Sub-Fractions of Red Blood Cells Respond Differently to Shear Exposure Following Superoxide Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Selection of Participants and Blood Sample Collection
2.2. Experimental Protocol
2.2.1. Overview of Experiment One
2.2.2. Overview of Experiment Two
2.3. Oxidative Status
2.4. RBC Deformability
2.5. Immunohistochemical Staining
2.6. S-Nitrosylation Assay
2.7. Statistical Analysis
3. Results
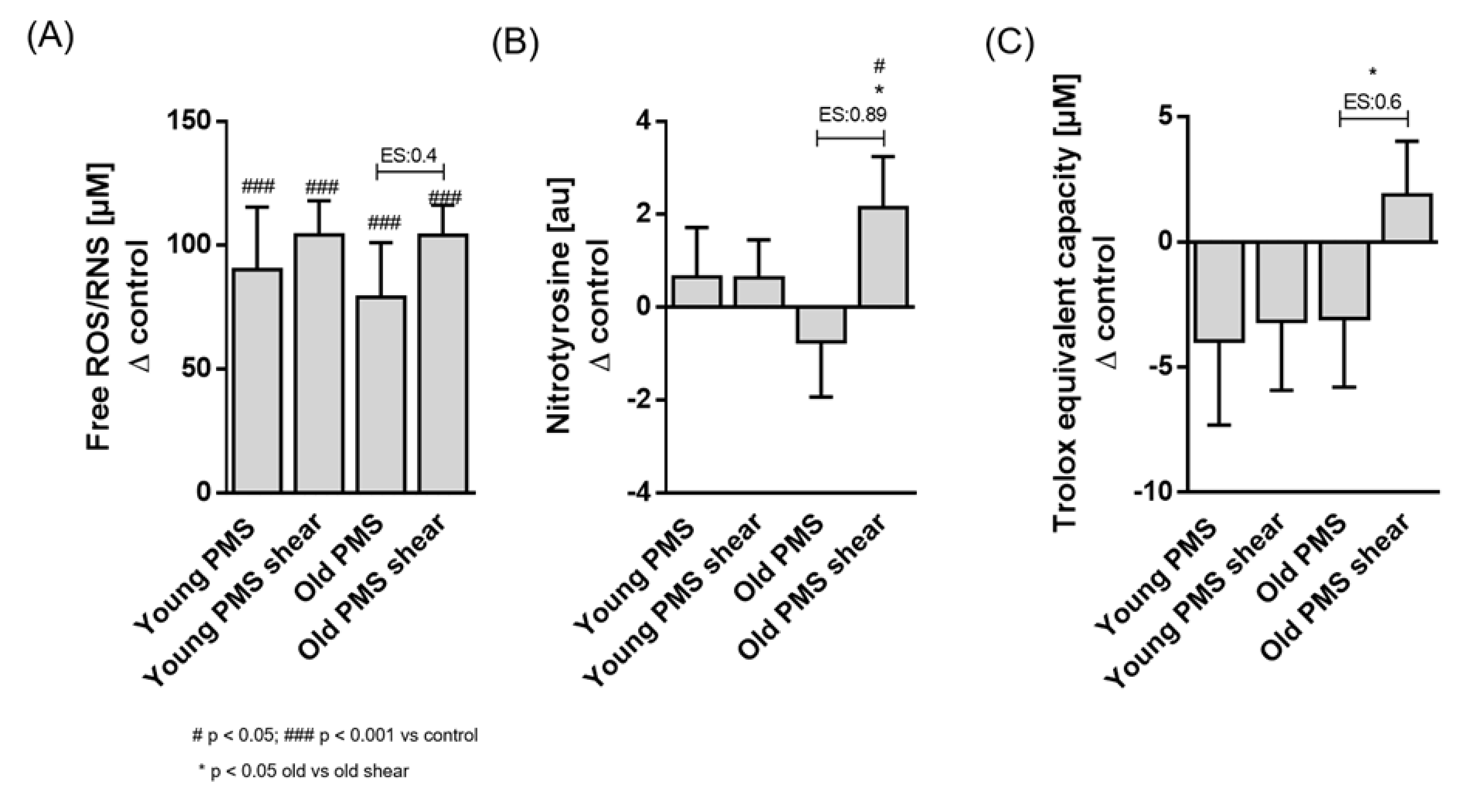
3.1. Oxidative Status
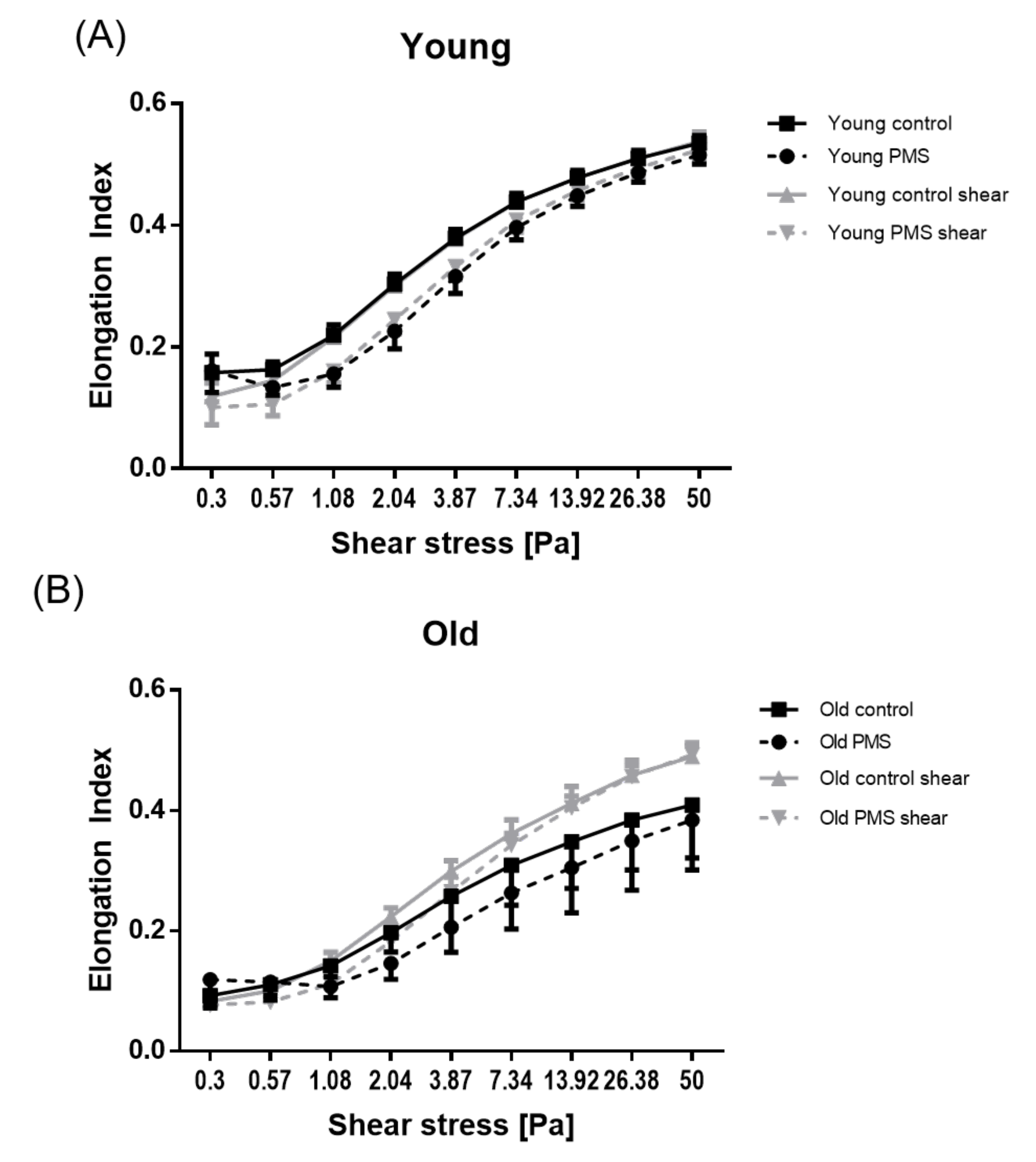
3.2. RBC Deformability
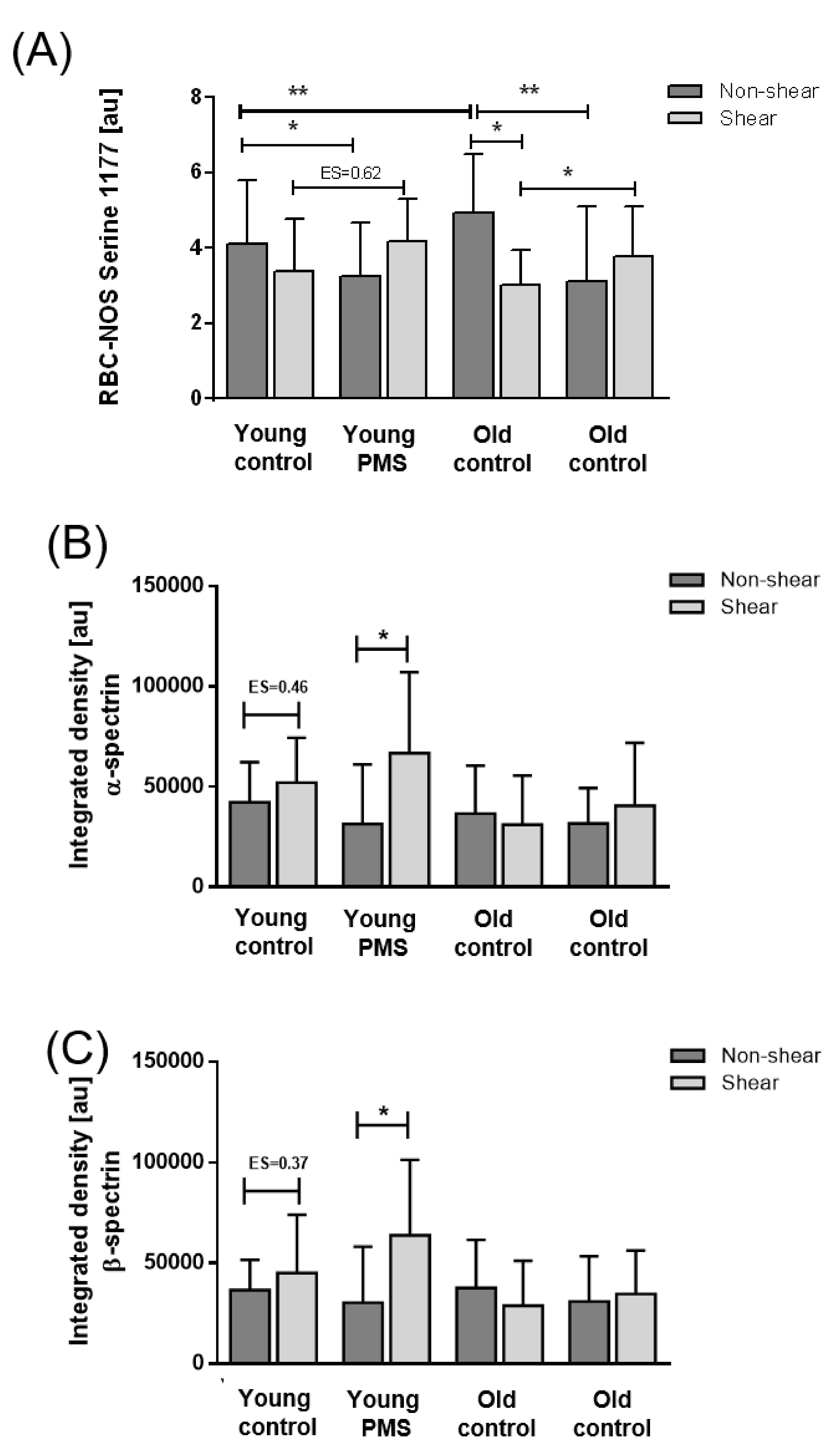
3.3. RBC-NOS Activation
3.4. S-Nitrosylation of Cytoskeletal Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohandas, N.; Chasis, J.A. Red blood cell deformability, membrane material properties and shape: Regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin. Hematol. 1993, 30, 171–192. [Google Scholar] [PubMed]
- Simmonds, M.J.; Meiselman, H.J.; Marshall-Gradisnik, S.M.; Pyne, M.; Kakanis, M.; Keane, J.; Brenu, E.; Christy, R.; Baskurt, O.K. Assessment of oxidant susceptibility of red blood cells in various species based on cell deformability. Biorheology 2011, 48, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Xiong, Y.-L.; Xiong, Y.-L.; Li, Y.-J.; Tang, F.-Z.; Wang, R.-F.; Zhao, Y.-J.; Wang, X. Effects of exhaustive exercise-induced oxidative stress on red blood cell deformability. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2014, 30, 289–293. [Google Scholar] [PubMed]
- McNamee, A.P.; Horobin, J.T.; Tansley, G.D.; Simmonds, M.J. Oxidative Stress Increases Erythrocyte Sensitivity to Shear-Mediated Damage. Artif. Organs 2018, 42, 184–192. [Google Scholar] [CrossRef]
- Koutsiaris, A.G.; Tachmitzi, S.V.; Batis, N. Wall shear stress quantification in the human conjunctival pre-capillary arterioles in vivo. Microvasc. Res. 2013, 85, 34–39. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Stefanidis, C. Vascular wall shear stress: Basic principles and methods. Hell. J. Cardiol. 2005, 46, 9–15. [Google Scholar]
