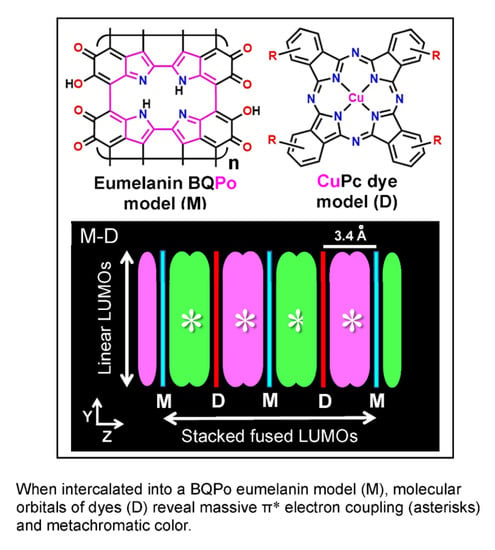
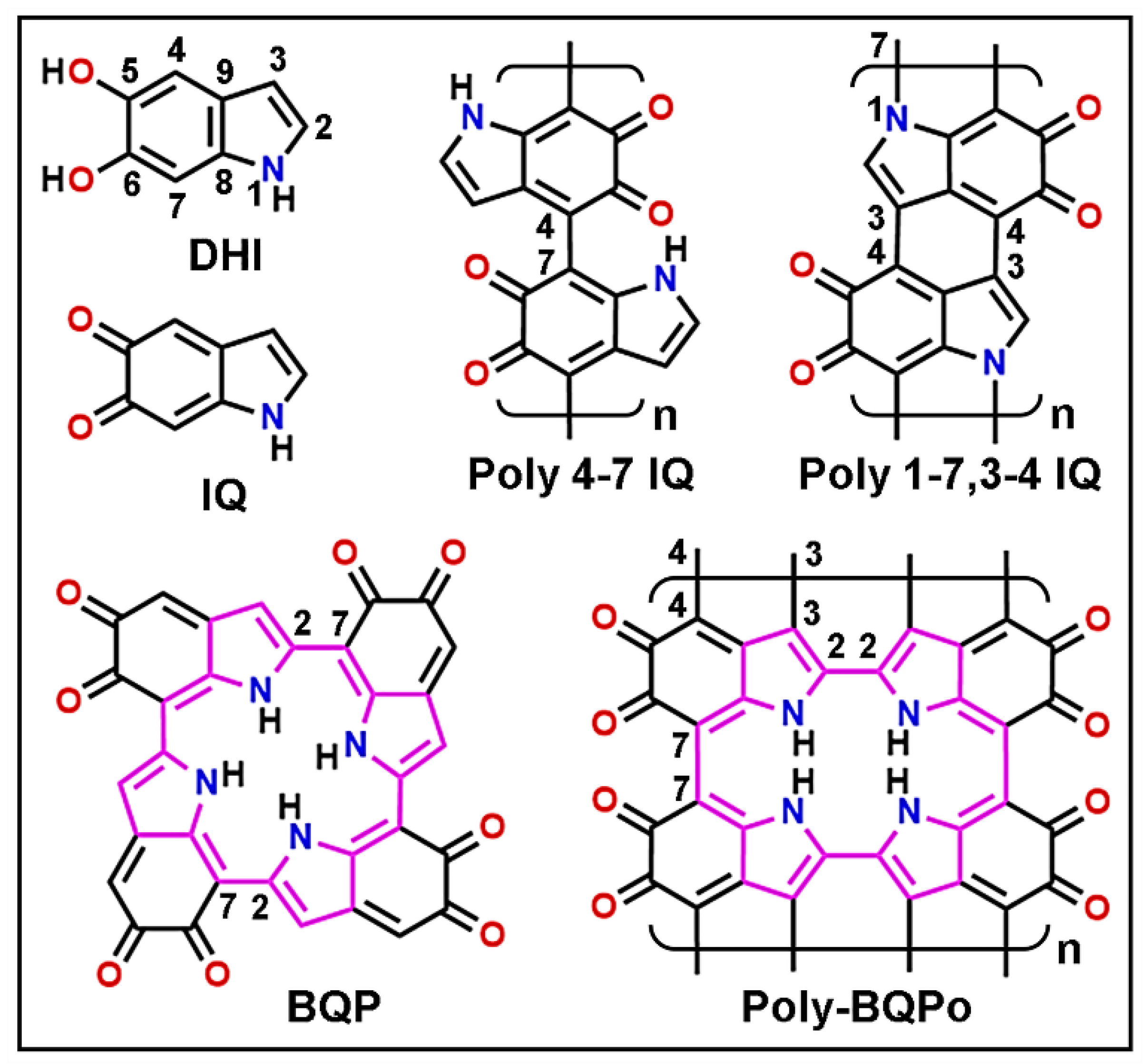
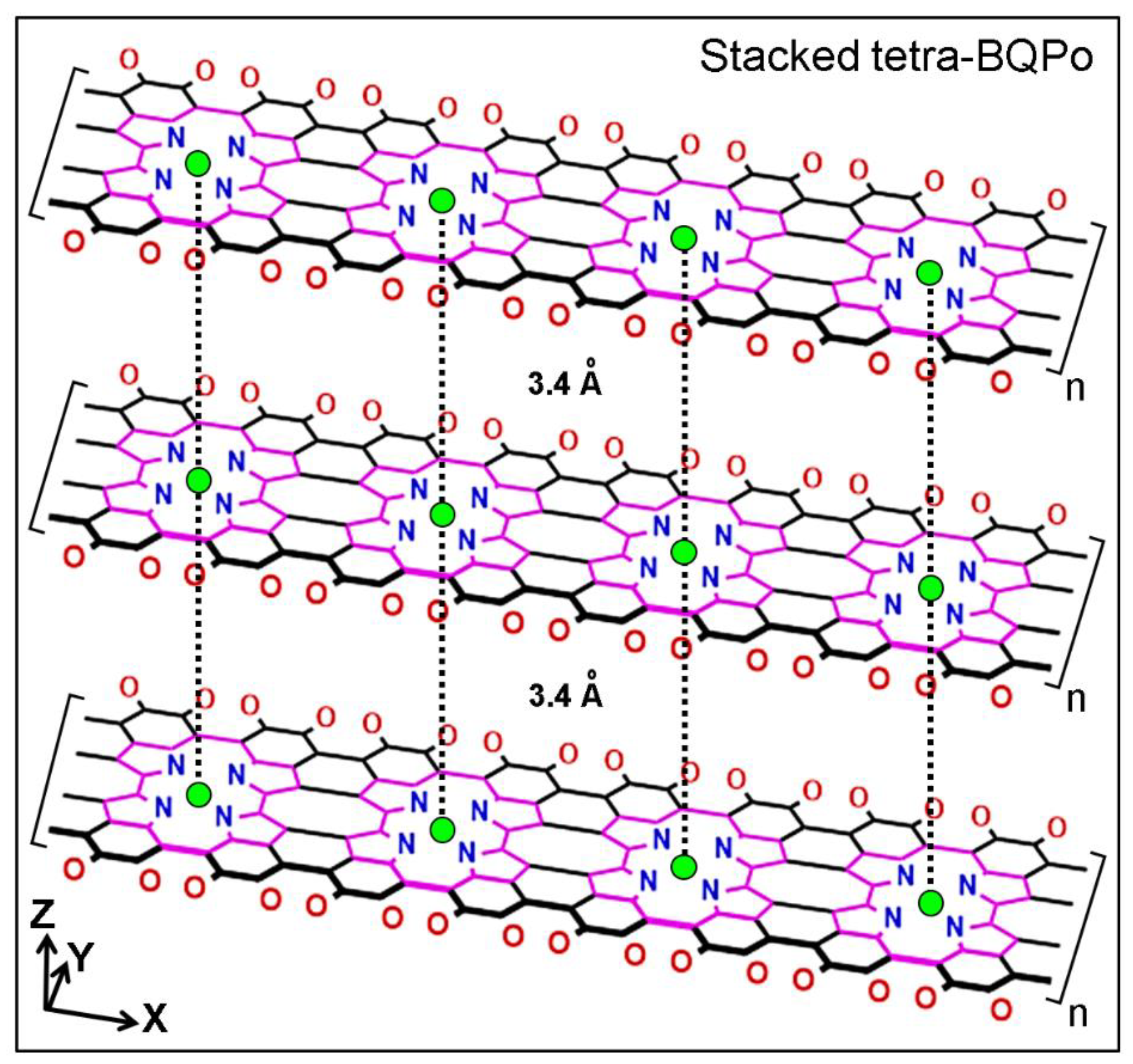
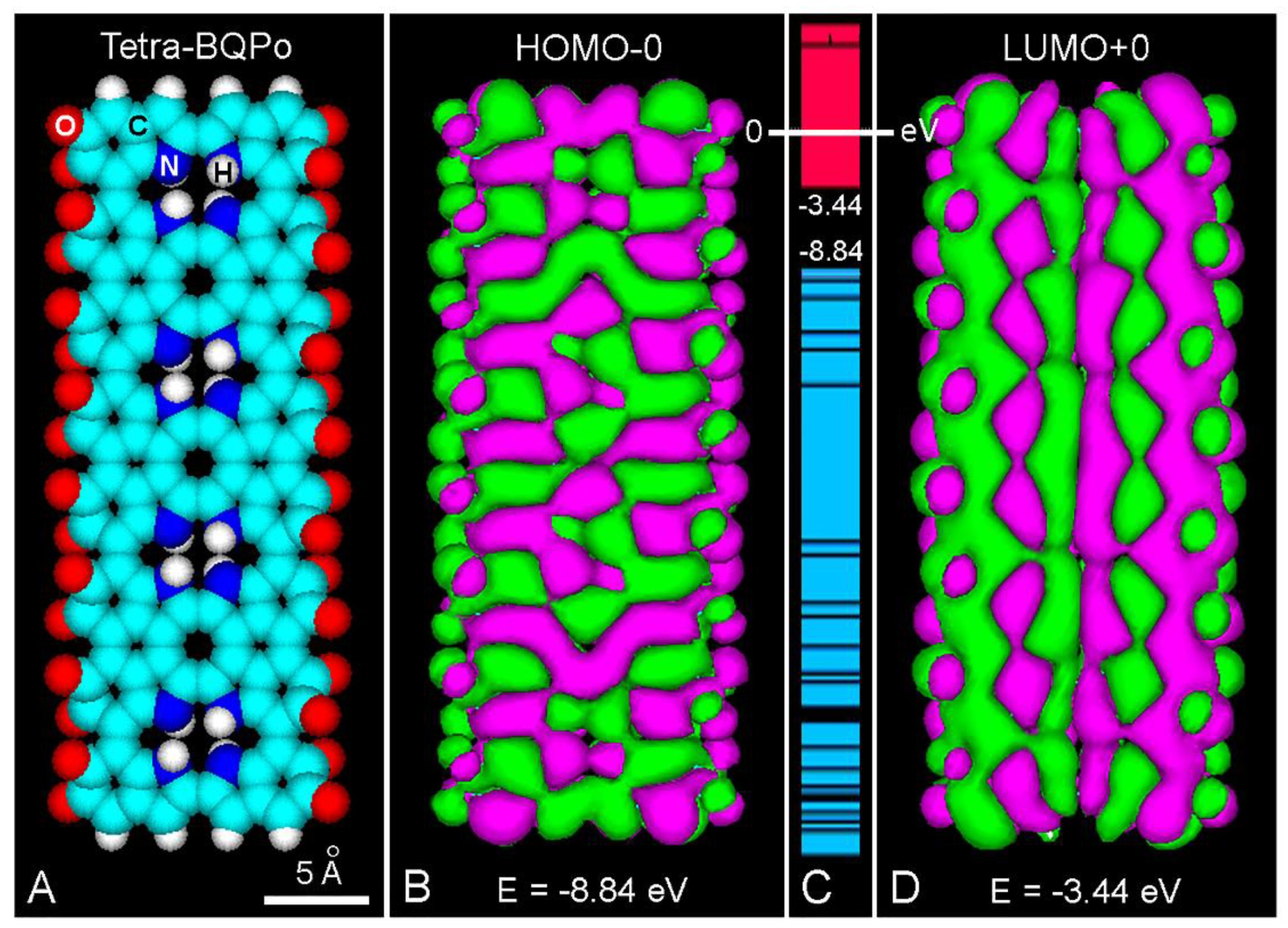
Melanin-Binding Colorants: Updating Molecular Modeling, Staining and Labeling Mechanisms, and Biomedical Perspectives
Abstract
1. Introduction
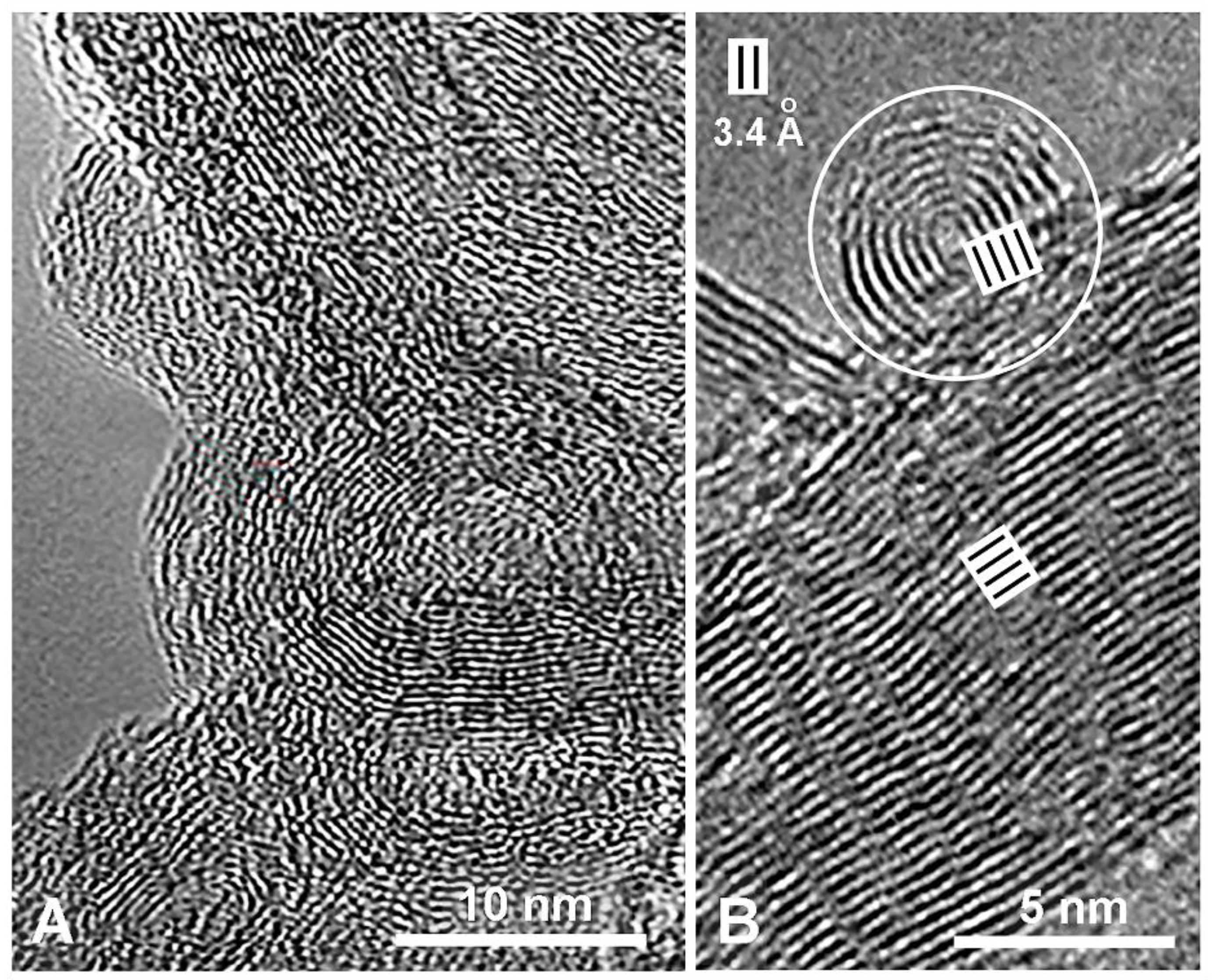
2. Molecular Structure of Eumelanin
3. Spectroscopy and Fluorescence of Melanins
4. Staining Mechanisms of Melanin
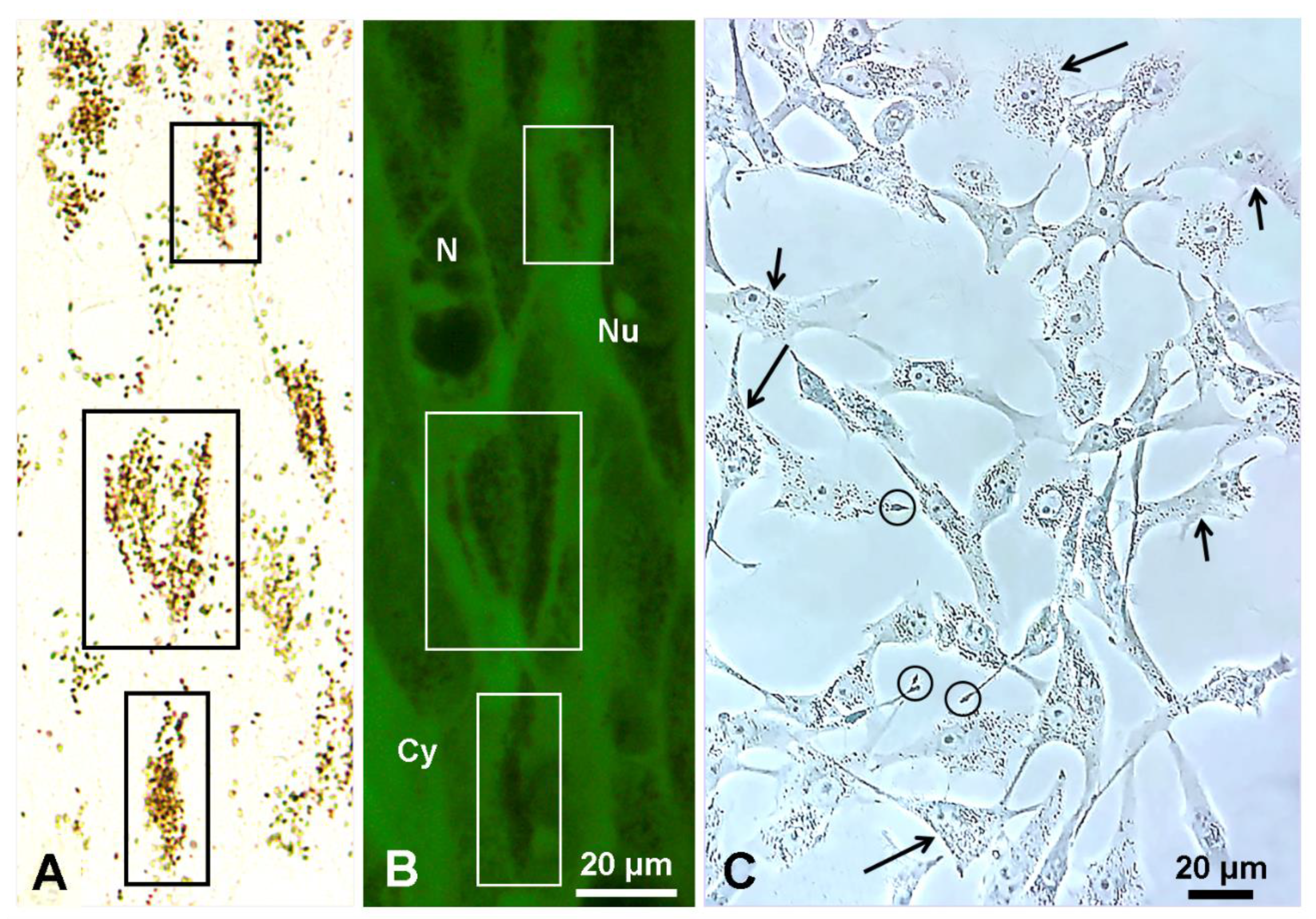
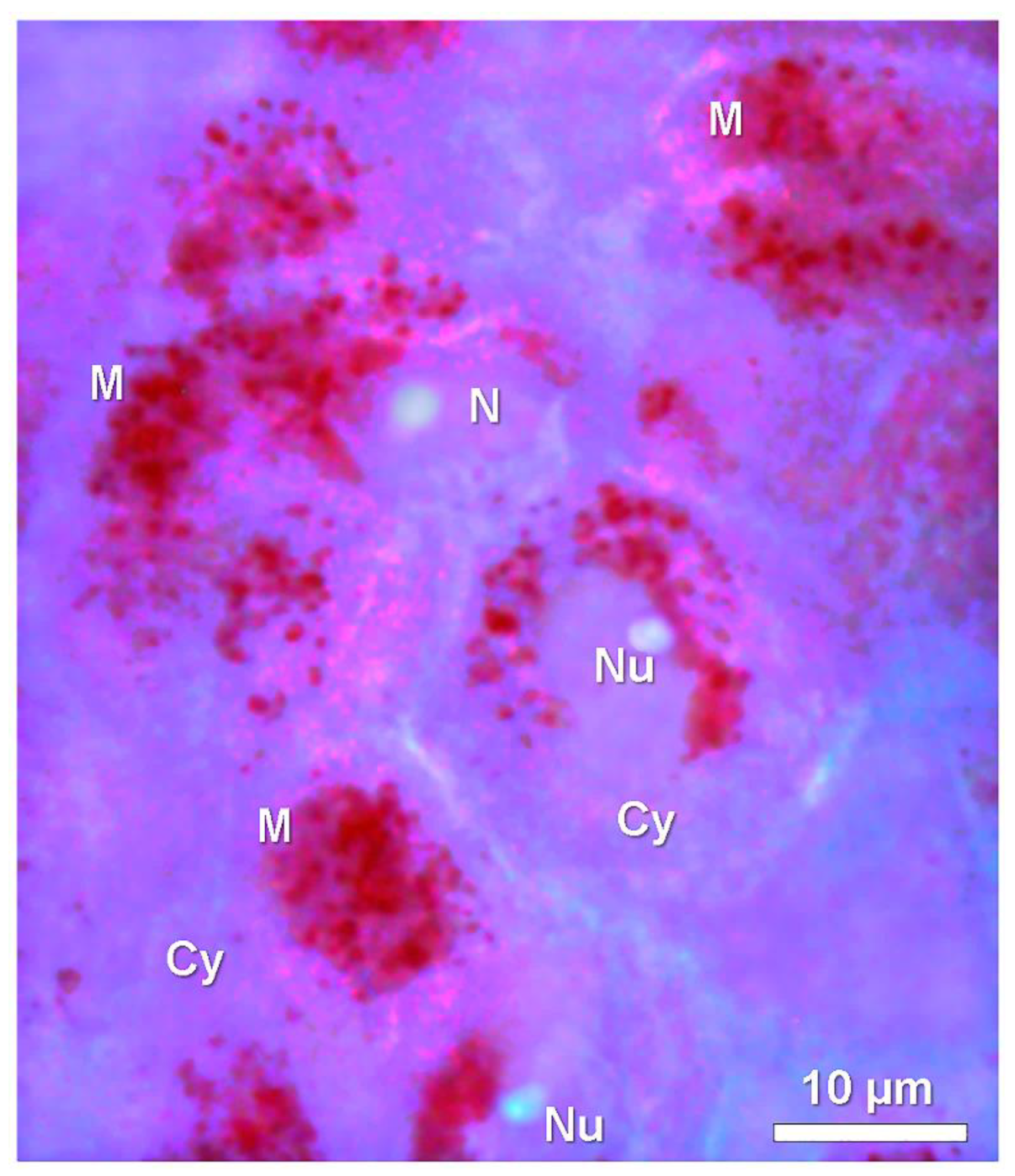
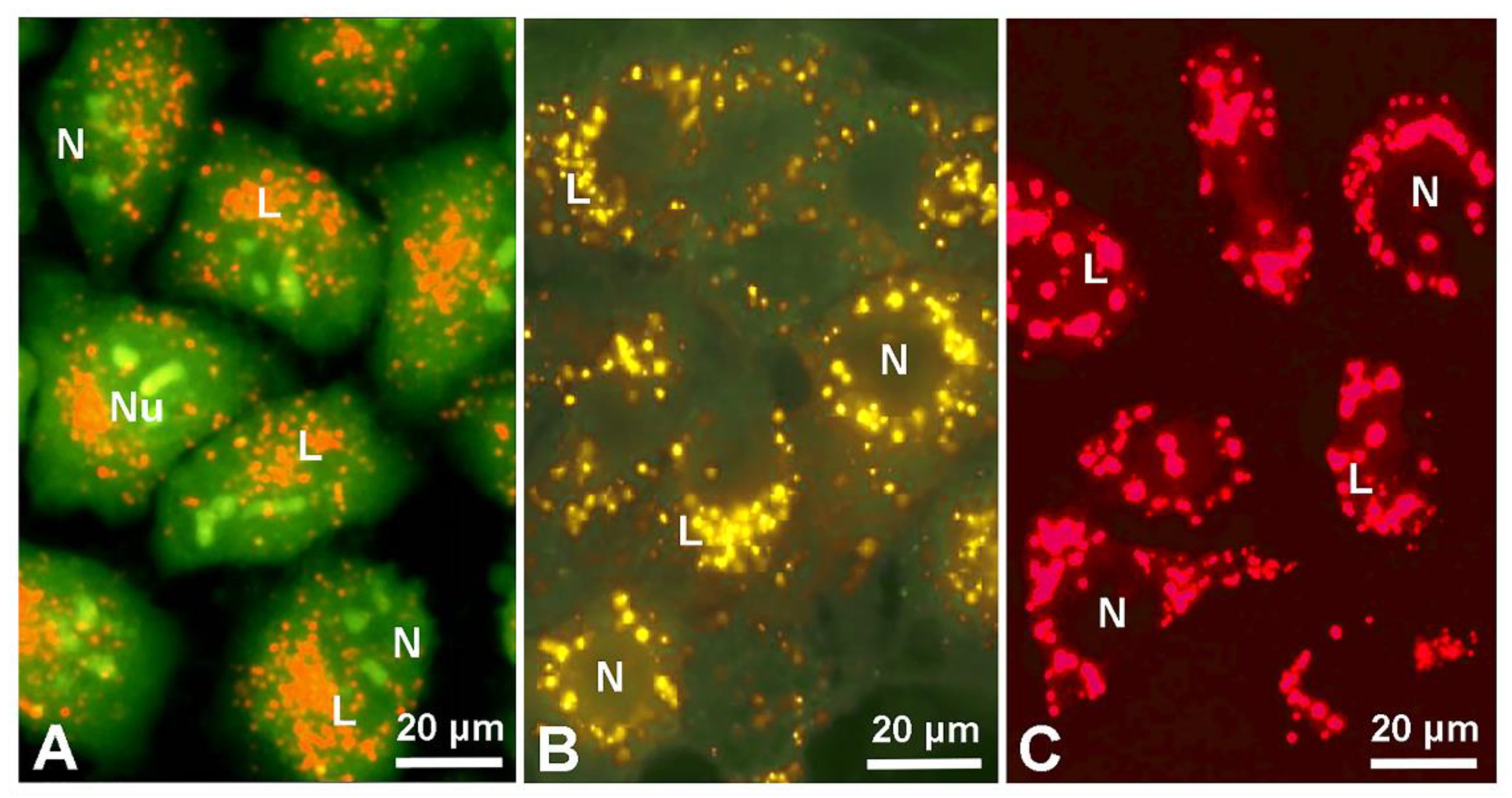
5. Lysosome and Melanosome Labeling
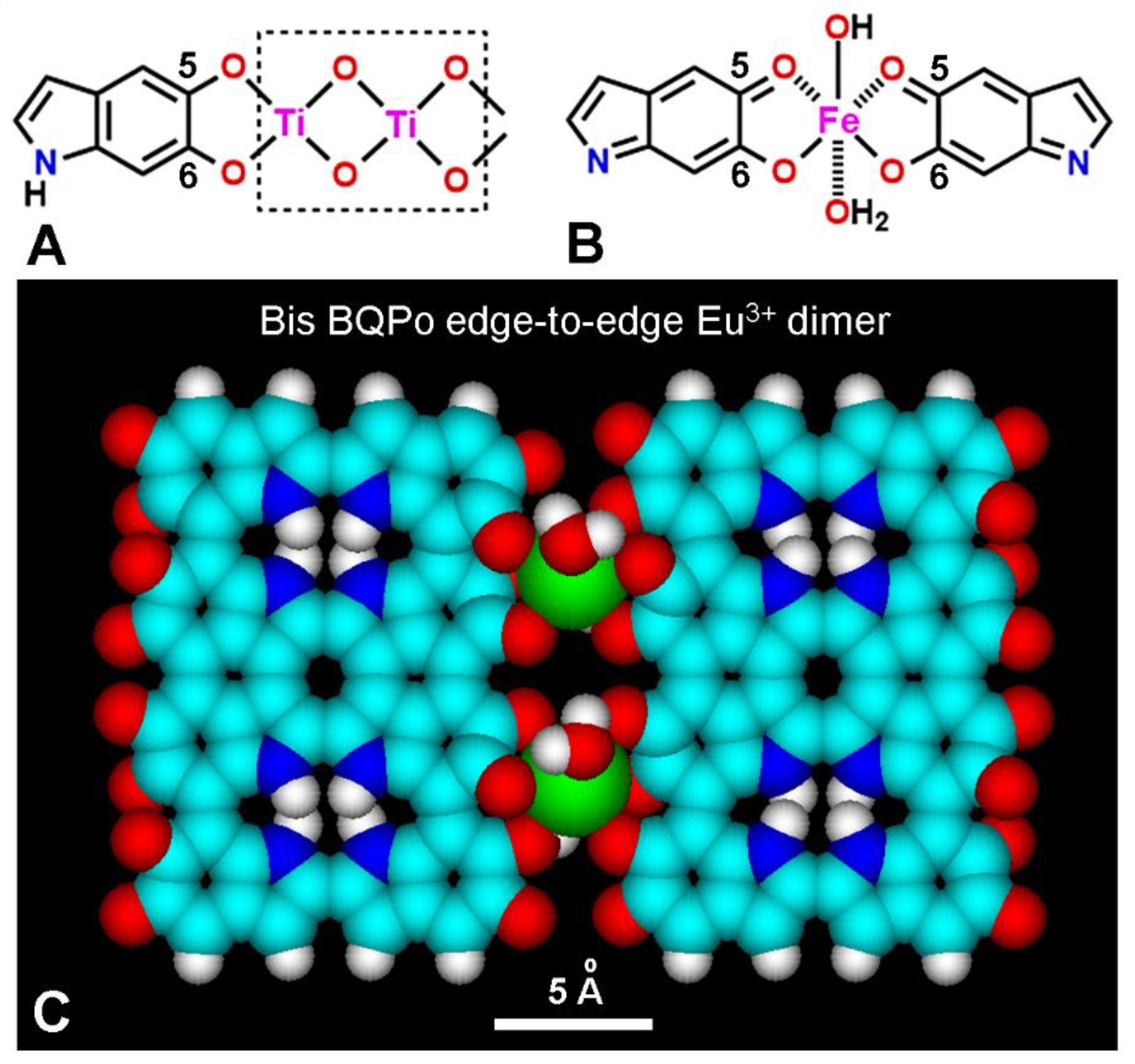
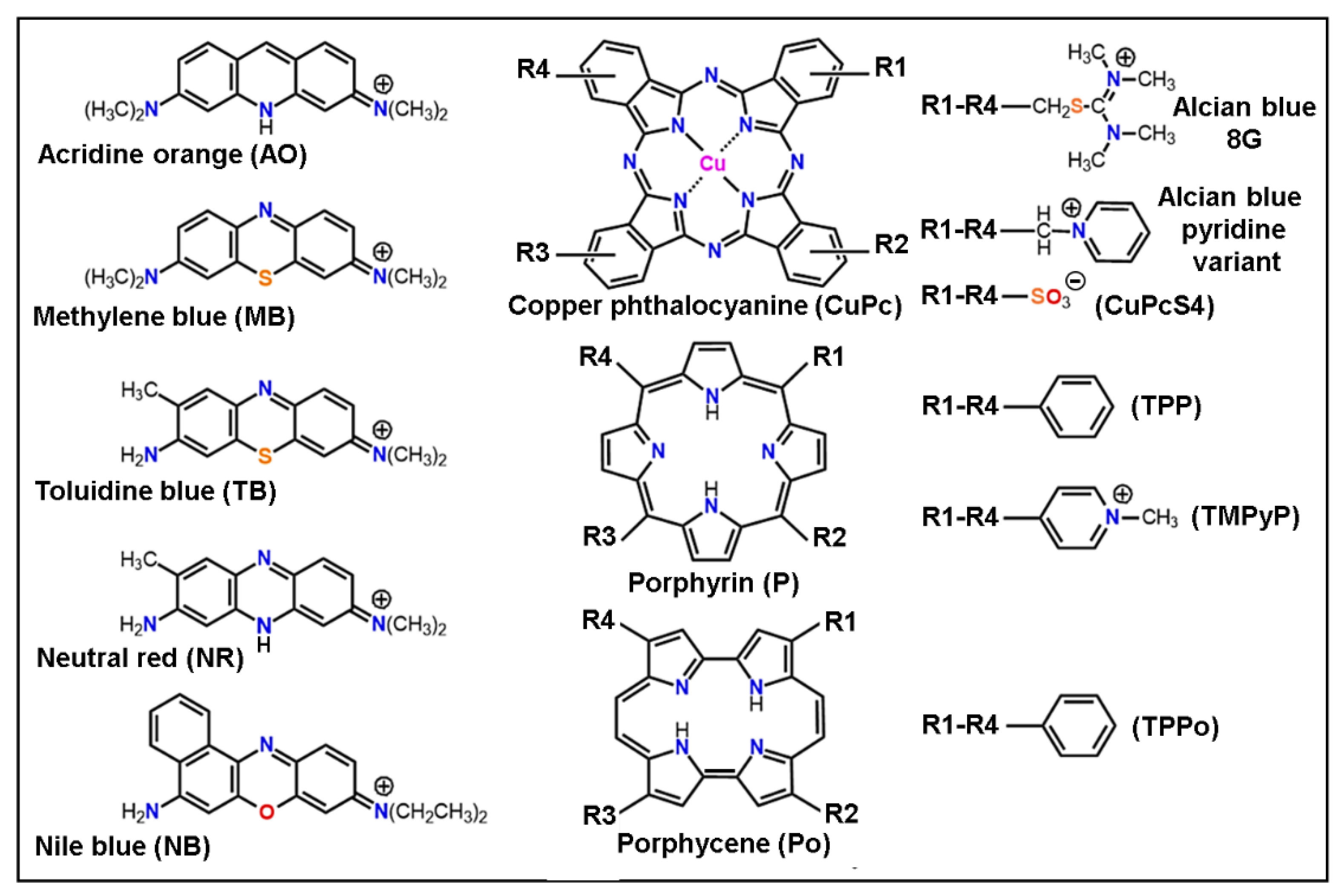
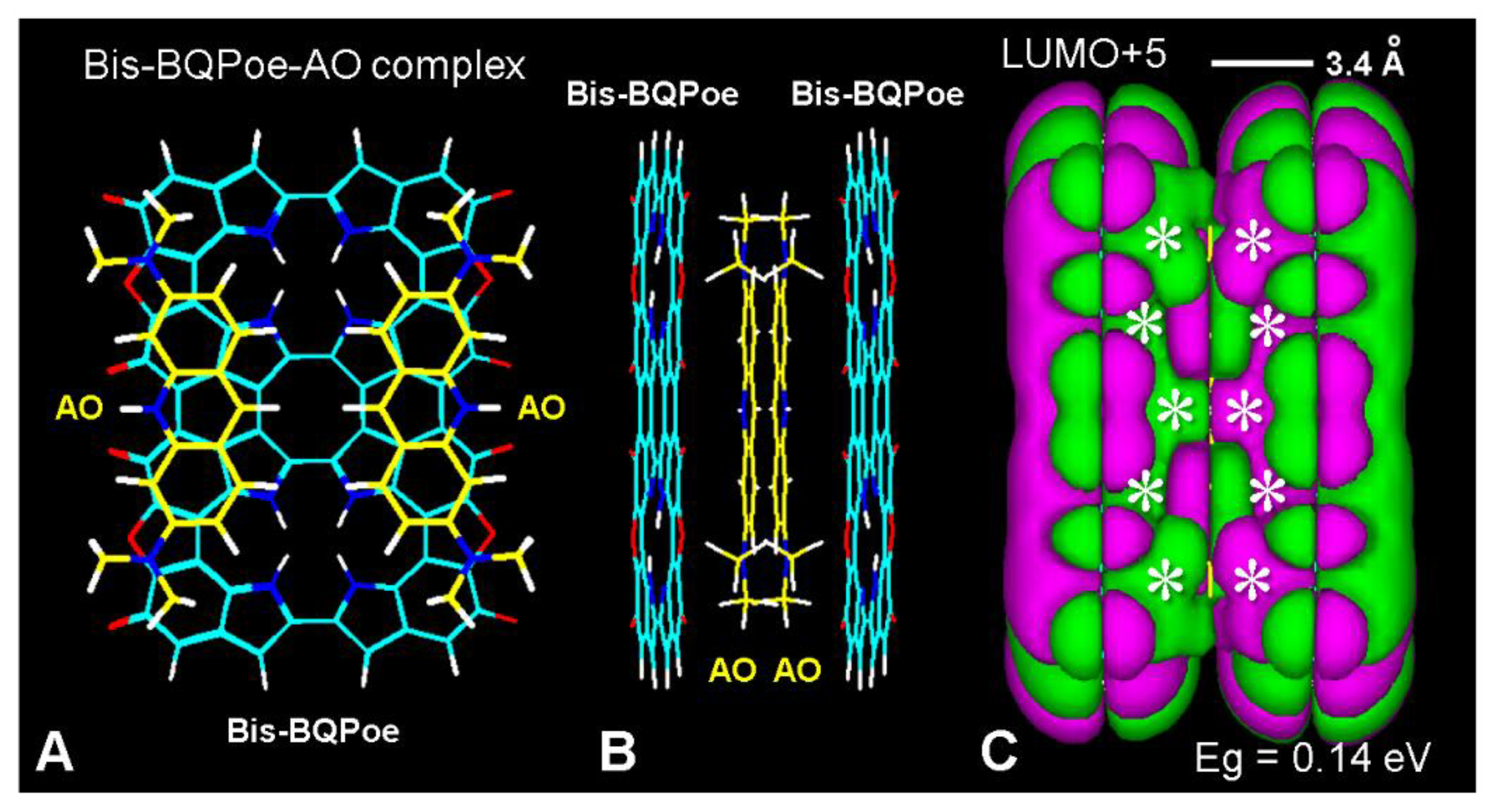
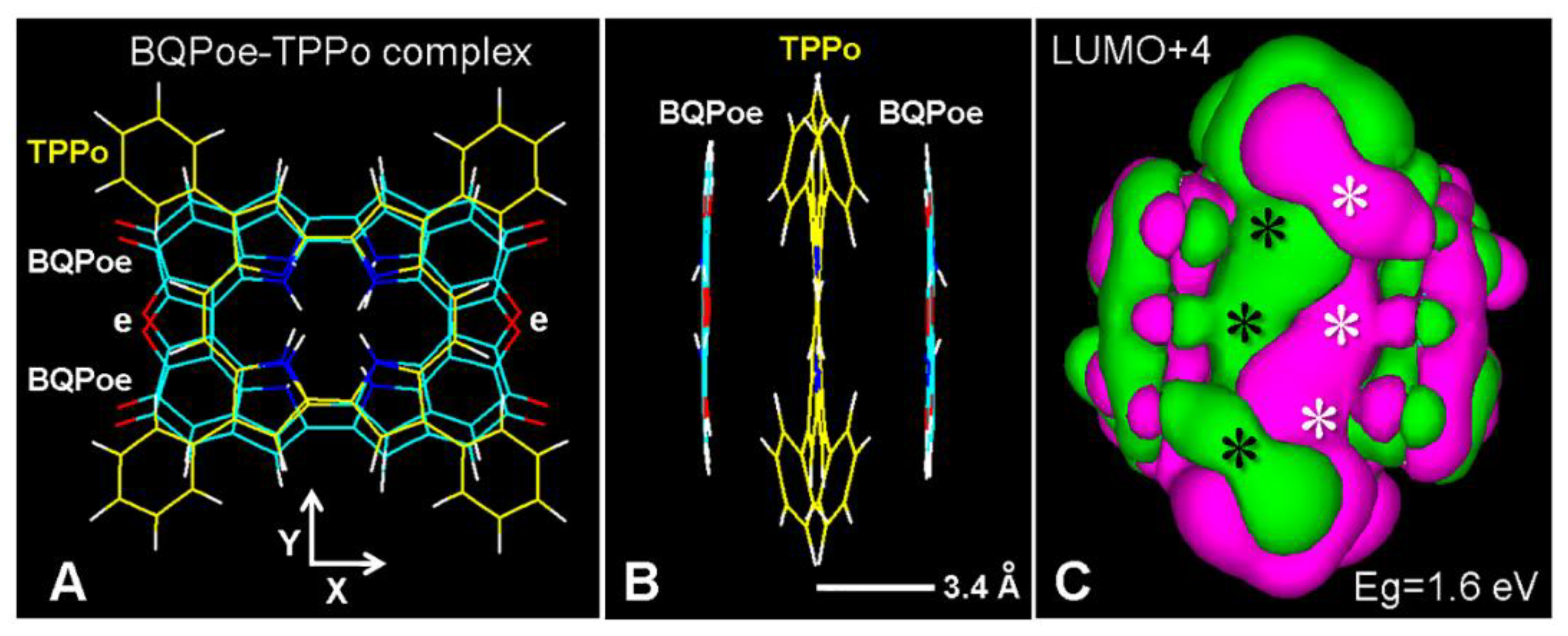
6. Binding of Colorants and Drugs to Melanin
7. Melanoma and Ultrasound Therapy
8. Biomedical Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Towns, A. Colorants: General survey. Phys. Sci. Rev. 2019, 4, 1–17. [Google Scholar] [CrossRef]
- Harriman, A. Colorants: A new journal bringing colour to life. Colorants 2022, 1, 1. [Google Scholar] [CrossRef]
- Wainwright, M. The use of dyes in modern biomedicine. Biotech. Histochem. 2003, 78, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Green, F.J. The Sigma-Aldrich Handbook of Stains, Dyes and Indicators; Aldrich Chemical Company: Milwaukee, WI, USA, 1990. [Google Scholar]
- Horobin, R.W.; Kiernan, J.A. Conn’s Biological Stains. A handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine, 10th ed.; Bios Scientific Publishers: Oxford, UK, 2002. [Google Scholar]
- Whitby, G.S. Dyes and dye chemistry fight disease. Text. Colorist 1942, 64, 119–121. [Google Scholar]
- Lillie, R.D. H.J. Conn’s Biological Stains, 9th ed.; Williams & Wilkins: Baltimore, MD, USA, 1977. [Google Scholar]
- Kafarski, P.; Lipok, M. Structural analogy—Direct similarity versus topographical complementarity. In Drug Discovery and Development. From Molecules to Medicine; Vallisuta, O., Ed.; IntechOpen Book Series; IntechOpen Limited: London, UK, 2015; Chapter 11. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Makler, M.T.; Angerhofer, C.K.; Williams, J.A. Antimalarial dyes revisited: Xanthenes, azines, oxazines, and thiazines. Antimicrob. Chemother. 1995, 39, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Denny, W.A. Acridine derivatives as chemotherapeutic agents. Curr. Med. Chem. 2002, 9, 1655–1665. [Google Scholar] [CrossRef]
