A Review of Natural Fibers: Classification, Composition, Extraction, Treatments, and Applications
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Selection Process
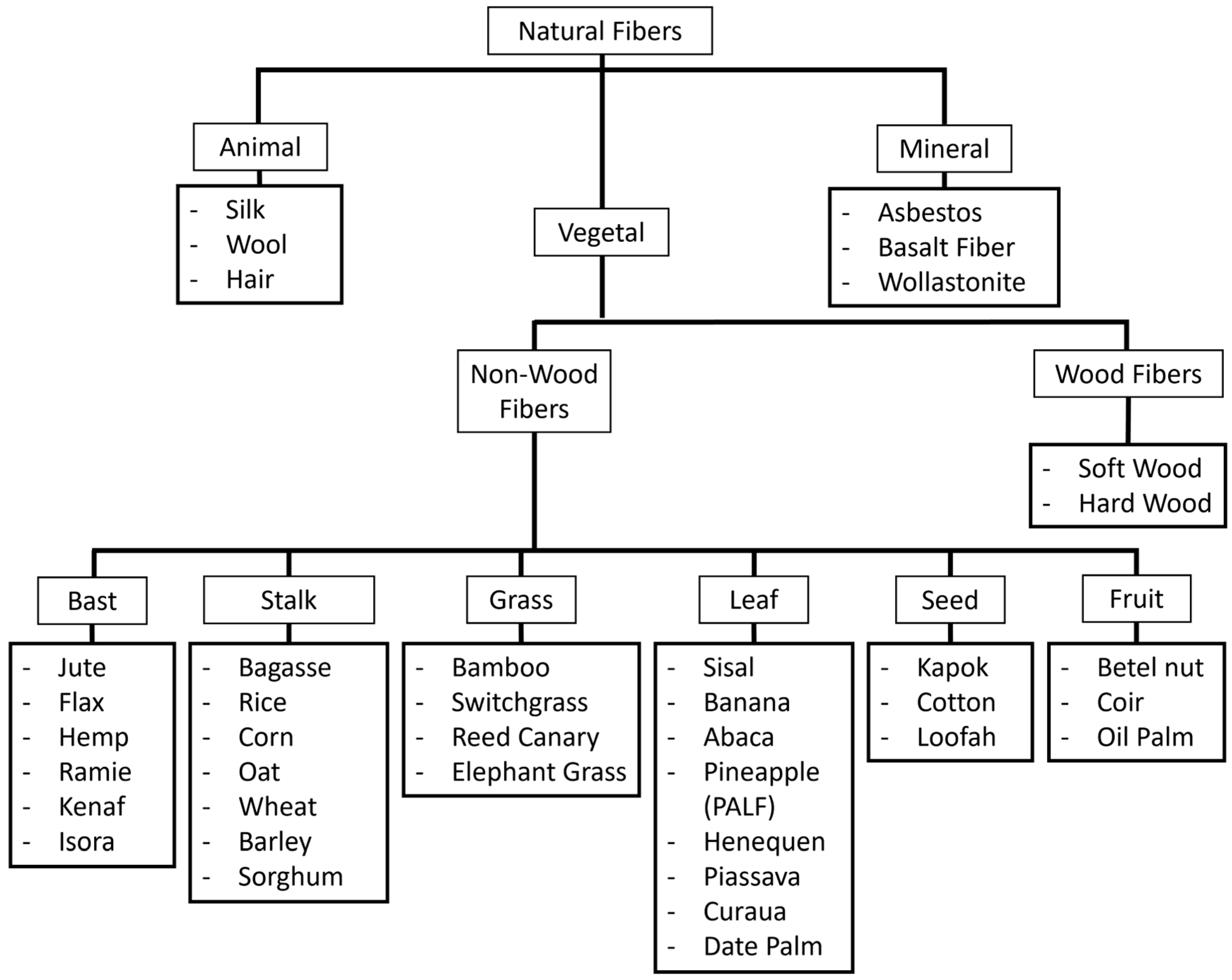
3. Classification of Natural Fibers
3.1. Animal Fibers
3.2. Mineral Fibers
3.3. Vegetal Fibers
4. Fiber Morphology, Composition, and Physical Properties
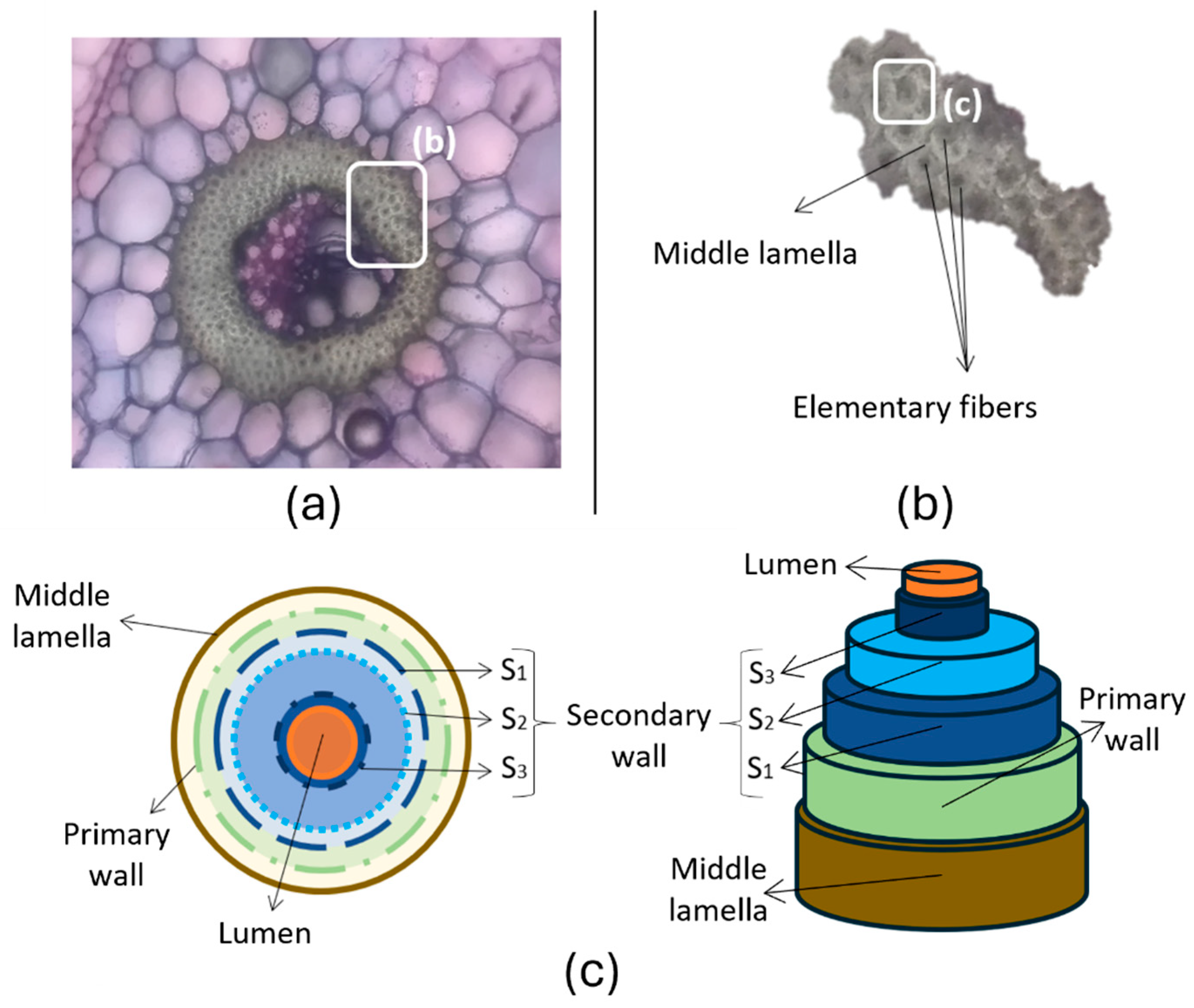
4.1. Morphology
4.2. Composition
4.2.1. Cellulose
4.2.2. Hemicellulose
4.2.3. Lignin
4.2.4. Pectins
4.2.5. Waxes
| Fiber Name | Cellulose (wt%) | Hemicellulose (wt%) | Lignin (wt%) | Extractables (wt%) | Ashes (wt%) | References |
|---|---|---|---|---|---|---|
| Abaca | 56–64 | 15–25 | 7–13 | 0.8–3 | 3 | [43,69,70,99] |
| Bagasse | 32–55.2 | 16.8–30 | 19–25.3 | 10 | - | [30,31,70,99] |
| Bamboo | 26–65 | 30 | 1–31 | - | - | [31,43,70,99] |
| Banana | 60–83 | 6–16 | 5–10 | 3–5 | - | [30,63,70,99] |
| Coir | 32–43 | 0.15–20 | 40–45 | 3–4 | - | [43,63,70,99] |
| Cotton | 75–92 | 2–5.7 | 0.1–2 | 0.1–0.6 | 0.8–2 | [43,63,99] |
| Curaua | 70.7–74 | 9.9–21 | 7.5–11 | 0.2–10 | - | [31,43,70,99] |
| Flax | 60–81 | 14–20.6 | 0.9–2.3 | 0.9–2.3 | - | [43,70,100,101] |
| Hemp | 57–90 | 14–22.4 | 3.7–13 | 0.8–0.9 | 0.8 | [31,63,99,100] |
| Jute | 45–84 | 12–22 | 5–26 | 0.2–5 | 0.5–2 | [43,70,100] |
| Kenaf | 31–72 | 8–21 | 8–21.5 | 0.3–5 | 2–5 | [43,70,100,102] |
| Mesta Jute | 60 | 15 | 10 | - | - | [88] |
| Nettle | 79–86 | 6.5–12.5 | 3.5–4.4 | 4 | - | [43,63,99] |
| Phormium tenax | 45.1–72 | 30.1 | 11.2 | 0.7 | - | [43] |
| Pineapple Leaf | 70–83 | 16–19 | 5–12.7 | 2–2.5 | - | [43,63,99] |
| Ramie | 68.6–91 | 5–16.7 | 0.5–1 | 0.3–10 | - | [30,31,100] |
| Rice Straw | 36–57 | 33 | 8–19 | - | - | [31,43,70,88] |
| Rice Husk | 35–45 | 12–25 | 20 | 14–17 | - | [31,43,70,88] |
| Roselle | 70.2 | 7.21 | 14.91 | - | - | [88,103] |
| Softwood | 30–60 | 20–30 | 21–37 | - | - | [88] |
| Hardwood | 31–64 | 25–42 | 14–34 | - | - | [88] |
| Sisal | 43–78 | 10–24 | 4–14 | 0.4–10 | 0.6–1 | [43,63,100] |
| Wheat straw | 38–51 | 15–31 | 12–20 | - | - | [31,43,70] |
| Luffa aegyptiaca | 63 | 19.4 | 11.2 | 3 | - | [63] |
| Crotalaria juncea | 41–48 | 8.3–13 | 22.7 | - | - | [63] |
| Sansevieria cylindrica | 79.7 | 10.13 | 3.8 | 0.09 | - | [43] |
| Sansevieria ehrenbergii | 80 | 11.25 | 7.8 | 0.45 | 0.6 | [43] |
| Attalea funifera | 28.6 | 25.8 | 45 | - | - | [43,63,99] |
