Diphenyl-Furanones and Diphenyl-Oxopyrrole Derivatives: From Analytical Reagents for Amino Groups to New Fluorochromes for Cytochemical Staining of Chromatin DNA and Chromosomes: Proposal for Intercalative Binding and Fluorescence Mechanism
Abstract
1. Introduction
2. Materials and Methods
3. Results
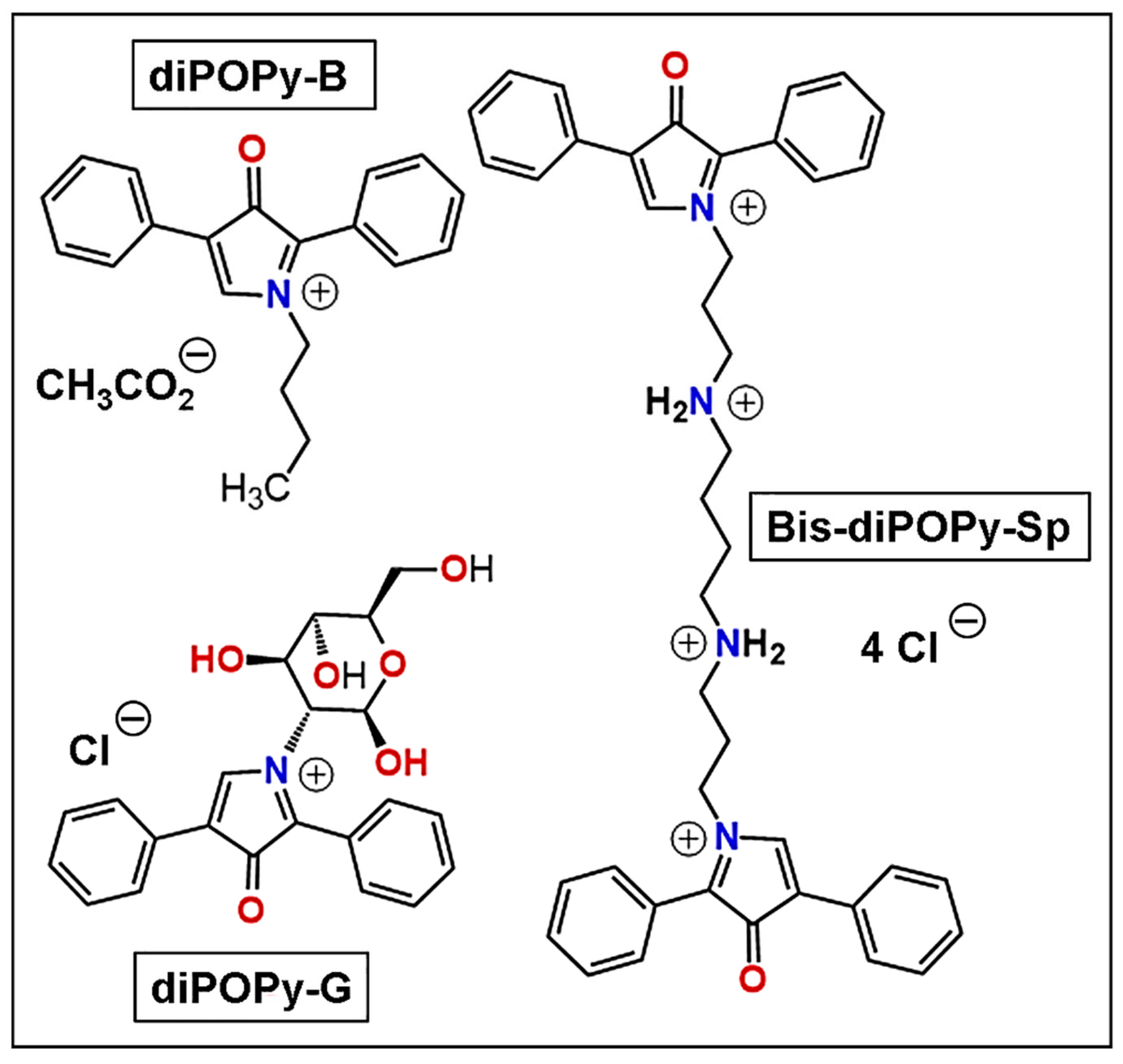
3.1. DiPOPy Fluorochromes from MDPF
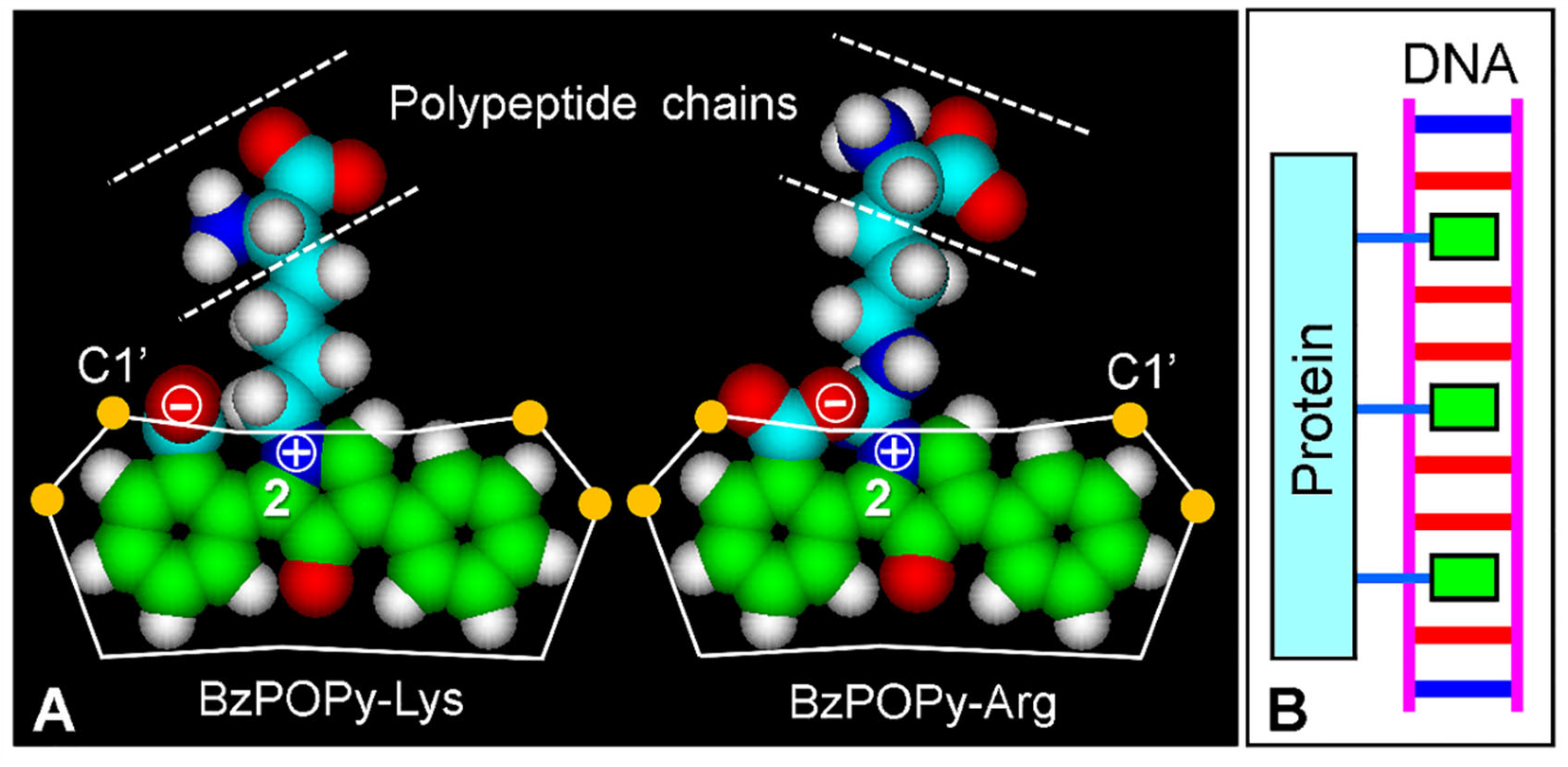
3.2. BzPOPy Fluorochromes from Fluorescamine
4. Discussion
4.1. DiPOPy Derivatives as Fluorochromes
4.2. Bis-Intercalative Binding
4.3. Fluorescence Mechanisms of Fluorescamine Products
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Udenfriend, S.; Stein, S.; Böhlen, P.; Dairman, W.; Leimgruber, W.; Weigele, M. Fluorescamine: A reagent for assay of amino acids, peptides, proteins and primary amines in the picomole range. Science 1972, 178, 871–872. [Google Scholar] [CrossRef] [PubMed]
- Weigele, M.; De Bernardo, S.L.; Tengi, J.P.; Leimgruber, W. A novel reagent for the fluorometric assay of primary amines. J. Am. Chem. Soc. 1972, 94, 5927–5928. [Google Scholar] [CrossRef]
- Böhlen, P.; Stein, S.; Dairman, W.; Udenfriend, S. Fluorometric assay of proteins in the nanogram range. Arch. Biochem. Biophys. 1973, 155, 213–220. [Google Scholar] [CrossRef]
- Lai, C.Y. Detection of peptides by fluorescent methods. Meth. Enzymol. 1977, 47, 236–243. [Google Scholar]
- Toome, V.; Wegrzynski, B. Application of MDPF and fluorescamine. XV. Chiroptical properties of MDPF condensation compounds with dipeptides in situ. Biochem. Biophys. Res. Commun. 1983, 114, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.M.; Jimenez, M.H. Usage of fluorescamine as a spray reagent for thin-layer chromatography. J. Chromatogr. 1974, 89, 361–364. [Google Scholar] [CrossRef]
- Samejima, K. Separation of fluorescamine derivatives of aliphatic diamines and polyamines by high-speed liquid chromatography. J. Chromatogr. 1974, 96, 250–254. [Google Scholar] [CrossRef]
- Tata, S.J.; Moir, G.F.J. Fluorescamine as a reagent for location of proteins after electrophoresis in starch gel or on paper. Anal. Biochem. 1976, 70, 495–498. [Google Scholar] [CrossRef]
- Nakamura, H.; Takagi, K.; Tamura, Z.; Yoda, R.; Yamamoto, Y. Stepwise fluorometric determination of primary and secondary amines by liquid chromatography after derivatization with 2 methoxy 2,4-diphenyl 3(2H)-furanone. Anal. Chem. 1984, 56, 919–922. [Google Scholar] [CrossRef]
- Bodhe, A.M.; Vartak, H.G.; Rele, M.V.; Dhamankar, V.S. Location and purification of enzymes on polyacrylamide gels using dialyzable fluorescent markers. Anal. Biochem. 1987, 164, 39–43. [Google Scholar] [CrossRef]