- Wagner, S.J.; Skripchenko, A. Investigation of photosensitizing dyes for pathogen reduction in red cell suspensions. Biotech. Histochem. 2003, 78, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Sugden, J.K. Photochemistry of dyes and fluorochromes used in biology and medicine: Some physicochemical background and current applications. Biotech. Histochem. 2004, 79, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The development of drugs for treatment of sleeping sickness: A historical review. Paras. Vect. 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Dapson, R.W. Dye-tissue interactions: Mechanisms, quantification and bonding parameters for dyes used in biological staining. Biotech. Histochem. 2005, 80, 49–72. [Google Scholar] [CrossRef]
- Prentø, P. Staining of macromolecules: Possible mechanisms and examples. Biotech. Histochem. 2009, 84, 139–158. [Google Scholar] [CrossRef]
- Stockert, J.C.; Abasolo, M.I. Inaccurate chemical structure of dyes and fluorochromes found in the literature can be problematic for teaching and research. Biotech. Histochem. 2011, 86, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Horobin, R.W.; Stockert, R.W.; Rashid-Doubell, F. Uptake and localization mechanisms of fluorescent and colored lipid probes. Part 2. QSAR models that predict localization of fluorescent probes used to identify (“specifically stain”) various biomembranes and membranous organelles. Biotech. Histochem. 2015, 90, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Blázquez-Castro, A. Fluorescence Microscopy in Life Sciences; Bentham Science Publishers: Sharjah, United Arab Emirates, 2017. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Cicoira, F.; di Mauro, E.; Garcia-Borron, J.C.; Commo, S. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pig. Cell Melan. Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [PubMed]
