Review of Venoms of Non-Polydnavirus Carrying Ichneumonoid Wasps
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Difficulties Working on Parasitoid Wasp Venoms
1.2. The Disparity between Physiologists, Taxonomists and Natural Historians
1.3. Non-Venom Sources of Chemical Interaction between Host and Parasitoid
1.4. Transcriptome Studies
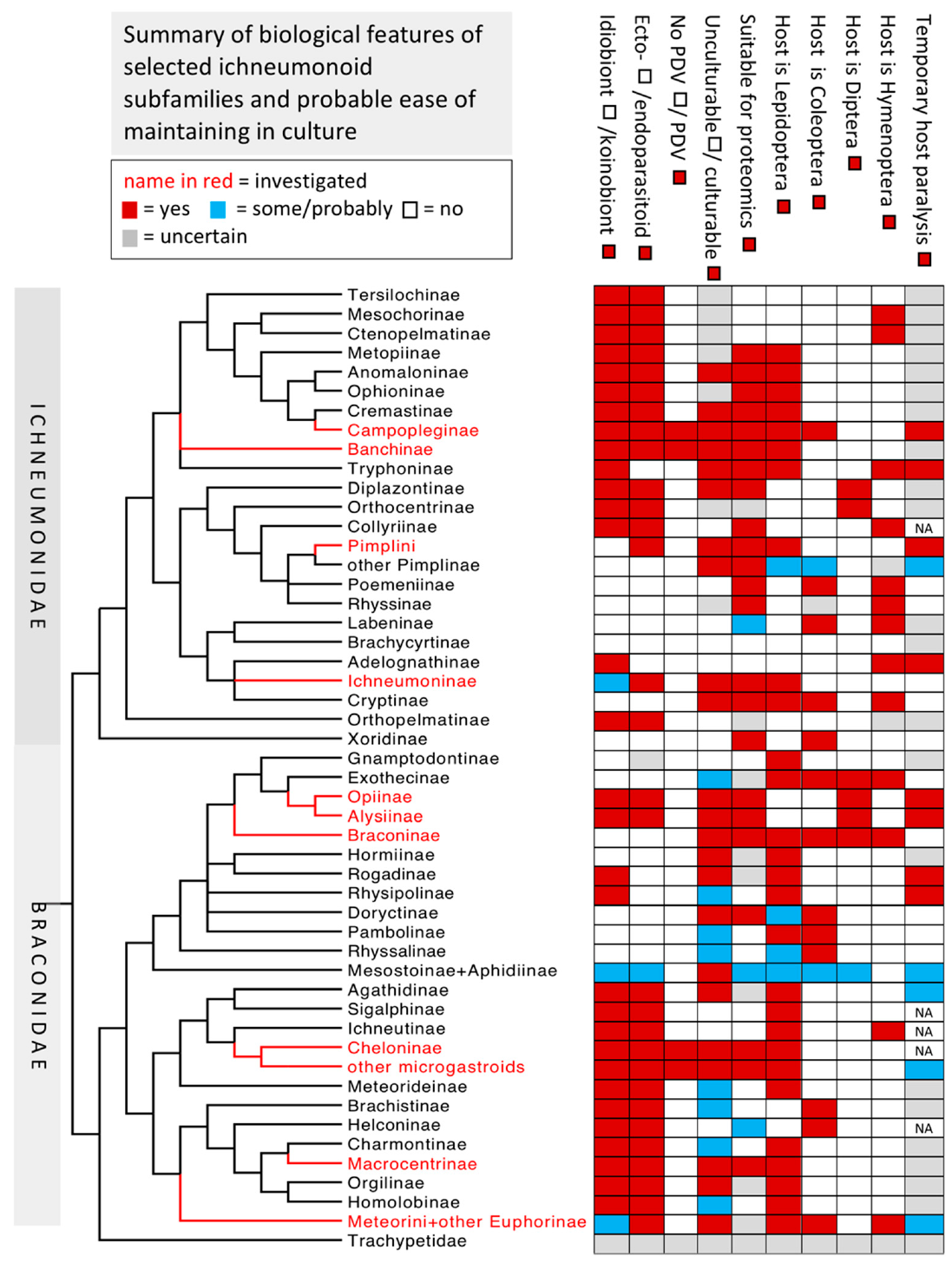
1.5. Taxonomic Notes
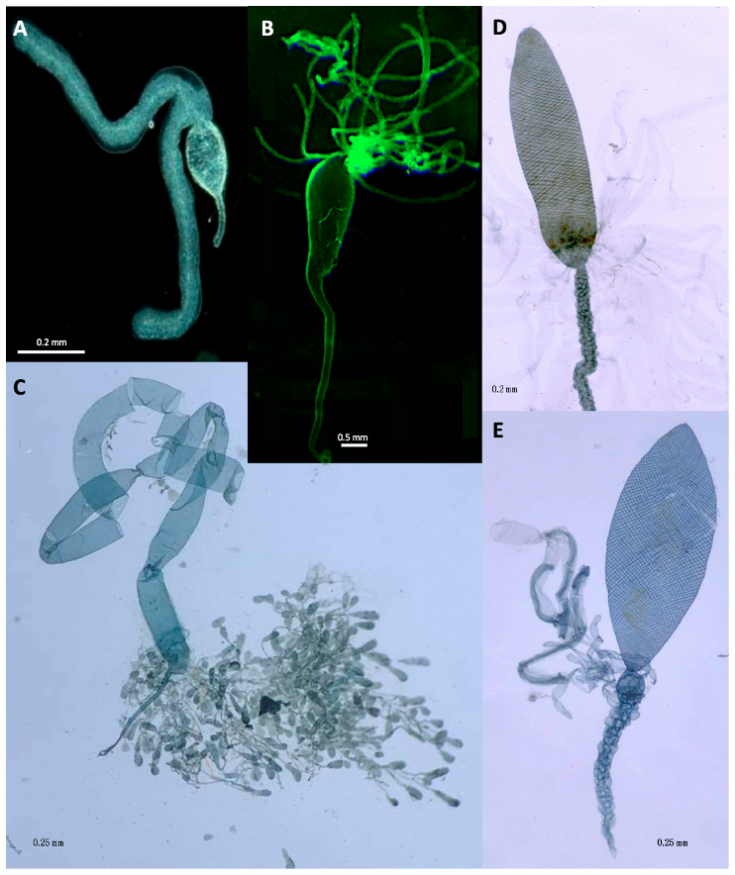
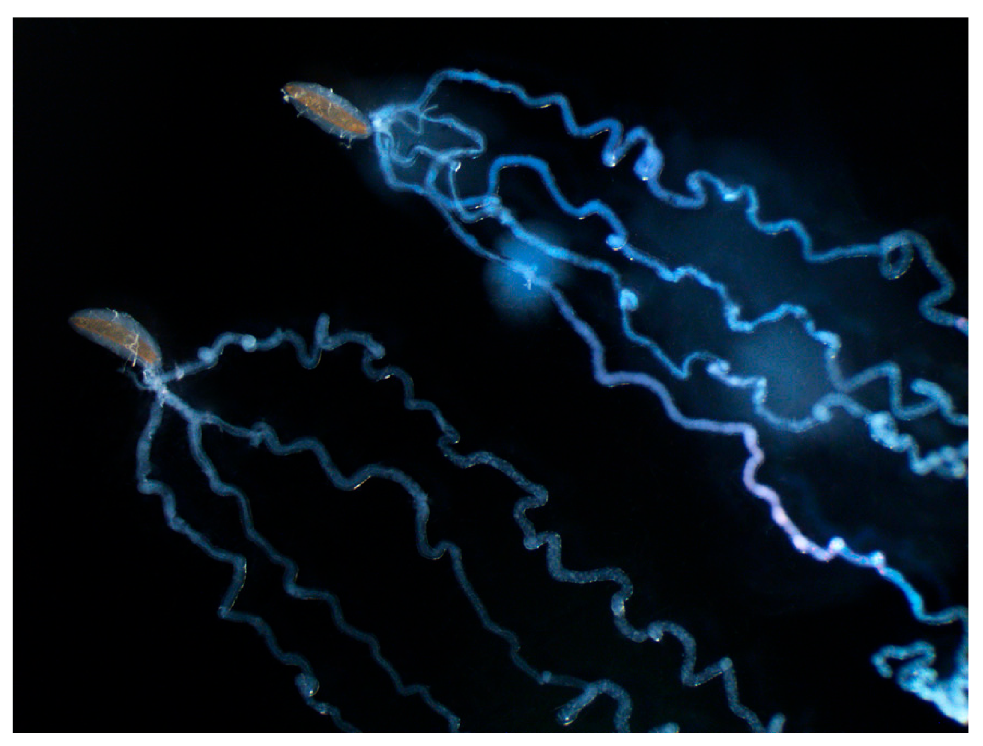
2. Implications Based on Venom Apparatus Morphology
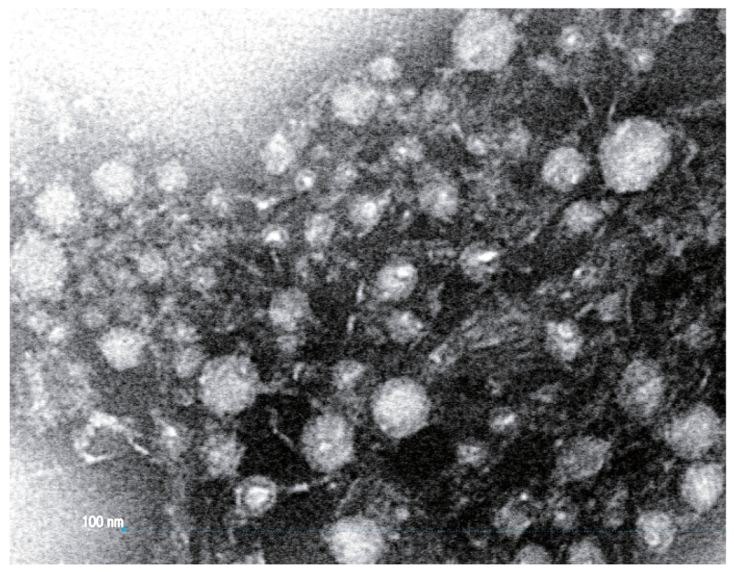
Viruses and Virus-Like Particles Produced in Venom Glands
3. Overview of Venom Effects
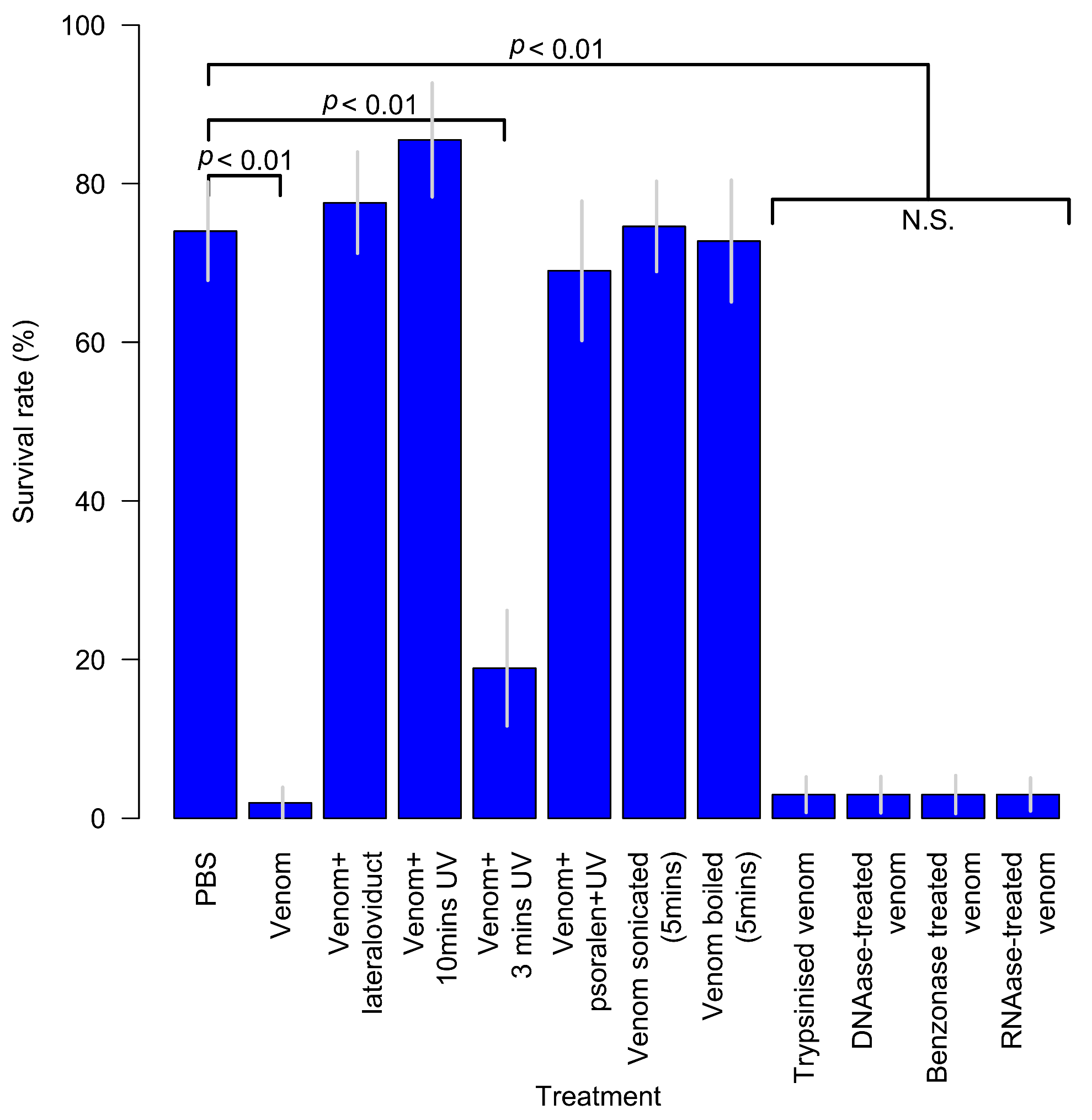
3.1. Paralysing Effects of Venoms
3.1.1. Temporary (Transient) Paralysis
3.1.2. Long Term/Permanent Paralysis
3.2. Venoms and Nutritional Regulation
Host Castration
3.3. General Effects on Host Immune System
4. Review of Studies on Ichneumonoid Wasp Venoms
4.1. Braconidae
4.1.1. Alysiinae
4.1.2. Aphidiinae
4.1.3. Braconinae
4.1.4. Euphorinae—Meteorini
4.1.5. Euphorinae—Perilitini
4.1.6. Macrocentrinae
4.1.7. Opiinae
4.1.8. Rogadinae
4.2. Review of Studies on Ichneumonid Wasp Venoms
4.2.1. Ichneumoninae
4.2.2. Pimplinae
4.2.3. Rhyssinae
5. Suggestions for Further Research Opportunities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Quicke, D.L.J. Parasitic Wasps; Chapman & Hall: London, UK, 1997; pp. 1–470. [Google Scholar]
- Yu, D.S.K.; van Achterberg, C.; Horstmann, K. Taxapad 2016, Ichneumonoidea 2015. Database on Flash-Drive, Nepean, ON, USA. 2016. Available online: http://www.taxapad.com (accessed on 23 September 2017).