- Miedel, M.C.; Hulmes, J.D.; Pan, Y.C. The use of fluorescamine as a detection reagent in protein microcharacterization. J. Biochem. Biophys. Meth. 1989, 18, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Kok, W.T. Postcolumn derivatization of peptides with fluorescamine in capillary electrophoresis. J. Chromatogr. A 1998, 814, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Weigele, M.; De Bernardo, S.L.; Leimgruber, W.; Cleeland, R.; Grunberg, E. Fluorescent labeling of proteins. A new methodology. Biochem. Biophys. Res. Commun. 1973, 54, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.I.; Grosso, L. Fluorescent labeling of proteins in sodium dodecyl sulfate complexes with fluorescamine. Biochem. Biophys. Res. Commun. 1976, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Barger, B.O.; White, F.C.; Pace, J.L.; Kemper, D.L.; Ragland, W.L. Estimation of molecular weight by polyacrylamide gel electrophoresis using heat stable fluorophors. Anal. Biochem. 1976, 70, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.A.; Hagen, S.; Trotter, J.L.; O’Connell, K.; Agrawal, H.C. Use of a stable fluorescent reagent, 2-methoxy-2,4-diphenyl-3(2H)-furanone, for the visualization and purification of myelin proteins. J. Neurochem. 1979, 32, 1077–1083. [Google Scholar] [CrossRef]
- Falk, B.W.; Elliott, C. Fluorescent monitoring of proteins during sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. Anal. Biochem. 1985, 144, 537–541. [Google Scholar] [CrossRef]
- Jackson, P.; Urwin, V.E.; Mackay, C.D. Rapid imaging, using a cooled charge-coupled-device, of fluorescent two-dimensional polyacrylamide gels produced by labeling proteins in the first-dimensional isoelectric focusing gel with the fluorophore 2-methoxy-2,4-diphenyl-3(2H)furanone. Electrophoresis 1988, 9, 330–339. [Google Scholar] [CrossRef]
- Alba, F.J.; Daban, J.R. Nonenzymatic chemiluminescent detection and quantitation of total protein on Western and slot blots allowing subsequent immunodetection and sequencing. Electrophoresis 1997, 18, 1960–1966. [Google Scholar] [CrossRef]
- Sprinzl, M.; Faulhammer, H.G. Participation of X47-fluorescamine modified E. coli tRNAs in in vitro protein biosynthesis. Nucleic Acids Res. 1978, 5, 4837–4853. [Google Scholar] [CrossRef]
- Kasai, H.; Hayami, H.; Yamaizumi, Z.; Saito, H.; Nishimra, S. Detection and identification of mutagens and carcinogens as their adducts with guanosine derivatives. Nucleic Acids Res. 1984, 12, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, S.P.; Meehan, T.D.; Bissell, M.J. The use of fluorescamine as a probe for labeling the outer surface of the plasma membrane. Biochem. Biophys Res. Commun. 1976, 68, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.W.; Briggs, W.R. Labeling of membranes from erythrocytes and corn with fluorescamine. Biochim. Biophys. Acta 1977, 471, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Håkanson, R.; Larsson, L.I.; Sundler, F. Fluorescamine: A novel reagent for the histochemical detection of amino groups. Histochemistry 1974, 39, 15–23. [Google Scholar] [CrossRef]
- Larsson, L.I.; Sundler, F.; Håkanson, R. Fluorescamine as a histochemical reagent: Demonstration of polypeptide hormone-secreting cells. Histochemistry 1975, 44, 245–251. [Google Scholar] [CrossRef]
- Bruni, A.; Fasulo, M.P.; Tosi, B.; Dall’Olio, G.; Vannini, G.L. Fluorogenic detection of primary amines in plant histochemistry with fluorescamine: A comparative study on the effects of coagulant and non-coagulant fixatives. Histochemistry 1976, 48, 269–281. [Google Scholar] [CrossRef]