- D’Alba, L.; Shawkey, M.D. Melanosomes: Biogenesis, properties, and evolution of an ancient organelle. Physiol. Rev. 2019, 99, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Zhou, X.; McCallum, N.C.; Hu, Z.; Cao, W.; Gnanasekaran, K.; Feng, Y.; Stoddart, J.F.; Wang, Z.; Gianneschi, N.C. Artificial allomelanin nanoparticles. ACS Nano 2019, 13, 10980–10990. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, L.M. Melanin: A unique biopolymer. Pathobiol. Annu. 1971, 1, 309–324. [Google Scholar] [PubMed]
- Swan, G.A. Structure, chemistry, and biosynthesis of the melanins. Forts. Chem. Organ. Naturs. 1974, 31, 522–582. [Google Scholar]
- Simon, J.D.; Hong, L.; Peles, D.N. Insights into melanosomes and melanin from some interesting spatial and temporal properties. J. Phys. Chem. B 2008, 112, 13201–13217. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.L.; Double, K.L.; Gerber, J.P. Using sepia melanin as a PD model to describe the binding characteristics of neuromelanin—A critical review. J. Chem. Neuroanat. 2015, 64–65, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Travan, A.; Turco, G.; Borgogna, M.; Marsich, E.; Pasqua, M.; Paoletti, S.; Donati, I. Adhesive coatings based on melanin-like nanoparticles for surgical membranes. Coll. Surf. B Biointerfaces 2017, 155, 553–559. [Google Scholar] [CrossRef]
- Micillo, R.; Panzella, L.; Iacomino, M.; Prampolini, G.; Cacelli, I.; Ferretti, A.; Crescenzi, O.; Koike, K.; Napolitano, A.; d’Ischia, M. Eumelanin broadband absorption develops from aggregation modulated chromophore interactions under structural and redox control. Sci. Rep. 2017, 7, 41532. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications—Cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and melanin-like hybrid materials in regenerative medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-applications of multifunctional melanin nanoparticles: From nanomedicine to nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Castro, A.; Stockert, J.C. Biomedical overview of melanin. 1. Updating melanin biology and chemistry, physico-chemical properties, melanoma tumors, and photothermal therapy. Biocell 2021, 45, 849–862. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A. Biomedical overview of melanin. 2. Updating molecular modeling, synthesis mechanism, and supramolecular properties regarding melanoma therapy. Biocell 2022, 46, 1391–1415. [Google Scholar] [CrossRef]
- Li, G.; Herlyn, M. Dynamics of intercellular communication during melanoma development. Mol. Med. Today 2000, 6, 163–169. [Google Scholar] [CrossRef]