- Quicke, D.L.J.; Austin, A.D.; Fagan-Jeffries, E.P.; Hebert, P.D.; Butcher, B.A. Recognition of the Trachypetidae stat. n. as a new extant family of Ichneumonoidea (Hymenoptera), based on molecular and morphological evidence. Syst. Entomol. 2020, 45, 771–782. [Google Scholar]
- Quicke, D.L.J. The Braconid and Ichneumonid Parasitic Wasps: Biology, Systematics, Evolution and Ecology; Wiley Blackwell: Oxford, UK, 2015; pp. 1–688. [Google Scholar]
- Quicke, D.L.J.; Laurenne, N.M.; Fitton, M.G.; Broad, G.R. A thousand and one wasps: A 28S rDNA and morphological phylogeny of the Ichneumonidae (Insecta: Hymenoptera) with an investigation into alignment parameter space and elision. J. Nat. Hist. 2009, 43, 1305–1421. [Google Scholar] [CrossRef]
- Quicke, D.L.J.; Austin, A.D.; Fagan-Jeffries, E.P.; Hebert, P.D.N.; Butcher, B.A. Molecular phylogeny places the enigmatic subfamily Masoninae within the Ichneumonidae, not the Braconidae. Zool. Scripta 2020, 49, 64–71. [Google Scholar] [CrossRef]
- Sharanowski, B.J.; Dowling, A.P.G.; Sharkey, M.J. Molecular phylogenetics of Braconidae (Hymenoptera: Ichneumonoidea), based on multiple nuclear genes, and implications for classification. Syst. Entomol. 2011, 36, 549–572. [Google Scholar] [CrossRef]
- Bennett, A.M.R.; Cardinal, S.; Gauld, I.D.; Wahl, D.B. Phylogeny of the subfamilies of Ichneumonidae (Hymenoptera). J. Hymenopt. Res. 2019, 71, 1–156. [Google Scholar] [CrossRef]
- Furihata, S.X.; Kimura, M.T. Effects of Asobara japonica venom on larval survival of host and nonhost Drosophila species. Physiol. Entomol. 2009, 34, 292–295. [Google Scholar] [CrossRef]
- Strand, M.R.; Drezen, J.-M. Family Polydnaviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Nertherlands, 2012; pp. 237–248. [Google Scholar]
- Soller, M.; Lanzrein, B. Polydnavirus and venom of the egg-larval parasitoid Chelonus inanitus (Braconidae) induce developmental arrest in the prepupa of its host Spodoptera littoralis (Noctuidae). J. Insect Physiol. 1996, 42, 471–481. [Google Scholar] [CrossRef]
- Stoltz, D.B.; Guzo, D.; Belland, E.R.; Lucarotti, C.J.; MacKinnon, E.A. Venom promotes uncoating In Vitro and persistence In Vivo of DNA from a braconid polydnavirus. J. Gen. Virol. 1988, 69, 903–907. [Google Scholar] [CrossRef]
- Zhang, G.; Schmidt, O.; Asgari, S. A novel venom peptide from an endoparasitoid wasp is required for expression of polydnavirus genes in host hemocytes. J. Biol. Chem. 2004, 279, 41580–41585. [Google Scholar] [CrossRef]
- Webb, B.A.; Strand, M.R. The biology and genomics of polydnaviruses. Comp. Molec. Insect Sci. 2005, 6, 323–360. [Google Scholar]
- Asgari, S. Venom proteins from polydnavirus-producing endoparasitoids: Their role in host-parasite interactions. Arch. Insect Biochem. Physiol. 2006, 61, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, R.; Tanaka, K.; Barney, W.E.; Whitfield, J.B.; Banks, J.C.; Béliveau, C.; Stoltz, D.B.; Webb, B.A.; Cusson, M. Genomic and morphological features of a banchine polydnavirus: Comparison with bracoviruses and ichnoviruses. J. Virol. 2007, 81, 6491–6501. [Google Scholar] [CrossRef] [PubMed]
- Djoumad, A.; Stoltz, D.B.; Béliveau, C.; Boyle, B.; Kuhn, L.; Cusson, M. Ultrastructural and genomic characterization of a second banchine polydnavirus confirms the existence of shared features within this ichnovirus lineage. J. Gen. Virol. 2013, 94, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-W.; Peiffer, M.; Hoover, K.; Rosa, C.; Acevedo, F.E.; Felton, G.W. Symbiotic polydnavirus of a parasite manipulates caterpillar and plant immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 5199–5204. [Google Scholar] [CrossRef]
- Vinson, S.B.; Scott, J.R. Particles containing DNA associated with the oocyte of an insect parasitoid. J. Invertebr. Pathol. 1975, 25, 375–378. [Google Scholar] [CrossRef]
- Salt, G. Experimental studies in insect parasitism XIII. The haemocytic reaction of a caterpillar to eggs of its habitual parasite. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1965, 162, 303–318. [Google Scholar]
- Rotheram, S.M. Immune surface of eggs of a parasitic insect. Nature 1967, 214, 700. [Google Scholar] [CrossRef]
- Rotheram, S.M. The surface of the egg of a parasitic insect. II. The ultrastructure of the particulate coat on the egg of Nemeritis. Proc. R. Soc. B Biol. Sci. 1973, 183, 195–204. [Google Scholar]
- Stoltz, D.B.; Whitfield, J.M. Viruses and virus-like entities in the parasitic Hymenoptera. J. Hymenopt. Res. 1992, 1, 125–139. [Google Scholar]
- Dorémus, T.; Urbach, S.; Jouan, V.; Cousserans, F.; Ravallec, M.; Demettre, E.; Wajnberg, E.; Poulain, J.; Azéma-Dossat, C.; Darboux, I.; et al. Venom gland extract is not required for successful parasitism in the polydnavirus-associated endoparasitoid Hyposoter didymator (Hym. Ichneumonidae) despite the presence of numerous novel and conserved venom proteins. Insect Biochem. Mol. Biol. 2013, 43, 292–307. [Google Scholar]
- Guzo, D.; Stoltz, D.B. Obligatory multiparasitism in the tussock moth Orgyia Leucostigma. Parasitololgy 1985, 90, 1–10. [Google Scholar] [CrossRef]
- Lawrence, P.O. Purification and partical characterization of an entomopoxvirus (DIEPV) from a parasitic wasp of tephritid fruit flies. J. Insect Sci. 2002, 2, 1–12. [Google Scholar] [CrossRef]
- Manzoor, A.; Zain-ul-Abdin; Shaina, H.; Webb, B.A.; Jamil, A. Functional analysis of Bracon hebetor venom on target and non-target insect cell lines. Acta Entomol. Serb. 2017, 22, 103–111. [Google Scholar]
- Çim, S.; Altunas, H.; Ak, A. Cytotoxicity of venom from endoparasitoid Pimpla turionellae L. (Hymenoptera: Ichneumonidae) on glioblastoma cells. Eskişehir Tech. Univ. J. Sci. Technol. C Life Sci. Biotechnol. 2020, 9, 219–225. [Google Scholar]
- Yazgan, Ş.; House, H.L. An hymenopterous insect, the parasitoid Itoplectis conquisitor, reared axenically on a chemically defined synthetic diet. Can. Entomol. 1970, 102, 1304–1306. [Google Scholar] [CrossRef]
- Yazgan, Ş. A chemically-defined synthetic diet and larval nutritional requirements of the endoparasitoid Itoplectis conquisitor (Hymenoptera). J. Insect Physiol. 1972, 18, 2123–2141. [Google Scholar] [CrossRef]
- Yazgan, Ş. A meridic diet and quantitative effects of tween 80, fatty acid mixtures and inorganic salts on development and survival of the endoparasitoid Pimpla turionellae L. Z. Angew.Entomol. 1981, 91, 433–441. [Google Scholar] [CrossRef]
- Thompson, S.N. Defined meridic and holidic diets and aseptic feeding procedures for artificially rearing the ectoparasitoid, Exeristes roborator (Fabricius). Ann. Entomol. Soc. Am. 1975, 68, 220–226. [Google Scholar] [CrossRef]
- House, H.L. An artificial host: Encapsulated synthetic medium for in vitro oviposition and rearing the endoparasitoid Itoplectis conquisitor (Hymenoptera: Ichneumonidae). Can. Entomol. 1978, 110, 331–333. [Google Scholar] [CrossRef]
- House, H.L. Artificial diets for the adult parasitoid Itoplectis conquisitor (Hymenoptera: Ichneumonidae). Can. Entomol. 1980, 112, 315–320. [Google Scholar] [CrossRef]
- Awuitor, K.; Masselot, M.; Tersac, J. In Vitro rearing of Pimpla instigator [Hym.: Ichneumonidea] 2. Completion of development in semi-artificial conditions. Entomophaga 1984, 29, 331–339. [Google Scholar] [CrossRef]
- Yeargan, K.V.; Braman, K. Life history of the hyperparasitoid Mesochorus discitergus (Hymenoptera: Lchneumonidae) and tactics used to overcome the defensive behavior of the green cloverworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1989, 82, 393–398. [Google Scholar] [CrossRef]
- Heatwole, H.; Davis, D.M. Ecology of three sympatric species of parasitic insects of the genus Megarhyssa (Hymenoptera: Ichneumonidae). Ecology 1965, 246, 140–150. [Google Scholar] [CrossRef]
- Leluk, J.; Schmidt, J.; Jones, D. Comparative studies on the protein composition of hymenopteran venom reservoirs. Toxicon 1989, 27, 105–114. [Google Scholar] [CrossRef]
- Führer, E.; Willers, D. The anal secretion of the endoparasitic larva Pimpla turionellae: Sites of production and effects. J. Insect Physiol. 1986, 32, 361–365, 367. [Google Scholar]
- Hochuli, A.; Pfister-Wilhelm, R.; Lanzrein, B. Analysis of endoparasitoid-released proteins and their effects on host development in the system Chelonus inanitus (Braconidae)–Spodoptera littoralis (Noctuidae). J. Insect Physiol. 1999, 45, 823–833. [Google Scholar] [CrossRef]
- Gopalapillai, R.; Kadono-Okuda, K.; Okuda, T. Molecular cloning and analysis of a novel teratocyte-specific carboxylesterase from the parasitic wasp, Dinocampus coccinellae. Insect Biochem. Mol. Biol. 2005, 35, 1171–1180. [Google Scholar] [CrossRef]
- Poirié, M.; Carton, Y.; Dubuffet, A. Virulence strategies in parasitoid Hymenoptera as an example of adaptive diversity. C. R. Biol. 2009, 332, 311–320. [Google Scholar] [CrossRef]
- Poirié, M.; Colinet, D.; Gatti, J.L. Insights into function and evolution of parasitoid wasp venoms. Curr. Opin. Insect Sci. 2014, 6, 52–60. [Google Scholar] [CrossRef]
- Burke, G.R.; Strand, M.R. Systematic analysis of a wasp parasitism arsenal. Mol. Ecol. 2014, 23, 890–901. [Google Scholar] [CrossRef]
- Falabella, P.; Riviello, L.; Caccialupi, P.; Rossodivita, T.; Valente, M.T.; De Stradis, M.L.; Tranfaglia, A.; Varricchio, P.; Gigliotti, S.; Graziani, F.; et al. A γ-glutamyltranspeptidase of Aphidius ervi venom induces apoptosis in the ovaries of host aphids. Insect Biochem. Mol. Biol. 2007, 37, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Colinet, D.; Mathé-Hubert, H.; Allemand, R.; Gatti, J.-L.; Poirie, M. Variability of venom components in immune suppressive parasitoid wasps: From a phylogenetic to a population approach. J. Insect. Physiol. 2013, 59, 205–212. [Google Scholar] [CrossRef]
- Colinet, D.; Anselme, C.; Deleury, E.; Mancini, D.; Poulain, J.; Azema-Dossat, C.; Belghazi, M.; Tares, S.; Pennacchio, F.; Poirie, M.; et al. Identification of the main venom protein components of Aphidius ervi, a parasitoid wasp of the aphid model Acyrthosiphon pisum. BMC Genom. 2014, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Zain-ul-Abdin; Webb, B.A.; Arif, M.J.; Jamil, A. De novo sequencing and transcriptome analysis of female venom glands of ectoparasitoid Bracon hebetor (Say.) (Hymenoptera: Braconidae). Comp. Biochem. Physiol. D Genom. Proteom. 2016, 20, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; ul Abdin, Z.; Arif, M.J.; Jamil, A.; Li, X. Isolation and characterization of immune suppressive genes through bioinformatic analysis of venom glands transcriptome of Bracon hebetor (Hymenoptera: Braconidae). Intl. J. Agric. Biol. 2019, 21, 1189–1196. [Google Scholar]