| Pueraria spp. | 33 | 11.6 | 14 | - | - | [43,63] |
| Asclepia syriaca | 74.5 | - | 4.1 | - | 2.2 | [104] |
| Agave fourcroydes | 60–78 | 4–28 | 8–13.1 | 0.5–4 | - | [30,63,69] |
| Helicteres isora | 74 | - | 23 | 1.09 | - | [43,63,99] |
4.3. Mechanical and Physical Properties
| Fiber Type | WA (%) | MC (%) | Den (g/cm3) | Dia (µm) | CI (%) | MFA (º) | Elongation at Break (%) | Tensile Strength (MPa) | Young’s Modulus (GPa) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Abaca | - | 15 | 0.83–1.5 | 17–130 | 68.2 | - | 1–10 | 400–980 | 6.2–20 | [11,31,99,114] |
| Bagasse | - | 8.8 | 0.55–1.5 | 10–400 | 32–96.33 | - | 0.9–1.1 | 20–290 | 2.7–27.1 | [31,43,63,102] |
| Bamboo | - | 8.9–10.14 | 0.6–1.1 | 25–330 | 48.0 | 10–11 | 1.4–3.7 | 140–800 | 11–35.9 | [31,43,70,88,115] |
| Banana | 60 | 10.71 | 0.8–1.4 | 12–280 | 56.2–61.7 | 11–12 | 1.5–10 | 180–914 | 7.7–32 | [31,115,116,117] |
| Coir | 130–180 | 4.7–11.4 | 1.0–1.5 | 10–460 | 37.28 | 30.45 | 2.84–51.4 | 46.4–500 | 2.17–26 | [8,11,99,115] |
| Cotton | - | 7.8–8.5 | 1.5–1.6 | 10–45 | 65 | 20–30 | 2–10 | 287–800 | 3.44–12.6 | [11,31,43,70] |
| Date Palm | 60–65 | 9.6–10.7 | 0.46–1.2 | 155–250 | 38.5 | - | 2–4.5 | 97–459 | 1.91–70 | [8,43,115,118] |
| Flax | 136 | 7–12 | 1.4–1.5 | 7–600 | 70–80 | 5–10 | 0.2–3.3 | 343–2000 | 18.4–103 | [31,43,69,101] |
| Hemp | - | 6.2–15 | 1.4–1.5 | 16–500 | 88 | 2–6.2 | 1–4.7 | 58–1100 | 3–90 | [31,67,70,99,117] |
| Jute | 281 | 12–23 | 1.3–1.5 | 20–350 | 71 | 8 | 1–8 | 187–938 | 3–78 | [11,31,67,70,101] |
| Kenaf | - | 6.2–20 | 1.22–1.45 | 40–250 | 60 | 2–6.2 | 1.5–6.9 | 223–1191 | 14.5–53 | [8,30,101,119] |
| Pineapple Leaf | - | 11.8–13 | 0.8–1.6 | 5–194 | 32–78.7 | 6–14 | 0.8–14.5 | 144–1627 | 1.44–2.5 | [43,88,99] |
| Ramie | - | 8–9 | 1–1.5 | 20–80 | 58 | - | 1.2–4 | 56–1000 | 3.6–128 | [11,70,88] |
| Roselle | 286.5 | - | 0.75–0.8 | - | - | - | 5–8 | 147–350 | 2.76–17 | [8,120,121] |
| Sisal | 190–250 | 11 | 0.7–1.5 | 8–300 | 71 | 10–22 | 2–14 | 268–855 | 9–38 | [8,11,121,122] |
| Roystonea regia | - | 12.09 | 0.81 | 175–230 | - | - | 3.46 | 549 | 15.85 | [43,115] |
5. Natural and Synthetic Fiber Comparison
6. Natural Fiber Production and Economical Value
6.1. Global Production
| Fiber Name | Main Producers | World Production (103 ton) | References |
|---|---|---|---|
| Abaca | Philippines, Ecuador, Costa Rica | 70 | [31,70] |