- Orlow, S.J. Melanosomes are specialized members of the lysosomal lineage of organelles. J. Invest. Dermatol. 1995, 105, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Marks, M.S. Melanosomes—Dark organelles enlighten endosomal membrane transport. Nature Rev. Mol. Cell Biol. 2007, 8, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Goldschmidt, M.H.; McManus, P.M. A comparative review of melanocytic neoplasms. Vet. Pathol. 2002, 39, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Phagocytosis of melanin particles by human epidermal cells in vitro. J. Invest. Dermatol. 1968, 50, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Overwijk, W.W.; Restifo, N.P. B16 as a mouse model for human melanoma. Curr. Prot. Immunol. 2000, 39, 20–21. [Google Scholar] [CrossRef]
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer: Utility and limitations. Drug Des. Develop. Ther. 2014, 8, 1911–1922. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Nguyen, F.D.; Noory, M.A.; Sharma, A. Current state of animal (mouse) modeling in melanoma research. Cancer Growth Metast. 2015, 8s1, 81–94. [Google Scholar] [CrossRef]
- Sniegocka, M.; Podgórska, E.; Płonka, P.M.; Elas, M.; Romanowska-Dixon, B.; Szczygieł, M.; Zijewski, M.A.; Cichorek, M.; Markiewicz, A.; Brozyna, A.A.; et al. Transplantable melanomas in hamsters and gerbils as models for human melanoma. Sensitization in melanoma radiotherapy—From animal models to clinical trials. Int. J. Mol. Sci. 2018, 19, 1048. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.M.E.; Agnetti, L.; Fondello, C.; Glikin, G.C. Combination of cytokine-enhanced vaccine and chemo-gene therapy as surgery adjuvant treatments for spontaneous canine melanoma. Gene Ther. 2019, 26, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Nasiell, K.; Tani, E.; Skoog, L. Fine needle aspiration cytology and immunocytochemistry of metastatic melanoma. Cytopathology 1991, 2, 137–147. [Google Scholar] [CrossRef]
- Olivieri, M.; Nicolaus, R.A. Sulla DHI-melanina. Rend. Accad. Sci. Fis. Matem. Napoli 1999, 66, 85–96. [Google Scholar]
- Nicolaus, R.A. Perspectives in Melanin Chemistry. 2005. Available online: http://www.tightrope.it/nicolaus/link%2023.htm (accessed on 7 December 2018).