- Becchimanzi, A.; Avolio, M.; Bostan, H.; Colantuono, C.; Cozzolino, F.; Mancini, D.; Chiusano, M.L.; Pucci, P.; Caccia, S.; Pennacchio, F. Venomics of the ectoparasitoid wasp Bracon nigricans. BMC Genom. 2020, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Sano, T.; Suzuki, M.; Tanaka, T.J.; Minakuchi, C.; Miura, K. The major constituents of the venom gland of a braconid endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae). Appl. Entomol. Zool. 2017, 52, 271–285. [Google Scholar] [CrossRef]
- Crawford, A.M.; Brauning, R.; Smolenski, G.; Ferguson, C.; Barton, D.; Wheeler, T.T.; Mcculloch, A. The constituents of Microctonus sp. parasitoid venoms. Insect Molec. Biol. 2008, 17, 313–324. [Google Scholar] [CrossRef]
- Yin, C.; Li, M.; Hu, J.; Lang, K.; Chen, Q.; Liu, J.; Guo, D.; He, K.; Dong, Y.; Luo, J.; et al. The genomic features of parasitism, polyembryony and immune evasion in the endoparasitic wasp Macrocentrus cingulum. BMC Genom. 2018, 19, 420. [Google Scholar] [CrossRef]
- Mathé-Hubert, H.; Colinet, D.; Deleury, E.; Belghazi, M.; Ravallec, M.; Poulain, J.; Dossat, C.; Poirié, M.; Gatti, J.-L. Comparative venomics of Psyttalia lounsburyi and P. concolor, two olive fruit fly parasitoids: A hypothetical role for a GH1 β-glucosidase. Sci. Rep. 2016, 6, 35873. [Google Scholar]
- Zhao, W.; Shi, M.; Ye, X.; Li, F.; Wang, X.-W.; Chen, X.-X. Comparative transcriptome analysis of venom glands from Cotesia vestalis and Diadromus collaris, two endoparasitoids of the host Plutella xylostella. Sci. Rep. 2017, 7, 1298. [Google Scholar] [CrossRef] [PubMed]
- Özbek, R.; Wielsch, N.; Vogel, H.; Lochnit, G.; Foerster, F.; Vilcinskas, A.; von Reumont, B.M. Proteo-transcriptomic characterization of the venom from the endoparasitoid wasp Pimpla turionellae with aspects on its biology and evolution. Toxins 2019, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Pook, V.G. Investigating Ichneumonidae: Insights into species identification and venom composition. Ph.D. Thesis, College of Agriculture, University of Kentucky, Lexington, KY, USA, 2016. Unpublished. [Google Scholar]
- Wharton, R.A.; Marsh, P.M. New World Opiinae (Hymenoptera: Braconidae) parasitic on Tephritidae (Diptera). J. Wash. Acad. Sci. 1978, 68, 147–167. [Google Scholar]
- Quicke, D.L.J.; van Achterberg, C. Phylogeny of the subfamilies of Braconidae (Hymenoptera). Zool. Verh. Leiden 1990, 258, 1–95. [Google Scholar]
- Quicke, D.L.J.; Tunstead, J.; Falco, J.V.; Marsh, P.M. Venom gland and reservoir morphology in the Doryctinae and related braconid wasps (Insecta, Hymenoptera, Braconidae). Zool. Scr. 1992, 21, 403–416. [Google Scholar] [CrossRef]
- Quicke, D.L.J.; van Achterberg, C.; Godfray, H.C.J. Comparative morphology of the venom gland and reservoir in opiine and alysiine braconid wasps (Insecta, Hymenoptera, Braconidae). Zool. Scr. 1997, 26, 23–50. [Google Scholar] [CrossRef]
- Zaldivar-Riveron, A.; Areekul, B.; Shaw, M.R.; Quicke, D.L.J. Comparative morphology of the venom apparatus in the braconid wasp subfamily Rogadinae (Insecta, Hymenoptera, Braconidae) and related taxa. Zool. Scr. 2004, 33, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.J.M.; Vinchon, S.; Cherqui, A.; Prévost, G. Components of Asobara venoms and their effects on hosts. In Advances in Parasitology; Prévost, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 70, pp. 217–232. [Google Scholar]
- Togashi, I. A comparative morphology of the poison glands in the adults of ichneumon-flies (Hymenoptera: Ichneumonidae) (1). Kontyû 1963, 31, 297–304, (In Japanese with English Summary). [Google Scholar]
- Edson, K.M.; Vinson, S.B. A comparative morphology of the venom apparatus of female braconids (Hymenoptera: Braconidae). Canad. Ent. 1979, 111, 1013–1024. [Google Scholar] [CrossRef]
- Bender, J.C. Anatomy and histology of the female reproductive organs of Habrobrucon juglandis (Ashmead). Ann. Entomol. Soc. Am. 1943, 36, 537–545. [Google Scholar] [CrossRef]
- Venkatraman, T.V.; Subba Rao, B.R. The mechanism of ovipositor in Stenobracon deesae (Cam.) (Hymenoptera: Braconidae). Proc. R. Entomol. Soc. Lond. A 1954, 29, 1–9. [Google Scholar]
- Shaw, M.R. On evolution of endoparasitism; the biology of some genera of Rogadinae (Braconidae). Contrib. Amer. Entomol. Inst. 1983, 20, 307–328. [Google Scholar]
- Stavraki-Paulopoulou, H.G. Contribution a l’étude de la capacité reproductrice et de la fecondité reélle d’Opius concolor Szépl. (Hymenoptera-Braconidae). Ann. Epiphyt. 1966, 17, 391–435. [Google Scholar]
- van Marle, J. Structure and histochemistry of the venom glands of the wasps Microbracon hebetor Say and Philanthus triangulum F. Toxicon 1977, 15, 529–539. [Google Scholar] [CrossRef]
- Alves, T.J.S.; Wanderley-Teixeira, V.; Teixeira, Á.A.C.; Alves, L.C.; Araújo, B.C.; Barros, E.M.; Cunha, F.M. Morphological and histological characterization of production structures, storage and distribution of venom in the parasitic wasp Bracon vulgaris. Toxicon 2015, 108, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.M.; Still, L.-E.; Smith, P. A histological examination of the venom apparatus of Microctonus hyperodae Loan (Hymenoptera: Braconidae). N. Z. Entomol. 2006, 29, 103–105. [Google Scholar] [CrossRef]
- Wan, Z.-W.; Wang, H.Y.; Chen, X.-X. Venom apparatus of the endoparasitoid wasp Opius caricivorae Fischer (Hymenoptera: Braconidae): Morphology and ultrastructure. Microsc. Res. Technol. 2006, 69, 820–825. [Google Scholar] [CrossRef]
- Li, W.-D.; Chen, X.-X.; He, H.H. Venom gland of the ichneumonid Diadromus collaris: Morphology, ultrastructure and age-related changes. Insect Sci. 2006, 13, 137–143. [Google Scholar] [CrossRef]
- Blass, S.; Ruthmann, A. Fine structure of the accessory gland of the female genital tract of the ichneumonid Pimpla turionellae (Insecta, Hymenoptera). Zoomorphology 1989, 108, 367–377. [Google Scholar] [CrossRef]
- Güven, T.; Yel, M. The ultrastructure of the venom gland in Pimpla turionellae (Hymenoptera: Ichneumonidae). Doğa Türk Biyoloji Dergisi 1990, 14, 17–31. (In Turkish) [Google Scholar]
- Uçkan, F. The morphology of the venom apparatus and histology of venom gland of Pimpla turionellae (Hym.; Ichneumonidae) females. Tr. J. Zool. 1999, 23, 461–466. [Google Scholar]
- Edson, K.M.; Barlin, M.R.; Vinson, S.B. Venom apparatus of braconid wasps: Comparative ultrastructure of reservoirs and gland filaments. Toxicon 1982, 20, 553–562. [Google Scholar] [CrossRef]
- Noirot, C.; Quennedy, A. Fine structure of insect epidermal glands. Annu. Rev. Entomol. 1974, 19, 61. [Google Scholar] [CrossRef]
- Coffman, K.A.; Harrell, T.C.; Burke, G.R. A mutualistic poxvirus exhibits convergent evolution with other heritable viruses in parasitoid wasps. J. Virol. 2020. [Google Scholar] [CrossRef]
- Lawrence, P.O. Morphogenesis and cytopathic effects of the Diachasmimorpha longicaudata entomopoxvirus in host haemocytes. J. Insect Physiol. 2005, 51, 221–233. [Google Scholar] [CrossRef]
- Mwaengo, D.M.; Lawrence, P.O. A putative DNA helicase and novel oligoribonuclease in the Diachasmimorpha longicaudata entomopoxvirus (DlEPV). Arch. Virol. 2003, 148, 1431–1444. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Lawrence, P.O. Comparative analysis of selected genes from Diachasmimorpha longicaudata entomopoxvirus and other poxviruses. J. Insect Physiol. 2005, 51, 207–220. [Google Scholar] [CrossRef]
- Lawrence, P.O.; Dillard, B.E. A homolog of the vaccinia virus D13L rifampicin resistance gene is in the entomopoxvirus of the parasitic wasp, Diachasmimorpha longicaudata. J. Insect Sci. 2008, 8, 8. [Google Scholar] [CrossRef]
- Gatti, J.; Schmitz, A.; Colinet, D.; Poirié, M. Diversity of virus-like particles in parasitoids’ venom. In Parasitoid Viruses: Symbionts and Pathogens; Beckage, N.E., Drezen, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 181–192. [Google Scholar]
- Jacas, J.A.; Budia, F.; Rodriguez-Cerezo, E.; Vinuela, E. Virus-like particles in the poison gland of the parasitic wasp Opius concolor. Ann. Appl. Biol. 1997, 130, 587–592. [Google Scholar] [CrossRef]
- Suzuki, M.; Tanaka, T. Virus-like particles in venom of Meteorus pulchricornis induce host hemocyte apoptosis. J. Insect Physiol. 2006, 52, 602–613. [Google Scholar] [CrossRef]
- Suzuki, M.; Miura, K.; Tanaka, T. The virus-like particles of a braconid endoparasitoid wasp, Meteorus pulchricornis, inhibit hemocyte spreading in its noctuid host, Pseudaletia separata. J. Insect Physiol. 2008, 54, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Barratt, B.I.; Evans, A.A.; Stoltz, D.B.; Vinson, S.B.; Easingwood, R. Virus-like particles in the ovaries of Microctonus aethiopoides Loan (Hymenoptera: Braconidae), a parasitoid of adult weevils (Coleoptera: Curculionidae). J. Invertebr. Pathol. 1999, 73, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Barratt, B.I.; Murney, R.; Easingwood, R.; Ward, V.K. Virus-like particles in the ovaries of Microctonus aethiopoides Loan (Hymenoptera, Braconidae), comparison of biotypes from Morocco and Europe. J. Invertebr. Pathol. 2006, 91, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wharton, R.A. Bionomics of the Braconidae. Annu. Rev. Entomol. 1993, 38, 121–143. [Google Scholar] [CrossRef]
- Myers, J.G. Habits of Alysia manducator. Bull. Entomol. Res. 1927, 17, 219–229. [Google Scholar] [CrossRef]
- Piek, T.; Thomas, R.T.S. Paralyzing venoms of solitary wasps. Comp. Biochem. Physiol. 1969, 30, 13–31. [Google Scholar] [CrossRef]
- Calvert, D.J.; Van Den Bosch, R. Behavior and biology of Monoctonus paulensis (Hymenoptera: Braconidae), a parasite of dactynotine aphids. Ann. Entomol. Soc. Am. 1972, 65, 773–779. [Google Scholar] [CrossRef]
- Nelson, J.M.; Roitberg, B.D. Factors Governing Host Discrimination by Opius dimidiatus (Ashmead) (Hymenoptera: Braconidae). J. Insect. Behav. 1993, 6, 13–24. [Google Scholar] [CrossRef]
- Moreau, S.J.M.; Dingremont, A.; Doury, G.; Giordanengo, P. Effects of parasitism by Asobara tabida (Hymenoptera: Braconidae) on the development, survival and activity of Drosophila melanogaster larvae. J. Insect Physiol. 2002, 48, 337–347. [Google Scholar] [CrossRef]
- Montoya, P.; Benrey, B.; Barrera, J.F.; Zenil, M.; Ruiz, L.; Liedo, P. Oviposition behavior and conspecific host discrimination in Diachasmimorpha longicaudata (Hymenoptera: Braconidae), a fruit fly parasitoid. Biocontrol Sci. Technol. 2003, 13, 683–690. [Google Scholar] [CrossRef]
- Sishuba, N. Investigation of the larval parasitoids of the false codling moth, Cryptophlebia leucotreta (Meyrick) (Lepidoptera: Tortricidae), on citrus in South Africa. Master’s Thesis, Rhodes University, Grahamstown, South Africa, 2003. [Google Scholar]
- Prévost, G.; Eslin, P.; Doury, G.; Moreau, S.J.M.; Guillot, S. Asobara, braconid parasitoids of Drosophila larvae: Unusual strategies to avoid encapsulation without VLPs. J. Insect Physiol. 2005, 51, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.; Maeto, K. Temporary host paralysis and avoidance of selfsuperparasitism in the solitary endoparasitoid Meteorus pulchricornis. Entomol. Exp. Appl. 2009, 132, 250–255. [Google Scholar] [CrossRef]
