Can Biomarkers Respond Upon Freshwater Pollution?—A Moss-Bag Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Collection and Moss-Bags
2.2. Nucleic Acid Extraction
2.3. PCR and RT-qPCR
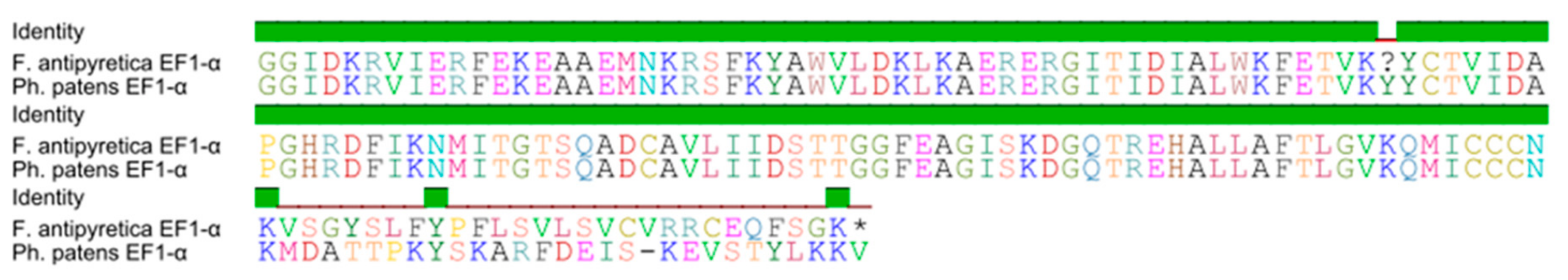
2.4. Sequencing
2.5. Non-Enzymatic Antioxidant Activities: Preparation of Aquatic Moss Extracts
2.6. Total Phenolic Content (TPC)
2.7. Antioxidant Activity Assay
2.8. Leaf Micromorphological Characteristics
2.9. Statistical Analysis
3. Results
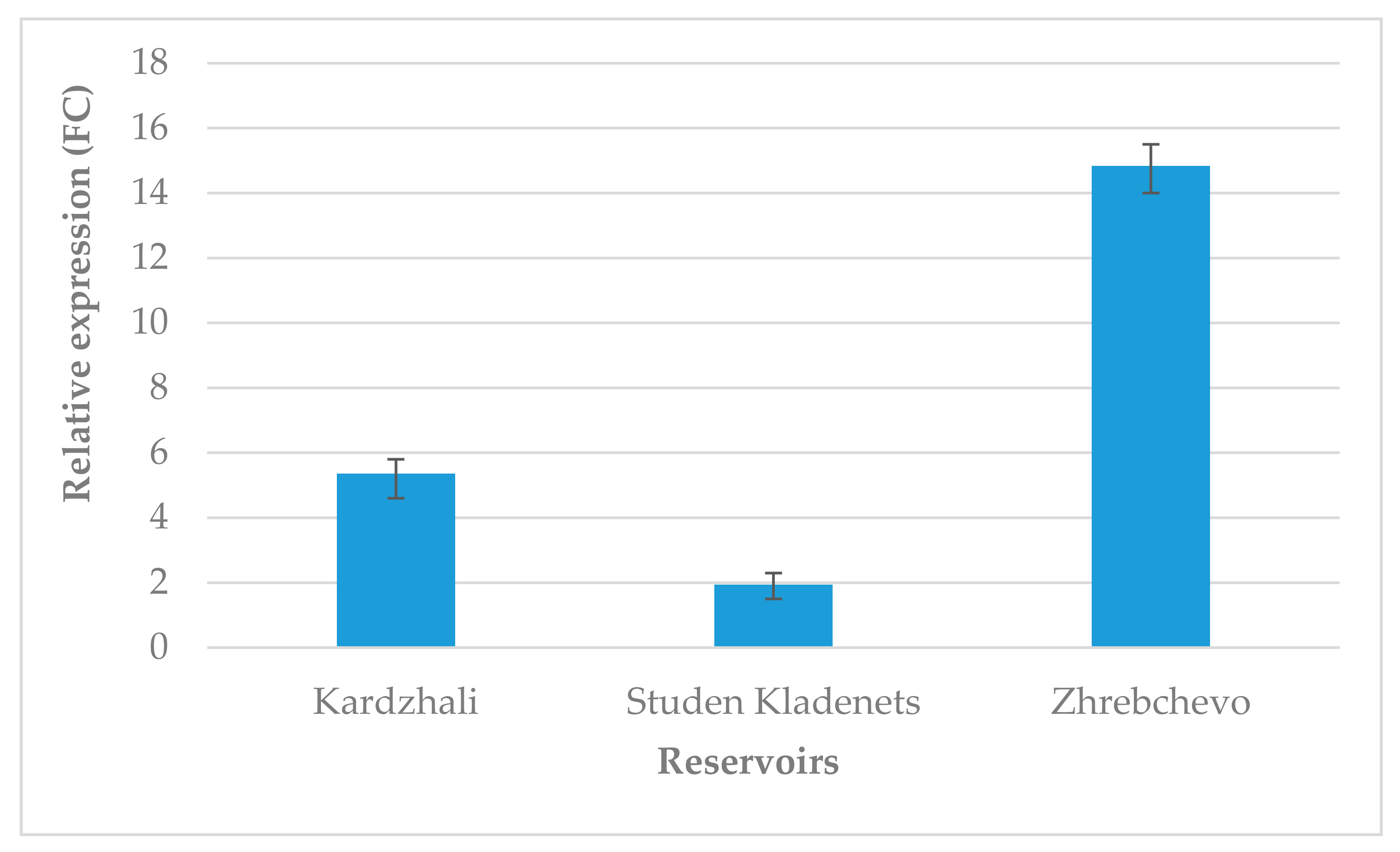
3.1. Expression Analysis of the Chloroplast rbcL Gene (Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase Large Subunit)
3.2. Non-Enzymatic Antioxidant Activities
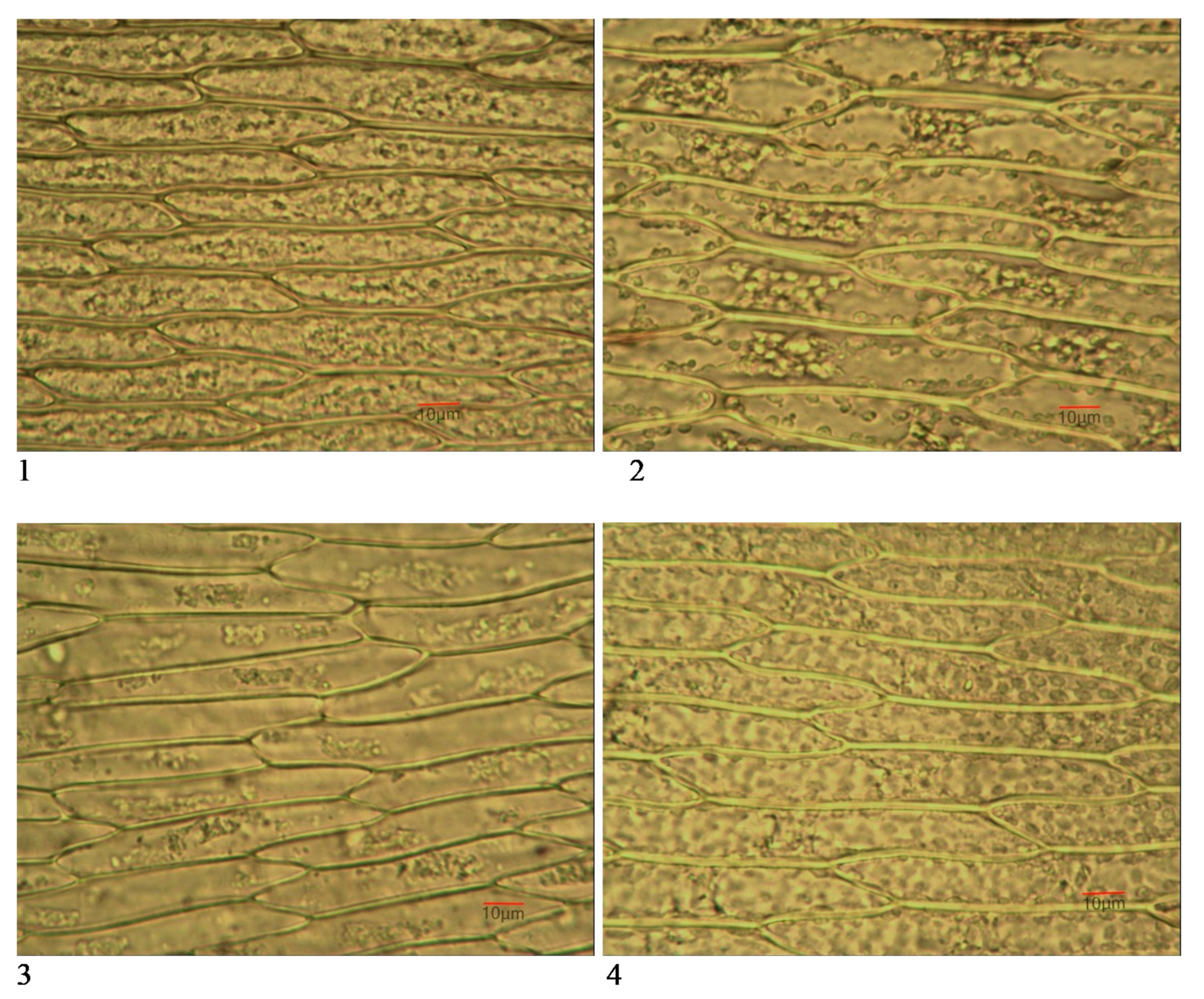
3.3. Leaf Micromorphological Characteristics
3.3.1. Number of Cells/mm2
3.3.2. Cell Dimensions
4. Discussion
4.1. Molecular Biomarker
4.2. Chemical Biomarkers
4.3. Micromorphological Biomarkers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartell, S.M. Biomarkers, bioindicators, and ecological risk assessment—a brief review and evaluation. Environ. Bioind. 2006, 1, 60–73. [Google Scholar] [CrossRef]
- Dey, A.; De, J.N. Antioxidative potential of bryophytes: Stress tolerance and commercial perspectives: A review. Pharmacologia 2012, 3, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, T.A.; Lampi, M.A.; Greenberg, B.M. Identification of six differentially expressed genes in response to copper exposure in the aquatic plant Lemna gibba (Duckweed). Environ. Toxicol. Chem. 2005, 24, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Portis, A.R., Jr. Regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1992, 43, 415–437. [Google Scholar] [CrossRef]
- Taylor, T.C.; Andersson, I. The structure of the complex between rubisco and its natural substrate ribulose 1,5-bisphosphate. J. Mol. Biol. 1997, 265, 432–444. [Google Scholar] [CrossRef]
- Keys, A.J. Rubisco: Its Role in Photorespiration. Philos. Trans. R. Soc. Lond. B. Biol. 1986, 313, 325–336. [Google Scholar] [CrossRef]
