from the rumen of dairy cattle, Effects of protein quantity; protein solubility; and feeding frequency. J. Dairy Sci. 1987, 70, 983–992. [Google Scholar] [CrossRef]
- Tagari, H.; Webb, K., Jr.; Theurer, B.; Huber, T.; DeYoung, D.; Cuneo, P.; Santos, J.E.P.; Simas, J.; Sadik, M.; Alio, A.; et al. Portal drained visceral flux; hepatic metabolism; and mammary uptake of free and peptide-bound amino acids and milk amino acid output in dairy cows fed diets containing corn grain steam flaked at 360 or 490 g/L. J. Dairy Sci. 2004, 87, 413–430. [Google Scholar] [CrossRef]
- Backwell, F.R.C.; Hipolito-Reis, M.; Wilson, D.; Bruce, L.A.; Buchan, V.; MacRae, J.C. Quantification of circulating peptides and assessment of peptide uptake across the gastrointestinal tract of sheep. J. Anim. Sci. 1997, 75, 3315–3322. [Google Scholar] [CrossRef]
- Bequette, B.J.; Backwell, F.R.C.; Kyle, C.E.; Calder, A.G.; Buchan, V.; Crompton, L.A.; France, J.; MacRae, J.C. Vascular sources of phenylalanine; tyrosine; lysine; and methionine for casein synthesis in lactating goats. J. Dairy Sci. 1999, 82, 362–377. [Google Scholar] [CrossRef]
- Wu, H.H.; Yang, J.Y.; Zhao, K.; Liu, H.Y.; Wu, Y.M.; Liu, J.X. Effects of methionine-containing dipeptides on casein αs1 expression in bovine mammary epithelial cells. J. Anim. Feed Sci. 2007, 16, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.X.; Wang, C.H.; Xu, Q.B.; Zhao, F.Q.; Liu, J.X.; Liu, H.Y. Methionyl-Methionine promotes as1 casein synthesis in bovine mammary gland explants by enhancing intracellular substrate availability and activating JAK2-STAT5 and mTOR-mediated signaling pathways. J. Nutr. 2015, 145, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Zhao, F.Q.; Liu, J.X.; Liu, H.Y. Dipeptide (methionyl-methionine) transport and its effect on β-casein synthesis in bovine mammary epithelial cells. Cell Physiol. Biochem. 2018, 49, 479–488. [Google Scholar] [CrossRef]
- Zhou, M.M.; Wu, Y.M.; Liu, H.Y.; Zhao, K.; Liu, J.X. Effect of tripeptides and lactogenic hormones on oligopeptide transporter 2 in bovine mammary gland. J. Anim. Physiol. Anim. Nutr. 2011, 95, 781–789. [Google Scholar] [CrossRef]
- Zhou, M.M.; Wu, Y.M.; Liu, H.Y.; Liu, J.X. Effects of phenylalanine and threonine oligopeptides on milk protein synthesis in cultured bovine mammary epithelial cells. J. Anim. Physiol. Anim. Nutr. 2015, 99, 215–220. [Google Scholar] [CrossRef]
- Baumrucker, C.R. Cationic amino-acid-transport by bovine mammary tissue. J. Dairy Sci. 1984, 67, 2500–2506. [Google Scholar] [CrossRef]
- Shennan, D.B.; Mcneillie, S.A. High-affinity (Na++Cl-)-dependent taurine transport by lactating mammary tissue. J. Dairy Res. 1994, 61, 335–343. [Google Scholar] [CrossRef]
- Shennan, D.B.; Boyd, C.A.R. The functional and molecular entities underlying amino acid and peptide transport by the mammary gland under different physiological and pathological conditions. J. Mammary Gland Biol. Neoplasia 2014, 19, 19–33. [Google Scholar] [CrossRef]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef]
- Baik, M.; Etchebarne, B.E.; Bong, J.; VandeHaar, M.J. Gene expression profiling of liver and mammary tissues of lactating dairy cows. Asian Austral. J. Anim. Sci. 2009, 6, 871–884. [Google Scholar] [CrossRef]
- Sciascia, Q.; Pacheco, D.; McCoard, S.A. Administration of exogenous growth hormone is associated with changes in plasma and intracellular mammary amino acid profiles and abundance of the mammary gland amino acid transporter SLC3A2 in midlactation dairy cows. PLoS ONE 2015, 10, e0134323. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Finucane, K.A.; McFadden, T.B.; Bond, J.P.; Kenelly, J.J.; Zhao, F. Onset of lactation in the bovine mammary gland: Gene expression profiling indicates a strong inhibition of gene expression in cell proliferation. Funct. Integr. Genomics. 2008, 8, 251–264. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform. Biol. Insights 2011, 5, 83–98. [Google Scholar] [CrossRef]