- Shaw, M.R.; Sims, I. Notes on the biology, morphology, nomenclature and classification of Pseudavga flavicoxa Tobias, 1964 (Hymenoptera, Braconidae, Rhysipolinae), a genus and species new to Britain parasitizing Bucculatrix thoracella (Thunberg) (Lepidoptera, Bucculatricidae). J. Hymenopt. Res. 2015, 42, 21–32. [Google Scholar] [CrossRef][Green Version]
- van Achterberg, C.; Shaw, M.R. Revision of the western Palaearctic species of Aleiodes Wesmael (Hymenoptera, Braconidae, Rogadinae). Part 1: Introduction, key to species groups, outlying distinctive species, and revisionary notes on some further species. ZooKeys 2016, 639, 1–164. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.R. Further notes on the biology of Pseudavga flavicoxa Tobias, 1964 (Hymenoptera, Braconidae, Rhysipolinae). J. Hymenopt. Res. 2017, 54, 113–128. [Google Scholar] [CrossRef][Green Version]
- Cushman, R.A. The genus Lysiognatha Ashmead. J. Wash. Acad. Sci. 1937, 10, 438–444. [Google Scholar]
- Piek, T. Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioral Aspects; Academic Press: London, UK; Orlando, FL, USA; San Diego, CA, USA, 1986. [Google Scholar]
- Shaw, M.R. Interactions between adults of some species of Netelia Gray (Hymenoptera: Ichneumonidae: Tryphoninae) and their caterpillar hosts (Lepidoptera). J. Hymenopt. Res. 2001, 10, 101–111. [Google Scholar]
- Takasuka, M.; Matsumoto, M.; Ohbayashi, N. Oviposition behavior of Zatypota albicoxa (Hymenoptera, Ichneumonidae), an ectoparasitoid of Achaearanea tepidariorum (Araneae, Theridiidae). Entomol. Sci. 2009, 338, 232–237. [Google Scholar]
- Shaw, M.R.; Wahl, D.B. Biology, early stages and description of a new species of Adelognathus Holmgren (Hymenoptera: Ichneumonidae: Adelognathinae). Zootaxa 2014, 3884, 235–252. [Google Scholar] [CrossRef]
- Kloss, T.G.; Gonzaga, M.O.; Roxinol, J.A.M.; Sperber, C.F. Attack behavior of two species of the Polysphincter genus group (Hymenoptera, Ichneumonidae) on their orb-weaver spider hosts (Araneae, Araneidae). J. Insect Behav. 2016, 29, 315–324. [Google Scholar] [CrossRef]
- Vinson, S.B.; Iwantch, G.F. Host suitability for insect parasitoids. Annu. Rev. Entomol. 1980, 25, 397–419. [Google Scholar] [CrossRef]
- Desneux, N.; Barta, R.J.; Delebecque, C.J.; Heimpel, G.E. Transient host paralysis as a means of reducing self-superparasitism in koinobiont endoparasitoids. J. Insect Physiol. 2009, 55, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Strazanac, J.S.; Marsh, P.M.; van Achtereerg, C.; Choi, W.Y. First recorded parasitoid from China of Agrilus planipennis: A new species of Spathius (Hymenoptera: Braconidae: Doryctinae). Ann. Entomol. Soc. Am. 2005, 98, 636–642. [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Strazanac, J.S.; Reardon, R.C.; Zhang, Y.N.; Liu, G.J.; Liu, E.S. Biology of Spathius agrili Yang (Hymenoptera: Braconidae) a parasite of the emerald ash borer (Coleoptera: Buprestidae) in China. J. Entomol. Sci. 2008, 10, 30. [Google Scholar]
- Wang, X.-Y.; Yang, Z.-Q.; Gould, J.R.; Wu, H.; Ma, J.-H. Host-seeking behavior and parasitism by Spathius agrili Yang (Hymenoptera: Braconidae), a parasitoid of the emerald ash borer. Biol. Control 2010, 52, 24–29. [Google Scholar] [CrossRef]
- Ranjith, A.P.; Quicke, D.L.J.; Saleem, U.K.A.; Butcher, B.A.; Zaldivar-Riveron, A.; Nasser, M. Entomophytophagy in an Indian braconid ‘parasitoid’ wasp (Hymenoptera): Specialized larval morphology, biology and description of a new species. PLoS ONE 2016, 11, e0156997. [Google Scholar] [CrossRef]
- Guerra, A.A.; Robacker, K.M.; Martinez, S. Free amino acid and protein titers in Anthonomus grandis larvae venomized by Bracon mellitor. Entomophaga 1993, 38, 519–525. [Google Scholar] [CrossRef]
- Sak, O.; Ergin, E.; Uçkan, F.; Rivers, D.B.; Er, A. Changes in the hemolymph total protein of Galleria mellonella (Lepidoptera: Pyralidae) after parasitism and envenomation by Pimpla turionellae (Hymenoptera: Ichneumonidae). Tr. J. Biol. 2011, 35, 425–432. [Google Scholar]
- Sak, O.; Uçkan, F.; Ergin, E.; Altuntas, H.; Er, A. Effects of parasitism and envenomation by Pimpla turionellae (Hymenoptera: Ichneumonidae) on hemolymph free amino acids of Galleria mellonella (Lepidoptera: Pyralidae). J. Entomol. Res. Soc. 2014, 16, 1–20. [Google Scholar]
- Kuleli, S.; Er, A.; Uçkan, F. Alterations in hemolymph carbohydrate and lipid composition of Galleria mellonella (Lepidoptera: Pyralidae) induced by parasitism and venom of Pimpla turionellae (Hymenoptera: Ichneumonidae). Ann. Entomol. Soc. Am. 2017, 110, 221–226. [Google Scholar]
- Ergin, E.; Altuntaş, H.; Uçkan, F. Effects of parasitization and envenomation by the endoparasitic wasp Pimpla turionellae L. (Hymenoptera: Ichneumonidae) on hemolymph protein profile of its host Galleria mellonella L. (Lepidoptera: Pyralidae). Biol. Divers. Conserv. 2013, 6, 62–70. [Google Scholar]
- Baker, J.E.; Fabrick, J.A. Host hemolymph proteins and protein digestion in larval Habrobracon hebetor (Hymenoptera: Braconidae). Insect Biochem. Mol. Biol. 2000, 30, 937–946. [Google Scholar] [CrossRef]
- Altuntaş, H.; Kiliç, A.Y.; Sivas Zeytinoğlu, H. The effects of parasitism by the ectoparasitoid Bracon hebetor Say (Hymenoptera: Braconidae) on host hemolymph proteins in the Mediterranean flour moth Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Tr. J. Zool. 2010, 34, 409–416. [Google Scholar]
- Gao, X.; Xue, H.; Luo, J.; Ji, J.; Zhang, L.; Niu, L.; Zhu, X.; Wang, L.; Zhang, S.; Cui, J. Molecular evidence that Lysiphlebia japonica regulates the development and physiological metabolism of Aphis gossypii. Int. J. Mol. Sci. 2020, 21, 4610. [Google Scholar] [CrossRef]
- Polaszek, A. The effects of two species of hymenopterous parasitoid on the reproductive system of the pea aphid, Acyrthosiphon pisum. Entomol. Exp. Appl. 1986, 40, 285–292. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Adriaanse, I.C.T.; van den Bergh, B. Parasitoid size as a function of host sex: Potential for different sex allocation strategies. Entomol. Exp. Appl. 1999, 92, 289–294. [Google Scholar] [CrossRef]
- Goldson, S.L.; Proffitt, J.R.; Baird, D.B. Establishment and phenology of the parasitoid Microctonus hyperodae (Hymenoptera: Braconidae) in New Zealand. Environ. Entomol. 1998, 27, 1386–1392. [Google Scholar] [CrossRef]
- Marris, G.C.; Bell, H.A.; Naylor, J.M.; Edwards, J.P. The role of Pimpla hypochondriaca venom in the suppression of pupal noctuid host immunity. Entomol. Exp. Appl. 1999, 93, 289–296. [Google Scholar] [CrossRef]
- Dani, M.P.; Richards, E.H.; Edwards, J.P. Venom from the pupal endoparasitoid, Pimpla hypochondriaca, increases susceptibility of larval Lacanobia oleracea to the entomopathogens Bacillus cereus and Beauveria bassiana. J. Invert. Pathol. 2004, 86, 19–25. [Google Scholar] [CrossRef]
- Moreau, S.J.; Eslin, P.; Giordanengo, P.; Doury, G. Comparative study of the strategies evolved by two parasitoids of the genus Asobara to avoid the immune response of the host, Drosophila melanogaster. Dev. Comp. Immunol. 2003, 27, 273–282. [Google Scholar] [CrossRef]
- Mabiala-Moundoungou, A.D.N.; Doury, G.; Eslin, P.; Cherqui, A.; Prévost, G. Deadly venom of Asobara japonica parasitoid needs ovarian antidote to regulate host physiology. J. Insect Physiol. 2010, 56, 35–41. [Google Scholar] [CrossRef] [PubMed]
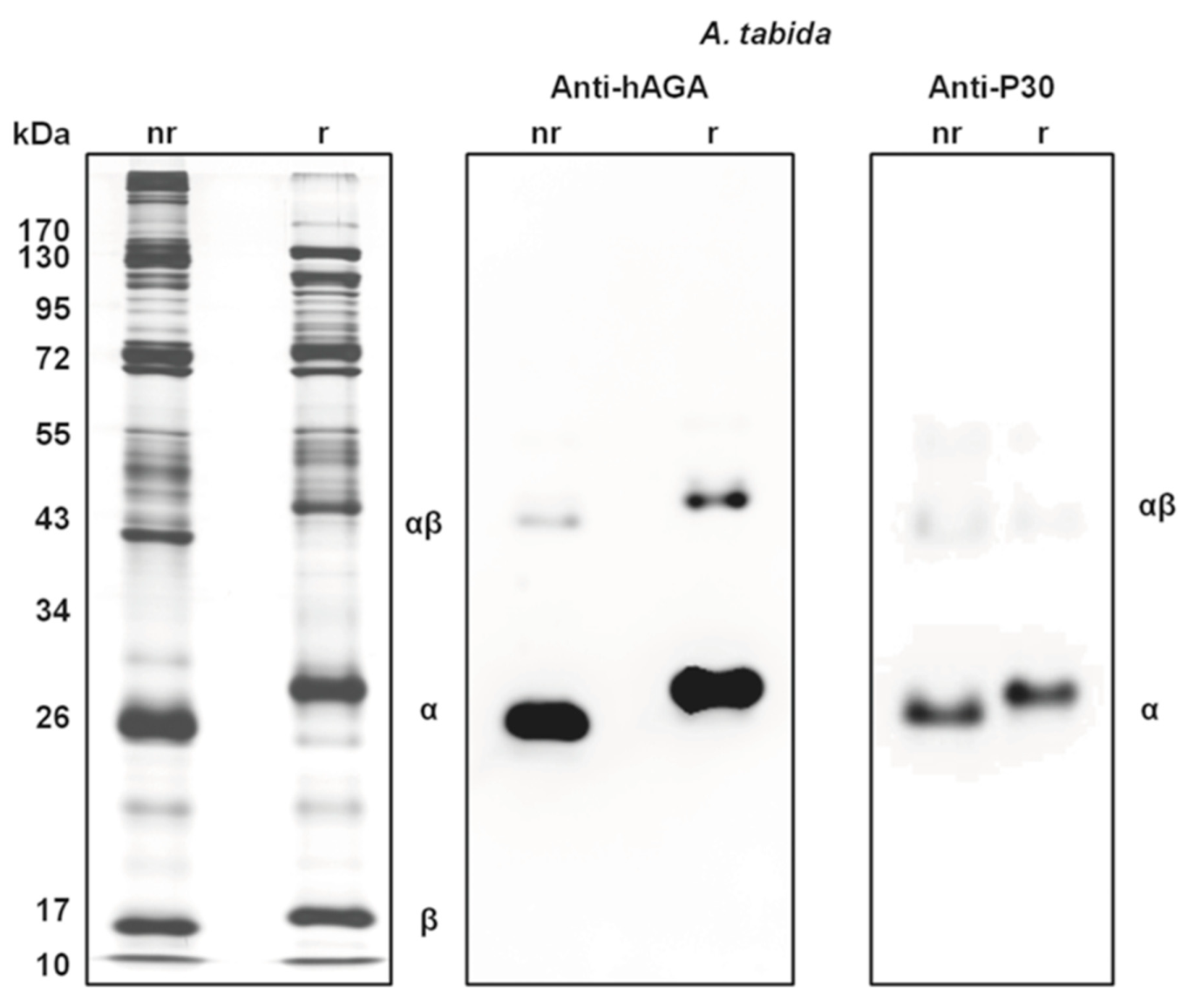
- Moreau, S.J.M.; Cherqui, A.; Doury, G.; Dubois, F.; Fourdrain, Y.; Sabatier, L.; Bulet, P.; Saarela, J.; Prévost, G.; Giordanengo, P. Identification of an aspartylglucosaminidase-like protein in the venom of the parasitic wasp Asobara tabida (Hymenoptera: Braconidae). Insect Biochem. Mol. Biol. 2004, 34, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Eslin, P.; Giordanengo, P.; Fourdrain, Y.; Prévost, G. Avoidance of encapsulation in the absence of VLP by a braconid parasitoid of Drosophila larvae: An ultrastructural study. Can. J. Zool. 1996, 74, 2193–2198. [Google Scholar] [CrossRef]
- Eslin, P.; Prévost, G. Racing against host’s immunity defenses: A likely strategy for passive evasion of encapsulation in Asobara tabida parasitoids. J. Insect Physiol. 2000, 46, 1161–1167. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; van Alphen, J.M. Geographical variation in resistance of the parasitoid Asobara tabida against encapsulation by Drosophila melanogaster larvae: The mechanism explored. Phys. Entomol. 1994, 19, 9–14. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Nowee, B.; Najem, R.W. Adaptative variation of host selection behaviour of Asobara tabida, a parasitoid of Drosophila larvae. Funct. Ecol. 1995, 9, 113–118. [Google Scholar] [CrossRef]
- Eslin, P.; Prévost, G. Variation in Drosophila concentration of haemocytes associated with different ability to encapsulate Asobara tabida larval parasitoid. J. Insect Physiol. 1996, 42, 549–555. [Google Scholar] [CrossRef]
- Eslin, P.; Prévost, G. Haemocyte load and immune resistance to Asobara tabida are correlated in species of the Drosophila melanogaster subgroup. J. Insect Physiol. 1998, 44, 807–816. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Godfray, H.C.J. Evolution of host resistance and parasitoid counter-resistance. Adv. Parasitol. 2009, 70, 257–280. [Google Scholar]
- Prince, G.J. Laboratory biology of Phaenocarpa persimilis Papp (Braconidae: Alysiinae), a parasitoid of Drosophila. Austral. J. Zool. 1976, 24, 249. [Google Scholar] [CrossRef]
- Vinchon, S.; Moreau, S.J.M.; Drezen, J.M.; Prévost, G.; Cherqui, A. Molecular and biochemical analysis of an aspartylglucosaminidase from the venom of the parasitoid wasp Asobara tabida (Hymenoptera: Braconidae). Insect Biochem. Mol. Biol. 2010, 40, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Coulette, Q.; Lemauf, S.; Colinet, D.; Prévost, G.; Anselme, C.; Poirié, M.; Gatti, J.L. Biochemical characterization and comparison of aspartylglucosaminidases secreted in venom of the parasitoid wasps Asobara tabida and Leptopilina heterotoma. PLoS ONE 2017, 12, e0181940. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.J.M.; Guillot, S. Advances and prospects on biosynthesis, structures and functions of venom proteins from parasitic wasps. Insect Biochem. Mol. Biol. 2005, 35, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.-T.; Soustelle, L.; Birman, S. Selective high-affinity transport of aspartate by a Drosophila homologue of the excitatory amino-acid transporters. Curr. Biol. 2000, 10, 207–210. [Google Scholar] [CrossRef][Green Version]
- Tomlin, E.; McLean, H.; Caveney, S. Active accumulation of glutamate and aspartate by insect epidermal cells. Insect Biochem. Mol. Biol. 1993, 23, 561–569. [Google Scholar] [CrossRef]