- Portis, A.R., Jr. Rubisco activase—Rubisco’s catalytic chaperone. Photosynth. Res. 2003, 75, 11–27. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Strzałka, K. Impact of Heavy Metals on Photosynthesis. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 1999; pp. 117–138. [Google Scholar] [CrossRef]
- Monnet, F.; Vaillant, N.; Vernay, P.; Coudret, A.; Sallanon, H.; Hitmi, A. Relationship between PSII activity, CO2 fixation, and Zn, Mn and Mg contents of Lolium perenne under zinc stress. J. Plant. Physiol. 2001, 158, 1137–1144. [Google Scholar] [CrossRef]
- Muthuchelian, K.; Bertamini, M.; Nedunchezhian, N. Triacontanol can protect Erythrina variegata from cadmium toxicity. J. Plant. Physiol. 2001, 158, 1487–1490. [Google Scholar] [CrossRef]
- Schafer, C.; Simper, H.; Hofmann, B. Glucose feeding results in coordinated changes of chlorophyll content, ribulose-1,5-biphosphate carboxylase-oxygenase activity and photosynthetic potential photoautotrophic suspension cultured cells of Chenopodium ruburum. Plant. Cell Environ. 1992, 15, 343–350. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Ilias, I. Hormone-induced protection of sunflower photosynthetic apparatus against copper toxicity. Biol. Plant. 2005, 49, 223–228. [Google Scholar] [CrossRef]
- Semane, B.; Dupae, J.; Cuypers, A.; Noben, J.P.; Tuomainen, M.; Tervahauta, A.; Kärenlampi, S.; Van Belleghem, F.; Smeets, K.; Vangronsveld, J. Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. J. Plant. Physiol. 2010, 167, 247–254. [Google Scholar] [CrossRef]
- Pankovic, D.; Plesnicar, M.; Arsenijeevic-Maksimovic, I.; Petrovic, N.; Sakac, Z.; Kastori, R. Effects of nitrogen nutrition on photosynthesis in Cd-treated sunflower plants. Ann. Bot. 2000, 86, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Khairy, A.I.H.; Oh, M.J.; Lee, S.M.; Kim, D.S.; Roh, K.S. Nitric oxide overcomes Cd and Cu toxicity in in vitro-grown tobacco plants through increasing contents and activities of rubisco and rubisco activase. Biochim. Open 2016, 2, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumaran, A.; Karunakaran, R.J. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Shahidi, F. Natural antioxidants: An overview. In Natural Antioxidants, Chemistry, Health Effects and Applications; Shahidi, F., Ed.; AOCS Press: Champaign, IL, USA, 1997; pp. 1–11. [Google Scholar]