- Connor, E.E.; Siferd, S.; Elsasser, T.H.; Evock-Clover, C.M.; Van Tassel, C.P.; Sonstegard, T.S.; Fernandes, V.M.; Capuco, A.V. Effects of increased milking frequency on gene expression in the bovine mammary gland. BMC Genom. 2008, 9, 362. [Google Scholar] [CrossRef]
- Shennan, D.; Millar, I.; Calvert, D. Mammary-tissue amino acid transport systems. Proc. Nutr. Soc. 1997, 56, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.; Torres, N.; Ortiz, V. Characterization and regulation of the gene expression of amino acid transport system A (SNAT2) in rat mammary gland. Am. J. Physiol. Endocrinol. Metab. 2006, 291, 1059–1066. [Google Scholar] [CrossRef]
- Lin, Y.; Duan, X.Y.; Lv, H.; Yang, Y.; Liu, Y.; Gao, X.J.; Hou, X.M. The effects of L-type amino acid transporter 1 on milk protein synthesis in mammary glands of dairy cows. J. Dairy Sci. 2018, 101, 1687–1696. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Control of protein synthesis by amino acid availability. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 63–67. [Google Scholar] [CrossRef]
- Appuhamy, J.A.D.R.N.; Nayananjalie, W.A.; England, E.M.; Gerrard, D.E.; Akers, R.M.; Hanigan, M.D. Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J. Dairy Sci. 2014, 97, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.C.; Zhao, S.G.; Wang, S.S.; Luo, C.C.; Gao, H.N.; Zheng, N.; Wang, J.Q. d-Glucose and amino acid deficiency inhibits casein synthesis through JAK2/STAT5 and AMPK/mTOR signaling pathways in mammary epithelial cells of dairy cows. J. Dairy Sci. 2018, 101, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Moshel, Y.; Rhoads, R.E.; Barash, I. Role of amino acids in translational mechanisms governing milk protein synthesis in murine and ruminant mammary epithelial cells. J. Cell Biochem. 2006, 98, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, L.S.; Kimball, S.R. Amino acids as regulators of gene expression at the level of mRNA translation. J. Nutr. 2003, 133, 2046S–2051S. [Google Scholar] [CrossRef] [PubMed]
- Edick, A.M.; Audette, J.; Burgos, S.A. CRISPR-Cas9-mediated knockout of GCN2 reveals a critical role in sensing amino acid deprivation in bovine mammary epithelial cells. J. Dairy Sci. 2021, 104, 1123–1135. [Google Scholar] [CrossRef]
- Arriola Apelo, S.I.; Singer, L.M.; Lin, X.Y.; McGilliard, M.L.; St-Pierre, N.R.; Hanigan, M.D. Isoleucine leucine methionine and threonine effects on mammalian target of rapamycin signaling in mammary tissue. J. Dairy Sci. 2014, 97, 1047–1056. [Google Scholar] [CrossRef]
- Nichols, K.; Doelman, J.; Kim, J.J.M.; Carson, M.; Metcalf, J.A.; Cant, J.P. Exogenous essential amino acids stimulate an adaptive unfolded protein response in the mammary glands of lactating cows. J. Dairy Sci. 2017, 100, 5909–5921. [Google Scholar] [CrossRef] [Green Version]
- Cant, J.P.; Kim, J.J.M.; Cieslar, S.R.L.; Doelman, J. Symposium review, Amino acid uptake by the mammary glands: Where does the control lie? J. Dairy Sci. 2018, 101, 5655–5666. [Google Scholar] [CrossRef] [Green Version]
- Mabjeesh, S.J.; Gal-Garber, O.; Milgram, J. Aminopeptidase N gene expression and abundance in caprine mammary gland is influenced by circulating plasma peptide. J. Dairy Sci. 2005, 88, 2055–2064. [Google Scholar] [CrossRef] [Green Version]
- Shennan, D.B. Peptide transport and metabolism by the lactating mammary gland. J. Nutr. 2016, 146, 384–385. [Google Scholar] [CrossRef] [Green Version]
- Adibi, S.A. The oligopeptide transporter (Pept-1) in human intestine: Biology and function. Gastroenterology 1997, 113, 332–340. [Google Scholar] [CrossRef]