- Stockert, J.C. Melanin and melanoma: Updating molecular structure and photothermal therapy. InVet 2021, 23, 1–15. Available online: http://www.fvet.uba.ar/archivos/publicaciones/invet/vol23-1-2021/art-4-vol23-1-2021.pdf (accessed on 15 February 2022).
- Li, X.Y.; Tan, L.C.; Dong, L.W.; Shen, X.X.; Lu, X.; Zheng, H.; Lu, Y.G. Susceptibility and resistance mechanisms during photodynamic therapy of melanoma. Front. Oncol. 2020, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Cranford, S. The ABCs of review articles. Matter 2021, 4, 1–3. [Google Scholar] [CrossRef]
- Prota, G. Pigment Cell Research: What directions? Pigm. Cell Res. 1997, 10, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Bridelli, M.G. Self-assembly of melanin studied by laser light scattering. Biophys. Chem. 1998, 73, 227–239. [Google Scholar] [CrossRef]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef]
- Panzella, L.; Ebato, A.; Napolitano, A.; Koike, K. The late stages of melanogenesis: Exploring the chemical facets and the application opportunities. Int. J. Mol. Sci. 2018, 19, 1753. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigm. Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Kaxiras, E.; Tsolakidis, A.; Zonios, G.; Meng, S. Structural model of eumelanin. Phys. Rev. Lett. 2006, 97, 218102. [Google Scholar] [CrossRef]
- Meng, S.; Kaxiras, E. Theoretical models of eumelanin protomolecules and their optical properties. Biophys. J. 2008, 94, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.S.; Caldas, M.J. Theoretical investigation of model polymers for eumelanins. I. Finite and infinite polymers. J. Chem. Phys. 1990, 92, 2630–2636. [Google Scholar] [CrossRef]
- Bridelli, M.G.; Crippa, P.R.; Ugozzoli, F. X-ray diffraction studies on melanins in lyophilized melanosomes. Pigm. Cell Res. 1990, 3, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Moss, S.C.; Eisner, M. X-ray characterization of melanins-II. Pigm. Cell Res. 1994, 7, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Watt, A.A.R.; Bothma, J.P.; Meredith, P. The supramolecular structure of melanin. Soft Matter. 2009, 5, 3754–3760. [Google Scholar] [CrossRef]
- Chen, C.T.; Ball, V.; de Almeida Gracio, J.J.; Singh, M.K.; Toniazzo, V.; Ruch, D.; Buehler, M.J. Self-assembly of tetramers of 5,6–dihydroxyindole explains the primary physical properties of eumelanin: Experiment, simulation, and design. ACS Nano 2013, 7, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Direct observation of the tetrahedral bonding in graphitized carbon black by high resolution electron microscopy. J. Crystal Growth 1980, 50, 675–683. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef]
- Diudea, M.V.; Bende, A.; Nagy, C.L. Carbon multi-shell cages. Phys. Chem. Chem. Phys. 2014, 16, 5260–5269. [Google Scholar] [CrossRef]
- Kroto, H.W.; McKay, K. The formation of quasi-icosahedral spiral shell carbon particles. Nature 1988, 331, 328–331. [Google Scholar] [CrossRef]
- Kroto, H.W. Fullerene cage clusters. The key to the structure of solid carbon. J. Chem. Soc. Faraday Trans. 1990, 86, 2465–2468. [Google Scholar] [CrossRef]
- Hamzah, M.A.N.; Johari, Z.; Hamid, F.K.A.; Ahmadi, M.T.; Ismail, R. Geometry effect on graphene nanoscrolls band gap. J. Comput. Theoret. Nanosci. 2013, 10, 581–586. [Google Scholar] [CrossRef]
- Khaledian, M.; Ismail, R.; Akbari, E. Band structures of graphene nanoscrolls and their dispersion relation near the Fermi point. RSC Adv. 2016, 6, 38753–38760. [Google Scholar] [CrossRef]
- Hedayat, S.N.; Ahmadi, M.T.; Sedghi, H.; Goudarzi, H. Quantum transmission modelling in graphene nano scrolls (GNSs) of double barrier. Int. J. New Tech. Res. 2017, 3, 12–19. [Google Scholar]
- Norouzi, S.; Fakhrabadi, M.M.S. Nanomechanical properties of single- and double-layer graphene spirals: A molecular dynamics simulation. Appl. Phys. A 2019, 125, 321. [Google Scholar] [CrossRef]
- Ahmadi, M.T.; Ahmadi, R.; Nguyen, T.K. Graphene nanoscroll geometry effect on transistor performance. J. Electr. Mater. 2019, 49, 544–550. [Google Scholar] [CrossRef]
- Longuet-Higgins, H.C. On the origin of the free radical property of melanins. Arch. Biochem. Biophys. 1960, 86, 231–232. [Google Scholar] [CrossRef]
- Büngeler, A.; Hämisch, B.; Strube, O.I. The supramolecular buildup of eumelanin: Structures, mechanisms, controllability. Int. J. Mol. Sci. 2017, 18, 1901. [Google Scholar] [CrossRef]
- Zeise, L.; Addison, R.B.; Chedekel, M.R. Bio-analytical studies of eumelanins. I. Characterization of melanin the particle. Pigm. Cell Res. 1992, 3 (Suppl. 2), 48–53. [Google Scholar] [CrossRef]
- Zajac, G.W.; Gallas, J.M.; Cheng, J.; Eisner, M.; Moss, S.C.; Alvarado-Swaisgood, A.E. The fundamental unit of synthetic melanin: A verification by tunneling microscopy of X-ray scattering results. Biochim. Biophys. General Subj. 1994, 1199, 271–278. [Google Scholar] [CrossRef]
- Zecca, L.; Bellei, C.; Costi, P.; Albertini, A.; Monzani, E.; Casella, L.; Gallorini, M.; Bergamaschi, L.; Moscatelli, A.; Turro, N.J.; et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc. Nat. Acad. Sci. USA 2008, 105, 17567–17572. [Google Scholar] [CrossRef]
- Sulzer, D.; Cassidy, C.; Horga, G.; Kang, U.J.; Fahn, S.; Casella, L.; Pezzoli, G.; Langley, J.; Hu, X.P.; Zucca, F.A.; et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Parkinson’s Dis. 2018, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Mostert, A.B. Melanin, the what, the why and the how: An introductory review for materials scientists interested in flexible and versatile polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Nicolaus, R.A. Coloured organic semiconductors: Melanins. Rend. Accad. Sci. Fis. Matem. Napoli 1997, 64, 325–360. [Google Scholar]
- Meredith, P.; Riesz, J. Radiative relaxation quantum yields for synthetic eumelanin. Photochem. Photobiol. 2004, 79, 211–216. [Google Scholar] [CrossRef]
- Reeb, D.; Best, P.B.; Kidson, S.H. Structure of the integument of southern right whales, Eubalaena australis. Anat. Rec. 2007, 290, 596–613. [Google Scholar] [CrossRef]
- Gallas, J.M.; Eisner, M. Fluorescence of melanin-dependence upon excitation wavelength and concentration. Photochem. Photobiol. 1987, 45, 595–600. [Google Scholar] [CrossRef]
- Kayatz, P.; Thumann, G.; Luther, T.T.; Jordan, J.F.; Bartz-Schmidt, K.U.; Esser, P.J.; Schraermeyer, U. Oxidation causes melanin fluorescence. Invest. Ophthalmol. Vis. Sci. 2001, 42, 241–246. [Google Scholar] [PubMed]
- Elleder, M.; Borovanský, J. Autofluorescence of melanins induced by ultraviolet radiation and near ultraviolet light. A histochemical and biochemical study. Histochem. J. 2001, 33, 273–281. [Google Scholar] [CrossRef]
- Fernandes, B.; Matamá, T.; Guimaraes, D.; Gomes, A.; Cavaco-Paulo, A. Fluorescent quantification of melanin. Pigm. Cell Melan. Res. 2016, 29, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; De Marco, C.; Fontana, M.; Rosei, M.A. Fluorescence properties of melanins from opioid peptides. Arch. Biochem. Biophys. 1999, 371, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Dysli, C.; Wolf, S.; Berezin, M.Y.; Sauer, L.; Hammer, M.; Zinkernagel, M.S. Fluorescence lifetime imaging ophthalmoscopy. Progr. Retin Eye Res. 2017, 60, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Parish, C.A.; Hashimoto, M.; Nakanishi, K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest. Ophthalm. Vis. Sci. 1999, 40, 2988–2995. [Google Scholar]
- Huang, Z.; Zeng, H.; Hamzavi, I.; Alajlan, A.; Tan, E.; McLean, D.I.; Lui, H. Cutaneous melanin exhibiting fluorescence emission under near-infrared light excitation. J. Biomed. Opt. 2006, 11, 034010–034016. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.; Jorge, A.; Ito, K.; Tabuchi, K.; Solano, F.; Wakamatsu, K. Raman spectroscopy as a non-invasive technique for the quantification of melanins in feathers and hairs. Pigm. Cell Melan. Res. 2013, 26, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Falck, B.; Jacobsson, S.; Olivecrona, H.; Rorsman, H. Pigmented nevi and malignant melanomas as studied with a specific fluorescence method. Science 1965, 149, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Rost, F.W.D.; Polak, J.M. Fluorescence microscopy and microspectrofluorimetry of malignant melanomas, naevi and normal melanocytes. Virchows Arch. Abt. A Pathol. Anat. 1969, 347, 321–326. [Google Scholar] [CrossRef]