- Smith, J.A. Exercise, training and red blood cell turnover. Sports Med. 1995, 19, 9–31. [Google Scholar] [CrossRef]
- Li, J.-H.; Luo, J.-F.; Jiang, Y.; Ma, Y.-J.; Ji, Y.-Q.; Zhu, G.-L.; Zhou, C.; Chu, H.-W.; Zhang, H.-D. Red Blood Cell Lifespan Shortening in Patients with Early-Stage Chronic Kidney Disease. Kidney Blood Press. Res. 2019, 44, 1158–1165. [Google Scholar] [CrossRef]
- Kuck, L.; Grau, M.; Bloch, W.; Simmonds, M.J. Shear Stress Ameliorates Superoxide Impairment to Erythrocyte Deformability With Concurrent Nitric Oxide Synthase Activation. Front. Physiol. 2019, 10, 36. [Google Scholar] [CrossRef]
- Suhr, F.; Brenig, J.; Müller, R.; Behrens, H.; Bloch, W.; Grau, M. Moderate exercise promotes human RBC-NOS activity, NO production and deformability through Akt kinase pathway. PLoS ONE 2012, 7, e45982. [Google Scholar] [CrossRef] [PubMed]
- Meram, E.; Yilmaz, B.D.; Bas, C.; Atac, N.; Yalcin, O.; Meiselman, H.J.; Baskurt, O.K. Shear stress-induced improvement of red blood cell deformability. Biorheology 2013, 50, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Monedero, D.; Robert, M.; Skinner, S.; Stauffer, E.; Cibiel, A.; Germain, M.; Hugonnet, J.; Scheer, A.; Joly, P.; et al. Impact of a 10 km running trial on eryptosis, red blood cell rheology, and electrophysiology in endurance trained athletes: A pilot study. Eur. J. Appl. Physiol. 2020, 120, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Koliamitra, C.; Holtkamp, B.; Zimmer, P.; Bloch, W.; Grau, M. Impact of training volume and intensity on RBC-NOS/NO pathway and endurance capacity. Biorheology 2017, 54, 37–50. [Google Scholar] [CrossRef]