| Bagasse | Brazil, India, China | 75,000 | [31,70] |
| Bamboo | India, China, Indonesia, Malaysia, Philippines | 30,000 | [31,70] |
| Banana | India | 200 | [38] |
| Coir | India, Sri Lanka, Philippines, Malaysia | 100 | [31,70] |
| Cotton | China, India, USA, Pakistan | 25,000 | [70] |
| Curaua | Brazil, Venezuela | >1 | [70] |
| Flax | Canada, China, Russia, France, Belgium | 830 | [31,70,130] |
| Hemp | China, France, Philippines | 214 | [31,70,130] |
| Henequen | Mexico | 30 | [70] |
| Jute | India, China, Bangladesh, | 2300 | [31,70,151] |
| Kapok | Indonesia | 123 | [38] |
| Kenaf | India, Bangladesh, USA | 970 | [31,38,70,130] |
| Oil Palm | Malaysia, Indonesia | 40 | [70] |
| Pineapple | Philippines, Thailand, Indonesia | 74 | [70] |
| Ramie | China, Brazil, Philippines, India | 100 | [31,38] |
| Roselle | Thailand, China | 250 | [120] |
| Sisal | Tanzania, Brazil, Kenya | 378 | [31,70] |
| Sunn Hemp | India, Bangladesh, Brazil | 70 | [120] |
| Rice Husk | China, India, Indonesia, Malaysia, Bangladesh | 160,000 | [152] |
| Rice Straw | China, India, Indonesia, Malaysia, Bangladesh | 579 | [153] |
| Wood Fiber | Canada, USA, China | 1,750,000 | [130] |
| Palm Date | Iran | 4200 | [154] |
6.2. Natural Fiber Economic Value
| Natural Fibers | Cost (USD/kg) | References |
|---|---|---|
| Abaca | 1.55–2.55 | [161,162] |
| Bamboo | 0.25–0.50 | [161] |
| Banana | 0.1 | [38] |
| Hemp | 0.30–1.60 | [38,161] |
| Coir | 0.20–0.84 | [38,161] |
| Cotton | 1.71–5.06 | [159,162,163] |
| Curaua | 0.44 | [155] |
| Jute | 0.25–0.50 | [161,162] |
| Flax | 0.30–1.55 | [38,161] |
| Kapok | 1.85–7.61 | [162] |
| Kenaf | 0.30–0.65 | [38,161] |
| Ramie | 1.50–2.40 | [161,162] |
| Piassava | 0.37 | [155] |
| Sisal | 0.35–1.30 | [38,161,162] |
| Sponge-gourd | 0.6 | [155] |
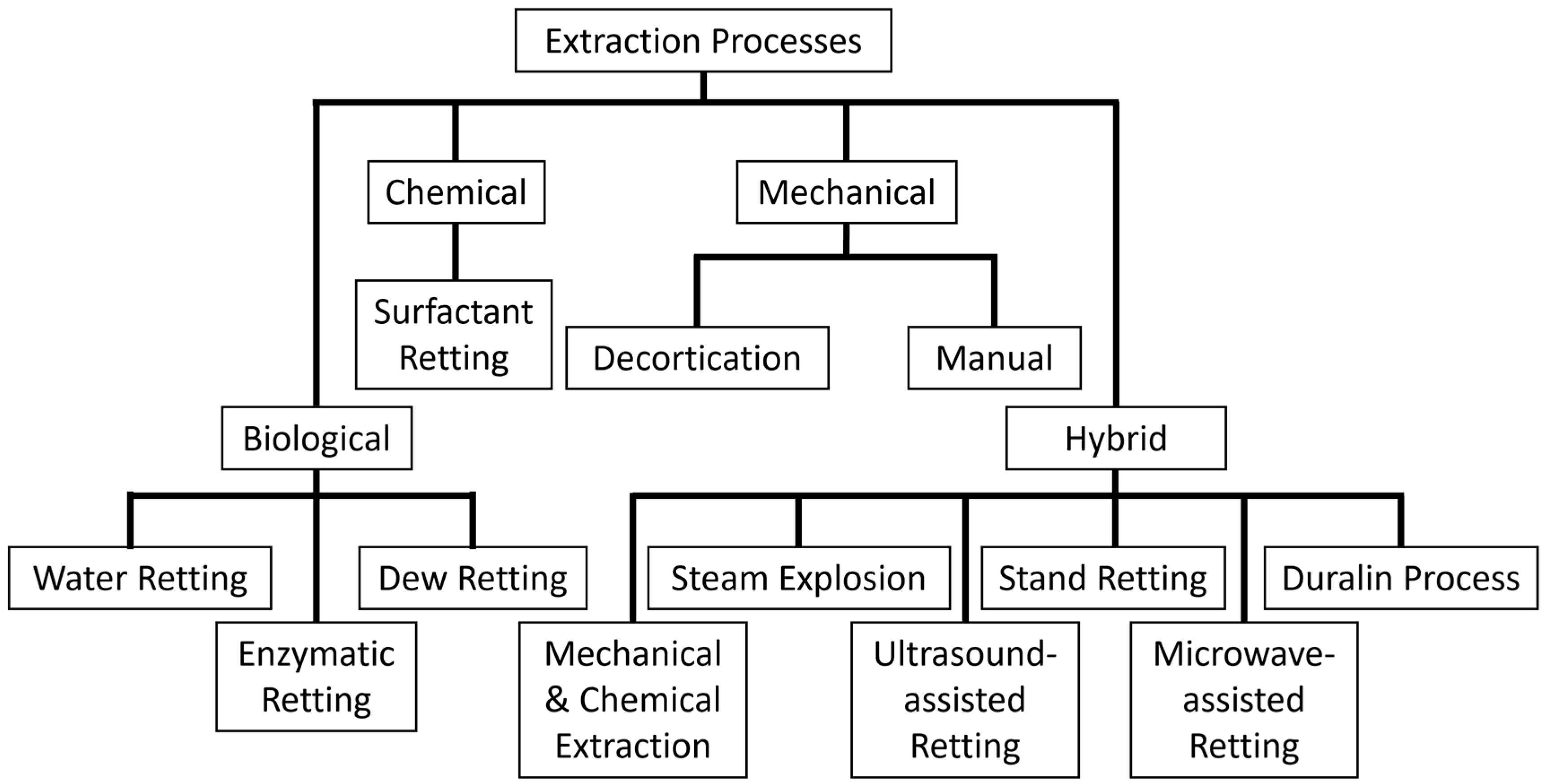
7. Natural Fiber Extraction Methods
7.1. Biological Extraction Methods
7.1.1. Dew Retting
7.1.2. Water Retting
7.1.3. Enzymatic Retting
7.2. Chemical Extraction Methods
Surfactant Retting
7.3. Mechanical Extraction Methods
7.3.1. Manual Extraction
7.3.2. Mechanical Extraction
7.4. Hybrid or Combined Extraction Methods
7.4.1. Mechanical and Chemical Extraction
7.4.2. Steam Explosion
7.4.3. Ultrasound Retting
7.4.4. Stand Retting
7.4.5. Microwave-Assisted Retting
7.4.6. The Duralin Process
8. Fiber Treatments
8.1. Chemical Treatments