- Sciorra, L.J.; Lee, M.L.; Wynnyckyj, H. Study of human chromosomes. IV. Labeling of chromosomal proteins with the amino group specific fluorescent reagent fluorescamine. J. Histochem. Cytochem. 1985, 33, 1252–1255. [Google Scholar] [CrossRef]
- Cuéllar, T.; Gosálvez, J.; Del Castillo, P.; Stockert, J.C. Fluram induces species-dependent C and G bands in mammalian chromosomes, revealing heterogeneous distribution of chromosomal proteins. Genome 1991, 34, 772–776. [Google Scholar] [CrossRef]
- Stockert, J.C.; Trigoso, C.I. Fluorescence of eosinophil leukocyte granules induced by the fluorogenic reagent 2 methoxy 2,4-diphenyl 3(2H)-furanone. Blood Cells 1993, 19, 423–430. [Google Scholar]
- Sundler, F.; Uddman, R.; Larsson, L.I.; Telenius-Berg, M.; Berg, B.; Håkanson, R. Fluorescamine in diagnosis of thyroid carcinomas. Acta Cytol. 1978, 22, 54–56. [Google Scholar]
- Uddman, R.; Larsson, L.I.; Sundler, F. Fluorescamine as a cytochemical detection reagent for mammary carcinoma cells. Acta Cytol. 1978, 22, 273–275. [Google Scholar]
- Lorch-Jorgensen, L.; Ingemansson, S.; Larsson, L.I. Formaldehyde-fluorescamine-induced fluorescence as a property of carcinoma cells. Virchows Arch. B Cell Pathol. 1979, 30, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Parry, G.; Blenis, J.; Hawkes, S.P. Detection of transformed cells using a fluorescent probe: The molecular basis for the differential reaction of fluorescamine with normal and transformed cells. Cytometry 1982, 3, 97–103. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, S.; Weigele, M.; Toome, V.; Manhart, K.; Leimgruber, W.; Böhlen, P.; Stein, S.; Udenfriend, S. Studies on the reaction of fluorescamine with primary amines. Arch. Biochem. Biophys. 1974, 163, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Weigele, M.; Tengi, J.P.; De Bernardo, S.; Czajkowski, R.; Leimgruber, W. Fluorometric reagents for primary amines. Synthesis of 2-alkoxy- and 2-acyloxy-3(2H)-furanones. J. Org. Chem. 1976, 41, 388–389. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A. Fluorescence Microscopy in Life Sciences; Bentham Science Publishers: Sharjah, United Arab Emirates, 2017. [Google Scholar] [CrossRef]
- Stockert, J.C. Reactive staining reagents and fluorescent labels. In Conn’s Biological Stains. A Handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine, 10th ed.; Horobin, R.W., Kiernan, J.A., Eds.; Bios Scientific Publishers: Oxford, UK, 2002; pp. 77–88. ISBN 859960995. [Google Scholar]
- Stockert, J.C.; Blázquez, A.; Galaz, S.; Juarranz, A. A mechanism for the fluorogenic reaction of amino groups with fluorescamine and 2 methoxy 2,4-diphenyl 3(2H)-furanone. Acta Histochem. 2008, 110, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Abasolo, M.I. Inaccurate chemical structure of dyes and fluorochromes found in the literature can be problematic for teaching and research. Biotech. Histochem. 2011, 86, 52–60. [Google Scholar] [CrossRef]
- Stockert, J.C.; Romero, S.A.; Felix-Pozzi, M.N.; Blázquez-Castro, A. In Vitro polymerization of the dopamine-borate melanin precursor: A proof-of-concept regarding 10boron neutron-capture therapy for melanoma. Biocell 2023, 47, 919–928. [Google Scholar] [CrossRef]