- Horobin, J.T.; Sabapathy, S.; Simmonds, M.J. Red blood cell tolerance to shear stress above and below the subhemolytic threshold. Biomech. Model. Mechanobiol. 2020, 19, 851–860. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Detterich, J.A.; Connes, P. Nitric oxide, vasodilation and the red blood cell. Biorheology 2014, 51, 121–134. [Google Scholar] [CrossRef]
- Bor-Kucukatay, M.; Wenby, R.B.; Meiselman, H.J.; Baskurt, O.K. Effects of nitric oxide on red blood cell deformability. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1577–H1584. [Google Scholar] [CrossRef]
- Jubelin, B.C.; Gierman, J.L. Erythrocytes may synthesize their own nitric oxide. Am. J. Hypertens. 1996, 9, 1214–1219. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Ozüyaman, B.; et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef]
- Horobin, J.T.; Watanabe, N.; Hakozaki, M.; Sabapathy, S.; Simmonds, M.J. Shear-stress mediated nitric oxide production within red blood cells: A dose-response. Clin. Hemorheol. Microcirc. 2019, 71, 203–214. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.W.; McMahon, T.J.; Stamler, J.S. S-nitrosylation in health and disease. Trends Mol. Med. 2003, 9, 160–168. [Google Scholar] [CrossRef]
- Grau, M.; Pauly, S.; Ali, J.; Walpurgis, K.; Thevis, M.; Bloch, W.; Suhr, F. RBC-NOS-dependent S-nitrosylation of cytoskeletal proteins improves RBC deformability. PLoS ONE 2013, 8, e56759. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.R. Nitric oxide: A brief overview of chemical and physical properties relevant to therapeutic applications. Future Sci. OA 2015, 1, FSO59. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Brinkmann, C.; Bloch, W.; Grau, M. Increase in Red Blood Cell-Nitric Oxide Synthase Dependent Nitric Oxide Production during Red Blood Cell Aging in Health and Disease: A Study on Age Dependent Changes of Rheologic and Enzymatic Properties in Red Blood Cells. PLoS ONE 2015, 10, e0125206. [Google Scholar] [CrossRef]
- McNamee, A.P.; Richardson, K.; Horobin, J.; Kuck, L.; Simmonds, M.J. Susceptibility of density-fractionated erythrocytes to subhaemolytic mechanical shear stress. Int. J. Artif. Organs 2019, 42, 151–157. [Google Scholar] [CrossRef]