8.1.1. Alkali Treatment
8.1.2. Acrylation Treatment
8.1.3. Acetylation Treatment
8.1.4. Silane Treatment
8.1.5. Peroxide Treatment
8.1.6. Sodium Chlorite Treatment
8.2. Physical Treatment
8.2.1. Ozone Treatment
8.2.2. Plasma Treatment
8.2.3. Corona Discharge
8.3. Biological Treatment
Fungal Treatment
8.4. Characterization of Treated Fibers
9. Natural Fiber Applications
9.1. Natural Fibers Applied in the Automotive Industry
9.2. Natural Fibers Applied in the Aerospace Industry
9.3. Natural Fibers Applied in the Construction Industry
9.4. Natural Fibers Applied in Geotextiles
9.5. Natural Fibers Applied in the Textile Industry
9.6. Natural Fibers Applied in Packaging and Bioplastics
9.7. Natural Fibers Applied in Biomedical Applications
9.8. Natural Fibers Processed into Nanocellulose
10. Challenges and Future Prospects
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kvavadze, E.; Bar-Yosef, O.; Belfer-Cohen, A.; Boaretto, E.; Jakeli, N.; Matskevich, Z.; Meshveliani, T. 30,000-Year-Old Wild Flax Fibers. Science 2009, 325, 1359. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, Biodegradable Polymers and Biocomposites: An Overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Macarthur, S.; Hemmings, F.J. Fibres, Yarns and Fabrics: An Introduction to Production, Structure and Properties. In Forensic Examination of Fibres; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-15658-3. [Google Scholar]
- Keya, K.N.; Kona, N.A.; Koly, F.A.; Maraz, K.M.; Islam, M.N.; Khan, R.A. Natural Fiber Reinforced Polymer Composites: History, Types, Advantages and Applications. Mater. Eng. Res. 2019, 1, 69–85. [Google Scholar] [CrossRef]
- Taj, S.; Munawar, M.A.; Khan, S. Natural Fiber-Reinforced Polymer Composites. Proc. Pak. Acad. Sci. 2007, 44, 129. [Google Scholar]
- Begum, K.; Islam, M.A. Natural Fiber as a Substitute to Synthetic Fiber in Polymer Composites: A Review. Res. J. Eng. Sci. 2013, 2, 46–53. [Google Scholar]
- Yahya, M.N.; Sheng Chin, D.D.V. A Review on the Potential of Natural Fibre for Sound Absorption Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012014. [Google Scholar] [CrossRef]
- Ashik, K.P.; Sharma, R.S. A Review on Mechanical Properties of Natural Fiber Reinforced Hybrid Polymer Composites. J. Miner. Mater. Charact. Eng. 2015, 3, 420. [Google Scholar] [CrossRef]