- Molero, M.L.; Hazen, M.J.; Pérez Gorroño, A.I.; Stockert, J.C. Simple ß-carboline alkaloids as nucleic acids fluorochromes. Acta Histochem. 1995, 97, 165–173. [Google Scholar] [CrossRef]
- Stockert, J.C.; Cañete, M.; Villanueva, A.; Juarranz, A.; Trigoso, C.I.; Braña, M.F. Fluorescence of chromatin DNA induced by antitumoral naphthalimides. Z. Nat. 1997, 52, 408–411. [Google Scholar] [CrossRef]
- Juarranz, A.; Villanueva, A.; Cañete, M.; Polo, S.; Domínguez, V.; Stockert, J.C. Microscopical and spectroscopic studies on the fluorescence of a daunomycin-aluminum complex. Histochem. J. 1999, 31, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Del Castillo, P.; Llorente, A.R.; Rasskin, D.M.; Romero, J.B.; Gómez, A. New fluorescence reactions in DNA cytochemistry. 1. Microscopic and spectroscopic studies on nonrigid fluorochromes. Anal. Quant. Cytol. Histol. 1990, 12, 1–10. [Google Scholar] [PubMed]
- Stockert, J.C.; Trigoso, C.I.; Cuéllar, T.; Bella, J.L.; Lisanti, J.L. A new fluorescence reaction in DNA cytochemistry: Microscopic and spectroscopic studies on the aromatic diamidino compound M&B 938. J. Histochem. Cytochem. 1997, 45, 97–105. [Google Scholar] [PubMed]
- Stockert, J.C.; Del Castillo, P.; Romero, J.B.; Tato, A.; Llorente, A.R.; Ferrer, J.M. Orientation of nucleosomes in the 30 nm chromatin fiber as revealed by microscopic studies of linear dichroism and polarized fluorescence. Biol. Zentralbl. 1990, 109, 471–480. [Google Scholar]
- Mello, M.L.S.; Vidal, B.C. Polarization microscopy of extended chromatin fibers. Meth. Mol. Biol. 2014, 1094, 71–78. [Google Scholar] [CrossRef]
- Stockert, J.C. Monomerizing effect of caffeine, o-phenanthroline and tannin on cationic dyes: A model system to analyze spectral characteristics of the intercalative binding to nucleic acids. Acta Histochem. 1989, 87, 33–42. [Google Scholar] [CrossRef]
- Trigoso, C.I.; Del Castillo, P.; Stockert, J.C. Influence of inorganic salts on the staining reaction of eosinophil lecocyte granules by anionic dyes. Acta Histochem. 1992, 93, 313–318. [Google Scholar] [CrossRef]
- Stockert, J.C. The horse eosinophil as a model leucocyte for morphological and cytochemical studies. Braz. J. Morphol. Sci. 2005, 22, 73–84. [Google Scholar]
- Del Castillo, P.; Horobin, R.W.; Blázquez-Castro, A.; Stockert, J.C. Binding of cationic dyes to DNA: Distinguishing intercalation and groove binding mechanisms using simple experimental and numerical models. Biotech. Histochem. 2010, 85, 247–256. [Google Scholar] [CrossRef]
- Stockert, J.C.; Espada, J.; Blázquez-Castro, A. Melanin-binding colorants: Updating molecular modeling, staining and labeling mechanisms, and biomedical perspectives. Colorants 2022, 1, 91–120. [Google Scholar] [CrossRef]
- Stockert, J.C. Molecular modeling of phenyl-boronic acids and catechol-borate esters: A mechanistic rationale for boron binding to melanin catechols, regarding the 10boron neutron-capture therapy of melanoma. Ann. Rev. Res. 2022, 8, 555726. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Horobin, R.W. Identifying different types of chromatin using Giemsa staining. Meth. Mol. Biol. 2014, 1094, 25–38. [Google Scholar]