- Moreau, S.J.M.; Doury, G.; Giordanengo, P. Intraspecific variation in the effects of parasitism by Asobara tabida on phenoloxidase activity of Drosophila melanogaster larvae. J. Invert. Pathol. 2000, 76, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, T.I.; Kimura, M.T. Toxicity of venom of Asobara and Leptopilina species to Drosophila species. Physiol. Entomol. 2015, 40, 304–308. [Google Scholar] [CrossRef]
- Furihata, S.X.; Matsumoto, H.; Kimura, M.T.; Hayakawa, Y. Venom components of Asobara japonica impair cellular immune responses of host Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2013, 83, 86–100. [Google Scholar] [CrossRef]
- Furihata, S.; Matsumura, T.; Hirata, M.; Mizutani, T.; Nagata, N.; Kataoka, M.; Katayama, Y.; Omatsu, T.; Matsumoto, H.; Hayakawa, Y. Characterization of venom and oviduct components of parasitoid wasp Asobara japonica. PLoS ONE 2016, 11, e0160210. [Google Scholar] [CrossRef]
- Henter, H.J.; Via, S. The potential for coevolution in a host-parasitoid system, I. Genetic variation within an aphid population insusceptibility to a parasitic wasp. Evolution 1995, 49, 427–438. [Google Scholar] [CrossRef]
- Chau, A.; Mackauer, M. Preference of the aphid parasitoid Monoctonus paulensis (Hymenoptera: Braconidae, Aphidiinae) for different aphid species: Female choice and offspring survival. Biol. Control 2001, 20, 30–38. [Google Scholar] [CrossRef]
- Digilio, M.C.; Pennacchio, F.; Tremblay, E. Host regulation effects of ovary fluid and venom of Aphidius ervi (Hymenoptera: Braconidae). J. Insect Physiol. 1998, 44, 779–784. [Google Scholar] [CrossRef]
- Digilio, M.C.; Isidoro, N.; Tremblay, E.; Pennacchio, F. Host castration by Aphidius ervi venom proteins. J. Insect Physiol. 2000, 46, 1041–1050. [Google Scholar] [CrossRef]
- Pennacchio, F.; Mancini, D. Aphid parasitoid venom and its role in host regulation. In Parasitoid Viruses: Symbionts and Pathogens; Beckage, N.E., Drezen, J.-M., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 247–254. [Google Scholar]
- Nguyen, T.T.A.; Magnoli, I.; Cloutier, C.; Michaud, D.; Muratori, F.; Hance, T. Early presence of an enolase in the oviposition injecta of the aphid parasitoid Aphidius ervi analyzed with chitosan beads as artificial hosts. J. Insect Physiol. 2013, 59, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Piek, T.; Spanjer, W.; Njio, K.D.; Veenendaal, R.L.; Mantel, P. Paralysis caused by the venom of the wasp, Microbracon Gelechiae. J. Insect Physiol. 1974, 20, 2307–2319. [Google Scholar] [CrossRef]
- Avolio, M. Functional and Molecular Analysis of Venom Produced by the Ectoparasitoid Bracon nigricans. Ph.D. Thesis, Faculty of Agriculture, University of Naples Federico II, Naples, Italy, 2015. [Google Scholar]
- Kittel, R.N.; Maeto, K. Revalidation of Habrobracon brevicornis stat. rest. (Hymenoptera: Braconidae) based on the CO1, 16S, and 28S gene fragments. J. Econ. Entomol. 2019, 112, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Heimpel, G.E.; Antolin, M.F.; Franqui, R.A.; Strand, M.R. Reproductive isolation and genetic variation between two “strains” of Bracon hebetor (Hymenoptera: Braconidae). Biol. Control 1997, 9, 149–156. [Google Scholar] [CrossRef]
- von Borstel, R.C.; Smith, R.H.; Grosch, D.S.; Whiling, A.R.; Amy, R.L.; Baird, M.B.; Buchanan, P.D.; Cain, K.T.; Carpenter, R.A.; Clark, A.M.; et al. Mutational response of Habrobracon in the BIOSATELLITE II experiment. Bioscience 1968, 18, 598–601. [Google Scholar] [CrossRef]
- Beard, R.L. The toxicology of Habrobracon venom: A study of a natural insecticide. Conn. Agr. Exp. Sta. Bull. 1952, 562, 1–27. [Google Scholar]
- Piek, T. Site of action of venom of Microbracon hebetor Say (Braconidae, Hymenoptera). J. Insect Physiol. 1966, 12, 561–562. [Google Scholar] [CrossRef]
- Piek, T. Neurotoxins from venoms of the Hymenoptera—Twenty-five years of research in Amsterdam. Comp. Biochem. Physiol. C Comp. Pharmacol. 1990, 96, 223–233. [Google Scholar] [CrossRef]
- Quistad, G.B.; Leisy, D.J. Insecticidal Toxins from the Parasitic Wasp, Bracon hebetor. U.S. Patent 554592A, 11 February 1996. [Google Scholar]
- Windass, J.D.; Duncan, R.E.; Christian, P.D.; Baule, V.J. Toxins from the Parasitic Wasp Bracon hebetor. International Patent WO 96/16171, 30 May 1996. [Google Scholar]
- Weaver, R.J.; Marris, G.C.; Bell, H.A.; Edwards, J.P. Identity and mode of action of the host endocrine disrupters from the venom of parasitoid wasps. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, S101. [Google Scholar] [CrossRef]
- Piek, T. Arthropod venoms as tools for the study of neuromuscular transmission. Comp. Biochem. Physiol. C Comp. Pharmacol. 1981, 68, 75–84. [Google Scholar] [CrossRef]
- Ghimire, M.N.; Phillips, T.W. Suitability of different Lepidopteran host species for development of Bracon hebetor (Hymenoptera: Braconidae). Environ. Entomol. 2010, 39, 449–458. [Google Scholar] [CrossRef]
- Beard, R.L. Effectiveness of paralyzing venom and its relation to host discrimination by braconid wasps. Ann. Entomol. Soc. Am. 1972, 65, 90–93. [Google Scholar] [CrossRef]
- Quistad, G.B.; Nguyen, Q.; Bernasconi, P.; Leisy, D.J. Purification and characterization of insecticidal toxins from venom glands of the parasitic wasp, Bracon hebetor. Insect Biochem. Mol. Biol. 1994, 24, 955–961. [Google Scholar] [CrossRef]
- Madyarov, S.R.; Mirzaeva, G.S.; Otarbaev, D.O.; Khamidi, K.S.; Kamilova, S.I.; Akhmerov, S.I.; Khamraev, A.S. Mulberry silkwork, Bombyx mori L., as a host for neurotoxic Braconidae, I. Insect-toxic properties of Bracon venom gland extract and its fractions. Int. J. Indust. Entomol. 2003, 7, 235–239. [Google Scholar]
- Temerak, S.A. Host preferences of the parasitoid Bracon brevicornis Wesmael (Hym., Braconidae) and host sensitivity to its venom. Z. Angew. Entomol. 2009, 96, 37–41. [Google Scholar] [CrossRef]
- Gerling, D.; Rotary, N. Hypersensitivity, resulting from host-unsuitability, as exemplified by two parasite species attacking Spodoptera littoralis. Entomophaga 1973, 18, 391–396. [Google Scholar] [CrossRef]
- Masler, E.P.; Kovaleva, E.S. Inhibition of larval growth in the gypsy moth (Lepidoptera: Lymantriidae) by venom from the parasitic wasp Microbracon hebetor (Hymenoptera: Braconidae). J. Entomol. Sci. 1999, 34, 435–444. [Google Scholar] [CrossRef]
- Drenth, D. Susceptibility of different species of insects to an extract of the venom gland of the wasp Microbracon hebetor (Say). Toxicon 1974, 12, 189–192. [Google Scholar] [CrossRef]
- Petters, R.M.; Stefaneli, J. Developmental arrest of endoparasitoid wasp larvae (Nemeritis canescens Grav.) caused by an ectoparasitoid wasp (Bracon hebetor Say). J. Exp. Zool. 1983, 225, 459–465. [Google Scholar] [CrossRef]
- Tamashiro, M. A biological study of the venoms of two species of Bracon. Hawaii Agric. Exp. Station Techn. Bull. 1971, 70, 52. [Google Scholar]
- Waller, J.B. The effect of the venom of Bracon hebetor on the respiration of the wax moth Galleria mellonella. J. Insect Physiol. 1965, 11, 1595–1599. [Google Scholar] [CrossRef]
- Sláma, K.; Lukáš, J. Myogenic nature of insect heartbeat and intestinal peristalsis, revealed by neuromuscular paralysis caused by the sting of a braconid wasp. J. Insect Physiol. 2011, 57, 251–259. [Google Scholar] [CrossRef]
- Sláma, K. Neuromuscular paralysis induced in insect larvae by the proteinic venom of a parasitic wasp. In Paralysis: Causes, Classification and Treatments, Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Science Publishers: New York, NY, USA, 2012; Volume 43, pp. 195–212. [Google Scholar]
- Piek, T.; Mantel, P. The effect of the venom of Microbracon hebetor (Say) on the hyperpolarizing potentials in a skeletal muscle of Philosamia cynthia Hübn. Comp. Gen. Pharmacol. 1970, 1, 87–92. [Google Scholar] [CrossRef]
- Walther, C.; Rathmayer, W. The effect of Habrobracon venom on excitatory neuromuscular transmission in insects. J. Comp. Physiol. 1974, 89, 23–38. [Google Scholar] [CrossRef]
- Spanjer, W.; Grosu, I.; Piek, T. Two different paralysing preparations obtained from a homogenate of the wasp Microbracon hebetor Say. Toxicon 1977, 15, 413–421. [Google Scholar] [CrossRef]
- Piek, T.; Engels, E. Action of the venom of Microbracon hebetor say on larvae and adults of Philosamia cynthia Hübn. Comp. Biochem. Physiol. 1969, 28, 603–606. [Google Scholar] [CrossRef]
- Rathmayer, W.; Walther, C. Mode of action and specificity of Habrobracon venom (Hymenoptera, Braconidae). In Animal, Plant, and Microbial Toxins; Ohsaka, A., Hayashi, K., Sawai, Y., Murata, R., Funatsu, M., Tamiya, N., Eds.; Springer: Boston, MA, USA, 1976. [Google Scholar]
- Walther, C.; Reinecke, M. Block of synaptic vesicle exocytosis without block of Ca+2 influx on locust motor nerve terminals. An ultrastructural analysis of the paralysing action of Habrobracon venom on locust motor nerve terminals. Neuroscience 1983, 9, 213–224. [Google Scholar] [CrossRef]
- El-Sawaf, B.M.; Zohdy, N.Z.M. Host-parasite relationship. 3-Cholinesterase activity of the larvae of the rice moth Corcyra cephalanica (Lep.: Pyralidae) parasitized by Bracon hebetor (Hym.: Braconidae). Entomophaga 1976, 21, 99–101. [Google Scholar] [CrossRef]
- Visser, B.J.; Spanjer, W.; De Klonia, H.; Piek, T.; Van Der Meer, C.; Van Der Drift, A.C.M. Isolation and some biochemical properties of a paralysing toxin from the venom of the wasp Microbracon hebetor (Say). Toxicon 1976, 14, 357–370. [Google Scholar] [CrossRef]
- Visser, B.J.; Labruyere, W.T.; Spanjer, W.; Piek, T. Characterization of two paralysing protein toxins (A-MTX, and B-MTX), isolated from a homogenate of the wasp Microbracon hebetor (Say). Comp. Biochem. Physiol. 1983, 75, 523–530. [Google Scholar] [CrossRef]
- Beard, R.L. Venoms of Braconidae. In Arthropod Venoms. Handbook of Experimental Pharmacology; Bettini, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1978; Volume 48. [Google Scholar]
- Edwards, S.; Sernka, T.J. On the action of Bracon venom. Toxicon 1969, 6, 303–305. [Google Scholar] [CrossRef]
- Slavnova, T.I.; Antonov, S.M.; Magazanik, L.G.; Tonkikh, A.K.; Kosovskii, A.V.; Sadykov, A.A.; Abduvakhabov, A.A. Effect of toxin from the venom of the ichneumon Habrobracon hebetor (Say) on neuromuscular transmission in insects. Dok. Akad. Nauk. SSR 1987, 297, 492–494. [Google Scholar]
- Kryukova, N.A.; Chertkova, E.A.; Semenova, A.D.; Glazachev, Y.I.; Slepneva, I.A.; Glupov, V.V. Venom from the ectoparasitic wasp Habrobracon hebetor activates calcium-dependent degradation of Galleria mellonella larval haemocytes. Arch. Insect Biochem. Physiol. 2015, 90, 117–130. [Google Scholar] [CrossRef]