- Paul, E. Malignant melanoma and nevocellular nevi. Histogenesis and relationships. Fluorescence-microscopic and catamnestic photographic studies. Norm. Pathol. Anat. 1984, 48, 1–112. [Google Scholar]
- Büssow, H.; Baumgarten, H.G.; Hansson, C. The tapetal cell: A unique melanocyte in the tapetum lucidum cellulosum of the cat (Felis domestica L.). Anat. Embryol. 1980, 158, 289–302. [Google Scholar] [CrossRef]
- Lee, E.E.L.; Bezanilla, F. Methodological improvements for fluorescence recordings in Xenopus laevis oocytes. J. Gen. Physiol. 2019, 151, 264–272. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A graphene nanoprobe for rapid, sensitive, and multicolor fluorescent DNA analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Benito-Martínez, S.; Zhu, Y.; Jani, R.A.; Harper, D.C.; Marks, M.S.; Delevoye, C. Research techniques made simple: Cell biology methods for the analysis of pigmentation. J. Invest. Dermatol. 2020, 140, 257–268.e8. [Google Scholar] [CrossRef]
- Hessler, M.; Jalilian, E.; Xu, Q.; Reddy, S.; Horton, L.; Elkin, K.; Manwar, R.; Tsoukas, M.; Mehregan, D.; Avanaki, K. Melanoma biomarkers and their potential application for in vivo diagnostic imaging modalities. Int. J. Mol. Sci. 2020, 21, 9583. [Google Scholar] [CrossRef]
- Thompson, S.W. Selected Histochemical and Histopathological Methods; C.C. Thomas: Springfield, IL, USA, 1996. [Google Scholar]
- Joly-Tonetti, N.; Wibawa, J.I.D.; Bell, M.; Tobin, D. Melanin fate in the human epidermis: A reassessment of how best to detect and analyze histologically. Exp. Dermatol. 2016, 25, 501–504. [Google Scholar] [CrossRef] [PubMed]
- D’ Ischia, M.; Napolitano, A.; Michalczyk-Wetula, D.; Płonka, P.M. Melanin “dust” or “ghost”? Exp. Dermatol. 2016, 25, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Impedance spectroscopy and zeta potential titration of dopa-melanin films produced by oxidation of dopamine. Coll. Surf. A 2010, 363, 92–97. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Molina, D.; Saiz Poseu, J.; Busque, F.; Nador, F.; Mancebo, J. The chemistry behind catechol-based adhesion. Ang. Chem. Int. Ed. 2018, 58, 696–714. [Google Scholar] [CrossRef]
- Chen, L.; Malollari, K.G.; Uliana, A.; Sanchez, D.; Phillip, B.; Messersmith, P.B.; Hartwig, J.F. Selective, catalytic oxidations of C–H bonds in polyethylenes produce functional materials with enhanced adhesion. Chem 2021, 7, 137–145. [Google Scholar] [CrossRef]
- Sarna, T.; Hyde, J.; Swartz, H. Ion-exchange in melanin: An electron spin resonance study with lanthanide probes. Science 1976, 192, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Park, W.; Kim, D.H. Silica-coated metal chelating-melanin nanoparticles as a dual-modal contrast enhancement imaging and therapeutic agent. ACS Appl. Mater. Interf. 2016, 9, 101–111. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Calcagno, V.; Silvestri, B.; Raiola, L.; D’Errico, G.; Luciani, G. 5,6-Dihydroxyindole-2-carboxylic acid–TiO2 charge transfer complexes in the radical polymerization of melanogenic precursor(s). J. Phys. Chem. C 2016, 120, 6262–6268. [Google Scholar] [CrossRef]
- Di Mauro, E.; Xu, R.; Soliveri, G.; Santato, C. Natural melanin pigments and their interfaces with metal ions and oxides: Emerging concepts and technologies. MRS Comm. 2017, 7, 141–151. [Google Scholar] [CrossRef]
- Enochs, W.S.; Petherick, P.; Bogdanova, A.; Mohr, U.; Weissleder, R. Paramagnetic metal scavenging by melanin: MR imaging. Radiology 1997, 204, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Colombo, L.L.; Vanzulli, S.I.; Blázquez-Castro, A.; Sanchez Terrero, C.; Stockert, J.C. Photothermal effect by 808-nm laser irradiation of melanin: A proof-of-concept study of photothermal therapy using B16-F10 melanotic melanoma growing in BALB/c mice. Biomed. Optics Exp. 2019, 10, 2932–2941. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.J.; Wilson, J.W.; Phipps, M.A.; Robles, F.E.; Selim, M.A.; Warren, S. Nonlinear microscopy of eumelanin and pheomelanin with sub-cellular resolution. J. Invest. Dermatol. 2013, 133, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C. Cytochemistry of nucleic acids: Binding mechanisms of dyes and fluorochromes. Biocell 1985, 9, 89–131. [Google Scholar]
- Whittingham, M.S.; Jacobson, A.J. (Eds.) Intercalation Chemistry; Academic Press: New York, NY, USA; London, UK, 1982. [Google Scholar]
- Stockert, J.C.; Cañete, M.; Colman, O.D. Histochemical mechanism for the orthochromatic staining and fluorescence reaction of lignified tissues. Cell. Mol. Biol. 1984, 30, 503–508. [Google Scholar] [PubMed]
- Armas-Portela, R.; Stockert, J.C. A reaction mechanism for the orthochromatic staining of polysaccharides: Monomerizing interactions with basic dyes. Cell. Mol. Biol. 1986, 32, 449-453. [Google Scholar]
- Lerman, L.S. Acridine mutagens and DNA structure. J. Cell. Comp. Physiol. 1964, 64 (Suppl. 1), 1–18. [Google Scholar] [CrossRef]
- Stockert, J.C. Monomerizing effect of caffeine, o-phenanthroline and tannin on cationic dyes: A model system to analyze spectral characteristics of the intercalative binding to nucleic acids. Acta Histochem. 1989, 87, 33–42. [Google Scholar] [CrossRef]
- Stockert, J.C.; Gosálvez, J.; Del Castillo, P.; Pelling, C.; Mezzanotte, R. X-ray microanalysis of toluidine blue stained chromosomes: A quantitative study of the metachromatic reaction of chromatin. Histochemitry 1991, 95, 289–295. [Google Scholar] [CrossRef]
- Barrera, C.; Mazzolli, A.B.; Pelling, C.; Stockert, J.C. Metachromatic staining of human sperm nuclei after reduction of disulphide bonds. Acta Histochem. 1993, 94, 141–149. [Google Scholar] [CrossRef]
- Stockert, J.C.; Lisanti, J.A. Acridine orange differential fluorescence of fast and slow reassociating DNA after in situ DNA denaturation and reassociation. Chromosoma 1972, 37, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Hatton, R.A.; Blanchard, N.P.; Stalojan, V.; Miller, A.J.; Silva, S.R.P. Nanostructured copper phthalocyanine-sensitized multiwall carbon nanotubes films. Langmuir 2007, 23, 6424–6430. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. The molecular biology of histochemical staining by cationic phthalocyanine dyes: The design of replacements for Alcian blue. J. Microsc. 1980, 119, 373–381. [Google Scholar] [CrossRef]
- Juarranz, A.; Cañete, M.; Stockert, J.C. Colour differences in the chromatin staining by cuprolinic blue. Zeit. Mikr. Anat. Forsch. 1987, 101, 532–536. [Google Scholar]
- Tempesti, T.C.; Stockert, J.C.; Durantini, E.N. Photosensitization ability of a water soluble zinc(II) tetramethyltetrapyridino porphyrazinium salt in aqueous solution and biomimetic reverse micelles medium. J. Phys. Chem. B. 2008, 112, 15701–15707. [Google Scholar] [CrossRef]
- Juarranz, A.; Stockert, J.C. Monastral fast blue. Cytochemical properties of a reaction product from Alcian blue stained chromatin. Acta Histochem. 1982, 70, 130–134. [Google Scholar] [CrossRef]
- Scott, J.E. Alcian blue. Now you see it, now you don’t. Eur. J. Oral Sci. 1996, 104, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Macii, F.; Arnaiz, C.P.; Arrico, L.; Busto, N.; Garcia, B.; Biver, T. Alcian blue pyridine variant interaction with DNA and RNA polynucleotides and G-quadruplexes: Changes in the binding features for different biosubstrates. J. Inorg. Biochem. 2020, 212, 111199. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C. Effects of drugs and toxic agents on lysosomes. In Interaction of Drugs and Subcellular Compartments in Animal Cells; Campbell, P.N., Ed.; J. & A. Churchill Ltd.: London, UK, 1968; pp. 218–236. [Google Scholar]
- Allison, A.C.; Young, M.R. Vital staining and fluorescence microscopy of lysosomes. In Lysosomes in Biology and Pathology; Dingle, J.T., Fell, H.B., Eds.; North-Holland Publishing Co.: Amsterdam, The Netherlands; London, UK, 1969; Volume 2, Chapter 22; pp. 600–628. [Google Scholar]