- Stockert, J.C. Cytochemistry of nucleic acids: Binding mechanism of dyes and fluorochromes. Biocell 1985, 9, 89–131. [Google Scholar]
- Blackburn, G.M.; Gait, M.J. (Eds.) Nucleic Acids in Chemistry and Biology; IRL Press: Oxford, UK; New York, NY, USA, 1990; pp. 313–326. [Google Scholar]
- Mason, S.F. Color and the Electronic States of Organic Molecules. Chemistry of the Synthetic Dyes; J. Wiley and Sons: New York, NY, USA, 1970; pp. 169–221. [Google Scholar]
- Stockert, J.C. Lipid peroxidation assay using BODIPY-phenylbutadiene probes: A methodological overview. Meth. Mol. Biol. 2021, 2202, 199–214. [Google Scholar] [CrossRef]
- Stockert, J.C.; Pinna-Senn, E.; Bella, J.L.; Lisanti, J.A. DNA-binding fluorochromes: Correlation between C-banding of mouse metaphase chromosomes and hydrogen bonding to adenine-thymine base pairs. Acta Histochem. 2005, 106, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Horobin, R.W.; Kiernan, J.A. Conn’s Biological Stains. A Handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine, 10th ed.; Bios Scientific Publishers: Oxford, UK, 2002; ISBN 859960995. [Google Scholar]
- Moutschen, J.; Degraeve, N.; Moutschen-Dahmen, M. Chromosome fluorescence with berberine. Cytobiologie 1973, 8, 112–117. [Google Scholar]
- Cañete, M.; Villanueva, A.; Juarranz, A.; Stockert, J.C. A study of interaction of thioflavine T with DNA: Evidence for intercalation. Cell. Mol. Biol. 1987, 33, 191–199. [Google Scholar]
- Stockert, J.C.; Trigoso, C.I.; Llorente, A.R.; Del Castillo, P. DNA fluorescence induced by polymethine cation pyrvinium binding. Histochem. J. 1991, 23, 548–552. [Google Scholar] [CrossRef]
- Glazer, A.N.; Rye, H.S. Stable dye-DNA intercalation complexes as reagents for high-sensitivity fluorescence detection. Nature 1992, 359, 859–861. [Google Scholar] [CrossRef]
- Stockert, J.C. 2,5-bis(4-aminophenyl)-1,3,4-oxadiazol: Fluorescence reaction of chromatin and basophilic cytoplasm. Acta Histochem. Cytochem. 1983, 16, 66–69. [Google Scholar] [CrossRef]
- Stockert, J.C.; Pelling, C.; Espada, J. New cationic fluorochromes from diaryloxazole scintillators: Fluorescence of chromatin DNA induced by N-quaternary POPOP derivatives. Acta Histochem. 1997, 99, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Pinna-Senn, E.; Lisanti, J.A.; Ortiz, M.I.; Dalmasso, G.; Bella, J.L.; Gosálvez, J.; Stockert, J.C. Specific heterochromatic banding of metaphase chromosomes using Nuclear Yellow. Biotech. Histochem. 2000, 75, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Del Castillo, P. Linear dichroism and polarized fluorescence of dye- complexed DNA fibers. Histochemistry 1989, 91, 263–264. [Google Scholar] [CrossRef]
- Saitoh, Y.; Laemmli, U.K. Metaphase chromosome structure: Bands arise from a differential folding path of the highly AT-rich scaffold. Cell 1994, 76, 609–622. [Google Scholar] [CrossRef]