- Arenas, J.P.; Crocker, M.J. Recent Trends in Porous Sound-Absorbing Materials. Sound Vib. 2010, 44, 12–18. [Google Scholar]
- Barth, M.; Carus, M. Carbon Footprint and Sustainability of Different Natural Fibres for Biocomposites and Insulation Material; Nova-Institute: Hürth, Germany, 2015; Available online: http://eiha.org/media/2017/01/15-04-Carbon-Footprint-of-Natural-Fibres-nova1.pdf (accessed on 5 September 2017).
- Khan, T.; Hameed Sultan, M.T.B.; Ariffin, A.H. The Challenges of Natural Fiber in Manufacturing, Material Selection, and Technology Application: A Review. J. Reinf. Plast. Compos. 2018, 37, 770–779. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun Cellulose Acetate Fiber Mats Containing Curcumin and Release Characteristic of the Herbal Substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, I.Y.; Song, W.S. Biodegradation of Polylactic Acid (PLA) Fibers Using Different Enzymes. Macromol. Res. 2014, 22, 657–663. [Google Scholar] [CrossRef]
- Zhu, K.; Tu, H.; Yang, P.; Qiu, C.; Zhang, D.; Lu, A.; Luo, L.; Chen, F.; Liu, X.; Chen, L.; et al. Mechanically Strong Chitin Fibers with Nanofibril Structure, Biocompatibility, and Biodegradability. Chem. Mater. 2019, 31, 2078–2087. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Z.; Tang, R. Structure and Thermal Properties of Bamboo Viscose, Tencel and Conventional Viscose Fiber. J. Therm. Anal. Calorim. 2006, 89, 197–201. [Google Scholar] [CrossRef]
- Yang, Y.; Reddy, N. Properties and Potential Medical Applications of Regenerated Casein Fibers Crosslinked with Citric Acid. Int. J. Biol. Macromol. 2012, 51, 37–44. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, X.; Liu, C.; Dong, Z.; Wang, F.; Wang, X.; Hu, W.; Zhang, X.; Zhao, P.; Xia, Q. Structural and Mechanical Properties of Silk from Different Instars of Bombyx Mori. Biomacromolecules 2019, 20, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Kurien, R.A.; Biju, A.; Raj, K.A.; Chacko, A.; Joseph, B.; Koshy, C.P. Chicken Feather Fiber Reinforced Composites for Sustainable Applications. Mater. Today Proc. 2022, 58, 862–866. [Google Scholar] [CrossRef]
- Barlow, C.A.; Grespin, M.; Best, E.A. Asbestos Fiber Length and Its Relation to Disease Risk. Inhalation Toxicol. 2017, 29, 541–554. [Google Scholar] [CrossRef]
- Bernstein, D.M. Synthetic Vitreous Fibers: A Review Toxicology, Epidemiology and Regulations. Crit. Rev. Toxicol. 2007, 37, 839–886. [Google Scholar] [CrossRef]