- Pitt, D. Lysosomes and Cell Function; Longman: London, UK; New York, NY, USA, 1975; pp. 24–26. [Google Scholar]
- Swanson, J. Fluorescent labeling of endocytic comartments. Meth. Cell Biol. 1989, 29, 137–151. [Google Scholar]
- Rashid, F.; Horobin, R.W.; Williams, M.A. Predicting the behavior and selectivity of fluorescent probes for lysosomes and related structures by means of structure-activity models. Histochem. J. 1991, 23, 450–459. [Google Scholar] [CrossRef]
- Rice, L.; Wainwright, M.; Phoenix, D.A. Phenothiazine photosensitizers. III. Activity of methylene blue derivatives against pigmented melanoma cell lines. J. Chemother. 2000, 12, 94–104. [Google Scholar] [CrossRef]
- Walker, I.; Gorman, S.A.; Cox, R.D.; Vernon, D.I.; Griffiths, J.; Brown, S.B. A comparative analysis of phenothiazinium salts for the photosensitisation of murine fibrosarcoma (RIF-1) cells in vitro. Photochem. Photobiol. Sci. 2004, 3, 653–659. [Google Scholar] [CrossRef]
- Blázquez-Castro, A.; Stockert, J.C.; Sanz-Rodríguez, F.; Zamarrón, A.; Juarranz, A. Differential photodynamic response of cultured cells to methylene blue and toluidine blue: Role of dark redox processes. Photochem. Photobiol. Sci. 2009, 8, 371–376. [Google Scholar] [CrossRef]
- Horobin, R.W. Can QSAR models describing small-molecule xenobiotics give useful tips for predicting uptake and localization of nanoparticles in living cells? And if not, why not? In Organelle-Specific Pharmaceutical Nanotechnology; Weissig, V., D’Souza, G.G.M., Eds.; Wiley: New York, NY, USA, 2010; pp. 193–206. [Google Scholar]
- Espada, J.; Horobin, R.W.; Stockert, J.C. Fluorescent cytochemistry of acid phosphatase and demonstration of fluid-phase endocytosis using an azo dye method. Histochem. Cell Biol. 1997, 108, 481–487. [Google Scholar] [CrossRef]
- Stockert, J.C.; Juarranz, A.; Villanueva, A.; Nonell, S.; Horobin, R.W.; Soltermann, A.T.; Durantini, E.N.; Rivarola, V.; Colombo, L.L.; Espada, J.; et al. Photodynamic therapy: Selective uptake of photosensitizing drugs into tumor cells. Curr. Top. Pharmacol. 2004, 8, 185–217. [Google Scholar]
- Stockert, J.C.; Cañete, M.; Juarranz, A.; Villanueva, A.; Horobin, R.H.; Borrell, J.I.M.; Teixidó, J.; Nonell, S. Porphycenes: Facts and prospects in photodynamic therapy of cancer. Curr. Med. Chem. 2007, 14, 997–1026. [Google Scholar] [CrossRef]
- Niederau, C.; Van Dyke, R.W.; Scharschmidt, B.F.; Grendell, J.H. Rat pancreatic zymogen granules. An actively acidified compartment. Gastroenterology 1986, 91, 1433–1442. [Google Scholar] [CrossRef]
- De Lisle, R.C.; Steinberg, R.; Williams, J.A. Zymogen granules of mouse parotid acinar cells are acidified in situ in an ATP-dependent manner. Cell Tissue Res. 1988, 253, 267–269. [Google Scholar] [CrossRef]
- Stockert, J.C. The horse eosinophil as a model leucocyte for morphological and cytochemical studies. Braz. J. Morphol. Sci. 2005, 22, 73–84. [Google Scholar]
- García-Díaz, M.; Nonell, S.; Villanueva, A.; Stockert, J.C.; Cañete, M.; Casadó, A.; Mora, M.; Sagristá, M.L. Do folate-receptor targeted liposomal photosensitizers enhance photodynamic therapy selectivity? Biochim. Biophys. Acta 2011, 1808, 1063–1071. [Google Scholar] [CrossRef]
- Villanueva, A.; Durantini, E.N.; Stockert, J.C.; Rello, S.; Vidania, R.; Cañete, M.; Juarranz, A.; Arranz, R.; Rivarola, V. Photokilling of cultured tumour cells by the porphyrin derivative CF3. Anti-Cancer Drug Des. 2001, 16, 279–290. [Google Scholar]
- Ruiz-González, R.; Acedo, P.; Sánchez-García, D.; Nonell, S.; Cañete, M.; Stockert, J.C.; Villanueva, A. Efficient induction of apoptosis in HeLa cells by a novel cationic porphycene photosensitizer. Eur. J. Med. Chem. 2013, 63, 401–414. [Google Scholar] [CrossRef]
- Boix-Garriga, E.; Acedo, P.; Casadó, A.; Villanueva, A.; Stockert, J.C.; Cañete, M.; Mora, M.; Sagristá, M.L.; Nonell, S. Poly(D,L-lactide-co-glycolide) nanoparticles as delivery agents for photodynamic therapy: Enhancing singlet oxygen release and phototoxicity by surface PEG coating. Nanotechnology 2015, 26, 365104. [Google Scholar] [CrossRef]
- Valli, F.; García Vior, M.C.; Roguin, L.P.; Marino, J. Oxidative stress generated by irradiation of a zinc(II) phthalocyanine induces a dual apoptotic and necrotic response in melanoma cells. Apoptosis 2019, 24, 119–134. [Google Scholar] [CrossRef]
- Rashid-Doubell, F.; Horobin, R.W. Selection of fluorescent Golgi complex probes using structure-activity relationship models. In Biotechnology Applications of Microinjection, Microscopic Imaging, and Fluorescence; Bach, P.H., Ed.; Plenum Press: New York, NY, USA, 1991; pp. 73–78. [Google Scholar]
- Ginevra, F.; Biffanti, S.; Pagnan, A.; Biolo, R.; Reddi, E.; Jori, G. Delivery of the tumour photosensitizer zinc(II)-phthalocyanine to serum proteins by different liposomes: Studies in vitro and in vivo. Cancer Lett. 1990, 49, 59–65. [Google Scholar] [CrossRef]
- Bossi, A.; Waluk, J.; Yivlialin, R.; Penconi, M.; Campione, M.; Bussetti, G. Porphycene protonation: A fast and reversible reaction enabling optical transduction for acid sensing. ChemPhotoChem 2020, 4, 5264–5270. [Google Scholar] [CrossRef]
- Rossi, U.A.; Gil-Cardeza, M.L.; Villaverde, M.S.; Finocchiaro, L.M.E.; Glikin, G.C. Interferon-β gene transfer induces a strong cytotoxic bystander effect on melanoma cells. Biomed. Pharmacother. 2015, 72, 44–51. [Google Scholar] [CrossRef]
- Filatovs, J.; McGinness, J.; Corry, P. Thermal and electronic contributions to switching in melanins. Biopolymers 1976, 15, 2309–2312. [Google Scholar] [CrossRef] [PubMed]
- Corry, P.M.; McGinness, J.E.; Armour, E. Semiconductor properties of melanins related to preferential killing of melanoma cells. Pigment Cell 1976, 2, 321–326. [Google Scholar]
- Kinross-Wright, V. Clinical trial of a new phenothiazine compound: NP-207. Psych. Res. Rep. 1956, 4, 89–94. [Google Scholar]
- Burian, H.M.; Fletcher, M.C. Visual functions in patients with retinal pigmentary degeneration following the use of NP 207. AMA Arch. Ophthal. 1958, 60, 612–629. [Google Scholar] [CrossRef]
- Hobbs, H.E.; Sorsby, A.; Freedman, A. Retinopathy following chloroquine therapy. Lancet 1959, 274, 478–480. [Google Scholar] [CrossRef]
- Raghavan, P.R.; Zane, P.A.; Tripp, S.L. Calculation of drug-melanin binding energy using molecular modeling. Experientia 1990, 46, 77–80. [Google Scholar] [CrossRef]
- Lowrey, A.H.; Fameini, G.R.; Loumbev, V.; Wilson, L.Y.; Tosk, J.M. Modeling drug-melanin interaction with theoretical linear solvation energy relationships. Pigm. Cell Res. 1997, 10, 251–256. [Google Scholar] [CrossRef]
- Jakubiak, P.; Cantrill, C.; Urtti, A.; Alvarez-Sánchez, R. Establishment of an in vitro-in vivo correlation for melanin binding and the extension of the ocular half-life of small-molecule drugs. Mol. Pharm. 2019, 16, 4890–4901. [Google Scholar] [CrossRef]
- Hellinen, L.; Bahrpeyma, S.; Rimpelä, A.K.; Hagström, M.; Reinisalo, M.; Urtti, A. Microscale thermophoresis as a screening tool to predict melanin binding of drugs. Pharmaceutics 2020, 12, 554. [Google Scholar] [CrossRef]
- Potts, A.M. Uveal pigment and phenothiazine compounds. Trans. Am. Ophthal. Soc. 1962, 60, 517–552. [Google Scholar]
- Blois, M.S. On chlorpromazine binding in vivo. J. Invest. Dermatol. 1965, 45, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S.; Taskovich, L. The reversible binding of some aromatic and cyclic compounds to biopolymers in vitro. J. Invest. Dermatol. 1969, 53, 344–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindquist, N.G.; Ullberg, S. The melanin affinity of chloroquine and chlorpromazine studied by whole body autoradiography. Acta Pharmacol. Toxicol. 1972, 31, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. The binding properties of melanin: In vivo and in vitro. Adv. Biol. Skin 1972, 12, 65–79. [Google Scholar] [PubMed]
- Lindquist, N.G. Accumulation of drugs on melanin. Acta Radiol. Diagnosis 1973, 325, 1–92. [Google Scholar]
- Larsson, B.S. Melanin-affinic thioureas as selective melanoma seekers. Melanoma Res. 1991, 1, 85–90. [Google Scholar] [CrossRef]