- Maurya, P.K.; Kumar, P.; Chandra, P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J. Methodol. 2015, 5, 216–222. [Google Scholar] [CrossRef]
- Grau, M.; Bölck, B.; Bizjak, D.A.; Stabenow, C.J.A.; Bloch, W. The red-vine-leaf extract AS195 increases nitric oxide synthase-dependent nitric oxide generation and decreases oxidative stress in endothelial and red blood cells. Pharmacol. Res. Perspect. 2016, 4, e00213. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Hardeman, M.R.; Dobbe, J.G.; Ince, C. The Laser-assisted Optical Rotational Cell Analyzer (LORCA) as red blood cell aggregometer. Clin. Hemorheol. Microcirc. 2001, 25, 1–11. [Google Scholar]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of red blood cell elongation index--shear stress curves obtained by ektacytometry. Scand. J. Clin. Lab. Invest. 2009, 69, 777–788. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Pushkaran, S.; Konstantinidis, D.G.; Koochaki, S.; Malik, P.; Mohandas, N.; Zheng, Y.; Joiner, C.H.; Kalfa, T.A. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood 2013, 121, 2099–2107. [Google Scholar] [CrossRef]
- Arias, C.F.; Arias, C.F. How do red blood cells know when to die? R. Soc. Open Sci. 2017, 4, 160850. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Pieniazek, A.; Tabaczar, S.; Jegier, A.; Brzeszczynska, J. Investigation of oxidative stress parameters in different lifespan erythrocyte fractions in young untrained men after acute exercise. Exp. Physiol. 2017, 102, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.J.; Wung, B.S.; Shyy, J.Y.; Hsieh, H.J.; Wang, D.L. Reactive oxygen species are involved in shear stress-induced intercellular adhesion molecule-1 expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA. 