- Ghio, A.J.; Funkhouser, W.; Pugh, C.B.; Winters, S.; Stonehuerner, J.G.; Mahar, A.M.; Roggli, V.L. Pulmonary Fibrosis and Ferruginous Bodies Associated with Exposure to Synthetic Fibers. Toxicol. Pathol. 2006, 34, 723–729. [Google Scholar] [CrossRef]
- Dey, V.; Kachala, R.; Bonakdar, A.; Mobasher, B. Mechanical Properties of Micro and Sub-Micron Wollastonite Fibers in Cementitious Composites. Constr. Build. Mater. 2015, 82, 351–359. [Google Scholar] [CrossRef]
- Fareez, U.N.M.; Loudiy, A.; Erkartal, M.; Yilmaz, C. Basalt Fiber Reinforced Polymers: A Recent Approach to Electromagnetic Interference (EMI) Shielding. J. Polym. Sci. 2025, 63, 50–73. [Google Scholar] [CrossRef]
- Chowdhury, I.R.; Pemberton, R.; Summerscales, J. Developments and Industrial Applications of Basalt Fibre Reinforced Composite Materials. J. Compos. Sci. 2022, 6, 367. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Rangappa, S.M.; Siengchin, S. Basalt Fibers: An Environmentally Acceptable and Sustainable Green Material for Polymer Composites. Constr. Build. Mater. 2024, 436, 136834. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Ma, H.; Xu, X. Properties and Applications of Basalt Fiber and Its Composites. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012052. [Google Scholar] [CrossRef]
- Chellan, S.; Mondal, M.I.H.; Joseph, K. Chapter 21—Basalt Fibres and Their Applications in the Automotive Industry. In Technical Organic and Inorganic Fibres from Natural Resources; Mondal, M.I.H., Ed.; Woodhead Publishing in Materials; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2025; pp. 599–623. ISBN 978-0-443-15459-1. [Google Scholar]
- Parida, C.; Dash, S.K.; Mohanta, K.L.; Patra, S. Effect of Fiber Treatment and Fiber Content on Flexural Properties of Luffa Cylindrica—Reinforced Resorcinol Composites. Adv. Sci. Lett. 2016, 22, 454–457. [Google Scholar] [CrossRef]
- Biagiotti, J.; Puglia, D.; Kenny, J.M. A Review on Natural Fibre-Based Composites-Part I: Structure, Processing and Properties of Vegetable Fibres. J. Nat. Fibers 2004, 1, 37–68. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites Reinforced with Natural Fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Nayak, R.; Houshyar, S.; Khandual, A.; Padhye, R.; Fergusson, S. 14—Identification of Natural Textile Fibres. In Handbook of Natural Fibres, 2nd ed.; Kozłowski, R.M., Mackiewicz-Talarczyk, M., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2020; pp. 503–534. ISBN 978-0-12-818398-4. [Google Scholar]