- Weisblum, B.; De Haseth, P.L. Quinacrine, a chromosome stain specific for deoxyadenylate-deoxythymidylate rich regions in DNA. Proc. Natl. Acad. Sci. USA 1972, 69, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Korenberg, J.R.; Engels, W.R. Base ratio DNA content and quinacrine brightness of human chromosomes. Proc. Nat. Acad. Sci. USA 1978, 75, 3382–3386. [Google Scholar] [CrossRef]
- Ferrucci, L.; Mezzanotte, R. A cytological approach to the role of guanine in determining quinacrine fluorescence response in eukaryotic chromosomes. J. Histochem. Cytochem. 1982, 30, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Bickmore, W.; Craig, J. Chromosome Bands: Patterns in the Genome; Springer: New York, NY, USA, 1997. [Google Scholar]
- Diekmann, S.; Zarling, D.A. Unique poly(dA) poly(dT) B′-conformation in cellular and synthetic DNAs. Nucleic Acids Res. 1987, 15, 6063–6074. [Google Scholar] [CrossRef]
- Diekmann, S. DNA curvature. Nucleic Acids Mol. Biol. 1987, 1, 138–156. [Google Scholar]
- Hörz, W.; Altenburger, W. Nucleotide sequence of mouse satellite DNA. Nucleic Acids Res. 1981, 9, 683–696. [Google Scholar] [CrossRef]
- Redi, C.A.; Garagna, S.; Della Valle, G.; Bottiroli, G.; Dell’Orto, P.; Viale, G.; Peverali, F.A.; Raimondi, E.; Forejt, J. Differences in the organization and chromosomal allocation of satellite DNA between the European long tailed house mice Mus domesticus and Mus musculus. Chromosoma 1990, 99, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Saenger, W. Principles of Nucleic Acid Structure; Springer-Verlag: New York, NY, USA; Berlin, Germany, 1984; pp. 385–404. [Google Scholar]
- Assa-Munt, N.; Denny, W.A.; Leupin, W.; Kearns, R.D. Proton NMR study of the binding of bis(acridines) to d(AT)5·d(AT)5. 1. Mode of binding. Biochemistry 1985, 24, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Tekola, P.; Baak, J.P.; Beliën, J.A.; Brugghe, J. Highly sensitive, specific, and stable new fluorescent DNA stains for confocal laser microscopy and image processing of normal paraffin sections. Cytometry 1994, 17, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, L.; Dullens, H.F.J.; Huisman, A.; van Diest, P.J. Fluorescent stains for quantification of DNA by confocal laser scanning microscopy in 3-D. Biotech. Histochem. 2008, 83, 63–69. [Google Scholar] [CrossRef]
- Nygren, J.; Svanvik, N.; Kubista, M. The interactions between the fluorescent dye thiazole orange and DNA. Biopolymers 1998, 46, 39–51. [Google Scholar] [CrossRef]
- Prodhomme, S.; Demaret, J.P.; Vinogradov, S.; Asseline, U.; Morin-Allory, L.; Vigny, P. A theoretical and experimental study of two thiazole orange derivatives with single- and double-stranded oligonucleotides, polydeoxyribonucleotides and DNA. J. Photochem. Photobiol. B Biol. 1999, 53, 60–69. [Google Scholar] [CrossRef]
- Hendry, L.B.; Mahesh, V.B.; Edwin, D.; Bransome, E.D.; Ewing, D.E. Small molecule intercalation with double stranded DNA: Implications for normal gene regulation and for predicting the biological efficacy and genotoxicity of drugs and other chemicals. Mutat. Res. 2007, 623, 53–71. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Yang, G.Z.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids. Part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef]