- Dimitrova, I.; Koev, K.; Hristovska, N. Investigations on Linum ussitatissimum grown on heavy metal contaminated soils. II. Morphological and anatomical analysis of the vegetative organs. Travaux Sci. Univ. d’Plovdiv Plant. 2001, 37, 25–34. [Google Scholar]
- Dimitrova, I.; Koev, K.; Prisadashka, P. Anatomo-morphological response of Pisum sativum after treatment with purified waste water. In Proceedings of the Sixth National Conference of Botany; Temniskova, D., Ed.; Sofia University “St. Kliment Ohridski” Press: Sofia, Bulgaria, 2001; pp. 461–466. [Google Scholar]
- Dospatliev, L.K.; Lacheva, M.N.; Ivanova, M.T.; Radoukova, T.I. Determination of the Total Phosphorus (P) Content and Anatomical Study on Stomata of Plant Species in Urban and Mountainous Environments (Part 2). Ecol. Balk. 2018, 10, 15–26. [Google Scholar]
- Radoukova, T.I.; Lacheva, M.N.; Ivanova, M.T.; Dospatliev, L.K. Determination of the Total Nitrogen (N) Content and Anatomical Study on the Leaves Epidermal Cells of Plant Species in Urban and Mountainous Environments (Part 1). Ecol. Balk. 2018, 10, 7–14. [Google Scholar]
- Brain, R.A.; Cedergreen, N. Biomarkers in Aquatic Plants: Selection and Utility. In Reviews of Environmental Contamination and Toxicology. (Continuation of Residue Reviews); Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2008; Volume 198, pp. 49–109. [Google Scholar] [CrossRef]
- Gecheva, G.; Yurukova, L. Water pollutant monitoring with aquatic bryophytes: A review. Environ. Chem. Lett. 2014, 12, 49–61. [Google Scholar] [CrossRef]
- Gecheva, G.; Yancheva, V.; Velcheva, I.; Georgieva, E.; Stoyanova, S.; Arnaudova, D.; Stefanova, V.; Georgieva, D.; Genina, V.; Todorova, B.; et al. Integrated monitoring with moss-bag and mussel transplants in reservoirs. Water 2020, 12, 1800. [Google Scholar] [CrossRef]