- Kryukova, N.A.; Dubovskiy, I.; Chertkova, E.A.; Vorontsova, Y.; Slepneva, I.A.; Glupov, V.V. The effect of Habrobracon hebetor venom on the activity of the prophenoloxidase system, the generation of reactive oxygen species and encapsulation in the haemolymph of Galleria mellonella larvae. J. Insect Physiol. 2011, 57, 769–800. [Google Scholar] [CrossRef]
- Hussain, F.; ul Abdin, Z.; Arif, M.J.; Jamil, A. Parasitization and envenomation by the ectoparasitoid, Bracon hebetor affect cellular immune response of Galleria mellonella. Pak. J. Agric. Sci. 2019, 56, 687–692. [Google Scholar]
- Hartzer, K.L.; Zhu, K.Y.; Baker, J.E. Phenoloxidase in larvae of Plodia: Interpunctella (Lepidoptera: Pyralidae) molecular cloning of the proenzyme cDNA and enzyme activity in larvae paralyzed and parasitized by Habrobracon hebetor (Hymenoptera: Braconidae). Arch. Insect Biochem. Physiol. 2005, 59, 67–79. [Google Scholar] [CrossRef]
- Geib, S.M.; Liang, G.H.; Murphy, T.D.; Sim, S.B. Whole genome sequencing of the braconid parasitoid wasp Fopius arisanus, an important biocontrol agent of pest tepritid fruit flies. Genes. Genomes Genet. 2017, 7, 2407–2411. [Google Scholar] [CrossRef][Green Version]
- Tvedte, E.S.; Walden, K.K.O.; McElroy, K.E.; Werren, J.H.; Forbes, A.A.; Hood, G.R.; Logsdon, J.M.; Feder, J.L.; Robertson, H.M. Genome of the parasitoid wasp Diachasma alloeum, an emerging model for ecological speciation and transitions to asexual reproduction. Genome Biol. Evol. 2019, 11, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Arimoto, H.; Kinumi, T.; Oba, Y.; Uemura, D. Identification of proteins from venom of the paralytic spider wasp, Cyphononyx dorsalis. Insect Biochem. Mol. Biol. 2007, 37, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Jin, B.R. Molecular characterization of a venom acid phosphatase Acph-1-like protein from the Asiatic honeybee Apis cerana. J. Asia Pac. Entomol. 2014, 17, 695–700. [Google Scholar] [CrossRef]
- Bull, H.; Murray, P.G.; Thomas, D.; Fraser, A.M.; Nelson, P.N. Acid phosphatases. Mol. Pathol. 2002, 55, 65–72. [Google Scholar] [CrossRef]
- Suzuki, M.; Tanaka, T.J. Development of Meteorus pulchricornis and regulation of its noctuid host, Pseudaletia separata. J. Insect Physiol. 2007, 53, 1072–1078. [Google Scholar] [CrossRef]
- Harvey, J.A.; Sano, T.; Tanaka, T.J. Differential host growth regulation by the solitary endoparasitoid, Meteorus pulchricornis in two hosts of greatly differing mass. J. Insect Physiol. 2010, 56, 1178–1183. [Google Scholar] [CrossRef]
- Suzuki, M.; Miura, K.; Tanaka, T. Effects of the virus-like particles of a braconid endoparasitoid, Meteorus pulchricornis, on haemocytes and hematopoietic organs of its noctuid host, Pseudaletia separata. Appl. Entomol. Zool. 2009, 44, 115–125. [Google Scholar] [CrossRef]
- Magdaraog, P.M.; Tanaka, T.; Harvey, J.A. Wasp-associated factors act in interspecies competition during multiparasitism. Arch. Insect Biochem. Physiol. 2016, 92, 87–107. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, X.X.; Fu, W.J. Passive evasion of encapsulation in Macrocentrus cingulum Brischke (Hymenoptera: Braconidae), a polyembryonic parasitoid of Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae). J. Insect Physiol. 2003, 49, 367–375. [Google Scholar] [CrossRef]
- Lu, J.-F.; Feng, C.-J.; Hu, J.; Fu, W.-J. Extraembryonic membrane of the polyembryonic parasitoid Macrocentrus cingulum Brischke (Hym., Braconidae) is essential for evasion of encapsulation. J. Appl. Entomol. 2007, 131, 472–477. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.-F.; Ke, X.; Fu, W.-J. The SDS-PAGE analysis of venom and ovarian proteins from Macrocentrus cingulum. In Natural Enemies of Insects; Gai Kan Bianjibu: Chongqing, China, 2007; Volume 3, (In Chinese, English Abstract). [Google Scholar]
- Li, Y.; Lu, J.-F.; Feng, C.-J.; Ke, X.; Fu, W.-J. Role of venom and ovarian proteins in immune suppression of Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae parasitized by Macrocentrus cingulum (Hymenoptera: Braconidae), a polyembryonic parasitoid. Insect Sci. 2007, 14, 93–100. [Google Scholar] [CrossRef]
- Feng, C.J.; Huang, J.; Song, Q.; Stanley, D.; Lu, W.; Zhang, Y.; Huang, Y. Parasitization by Macrocentrus cingulum (Hymenoptera: Braconidae) influences expression of prophenoloxidase in asian corn borer Ostrinia furnacalis. Arch. Insect Biochem. Physiol. 2011, 77, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.J.; Qiu, H.G.; Qiu, Z.L.; Fu, W.J. Effects of parasitism by Macrocentrus cingulum Brisehke (Hymenoptera, Braconidae) on the activity of phenoloxidase in larvae of the Asian corn borer, Ostrinia furnacalis Guenee (Lepidoptera, Pyralidae). Acta Entomol. Sin. 2004, 47, 298–304. [Google Scholar]
- Shaw, M.R.; Huddleston, T. Classification and biology of braconid wasps (Hymenoptera: Braconidae). Handbks. Ident. Br. Insects 1991, 7, 1–126. [Google Scholar]
- Lawrence, P.O.; Akin, D. Virus-like particles from the poison glands of the parasitic wasp Biosteres longicaudatus (Hymenoptera: Braconidae). Canad. J. Zool. 1990, 68, 539–546. [Google Scholar] [CrossRef]
- Simmonds, T.J.; Carrillo, D.; Burke, G.R. Characterization of a venom gland-associated rhabdovirus in the parasitoid wasp Diachasmimorpha longicaudata. J. Insect Physiol. 2016, 91, 48–55. [Google Scholar] [CrossRef]
- Luo, L.; Zeng, L. A new rod-shaped virus from parasitic wasp Diachasmimorpha longicaudata (Hymenoptera: Braconidae). J. Invert. Pathol. 2010, 103, 165–169. [Google Scholar] [CrossRef]
- Lawrence, P.O.; Matos, L.F. Transmission of the Diachasmimorpha longicaudata rhabdovirus (DlRhV) to wasp offspring: An ultrastructural analysis. J. Insect Physiol. 2005, 51, 235–241. [Google Scholar] [CrossRef]
- Shaw, M.R. Delayed inhibition of host development by the nonparalysing venoms of parasitic wasps. J. Invert. Path. 1981, 37, 215–221. [Google Scholar] [CrossRef]
- Li, W.-D.; Huang, F.; Chen, Y.-F.; Chen, X.-X. Immunosuppression effects of venom of pupal endoparasitoid wasp, Diadromus collaris (Gravenhorst) on its host, Plutella xylostella pupae. Acta Entomol. Sin. 2006, 49, 206–212. [Google Scholar]
- Li, W.-D.; Shi, M.; Chen, X.-X. Effects of parasitism by Diadromus collaris (Hymenoptera: Ichneumonidae) on morphology and ultrastructure of fat body and adipocytes of host Plutella xylostella (Lepidoptera: Plutellidae) pupae. Acta Ecol. Sin. 2007, 50, 662–666. [Google Scholar]
- Bigot, Y.; Rabouille, A.; Doury, G.; Sizaret, P.Y.; Delbost, F.; Hamelin, M.H.; Periquet, G. Biological and molecular features of the relationships between Diadromus pulchellus ascovirus, a parasitoid hymenopteran wasp (Diadromus pulchellus) and its lepidopteran host, Acrolepiopsis assectella. J. Gen. Virol. 1997, 78, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.H.; Parkinson, N.M. Venom from the endoparasitic wasp Pimpla hypochondriaca adversely affects the morphology, viability and immune function of haemocytes from larvae of the tomato moth, Lacanobia oleracea. J. Invertebr. Pathol. 2000, 76, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Rivers, D.B.; Dani, M.P.; Richards, E.H. The mode of action of venom from the endoparasitic wasp Pimpla hypochondriaca (Hymenoptera: Ichneumonidae) involves Ca+2-dependent cell death pathways. Arch. Insect Biochem. Physiol. 2009, 71, 173–190. [Google Scholar] [CrossRef]
- Parkinson, N.M.; Weaver, R.J. Noxious components of venom from pupa-specific parasitoid Pimpla Hypocondriaca. J. Invertebr. Pathol. 1999, 73, 74–83. [Google Scholar] [CrossRef]
- Dani, M.P.; Richards, E.H. Cloning and expression of the gene for an insect haemocye anti-aggregation protein (VPr3), from the venom of the endoparasitic wasp, Pimpla hypochondriaca. Arch. Insect Biochem. Physiol. 2009, 71, 191–204. [Google Scholar] [CrossRef]
- Dani, M.P.; Richards, E.H. Identification, cloning and expression of a second gene (vpr1) from the venom of the endoparasitic wasp, Pimpla hypochondriaca that displays immunosuppressive activity. J. Insect Physiol. 2010, 56, 195–203. [Google Scholar] [CrossRef]
- Richards, E.H.; Dani, M.P. A recombinant immunosuppressive protein from Pimpla hypochondriaca (rVPr1) increases the susceptibility of Lacanobia oleracea and Mamestra brassicae larvae to Bacillus thuringiensis. J. Invertebr. Pathol. 2010, 104, 51–57. [Google Scholar] [CrossRef]
- Parkinson, N.; Smith, I.; Audsley, N.; Edwards, J. Purification of pimplin, a paralytic heterodimeric polypeptide from venom of the parasitoid wasp Pimpla hypochondriaca, and cloning of the cDNA encoding one of the subunits. Insect Biochem. Mol. Biol. 2002, 32, 1769–1773. [Google Scholar] [CrossRef]
- Dani, M.P.; Richards, E.H.; Isaac, R.E.; Edwards, J.P. Antibacterial and proteolytic activity in venom from the endoparasitic wasp Pimpla hypochondriaca (Hymenoptera: Ichneumonidae). J. Insect Physiol. 2003, 49, 945–954. [Google Scholar] [CrossRef]
- Asgari, S.; Rivers, D.B. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annu. Rev. Entomol. 2011, 56, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, N.M.; Smith, I.; Weaver, R.J.; Edwards, J.P. A new form of arthropod phenoloxidase is abundant in venom of the parasitoid wasp Pimpla hypochondriaca. Insect Biochem. Mol. Biol. 2001, 31, 57–63. [Google Scholar] [CrossRef]
- Dhananjaya, B.L.; D’Souza, C.J.M. The pharmacological role of phosphatases (acid and alkaline phosphomonoesterases) in snake venoms related to release of purines—A multitoxin. Basic Clin. Pharmacol. Toxicol. 2010, 108, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, N.M.; Conyers, C.; Keen, J.; MacNicoll, A.; Smith, I.; Audsley, N.; Weaver, R. Towards a comprehensive view of the primary structure of venom proteins from the parasitoid wasp Pimpla hypochondriaca. Insect Biochem. Mol. Biol. 2004, 34, 565–571. [Google Scholar] [CrossRef]
- Dani, M.P.; Edwards, J.P.; Richards, E.H. Hydrolase activity in the venom of the pupal endoparasitic wasp, Pimpla hypochondriaca. Comp. Biochem. Physiol. B 2005, 141, 373–381. [Google Scholar] [CrossRef]
- Parkinson, N.M.; Conyers, C.; Keen, J.N.; MacNicoll, A.D.; Smith, I.; Weaver, R.J. cDNAs encoding large venom proteins from the parasitoid wasp Pimpla hypocondriaca identified by random sequence analysis. Comp. Biochem. Physiol. C 2003, 134, 513–520. [Google Scholar]
- Parkinson, N.; Conyers, C.; Smith, I. A venom protein from the endoparasitoid wasp Pimpla hypochondriaca is similar to snake venom reprolysin-type metalloproteases. J. Invertebr. Pathol. 2002, 79, 129–131. [Google Scholar] [CrossRef]
- Parkinson, N.; Richards, E.H.; Conyers, C.; Smith, I.; Edwards, J.P. Analysis of venom constituents from the parasitoid wasp Pimpla hypochondriaca and cloning of cDNA encoding a venom protein. Insect Biochem. Mol. Biol. 2002, 32, 729–735. [Google Scholar] [CrossRef]