- Ughetto, G.; Wang, A.H.; Quigley, G.J.; van der Marel, G.A.; van Boom, J.H.; Rich, A. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 1985, 13, 2305–2323. [Google Scholar] [CrossRef]
- Lown, J.W.; Hanstock, C.C. Structure and function of the antitumor antibiotic carzinophilin A: The first natural intercalative bisalkylator. J. Am. Chem. Soc. 1982, 104, 3213–3214. [Google Scholar] [CrossRef]
- Huang, C.H.; Mong, S.; Crooke, S.T. Interactions of a new antitumor antibiotic BBM-928A with deoxyribonucleic acid. Bifunctional intercalative binding studied by fluorometry and viscometry. Biochemistry 1980, 19, 5537–5542. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Mirabelli, C.K.; Mong, S.; Crooke, S.T. Intermolecular cross-linking of DNA through bifunctional intercalation of an antitumor antibiotic, luzopeptin A (BBM-928A). Cancer Res. 1983, 43, 2718–2724. [Google Scholar] [PubMed]
- Dai, J.; Punchihewa, C.; Mistry, P.; Ooi, A.T.; Yang, D. Novel DNA bis-intercalation by MLN944, a potent clinical bisphenazine anticancer drug. J. Biol. Chem. 2004, 279, 46096–46103. [Google Scholar] [CrossRef] [PubMed]
- Jobson, A.G.; Willmore, E.; Tilby, M.J.; Mistry, P.; Charlton, P.; Austin, C.A. Effect of phenazine compounds XR11576 and XR5944 on DNA topoisomerases. Cancer Chemother. Pharmacol. 2009, 63, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Mathad, R.L.; Shang, Z.; Sidell, N.; Yang, D. Solution structure of a 2:1 complex of anticancer drug XR5944 with TFF1 estrogen response element: Insights into DNA recognition by a bis-intercalator. Nucleic Acids Res. 2014, 42, 6012–6024. [Google Scholar] [CrossRef]
- Moorthy, N.S.H.N.; Pratheepa, V.; Ramos, M.J.; Vasconcelos, V.; Fernandes, P.A. Fused aryl-phenazines: Scaffold for the development of bioactive molecules. Curr. Drug Targets 2014, 15, 681–688. [Google Scholar] [CrossRef]
- Antony, S.; Agama, Z.; Miao, K.K.; Hollingshead, M.; Holbeck, S.L.; Wright, M.H.; Varticovski, L.; Nagarajan, M.; Morrell, A.; Cushman, M.; et al. Bisindenoisoquinoline bis-1,3-{(5,6-dihydro-5,11-diketo-11H-indeno[1,2-c]isoquinoline)-6-propylamino}propane bis(trifluoroacetate) (NSC 727357), a DNA intercalator and topoisomerase inhibitor with antitumor activity. Mol. Pharmacol. 2006, 70, 1109–1120. [Google Scholar] [CrossRef]
- Gao, Q.; Williams, L.D.; Egli, M.; Rabinovich, D.; Chen, L.; Quigley, G.J.; Rich, A. Drug-induced DNA repair: X-ray structure of a DNA ditercalinium complex. Proc. Natl. Acad. Sci. USA 1991, 88, 2422–2426. [Google Scholar] [CrossRef]
- Braña, M.F.; Castellano, J.-M.; Morán, M.; Pérez de Vega, M.J.; Romerdahl, C.R.; Qian, X.D.; Bousquet, P.; Emling, F.; Schlick, E.; Keilhauer, G. Bis-naphthalimides: A new class of antitumor agents. Anticancer Drug Des. 1993, 8, 257–268. [Google Scholar]
- Wainwright, M. The use of dyes in modern biomedicine. Biotech. Histochem. 2003, 78, 147–155. [Google Scholar] [CrossRef]