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Linderkamp, O.; Wu, P.Y.; Meiselman, H.J. Deformability of density separated red blood cells in normal newborn infants and adults. Pediatr. Res. 1982, 16, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Bratosin, D.; Mazurier, J.; Tissier, J.P.; Estaquier, J.; Huart, J.J.; Ameisen, J.C.; Aminoff, D.; Montreuil, J. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie 1998, 80, 173–195. [Google Scholar] [CrossRef]
- Burger, P.; de Korte, D.; van den Berg, T.K.; van Bruggen, R. CD47 in Erythrocyte Ageing and Clearance—The Dutch Point of View. Transfus. Med. Hemotherapy 2012, 39, 348–352. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Nagababu, E. Hemoglobin redox reactions and red blood cell aging. Antioxidants Redox Signal. 2013, 18, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Lang, E.; Föller, M. Physiology and pathophysiology of eryptosis. Transfus. Med. Hemotherapy. 2012, 39, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Antonelou, M.H.; Kriebardis, A.G.; Stamoulis, K.E.; Economou-Petersen, E.; Margaritis, L.H.; Papassideri, I.S. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion 2010, 50, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, Y.; Topaz, O.; Cohen, L.A.; Yakov, L.D.; Haber, T.; Morgenstern, A.; Weiss, A.; Chait Berman, K.; Fibach, E.; Meyron-Holtz, E.G. Physiologically aged red blood cells undergo erythrophagocytosis in vivo but not in vitro. Haematologica 2012, 97, 994–1002. [Google Scholar] [CrossRef]
- Tomschi, F.; Bizjak, D.; Bloch, W.; Latsch, J.; Predel, H.G.; Grau, M. Deformability of different red blood cell populations and viscosity of differently trained young men in response to intensive and moderate running. Clin. Hemorheol. Microcirc. 2018, 69, 503–514. [Google Scholar] [CrossRef]
- Kuck, L.; Grau, M.; Simmonds, M.J. Recovery time course of erythrocyte deformability following exposure to shear is dependent upon conditioning shear stress. Biorheology 2018, 54, 141–152. [Google Scholar] [CrossRef]