- Dazy, M.; Masfaraud, J.F.; Ferard, J.F. Induction of oxidative stress biomarkers associated with heavy metal stress in Fontinalis antipyretica Hedw. Chemosphere 2009, 75, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sen, C.K.; Hanninen, O. Monitoring of polycyclic aromatic hydrocarbons using moss bags: Bioaccumulation and responses of antioxidant enzymes in Fontinalis antipyretica Hedw. Chemosphere 1996, 32, 2305–2315. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST: PAleontological STatistical software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Jaskulak, M.; Rorat, A.; Grobelak, A.; Kacprzak, M. Antioxidative enzymes and expression of rbcL gene as tools to monitor heavy metal-related stress in plants. J. Environ. Manag. 2018, 218, 71–78. [Google Scholar] [CrossRef]
- Mishra, S.; Dubey, R. Heavy metal toxicity induced alterations in photosynthetic metabolism in plants. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Lunić, T.M.; Oalđe, M.M.; Mandić, M.R.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.; Božić Nedeljković, B.D. Extracts Characterization and In Vitro Evaluation of Potential Immunomodulatory Activities of the Moss Hypnum cupressiforme Hedw. Molecules 2020, 25, 3343. [Google Scholar] [CrossRef]
| Sample | TPC mg GAE/100 g | RSA % | CUPRAC Assay mM TE/g |
|---|---|---|---|
| Background | 49.9 ± 1.6 | 77.9 | 1.45 ± 0.1 |
| Kardzhali | 29.3 ± 1.8 | 54.9 | 2.44 ± 0.14 |
| Studen Kladenets | 27.3 ± 0.4 | 80.2 | 1.34 ± 0.08 |
| Zhrebchevo | 29.0 ± 0.4 | 74.2 | 0.85 ± 0.05 |
| Leaf Samples | Stem Leaves | Twig Leaves | |||||
|---|---|---|---|---|---|---|---|
| min (X ± Sx) max | Sx % | CV % | min (X ± Sx ) max | Sx % | CV % | ||
| Background | Number of cells/mm2 | 851 (918.5 ± 7.6) 989 | 0.8 | 4.5 | 989 (1153.1 ± 12.5) 1265 | 1.1 | 5.9 |
| Cell length | 75 (95.5 ± 2.5) 132 | 2.6 | 14.6 | 66 (87.2 ± 2.6) 114 | 3 | 16.7 | |
| Cell width | 9 (11.8 ± 0.2) 14 | 1.8 | 10.1 | 5 (8.1 ± 0.2) 11 | 2.4 | 13.9 | |