- Kozlowski, R.M.; Mackiewicz-Talarczyk, M. Handbook of Natural Fibres; Woodhead Publishing: Cambridge, UK, 2020; Volume 2, ISBN 978-0-12-819070-8. [Google Scholar]
- Mańkowski, J.; Kołodziej, J.; Pudełko, K.; Kozłowski, R.M. 11—Bast Fibres: The Role of Hemp (Cannabis Sativa L.) in Remediation of Degraded Lands. In Handbook of Natural Fibres, 2nd ed.; Kozłowski, R.M., Mackiewicz-Talarczyk, M., Eds.; The Textile Institute Book Series; Woodhead Publishing: Cambridge, UK, 2020; pp. 393–417. ISBN 978-0-12-818782-1. [Google Scholar]
- Sadrmanesh, V.; Chen, Y. Bast Fibres: Structure, Processing, Properties, and Applications. Int. Mater. Rev. 2019, 64, 381–406. [Google Scholar] [CrossRef]
- Syduzzaman, M.; Rumi, S.S.; Fahmi, F.F.; Akter, M.; Dina, R.B. Mapping the Recent Advancements in Bast Fiber Reinforced Biocomposites: A Review on Fiber Modifications, Mechanical Properties, and Their Applications. Results Mater. 2023, 20, 100448. [Google Scholar] [CrossRef]
- Zhu, S.; Xie, J.; Sun, Q.; Zhang, Z.; Wan, J.; Zhou, Z.; Lu, J.; Chen, J.; Xu, J.; Chen, K. Recent Advances on Bast Fiber Composites: Engineering Innovations, Applications and Perspectives. Compos. Part B Eng. 2024, 284, 111738. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y. Review-of-the-History-Properties-and-Application-of-Plant-Fibres. Afr. J. Sci. Technol. 2006, 7, 120–133. [Google Scholar]
- Singh, K.; Rajput, B.S.; Umrao, R.; Kumar, S. E-Reading Manual on Dendrology; Banda University of Agriculture and Technology: Banda, India, 2024. [Google Scholar]
- Sharma, P.P.; Dinesh, D.V. Angiosperms, Histology, Anatomy and Embryology; Educational Publishers & Distributors: Aurangabad, India, 2020. [Google Scholar]
- Kellogg, E.A. Flowering Plants. Monocots: Poaceae; Springer International Publishing: Cham, Germany, 2015; ISBN 978-3-319-15331-5. [Google Scholar]
- Catling, D. Identification of Vegetable Fibres; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sathishkumar, T.; Navaneethakrishnan, P.; Shankar, S.; Rajasekar, R.; Rajini, N. Characterization of Natural Fiber and Composites—A Review. J. Reinf. Plast. Compos. 2013, 32, 1457–1476. [Google Scholar] [CrossRef]