- Suhr, F.; Porten, S.; Hertrich, T.; Brixius, K.; Schmidt, A.; Platen, P.; Bloch, W. Intensive exercise induces changes of endothelial nitric oxide synthase pattern in human erythrocytes. Nitric Oxide 2009, 20, 95–103. [Google Scholar] [CrossRef]
- Ulker, P.; Sati, L.; Celik-Ozenci, C.; Meiselman, H.J.; Baskurt, O.K. Mechanical stimulation of nitric oxide synthesizing mechanisms in erythrocytes. Biorheology 2009, 46, 121–132. [Google Scholar] [CrossRef]
- Ulker, P.; Meiselman, H.J.; Baskurt, O.K. Nitric oxide generation in red blood cells induced by mechanical stress. Clin. Hemorheol. Microcirc. 2010, 45, 169–175. [Google Scholar] [CrossRef]
- Grau, M.; Jerke, M.; Nader, E.; Schenk, A.; Renoux, C.; Collins, B.; Dietz, T.; Bizjak, D.A.; Joly, P.; Bloch, W.; et al. Effect of acute exercise on RBC deformability and RBC nitric oxide synthase signalling pathway in young sickle cell anaemia patients. Sci. Rep. 2019, 9, 11813. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau, M.; Kuck, L.; Dietz, T.; Bloch, W.; Simmonds, M.J. Sub-Fractions of Red Blood Cells Respond Differently to Shear Exposure Following Superoxide Treatment. Biology 2021, 10, 47. https://doi.org/10.3390/biology10010047
Grau M, Kuck L, Dietz T, Bloch W, Simmonds MJ. Sub-Fractions of Red Blood Cells Respond Differently to Shear Exposure Following Superoxide Treatment. Biology. 2021; 10(1):47. https://doi.org/10.3390/biology10010047
Chicago/Turabian StyleGrau, Marijke, Lennart Kuck, Thomas Dietz, Wilhelm Bloch, and Michael J. Simmonds. 2021. "Sub-Fractions of Red Blood Cells Respond Differently to Shear Exposure Following Superoxide Treatment" Biology 10, no. 1: 47. https://doi.org/10.3390/biology10010047
APA StyleGrau, M., Kuck, L., Dietz, T., Bloch, W., & Simmonds, M. J. (2021). Sub-Fractions of Red Blood Cells Respond Differently to Shear Exposure Following Superoxide Treatment. Biology, 10(1), 47. https://doi.org/10.3390/biology10010047