- Richards, E.H.; Dani, M.P. Biochemical isolation of an insect haemocyte anti-aggregation protein from the venom of the endoparasitic wasp, Pimpla hypochondriaca, and identification of its gene. J. Insect Physiol. 2008, 54, 1041–1049. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2605. [Google Scholar] [CrossRef]
- Messerschmidt, A. Multi-Copper Oxidases; World Scientific: Singapore, 1997. [Google Scholar]
- Dittmer, N.; Suderman, R.; Jiang, H.; Zhu, Y.; Gorman, M.; Kramer, K.; Kanost, M. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 2004, 34, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Muthukrishnan, S.; Beeman, R.; Kanost, M.; Kramer, K. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc. Natl. Acad. Sci. USA 2005, 102, 11337–11342. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.E. Die wirkung der sekrete der weiblichen genitalanhangsdru sen von Pimpla turionellae L. (Hym. Ichneumonidae) auf die hamocyten und die ein kapselungsreaktion von wirtspuppen. Z. Parasitenkd. 1978, 57, 89–100. [Google Scholar] [CrossRef]
- Ergin, E.; Uçkan, F.; Rivers, D.B.; Sak, O. In Vivo and In Vitro activity of venom from the endoparasitic wasp Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Arch. Insect Biochem. Physiol. 2006, 61, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Uçkan, F.; Gülel, A. Endoparazitoit Pimpla turionellae (Hymenoptera: Ichneumonidae) dis įlerinde zehir aparatõnõn yapõsõ ve zehirin bas ļõca kimyasal grubunun tayini. In Proceedings of the X Ulusal Biyoloji Kongresi, Erzurum, Turkey, 18–20 July 1990. [Google Scholar]
- Uçkan, F.; Sinan, S.; Savașçi, S.; Ergin, E. Determination of venom components from the endoparasitoid wasp Pimpla turionellae L. (Hymenoptera; Ichneumonidae). Ann. Entomol. Soc. Am. 2004, 97, 775–780. [Google Scholar]
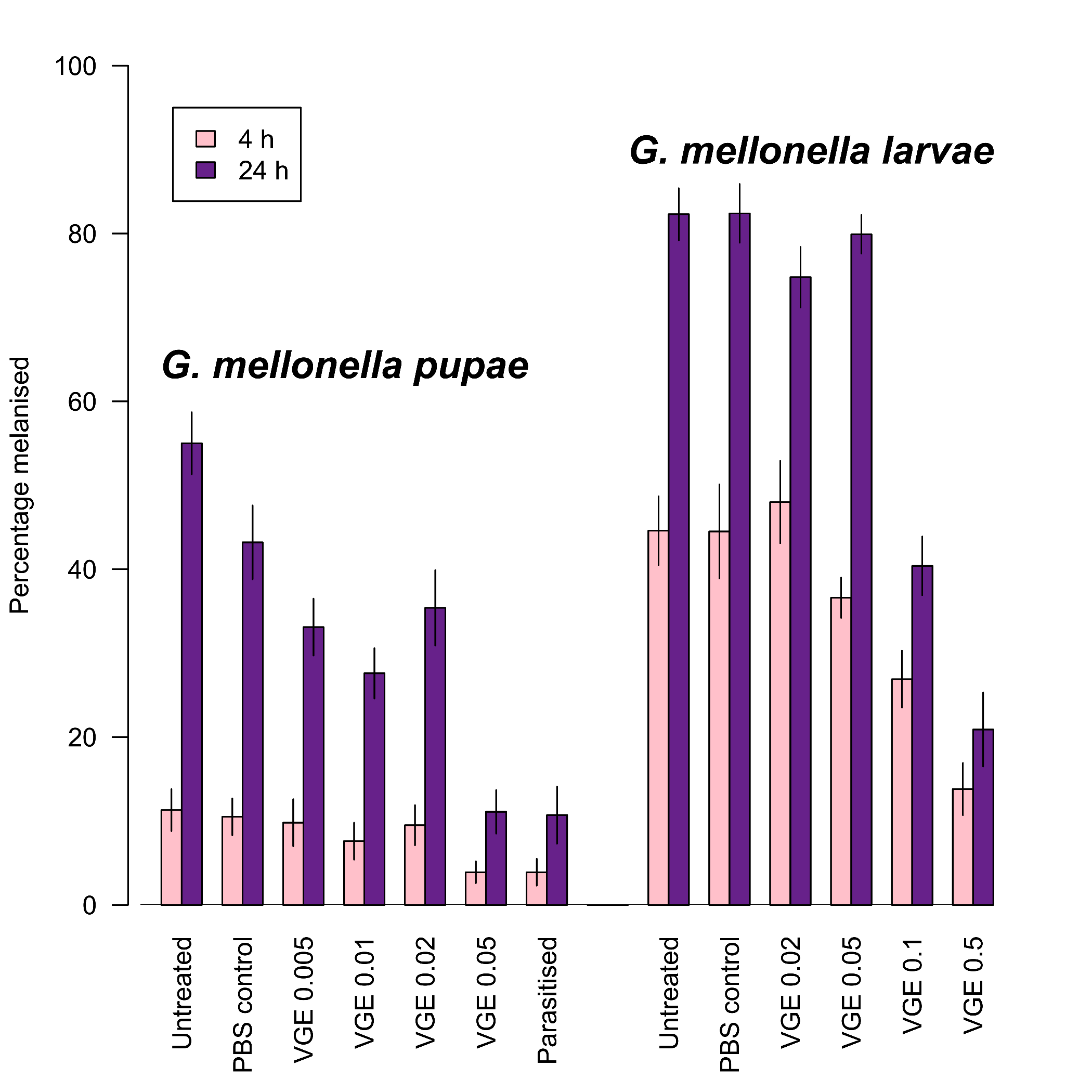
- Uçkan, F.; Er, A.; Ergin, E. Levels of encapsulation and melanization in Galleria mellonella (Lepidoptera: Pyralidae) parasitized and envenomated by Pimpla turionellae (Hymenoptera: Ichneumonidae). J. Appl. Entomol. 2010, 134, 718–726. [Google Scholar] [CrossRef]
- Er, A.; Uçkan, F.; Rivers, D.B.; Ergin, E.; Sak, O. Effects of parasitization and envenomation by the endoparasitic wasp Pimpla turionellae (Hymenoptera: Ichneumonidae) on haemocyte numbers, morphology, and viability of its host Galleria mellonella (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 2010, 103, 273–282. [Google Scholar] [CrossRef]
- Keenan, B.; Uçkan, F.; Ergin, E.; Rivers, D.B. Morphological and biochemical changes in cultured cells induced by venom from the endoparasitoid, Pimpla turionellae. In Recent Advances in the Biochemistry, Toxicity, and Mode of Action of Parasitic Wasp Venoms; Rivers, D., Yoder, J., Eds.; Research Signpost: Kerala, India, 2007; Volume 5, pp. 75–92. [Google Scholar]
- Er, A.; Sak, O.; Ergin, E.; Uçkan, F.; Rivers, D.B. Venom-induced immunosuppression: An overview of haemocyte-mediated responses. Psyche 2011. [Google Scholar] [CrossRef]
- Er, A.; Uçkan, F.; Rivers, D.B.; Sak, O. Cytotoxic effects of parasitism and application of venom from the endoparasitoid Pimpla turionellae on haemocytes of the host Galleria mellonella. J. Appl. Entomol. 2011, 135, 225–236. [Google Scholar] [CrossRef]
- Uçkan, F.; Ergin, E.; Rivers, D.B.; Gençer, N. Age and diet influence the composition of venom from the endoparasitic wasp Pimpla turionellae L. (Hymenoptera: Ichneumonidae). Arch. Insect. Biochem. Physiol. 2006, 63, 177–187. [Google Scholar]
- Banks, B.; Brown, C.; Burgess, G.; Burnstock, G.; Claret, M.; Cocks, T.M.; Jenkinson, D.H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature 1979, 282, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Zhang, G.; Zareie, R.; Schmidt, O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem. Mol. Biol. 2003, 33, 1017–1024. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, Z.Q.; Jiang, H.; Asgari, S. Negative regulation of prophenoloxidase (proPO) activation by a clip-domain serine proteinase homolog (SPH) from endoparasitoid venom. Insect Biochem. Mol. Biol. 2004, 34, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Asgari, S. Inhibition of melanization by a parasitoid serine protease homolog venom protein requires both the clip and the non-catalytic protease-like domains. Insects 2011, 2, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Huang, J.-M.; Zhao, Y.-J.; Xu, Z.-W.; Zhu, J.-Y. Venom serine proteinase homolog of the ectoparasitoid Scleroderma guani impairs host phenoloxidase cascade. Toxicon 2020, 183, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Nénon, J.-P.; Kacem, N.; le Lannic, J.L. Structure, sensory equipment, and secretions of the ovipositor in a giant species of Hymenoptera: Megarhyssa atrata F. (Ichneumonidae, Pimplinae). Can. Entomol. 1997, 129, 789–799. [Google Scholar]
- Whistlecraft, J.W.; Harris, C.R.; Tomlin, A.D.; Tolman, J.H. Mass rearing technique for a braconid parasite, Aphaereta pallipes (Say) (Hymenoptera: Braconidae). J. Econ. Entomol. 1984, 77, 814–816. [Google Scholar] [CrossRef]
- Melton, C.W.; Browning, H.W. Life history and reproductive biology of Allorhogas pyralophagus (Hymenoptera: Braconidae), a parasite imported for release against Eoreuma loftini (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 1986, 79, 402–406. [Google Scholar] [CrossRef]
- Martinez, A.J.; Bard, J.; Holler, T. Mass rearing sugarcane borer and Mexican rice borer for production of parasites Allorhogas pyralophagus and Rhaconotus roslinensis. USDA APHIS 1988, 83, 1. [Google Scholar]
- Auer, J.; Kassel, A. Braconid wasps: A biological control method for the common furniture beetle (Coleoptera: Anobiidae). In Proceedings of the Eighth International Conference on Urban Pests, Zurich, Switzerland, 20–23 July 2014; Müller, G., Pospischil, R., Robinson, W.H., Eds.; OOK-Press Kft.: Veszprém, Hungary, 2014. [Google Scholar]
- Francati, S. Rearing of parasitoid braconid wasp Dinocampus coccinellae in a simplified tritrophic system. Bull. Insectol. 2018, 71, 287–293. [Google Scholar]
- Oatman, E.R.; Platner, G.R.; Greany, P.D. The biology of Orgilus lepidus (Hymenoptera: Braconidae), a primary parasite of the potato tuberworm. Ann. Entomol. Soc. Am. 1969, 62, 1407–1414. [Google Scholar] [CrossRef]
- Greany, P.D.; Oatman, E.R. Analysis of host discrimination in the parasite Orgilus lepidus (Hymenoptera: Braconidae). Ann. Entomol. Soc. Am. 1972, 65, 377–383. [Google Scholar] [CrossRef]
- Johns, C.V.; Whitehouse, M.E.A. Mass rearing of two larval parasitoids of Helicoverpa spp. (Lepidoptera: Noctuidae): Netelia producta (Brullé) and Heteropelma scaposum (Morley) (Hymenoptera: Ichneumonidae) for field release. Austral. J. Entomol. 2004, 43, 83–87. [Google Scholar] [CrossRef]
- Pair, S.D. Biology and rearing of Diapetimorpha introita (Cresson) (Hymenoptera: Ichneumonidae) on host and non-host noctuid pupae. J. Entomol Sci. 1995, 3, 468–480. [Google Scholar] [CrossRef]
- Ferkovich, S.M.; Morales-Ramos, J.A.; Rojas, M.G.; Oberlander, H.; Carpenter, J.E.; Greany, P. Rearing of ectoparasitoid Diapetimorpha introita on an artificial diet: Supplementation with insect cell line-derived factors. BioControl 1999, 44, 29–45. [Google Scholar] [CrossRef]
- Ueno, T. Effects of host size and laboratory rearing on offspring development and sex ratio in the solitary parasitoid Agrothereutes lanceolatus (Hymenoptera: Ichneumonidae). Eur. J. Entomol. 2015, 112, 281–287. [Google Scholar] [CrossRef]
- Puttler, B. Notes on the biology of Hemiteles graculus (Hymenoptera: Ichneumonidae) parasitizing the alfalfa weevil, Hypera postica. Ann. Entomol. Soc. Am. 1963, 56, 857–859. [Google Scholar] [CrossRef]
- Sandanayaka, W.R.M.; Charles, J.G.; Davis, V.A.; Chhagan, A.; Shaw, P.W.; Cole, L.M.; Colhoun, K.; Wallis, D.R. Mass rearing and release of Mastrus ridens (Hym: Ichneumonidae) a parasitoid for the biological control of codling moth Cydia pomonella. N. Z. Entomol. 2018, 41, 37–45. [Google Scholar] [CrossRef]
- Smith, J.W.; Rodriguez-Del-Bosque, L.A.; Agnew, C.W. Biology of Mallochia pyralidis (Hymenoptera: Ichneumonidae), an ectoparasite of Eoreuma loftini (Lepidoptera: Pyralidae) from Mexico. Ann. Entomol. Soc. Am. 1990, 83, 961–966. [Google Scholar] [CrossRef]
- Syed, A. New rearing device for Exeristes roborator (F.) (Hymenoptera: Ichneumonidae). J. Econ. Entomol. 1985, 78, 279–281. [Google Scholar] [CrossRef]


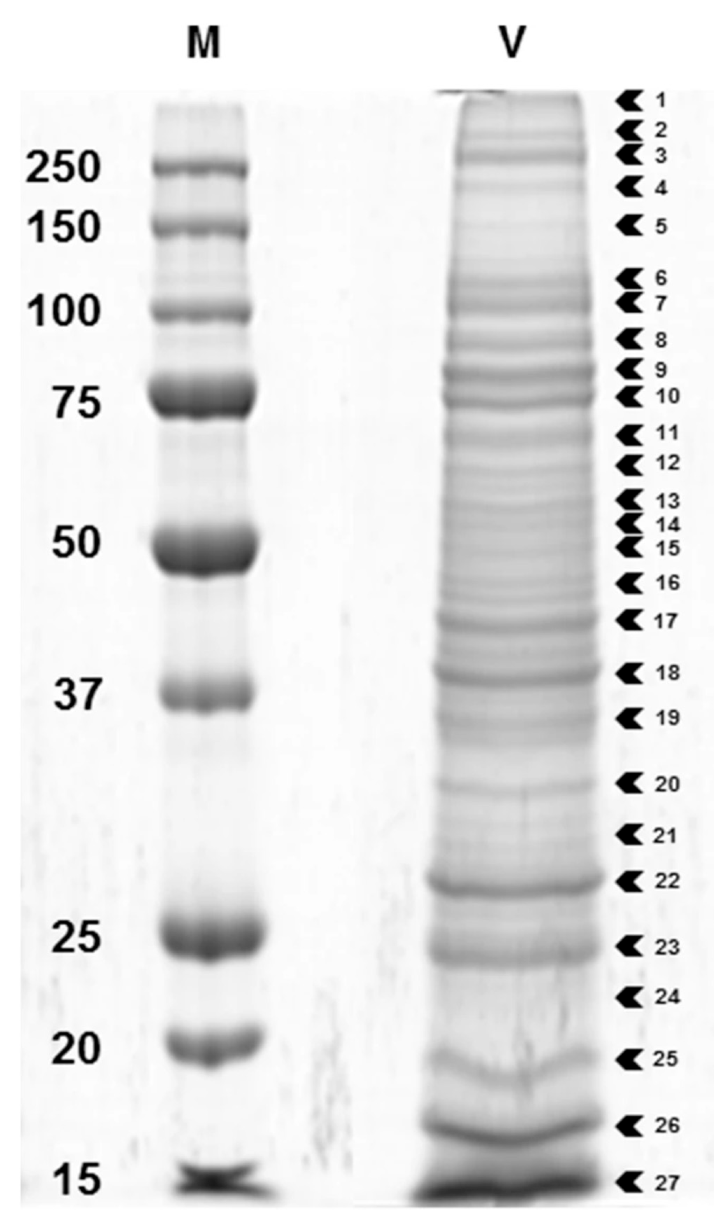
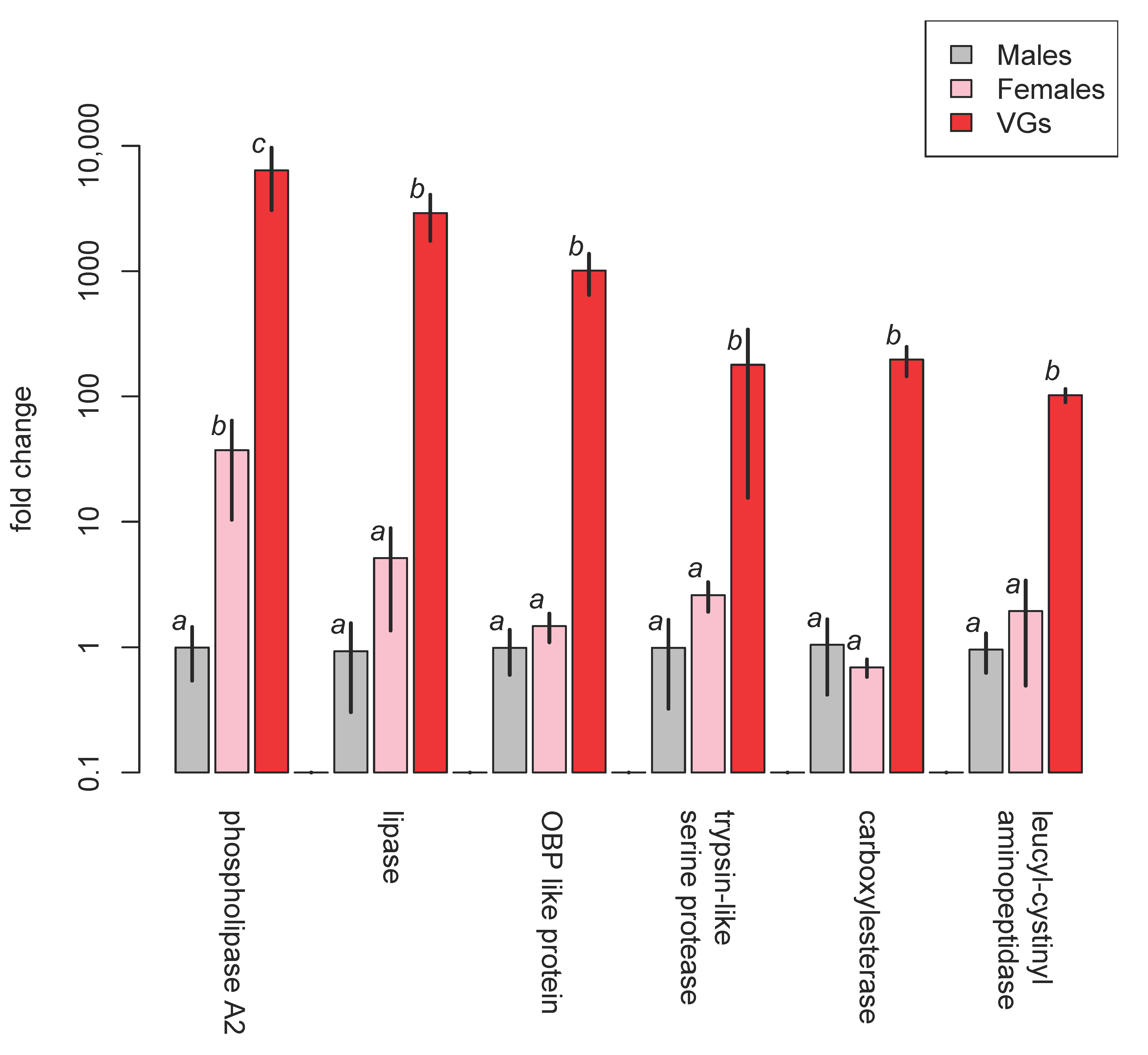

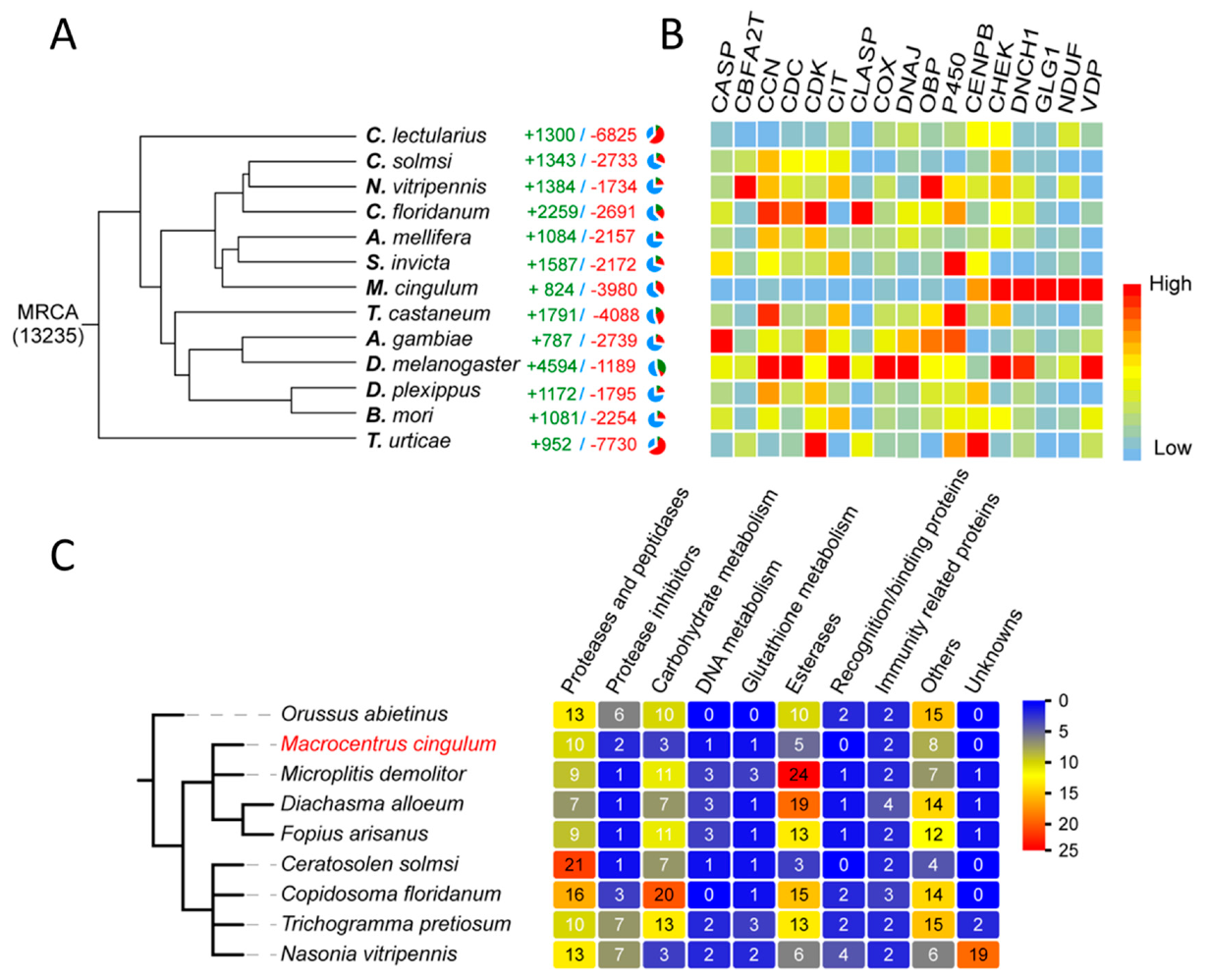
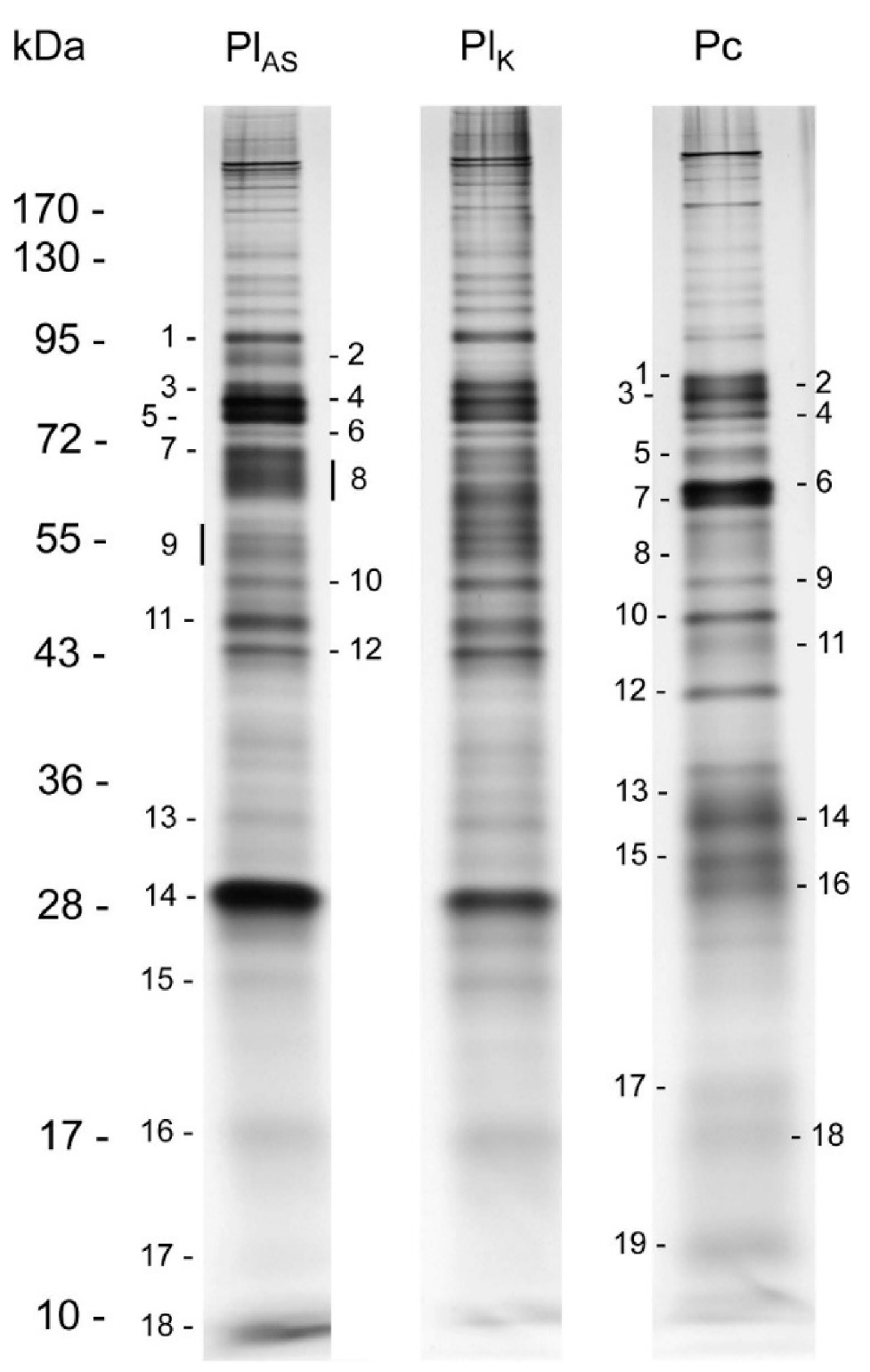

| Family | Subfamily | Species | Method and Comments | References |
|---|---|---|---|---|
| Braconidae | Aphidiinae | Aphidius ervi | proteomics | [48] |
| — | Braconinae | Habrobracon hebetor | de novo sequencing and transcriptome | [49,50] |
| — | — | Habrobracon nigricans | proteo-transcriptomics | [51] |
| Euphorinae | Meteorus puchricornis | VG transcriptomics and RNA interference | [52] | |
| — | — | Microctonus hyperodae & M. aethioiodes | transcriptomics and cloning, and pysosequencing of cDNA | [53] |
| — | Macrocentrinae | Macrocentrus cingulum | genome and whole body transcriptome sequencing | [54] |
| — | Opiinae | Psyttalia concolor & P. lounsburyi | [55] | |
| Ichneumonidae | Ichneumoninae | Diadromus collaris | transcriptomes of VGs and of bodies without VGs | [56] |
| — | Pimplinae | Pimpla turionellae | VG transcriptome | [57] |
| — | Rhyssinae | Megarhyssa greenei & M. macrurus | transcriptome of terminal three metasomal segments; venom proteomics using LC-ESI-MS/MS | [58] |
| Family | Subfamily | Previous (Incorrect) Combinations | Correct Scientific Name |
|---|---|---|---|
| Braconidae | Alysiinae | Phaenocarpa persimilis | Asobara persimilis |
| — | Braconinae | Bracon brevicornis | Habrobracon brevicornis |
| — | — | Bracon hebetor | Habrobracon hebetor |
| — | — | Microbracon gelechiae | Habrobracon gelechiae |
| — | — | Microbracon hebetor | Habrobracon hebetor |
| — | — | Bracon nigricans | Habrobracon nigricans |
| — | Opiinae | Biosteres longicaudatus | Diachasmimorpha longicaudata |
| — | — | Opius concolor | Psyttalia concolor |
| Ichneumonidae | Campopleginae | Nemeritis canescens | Venturia canescens |
| — | — | Coccygomimus turionellae | Pimpla turionellae |
| — | Pimplinae | Pimpla hypochondriaca | Pimpla rufipes |
| Protein | No. of ESTs |
|---|---|
| Elongation factor 2 | 6 |
| Endoplasmin | 19 |
| γ-glutamyl transpeptidase | 539 |
| Leucine rich repeat domain-containing protein | 30 |
| Serine protease homologue | 97 |
| Serpin | 26 |
| Target Insect | Toxin LD50 Per Target Weight (μg/g) | |
|---|---|---|
| Brh-I | Brh-V | |
| Galleria mellonella | 0.0023 | 0.0001 |
| Manduca sexta | 0.05 | 0.04 |
| Spodoptera exigua | 0.033 | 0.051 |
| Heliothis virescens | 0.18 | 0.26 |
| Heliothis zea | 0.045 | 0.085 |
| Trichoplusia ni | 0.019 | 0.0038 |