| Kardzhali | Number of cells/mm2 | 849 (935.9 ± 15.8) 1173 | 1.6 | 8.6 | 1111 (1195.3 ** ± 11.6) 1295 | 0.8 | 4.5 |
| Cell length | 61 (92.5 ± 3.4) 120 | 3.6 | 20 | 62 (86.3 ± 2.3) 111 | 2.6 | 14.4 | |
| Cell width | 9 (11.4 ± 0.2) 14 | 1.5 | 9.1 | 5 (7.5 * ± 0.2) 10 | 2.1 | 12.1 | |
| Studen Kladenets | Number of cells/mm2 | 874 (946.1 ± 14.1) 1127 | 1.4 | 8.1 | 1142 (1204 *** ± 6.2) 1257 | 0.4 | 2.6 |
| Cell length | 70 (94.2 ± 2.9) 127 | 3 | 16.7 | 69 (84.9 ± 2.9) 122 | 3.4 | 18.8 | |
| Cell width | 8 (10.8 ** ± 0.26) 14 | 2.4 | 13.2 | 5 (7.3 ** ± 0.2) 11 | 2.3 | 12.5 | |
| Zhrebchevo | Number of cells/mm2 | 851 (952.2 *** ± 6.8) 1012 | 0.7 | 3.9 | 1127 (1227.4 *** ± 12.1) 1357 | 0.9 | 5.4 |
| Cell length | 56 (85.3 ** ± 3.4) 119 | 4 | 22.1 | 61 (80.1 * ± 2.2) 110 | 2.7 | 15 | |
| Cell width | 7 (10.7 ** ± 0.3) 15 | 2.9 | 16.4 | 4 (7.4 ** ± 0.2) 10 | 2.9 | 16.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gecheva, G.; Mollov, I.; Yahubyan, G.; Gozmanova, M.; Apostolova, E.; Vasileva, T.; Nikolova, M.; Dimitrova-Dyulgerova, I.; Radoukova, T. Can Biomarkers Respond Upon Freshwater Pollution?—A Moss-Bag Approach. Biology 2021, 10, 3. https://doi.org/10.3390/biology10010003
Gecheva G, Mollov I, Yahubyan G, Gozmanova M, Apostolova E, Vasileva T, Nikolova M, Dimitrova-Dyulgerova I, Radoukova T. Can Biomarkers Respond Upon Freshwater Pollution?—A Moss-Bag Approach. Biology. 2021; 10(1):3. https://doi.org/10.3390/biology10010003
Chicago/Turabian StyleGecheva, Gana, Ivelin Mollov, Galina Yahubyan, Mariyana Gozmanova, Elena Apostolova, Tonka Vasileva, Mariana Nikolova, Ivanka Dimitrova-Dyulgerova, and Tzenka Radoukova. 2021. "Can Biomarkers Respond Upon Freshwater Pollution?—A Moss-Bag Approach" Biology 10, no. 1: 3. https://doi.org/10.3390/biology10010003
APA StyleGecheva, G., Mollov, I., Yahubyan, G., Gozmanova, M., Apostolova, E., Vasileva, T., Nikolova, M., Dimitrova-Dyulgerova, I., & Radoukova, T. (2021). Can Biomarkers Respond Upon Freshwater Pollution?—A Moss-Bag Approach. Biology, 10(1), 3. https://doi.org/10.3390/biology10010003