- Larsson, B.S. Interaction between chemicals and melanin. Pigm. Cell Res. 1993, 6, 127–133. [Google Scholar] [CrossRef]
- Oltmanns, D.; Eisenhut, M.; Mier, W.; Haberkorn, U. Benzamides as melanotropic carriers for radioisotopes, metals, cytotoxic agents and as enzyme inhibitors. Curr. Med. Chem. 2009, 16, 2086–2094. [Google Scholar] [CrossRef]
- Kowalska, J.; Banach, K.; Rok, J.; Beberok, A.; Rzepka, Z.; Wrzesniok, D. Molecular and biochemical basis of fluoroquinolones-induced phototoxicity—The study of antioxidant system in human melanocytes exposed to UV-A radiation. Int. J. Mol. Sci. 2020, 21, 9714. [Google Scholar] [CrossRef]
- Rimpelä, A.K.; Garneau, M.; Baum-Kroker, K.S.; Schönberger, T.; Runge, F.; Sauer, A. Quantification of drugs in distinctly separated ocular substructures of albino and pigmented rats. Pharmaceutics 2020, 12, 1174. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, Y.Y.; Lo, Y.H.; Lin, M.H.; Chang, C.H.; Chen, C.L.; Wang, H.E.; Wu, C.Y. Evaluation of radioiodinated fluoronicotinamide/fluoropicolinamide-benzamide derivatives as theranostic agents for melanoma. Int. J. Mol. Sci. 2020, 21, 6597. [Google Scholar] [CrossRef] [PubMed]
- Shaw, H.M.; Balch, C.M.; Soong, S.; Milton, G.W.; McCarthy, W.H. Prognostic histopathological factors in malignant melanoma. Pathology 1985, 17, 271–274. [Google Scholar] [CrossRef]
- Sarna, T.; Swartz, H.M. Identification and characterization of melanin in tissues and body fluids. Folia Histochem. Cytochem. 1978, 16, 275–286. [Google Scholar]
- Dadachova, E.; Casadevall, A. Melanin as a potential target for radionuclide therapy of metastatic melanoma. Future Oncol. 2005, 1, 541–549. [Google Scholar] [CrossRef]
- Panasiewicz, J.; Rybakow, Z.; Kaskiewicz, M.; Wiza, J. Preparation of 35S labelled methylene blue. Radiochem. Radioanal. Lett. 1978, 33, 397–402. [Google Scholar]
- Link, E.; Łukiewicz, S. A new radioactive drug selectively accumulating in melanoma cells. Eur. J. Nucl. Med. 1982, 7, 469–473. [Google Scholar] [CrossRef]
- Napolitano, A.; Palumbo, A.; d’ Ischia, M.; Prota, G. Mechanism of selective incorporation of the melanoma seeker 2-thiouracil into growing melanin. J. Med. Chem. 1996, 39, 5192–5201. [Google Scholar] [CrossRef] [PubMed]
- Link, E.M.; Brown, I.; Carpenter, R.N.; Mitchell, J.S. Uptake and therapeutic effectiveness of 125I- and 211At-methylene blue for pigmented melanoma in an animal model system. Cancer Res. 1989, 49, 4332–4337. [Google Scholar]
- Link, E.M. Targeting melanoma with 211At/131I-Methylene blue: Preclinical and clinical experience. Hybridoma 1999, 18, 77–84. [Google Scholar] [CrossRef]
- Sobal, G.; Rodrigues, M.; Sinzinger, H. Radioiodinated methylene blue—A promising agent for melanoma scintigraphy: Labelling, stability and in vitro uptake by melanoma cells. Anticancer Res. 2008, 28, 3691–3696. [Google Scholar]
- Gardette, M.; Viallard, C.; Paillas, S.; Guerquin-Kern, J.L.; Papon, J.; Moins, N.; Labarre, P.; Desbois, N.; Wong-Wah-Chung, P.; Palle, S.; et al. Evaluation of two 125I-radiolabeled acridine derivatives for Auger-electron radionuclide therapy of melanoma. Invest. New Drugs 2014, 32, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yuan, L.; Gai, Y.; Liu, Q.; Yin, L.; Jiang, Y.; Wang, Y.; Zhang, Y.; Lan, X. Targeted radiotherapy of pigmented melanoma with 131I-5-IPN. J. Exp. Clin. Cancer Res. 2018, 37, 306. [Google Scholar] [CrossRef]
- Wei, W.; Ehlerding, E.B.; Lan, X.; Luo, Q.; Cai, W. PET and SPECT imaging of melanoma: The state of the art. Eur. J. Nucl. Med. Mol. Imag. 2018, 45, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, S.; Calame-Christe, M.; Tanner, H.; Sumanovski, L.; Eberle, A.N. A novel DOTA-α-melanocytestimulating hormone analog for metastatic melanoma diagnosis. J. Nucl. Med. 2002, 43, 1699–1706. [Google Scholar]
- Bigliardi, P.L.; Rout, B.; Pant, A.; Krishnan-Kutty, V.; Eberle, A.N.; Srinivas, R.; Burkett, B.A.; Bigliardi-Qi, M. Specific targeting of melanotic cells with peptide ligated photosensitizers for photodynamic therapy. Sci. Rep. 2017, 7, 15750. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Wickramasinghe, H.K.; Lemons, R.A. Acoustic microscopy of the human retina and pigment epithelium. Invest. Ophthal. Visual Sci. 1977, 16, 660–666. [Google Scholar]
- Kou, J.Y.; Li, Y.; Zhong, Z.Y.; Jiang, Y.Q.; Li, X.S.; Han, X.B.; Liu, Z.N.; Tian, Y.; Yang, L.M. Berberine-sonodynamic therapy induces autophagy and lipid unloading in macrophage. Cell Death Dis. 2017, 8, e2558. [Google Scholar] [CrossRef] [PubMed]
- An, Y.W.; Jin, H.T.; Wang, Y.B.; Wang, J.C.; Liu, H.Q. Research progress of berberine mediated photodynamic therapy (Review). Oncol. Lett. 2021, 21, 359. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Ogawa, K.; Irie, Y.; Endo, H.; Feril, L.B.; Uemura, T.; Tachibana, K. Ultrasound activation of TiO2 in melanoma tumors. J. Contr. Release 2011, 149, 190–195. [Google Scholar] [CrossRef]
- Kono, R.; Yamaoka, T.; Yoshizaki, H.; McGinness, J. Anomalous absorption and dispersion of sound waves in diethylamine melanin. J. Appl. Phys. 1979, 50, 1236. [Google Scholar] [CrossRef]
- McGinness, J.E.; Corry, P.M.; Armour, E. Melanin-binding drugs and ultrasonic-induced cytotoxicity. Pigment Cell 1976, 2, 316–320. [Google Scholar]
- Lacy, M.E. Phonon-electron coupling as a possible transducing mechanism in bioelectronics processes involving neuromelanins. J. Theor. Biol. 1984, 111, 201–204. [Google Scholar] [CrossRef]
- Crippa, P.R.; Martini, F.; Viappiani, C. Direct evidence of electron-phonon interaction in melanins. J. Photochem. Photobiol. B Biol. 1991, 11, 371–375. [Google Scholar] [CrossRef]
- Migliaccio, A.; Gryszel, M.; Ðerek, V.; Pezzella, A.; Głowacki, E.D. Aqueous photo(electro)catalysis with eumelanin thin films. Mater. Horiz. 2018, 5, 984. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016, 6, 62–72. [Google Scholar] [CrossRef]
- Wang, Y.; You, W.; Li, X. Current status of gene therapy in melanoma treatment. Biocell 2020, 44, 167–174. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, H.; Shi, W.; Gan, L.; Zhong, L.; He, J.; Xie, L.; Wu, P.; Zhao, Y.; Deng, Z.; et al. Application of photodynamic therapy in cancer: Challenges and advancements. Biocell 2021, 45, 489–500. [Google Scholar] [CrossRef]
- Yoshino, K.; Kotaka, M.; Okamoto, M.; Kakihana, H. 11B-NMR study of the complex formation of borate with catechol and L-DOPA. Bull. Chem. Soc. Japan 1979, 52, 3005–3009. [Google Scholar] [CrossRef]
- Davids, L.M.; Kleemann, B.; Kacerovská, D.; Pizinger, K.; Kidson, S.H. Hypericin phototoxicity induces different modes of cell death in melanoma and human skin cells. J. Photochem. Photobiol. B Biol. 2008, 91, 67–76. [Google Scholar] [CrossRef]
- Theodossiou, T.; Spiro, M.D.; Jacobson, J.; Hothersall, J.S.; MacRobert, A.J. Evidence for intracellular aggregation of hypericin and the impact on its photocytotoxicity in PAM 212 murine keratinocytes. Photochem. Photobiol. 2004, 80, 438–443. [Google Scholar] [CrossRef]







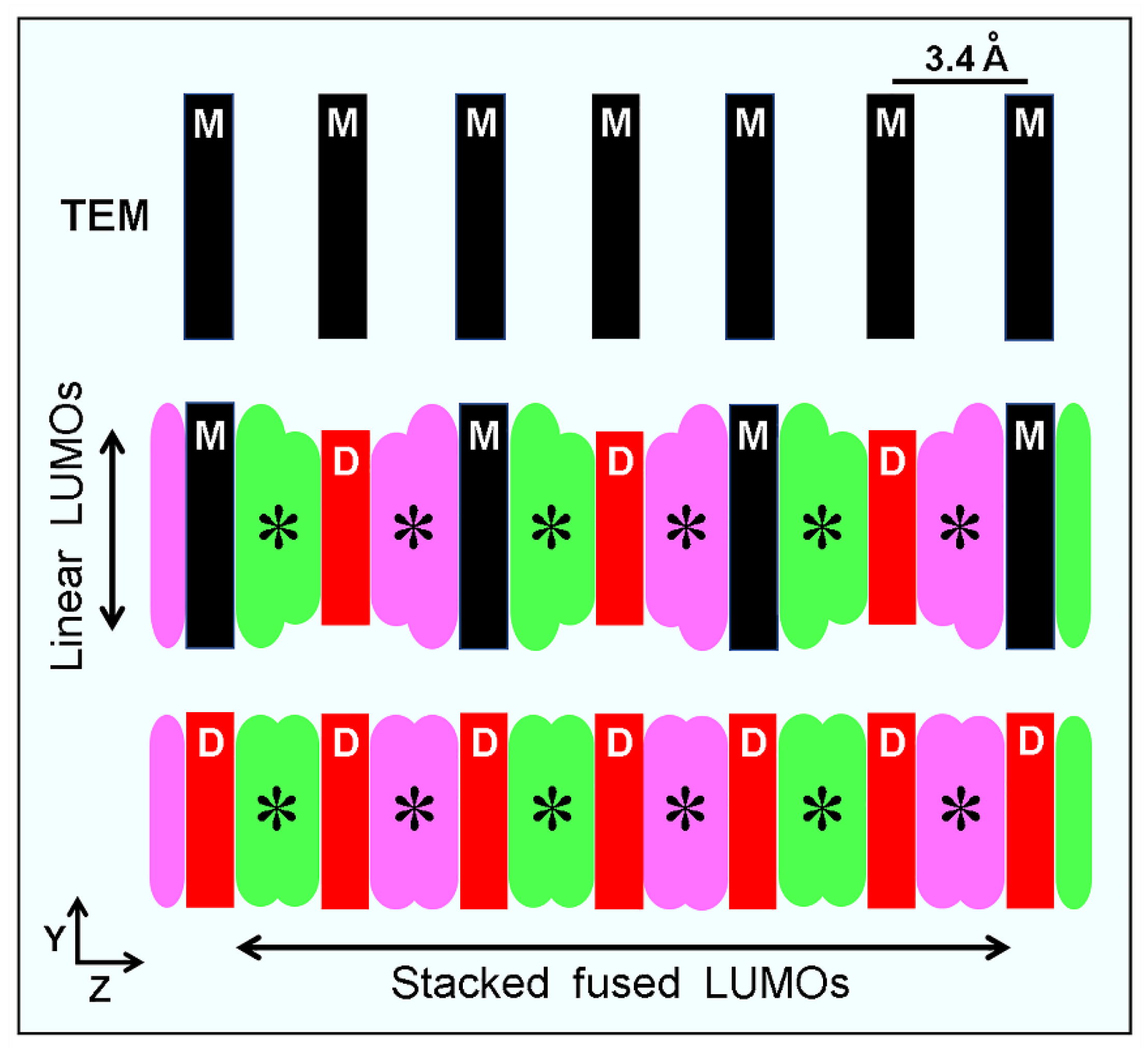
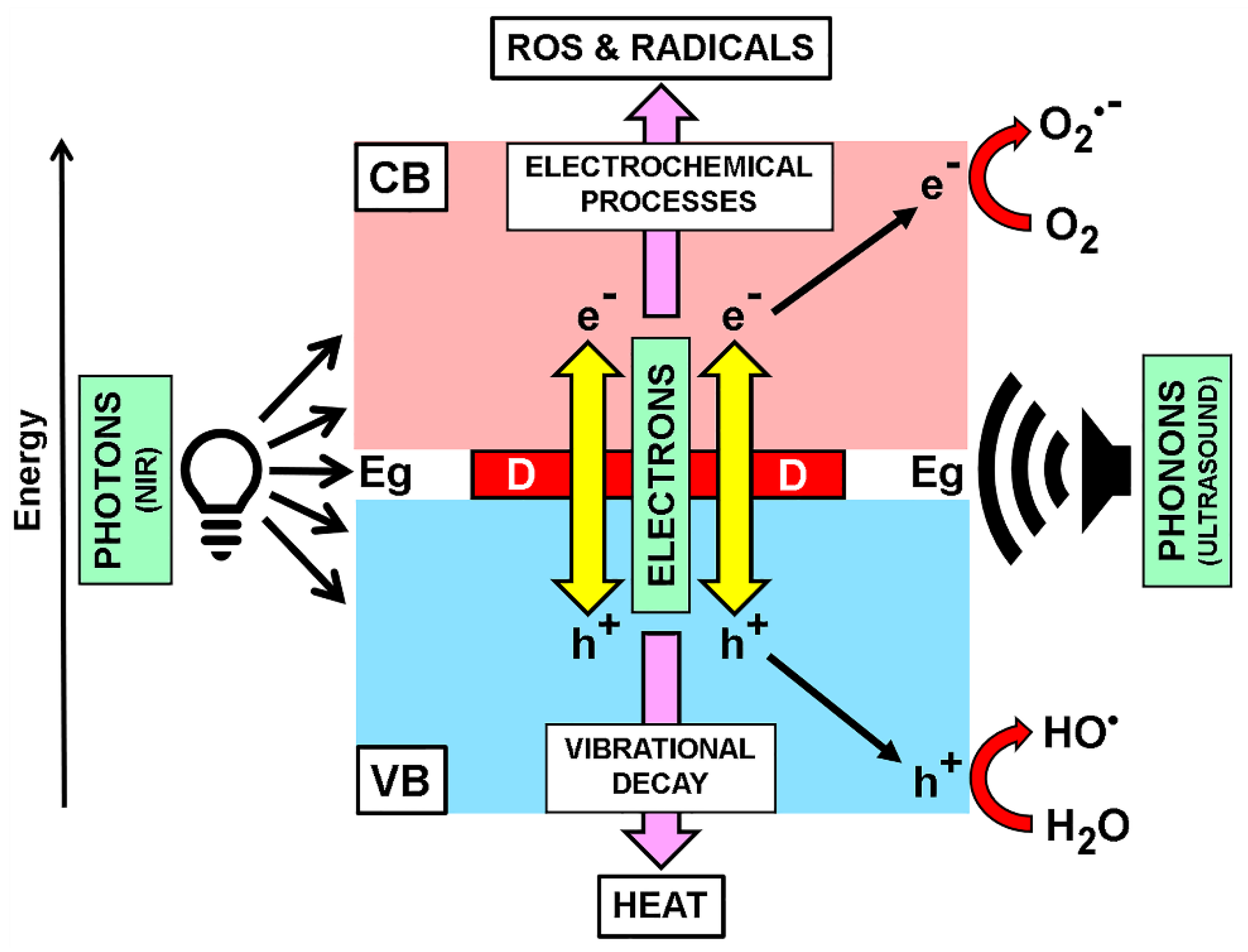
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stockert, J.C.; Espada, J.; Blázquez-Castro, A. Melanin-Binding Colorants: Updating Molecular Modeling, Staining and Labeling Mechanisms, and Biomedical Perspectives. Colorants 2022, 1, 91-120. https://doi.org/10.3390/colorants1010007
Stockert JC, Espada J, Blázquez-Castro A. Melanin-Binding Colorants: Updating Molecular Modeling, Staining and Labeling Mechanisms, and Biomedical Perspectives. Colorants. 2022; 1(1):91-120. https://doi.org/10.3390/colorants1010007
Chicago/Turabian StyleStockert, Juan C., Jesús Espada, and Alfonso Blázquez-Castro. 2022. "Melanin-Binding Colorants: Updating Molecular Modeling, Staining and Labeling Mechanisms, and Biomedical Perspectives" Colorants 1, no. 1: 91-120. https://doi.org/10.3390/colorants1010007
APA StyleStockert, J. C., Espada, J., & Blázquez-Castro, A. (2022). Melanin-Binding Colorants: Updating Molecular Modeling, Staining and Labeling Mechanisms, and Biomedical Perspectives. Colorants, 1(1), 91-120. https://doi.org/10.3390/colorants1010007