- Feughelman, M.; Langridge, R.; Seeds, W.E.; Stokes, A.R.; Wilson, H.R.; Hooper, C.W.; Wilkins, M.H.F.; Barclay, R.K.; Hamilton, L.D. Molecular structure of deoxyribose nucleic acid and nucleoprotein. Nature 1955, 175, 834–838. [Google Scholar] [CrossRef] [PubMed]
- De Santis, P.; Forni, E.; Rizzo, R. Conformational analysis of DNA-basic polypeptide complexes: Possible models of nucleoprotamines and nucleohistones. Biopolymers 1974, 13, 3113–3326. [Google Scholar] [CrossRef] [PubMed]
- Suau, P.; Subirana, J.A. X-ray diffraction studies of nucleoprotamine structure. J. Mol. Biol. 1977, 117, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Warrant, R.W.; Kim, S.H. α-Helix-double helix interaction shown in the structure of a protamine-transfer RNA complex and a nucleoprotamine model. Nature 1978, 271, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Fita, I.; Campos, J.L.; Puigjaner, L.C.; Subirana, J.A. X-ray diffraction study of DNA complexes with arginine peptides and their relation to nucleoprotamine structure. J. Mol. Biol. 1983, 167, 157–177. [Google Scholar] [CrossRef]
- Rohs, R.; West, S.M.; Sosinsky, A.; Liu, P.; Mann, R.S.; Honig, B. The role of DNA shape in protein–DNA recognition. Nature 2009, 461, 1248–1254. [Google Scholar] [CrossRef]
- Pullman, B. Electrostatics of polymorphic DNA. J. Biomol. Struct. Dyn. 1983, 1, 773–794. [Google Scholar] [CrossRef]
- Aymami, J.; Coll, M.; Frederick, C.A.; Wang, A.H.J.; Rich, A. The propeller DNA conformation of poly(dA)·poly(dT). Nucleic Acids Res. 1989, 17, 3229–3245. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stockert, J.C.; Romero, S.A.; Felix-Pozzi, M.N.; Blázquez-Castro, A. Diphenyl-Furanones and Diphenyl-Oxopyrrole Derivatives: From Analytical Reagents for Amino Groups to New Fluorochromes for Cytochemical Staining of Chromatin DNA and Chromosomes: Proposal for Intercalative Binding and Fluorescence Mechanism. Colorants 2023, 2, 245-263. https://doi.org/10.3390/colorants2020016
Stockert JC, Romero SA, Felix-Pozzi MN, Blázquez-Castro A. Diphenyl-Furanones and Diphenyl-Oxopyrrole Derivatives: From Analytical Reagents for Amino Groups to New Fluorochromes for Cytochemical Staining of Chromatin DNA and Chromosomes: Proposal for Intercalative Binding and Fluorescence Mechanism. Colorants. 2023; 2(2):245-263. https://doi.org/10.3390/colorants2020016
Chicago/Turabian StyleStockert, Juan C., Silvina A. Romero, Marcelo N. Felix-Pozzi, and Alfonso Blázquez-Castro. 2023. "Diphenyl-Furanones and Diphenyl-Oxopyrrole Derivatives: From Analytical Reagents for Amino Groups to New Fluorochromes for Cytochemical Staining of Chromatin DNA and Chromosomes: Proposal for Intercalative Binding and Fluorescence Mechanism" Colorants 2, no. 2: 245-263. https://doi.org/10.3390/colorants2020016
APA StyleStockert, J. C., Romero, S. A., Felix-Pozzi, M. N., & Blázquez-Castro, A. (2023). Diphenyl-Furanones and Diphenyl-Oxopyrrole Derivatives: From Analytical Reagents for Amino Groups to New Fluorochromes for Cytochemical Staining of Chromatin DNA and Chromosomes: Proposal for Intercalative Binding and Fluorescence Mechanism. Colorants, 2(2), 245-263. https://doi.org/10.3390/colorants2020016