| Gel Band | Best Identifications | Venom Proteins Found in other Parasitoid Wasps (Braconids Highlighted) * | |
|---|---|---|---|
| SwissProt | InterProScan | ||
| 28 | none | odorant-binding protein | Ac, Ci, Nv, Lh, Pp |
| 26 | phospholipase A2 | phospholipase A2 | Hh, Eo, Pc, Tn |
| 27 | none | prokaryotic membrane lipoprotein lipid attachment site | Hh |
| 11, 12 | venom carboxylesterase-6 (Apis mellifera) | carboxylesterase, type B | Ac, Dc, Hd, Nv |
| 23 | venom allergen 5 (Vespa crabro) | venom allergen 5-like | Ci, Hd, Lh, Mh, Nv, Tb |
| 20 | chymotrypsin-1 (Anopheles gambiae) | serine protease, peptidase, chymotrypsin | Ae, Hh, Ci, Cr, Es, Hd, Nv, Pr, Pp, Tn |
| 27 | none | odorant-binding protein | Ac, Ci, Nv, Lh, Pp |
| 6, 7 | aminopeptidase M1-A | aminopeptidase N-type | Cc, Lb, Pr, Pl |
| 13 | protein disulfide-isomerase | disulfide isomerase—PDI | Ae, Cc, De, Pc, Pl, Pp |
| 13 | platelet glycoprotein V | leucine-rich repeat | Ae, Pc, Pl |
| 16 | lipase 3 | lipase | Ci, Lb, Ma, Nv, Ot, Pr, Pp |
| 5 | lysosomal alpha-mannosidase | alpha mannosidase | De |
| Putative Function | Homology (Top Hits) | Estimated No. in Cluster | |
|---|---|---|---|
| BLAST2GO | BLAST | ||
| cell adhesion/fusion | none | laminin | 10 |
| — | CD63 antigen | 3 | |
| apoptosis induction/cell disruption | proteasome assembly chaperone 2 | 1 | |
| — | none | 1 | |
| cell motility | none | rabaptin | 3 |
| — | cytoplasmic fmr1-interacting protein | 1 | |
| — | Ras-like GTP-binding protein rho1 isoform x2 | 1 | |
| immune suppression | none | macroglobulin/complement-like protein | 25 |
| — | none | serine protease inhibitor | 1 |
| — | none | serine protease inhibitor | 1 |
| functional venom protein/enzyme | none | haemolysin-like | 16 |
| — | none | chitinase | 5 |
| — | hyaluronidase | none | 4 |
| — | bvpp41b protein | none | 1 |
| not determined | none | matrilin | 22 |
| — | none | none | 21 |
| — | none | methyl-accepting chemotaxis protein | 9 |
| — | none | iron transporter | 8 |
| — | none | ferrodoxin | 7 |
| — | none | none | 5 |
| M. aethiopoides Contig Sequence Reads | Putative Function |
|---|---|
| 1651 | Lipase based on automated gene annotation |
| 940 | Low density lipoprotein receptor involved in transmembrane lipid transport |
| 614 | Uncertain: could be an autophosphorylating protein tyrosine kinase |
| 611 | Neutral endopeptidase |
| 416 | Histidine acid phosphatase based on automated gene annotation |
| 296 | Protein involved in cell adhesion and motility |
| 253 | Uncertain: some resemblance to snake toxins; allergenic to humans |
| 213 | Uncertain: highly glycosilated protein; allergenic to humans |
| 183 | Intracellular Ca2+-binding protein |
| 155 | Fe+-binding protein |
| 96 | Resembles a gene involved in suppression of apoptosis |
| 94 | Lysosomal thiol reductase |
| 93 | Lysosome associated protease |
| 91 | Chitinase |
| RPKM (Rank) | Putative Function | Proteins Found in other Parasitoid Wasp Venoms (Braconids Highlighted) * | |
|---|---|---|---|
| P. lounsburyi | P. concolor | ||
| 574.51 (9) | 3110.46 (1) | DUF4803 domain-containing protein | Ci, Hn, Md, Mh |
| 1963.84 (1) | 1583.31 (3) | — | |
| 1865.04 (2) | — | Leucine-rich repeat protein | Ae, Hn, Mg |
| 738.87 (5) | 441.51 (12) | DUF4803 domain-containing protein | Ci, Hn, Md, Mh |
| 691.22 (6) | 947.92 (10) | DUF4803 domain-containing protein | Ci, Hn, Md, Mh |
| 627.83 (8) | 1178.46 (8) | Neprilysin-like metalloprotease | Ae, Mh |
| 476.04 (10) | 441.52 (12) | DUF4803 domain-containing protein | Ci, Hn, Md, Mh |
| 272.37 (11) | 1196.52 (7) | GH1 β-glucosidase | Ae, Md |
| 263.42 (12) | 236.32 (17) | Calreticulin | Hh, Mh |
| 242.5 (13) | 1507.73 (4) | — | |
| 186.32 (14) | — | Reprolysin-like metalloprotease | Ci, Md |
| 143.39 (15) | — | ||
| 135.9 (16) | — | Esterase/lipase-like | Ci, Hn, Md |
| 115.21 (17) | 1178.46 (8) | Neprilysin-like metalloprotease | Ae, Mh |
| 112.79 (18) | 259.63 (15) | Protein disulfide isomerase | Ae, Cc, De, Hn, Mg, Mm, Pp |
| 85.45 (19) | 227.98 (18) | Heat shock protein 70 | Ae |
| 63.77 (20) | — | Protein disulfide isomerase | Ae, Cc, De, Hn, Mg, Mm, Pp |
| 40.85 (21) | — | Endoplasmin | Ae |
| 35.95 (22) | — | DUF4803 domain-containing protein | Ci, Md, Mh |
| 22.74 (23) | 199.42 (19) | Protein disulfide isomerase | Ae, Cc, De, Hn, Mg, Mm, Pp |
| 21.08 (24) | — | Puromycin-sensitive aminopeptidase | |
| 17.02 (25) | 53.51 (22) | Enolase | |
| 6.13 (26) | — | Arginine kinase-like protein | Hh |
| 2.43 (28) | — | Esterase/lipase-like | |
| 2.28 (29) | 32.85 (25) | Serpin | Ae, Md |
| 202 (30) | — | Leucine rich repeat protein | Ae, Hn, Mg |
| 1.47 (31) | — | Neprilysin-like | Ae, Mh |
| 1.31 (32) | — | Glycogen phosphorylase | |
| — | 1326.3 (6) | Reprolysin-like metalloprotease | Pr |
| — | 580.77 (11) | Phospholipase A2 | Eo, Hh, Hn, Md, Mg, Tn |
| — | 1152.41 (9) | DUF4803 domain-containing protein | Ci, Hn, Md, Mh |
| — | 360.78 (13) | Annexin | |
| — | 346.13 (14) | Serine carboxypeptidase | Md |
| — | 102.94 (20) | Leucine-rich repeat protein | Ae, Hn |
| — | 60.85 (21) | Protein disulfide isomerase | Ae, Cc, De, Hn, Mg, Mm, Pp |
| — | 39.64 (23) | Leucine-rich repeat protein | Ae, Hn, Mg |
| — | 36.88 (24) | Ezrin/radixin/moesin family | |
| — | 29.37 (26) | Neprilysin-like metalloprotease | Ae, Mh |
| — | 12.69 (27) | Aldehyde dehydrogenase | |
| — | 5.03 (28) | Leucine-rich repeat protein | Ae, Hn, Mg |
| — | 4.49 (29) | Leucine-rich repeat protein | Ae, Hn, Mg |
| — | 2.31 (30) | Adenosylhomocysteinase | |
| Class | Protein Name | Putative Function | Comments | References |
|---|---|---|---|---|
| Neurotoxins | Pimplin | major paralytic factor in venom | no similarity to other known proteins | [227] |
| — | Cys-rich venom protein 3 | possible minor neurotoxin | atracotoxin-like | [232] |
| — | Cys-rich venom protein 5 | possible minor neurotoxin | conotoxin-like | [232] |
| Protease inhibitors | Cys-rich venom protein 1 | — | protease inhibitor | [232] |
| — | Cys-rich venom protein 2 | — | Kunitz type protease inhibitor | [232] |
| — | Cys-rich venom protein 4 | — | pacifastin; protease inhibitor | [232] |
| — | Cys-rich venom protein 6 | — | protease inhibitor | [232] |
| Other enzymes | Acid phosphatase | — | possibly related to purine release | [233] |
| — | Laccase, lac1, Phlac | oxidation | — | [234] |
| — | tre1 | — | similar to trehalase | [234] |
| — | Phenoloxidase I | putative haemocyte disruption | from VG cDNA library | [230] |
| — | Phenoloxidase II | putative haemocyte disruption | from VG cDNA library | [230] |
| — | Phenoloxidase III | putative haemocyte disruption | from VG cDNA library | [230] |
| — | Metalloprotease | — | similar to snake venom reprolysin-type metalloproteases | [235] |
| — | Serine protease | — | — | [236] |
| Haemocyte anti aggregation proteins | VPr1 | probably reduces host encapsulation ability | haemocyte inactivation | [224,225,226,234] |
| — | VPr3 | hydrolysis | Antihaemocyte aggregation | [224,237] |
| others | [228] |
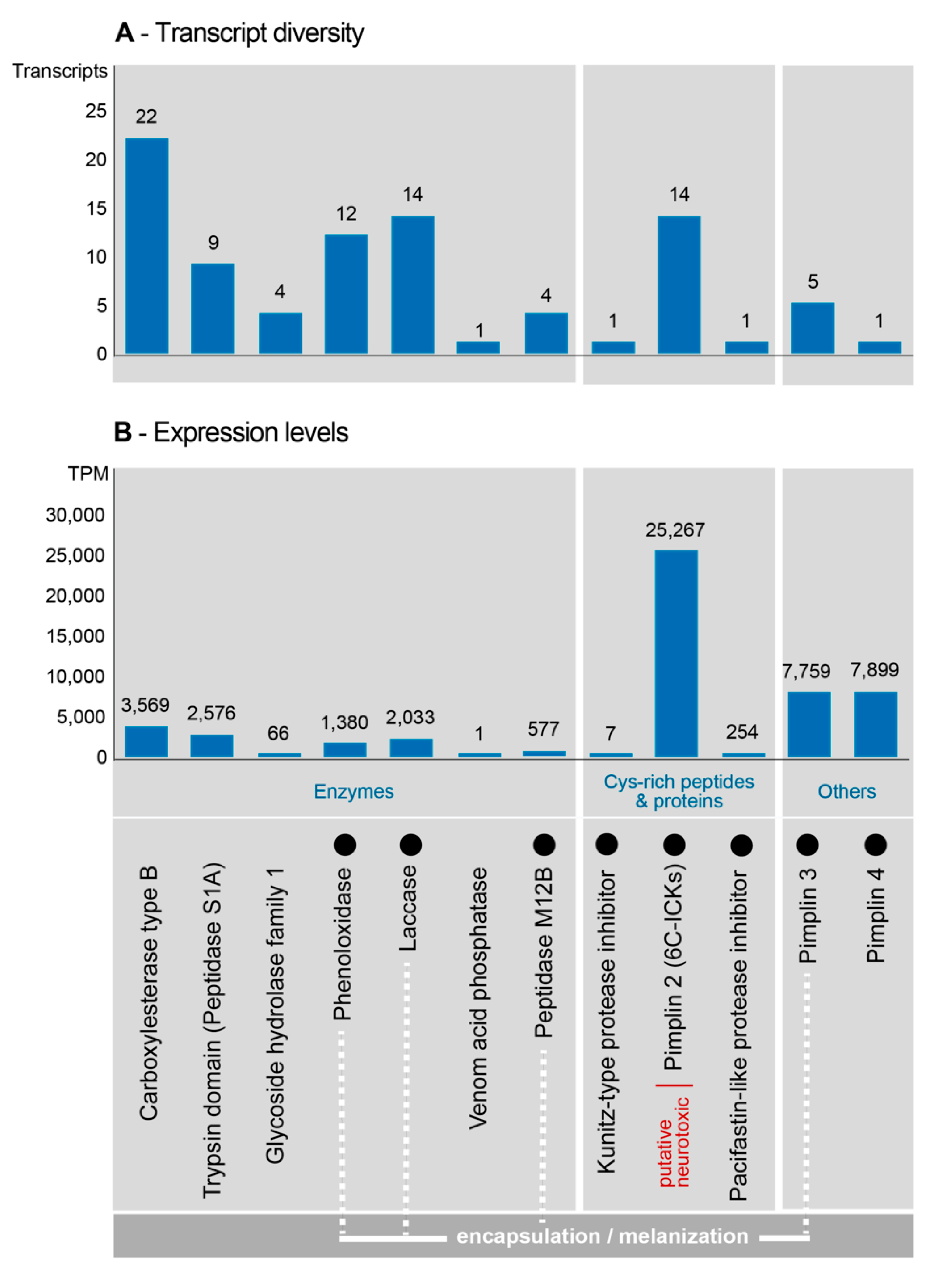
| Protein | Transcripts Per Million | Amino Acid Length | Scaffold | Putative Function/Comments |
|---|---|---|---|---|
| pimplin2 | 25,267 | 64–115 | X-CX7 -[C-X6 -C-X5–8-CC-X2–4 -C-X6–9]-X | possibly similar neurotoxic asilid1 sequences from robber flies though the Cys-scaffold is different to a typical ICK one |
| pimplin3 | 7759 | 167–315 | potential P and C scaffold | same family as venom protein1, Vpr1 from P. rufipes |
| pimplin4 | 7899 | 70–78 | no cysteine scaffold, 3 P residues | same family as small venom protein2, svp2 from P. rufipes |
| Family | Subfamily | Species | Host(s) | Notes | Reference |
|---|---|---|---|---|---|
| Braconidae | Agathidinae | Alabagrus stigma | Diatraea saccharalis | was cultured at Texas A&M in the 1980s by James Smith’s group | Nothing appears to have been published |
| — | Alysiinae | Aphaereta pallipes | onion maggot, Delia antiqua | — | [258] |
| — | Doryctinae | Allorhogas pyralophagus | Eoreuma loftini | gregarious idiobiont ectoparasitoid | [259,260] |
| — | — | Heterospilus prosopidis | various bruchid beetle larvae feeding within various beans | easily cultured, cyclostome idiobiont ectoparasitoid | |
| — | — | Spathius exarator | common furniture beetle, Anobium punctatum | idiobiont ectoparasitoid | [261] |
| — | Euphorinae | Dinocamptus coccinellae | many species of aphidophagous ladybird beetles (Coccinellidae) | cosmopolitan endoparasitoid | [262] |
| — | Orgilinae | Orgilus lepidus | potato tuber moth. Phthorimaea operculella | koinobiont larval endoparasitoid; sometimes sold commercially | [263,264] |
| Ichneumonidae | Anomaloninae | Heteropelma scaposum | Helicoverpa spp. (Lepidoptera: Noctuidae) | solitary koinobiont endoparasitoid | [265] |
| — | Cremastinae | Pristomerus vulnerator | codling moth, Cydia pomonella (Lepidoptera: Tortricidae) | koinobiont larval endoparasitoid | |
| — | Cryptinae | Diapetimorpha introita | Spodoptera frugiperda (Lepidoptera: Noctuidae) | pupal ectoparasitoid; can be reared on artificiual diet | [266,267] |
| — | — | Agrothereutes lanceolatus | various tortricids and pyralids, including Chilo suppressalis, Glyphodes pyloalis and Homona magnanima | solitary idiobiont ectoparasitoid of prepupal (and early pupal) host | [268] |
| — | — | Hemiteles graculus | alfalfa weevil, Hypera positica | solitary idiobiont ectoparasitoid of prepupal and pupal host | [269] |
| — | — | Mastrus ridens | codling moth, Cydia pomonella | gregarious idiobiont ectoparasitoid of cocooned mature larval host | [270] |
| — | — | Mallochia pyralidis | Eoreuma loftini (Pyralidae) | idiobiont ectoparasitoid | [271] |
| — | Pimplinae | Itoplectis naranyae | include Galleria mellonella, Chilo suppressalis | pupal endoparasitoid | |
| — | — | Exeristes roborator | include Galleria mellonella, Pectinophora gossypiella, Phthorimaea operculella, Larinus sturnus | highly polyphagous endoparasitoid | [272] |
| — | Tryphoninae | Netelia producta | Helicoverpa spp. (Lepidoptera: Noctuidae) | koinobiont larval ectoparasitoid | [265] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quicke, D.L.J.; Butcher, B.A. Review of Venoms of Non-Polydnavirus Carrying Ichneumonoid Wasps. Biology 2021, 10, 50. https://doi.org/10.3390/biology10010050
Quicke DLJ, Butcher BA. Review of Venoms of Non-Polydnavirus Carrying Ichneumonoid Wasps. Biology. 2021; 10(1):50. https://doi.org/10.3390/biology10010050
Chicago/Turabian StyleQuicke, Donald L. J., and Buntika A. Butcher. 2021. "Review of Venoms of Non-Polydnavirus Carrying Ichneumonoid Wasps" Biology 10, no. 1: 50. https://doi.org/10.3390/biology10010050
APA StyleQuicke, D. L. J., & Butcher, B. A. (2021). Review of Venoms of Non-Polydnavirus Carrying Ichneumonoid Wasps. Biology, 10(1), 50. https://doi.org/10.3390/biology10010050