- Chen, H.; Wong, E.A.; Webb, K.E., Jr. Tissue distribution of a peptide transporter mRNA in sheep dairy cows pigs and chickens. J. Anim. Sci. 1999, 77, 1277–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Smith, D.E.; Yang, T.; Huang, Y.G.; Schnermann, J.B.; Brosius, F.C., III. Localization of PEPT1 and PEPT2 proton coupled oligopeptide transporter mRNA and protein in rat kidney. Am. J. Physiol. Renal. Physiol. 1999, 276, F658–F665. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Sun, Y.L.; Zhao, F.Q.; Liu, J.X.; Liu, H.Y. Functional characterization of peptide transporters in bovine mammary epithelial cells. J. Agr. Food Chem. 2019, 67, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, F.Q.; Ren, Y.; Han, J.; Liu, J.; Li, Y.; Liu, H. Parenterally delivered methionyl-methionine dipeptide during pregnancy enhances mammogenesis and lactation performance over free methionine by activating PI3K-AKT Signaling in methionine-deficient mice. J. Nutr. 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
| EAA | Effects on MP Synthesis | Reference |
|---|---|---|
| Met | Increases in milk yield and MP yield | [24,34,35,36,37] |
| Increases milk yield | [38] | |
| Increases in β-casein synthesis | [39,40] | |
| No effect on milk yield and MP | [41,42] | |
| Lys | Increases in MP concentration | [22,35,43] |
| Promotes β-casein synthesis | [39] | |
| Increases milk yield and milk true protein | [44] | |
| Val | Increases in milk yield and MP synthesis | [23,43,45,46] |
| Increase in casein mRNA abundance | [40] | |
| No effect on milk yield and MP | [24,41,42] | |
| Decrease in MP yield | [14] | |
| Leu | Ppositively associated with milk yield and MP yield | [24,43] |
| Promotes β-casein synthesis | [39] | |
| Decrease in MP yield | [14] | |
| Ile | Increase in MP synthesis | [43,45] |
| Decrease in MP yield | [14] | |
| His | Increases in MP concentration and yield. | [24,35,47,48] |
| Promotes β-casein synthesis | [39] | |
| Increases milk yield and tendes to increase MP yield | [21] | |
| Increases milk and MP yield | [49] | |
| Phe | Positively associated with milk yield | [22] |
| Thr | Positively associated with milk yield | [24] |
| Arg | Positively associated with milk yield | [22] |
| No effects on MP synthesis | [45] | |
| Trp | Positively associated with milk yield | [24] |
| Gene | Protein | Associated Transport System | Substrates | Reference |
|---|---|---|---|---|
| SLC1A1 | EAAC1, EAAT3 | X− AG | Glutamate, aspartate | [93] |
| SLC1A2 | GLT-1, EAAT2 | X− AG | Glutamate, aspartate | [93] |
| SLC1A3 | GLAST, EAAT1 | X− AG | Glutamate, aspartate | [93] |
| SLC1A4 | ASCT1, SATT | ASC | Alanine, serine, cysteine, and threonine | [88,93] |
| SLC1A5 | ASCT2, AAAT | ASC | Neutral amino acid | [93] |
| SLC3A2 | 4F2hc | Heavy chain | Systems L, y+L, xc− and asc with light subunits SLC7A5-8 and SLC7A10-11 | [94,95] |
| SLC6A1 | GAT1 | GABA | Gamma-aminobutyric acid | [93] |
| SLC6A6 | TauT | System β | Beta-alanine | [93] |
| SLC6A9 | GlyT1 | System Gly | Glycine | [96] |
| SLC6A14 | ATB0,+ | B0,+ | Cationic amino acid | [88] |
| SLC7A1 | CAT-1 | y+ | Cationic amino acid | [95,97] |
| SLC7A2 | CAT-2 | y+ | Cationic amino acid | [88,95] |
| SLC7A3 | CAT-3 | y+ | Cationic amino acid | [93,95] |
| SLC7A5 | LAT1 | L | Large neutral amino acid | [95,98] |
| SLC7A7 | y+LAT1 | y+L | Na+ indep.: cationic amino acids; Na+/large neutral amino acids | [93,95] |
| SLC7A8 | LAT2 | L | Neutral L-amino acids | [93,95] |
| SLC16A10 | TAT1, MCT10 | T | Aromatic amino acid | [88] |
| SLC38A2 | SNAT2 | A | Neutral amino acid | [93] |
| SLC38A3 | SNAT3 | N | Neutral amino acid | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Xu, L.; Zhao, F.; Liu, H. Regulation of Milk Protein Synthesis by Free and Peptide-Bound Amino Acids in Dairy Cows. Biology 2021, 10, 1044. https://doi.org/10.3390/biology10101044
Zhou M, Xu L, Zhao F, Liu H. Regulation of Milk Protein Synthesis by Free and Peptide-Bound Amino Acids in Dairy Cows. Biology. 2021; 10(10):1044. https://doi.org/10.3390/biology10101044
Chicago/Turabian StyleZhou, Miaomiao, Lianbin Xu, Fengqi Zhao, and Hongyun Liu. 2021. "Regulation of Milk Protein Synthesis by Free and Peptide-Bound Amino Acids in Dairy Cows" Biology 10, no. 10: 1044. https://doi.org/10.3390/biology10101044
APA StyleZhou, M., Xu, L., Zhao, F., & Liu, H. (2021). Regulation of Milk Protein Synthesis by Free and Peptide-Bound Amino Acids in Dairy Cows. Biology, 10(10), 1044. https://doi.org/10.3390/biology10101044