- Bismarck, A.; Mishra, S.; Lampke, T. Plant Fibers as Reinforcement for Green Composites. In Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005; pp. 52–128. [Google Scholar]
- Reddy, N.; Yang, Y. Biofibers from Agricultural Byproducts for Industrial Applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P. Bamboo Availability and Utilization Potential as a Building Material. For. Res. Eng. Int. J. 2018, 2, 240–242. [Google Scholar] [CrossRef][Green Version]
- Dardick, C.; Callahan, A.M. Evolution of the Fruit Endocarp: Molecular Mechanisms Underlying Adaptations in Seed Protection and Dispersal Strategies. Front. Plant Sci. 2014, 5, 284. [Google Scholar] [CrossRef]
- Huss, J.C.; Gierlinger, N. Functional Packaging of Seeds. New Phytol. 2021, 230, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Iqbal, M. Loofa (Luffa Cylindrica) Sponge: Review of Development of the Biomatrix as a Tool for Biotechnological Applications. Biotechnol. Prog. 2013, 29, 573–600. [Google Scholar] [CrossRef]
- Khalil, H.; Alwani, M.S.; Omar, A.M. Chemical Composition, Anatomy, Lignin Distribution, and Cell Wall Structure of Malaysian Plant Waste Fibers. BioResources 2006, 1, 220–232. [Google Scholar] [CrossRef]
- Sarmin, S.N. Anatomical Structure of Coir Fibers. In Coir Fiber and Its Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 43–54. [Google Scholar]
- Francoz, E.; Lepiniec, L.; North, H.M. Seed Coats as an Alternative Molecular Factory: Thinking Outside the Box. Plant Reprod. 2018, 31, 327–342. [Google Scholar] [CrossRef]
- Western, T.L. The Sticky Tale of Seed Coat Mucilages: Production, Genetics, and Role in Seed Germination and Dispersal. Seed Sci. Res. 2012, 22, 1–25. [Google Scholar] [CrossRef]
- Dochia, M.; Sirghie, C.; Kozłowski, R.M.; Roskwitalski, Z. Cotton Fibres. In Handbook of Natural Fibres; Elsevier: Amsterdam, The Netherlands, 2012; pp. 11–23. [Google Scholar]
- Sangalang, R.H. Kapok Fiber-Structure, Characteristics and Applications: A Review. Orient. J. Chem. 2021, 37, 513–523. [Google Scholar] [CrossRef]
- Ištok, I.; Šefc, B.; Hasan, M.; Popović, G.; Sedlar, T. Fiber Characteristics of White Poplar (Populus alba L.) Juvenile Wood along the Drava River. Drv. Ind. 2017, 68, 241–247. [Google Scholar] [CrossRef]
- Xu, Y.; Su, Q.; Shen, H.; Xu, G. Physicochemical and Sorption Characteristics of Poplar Seed Fiber as a Natural Oil Sorbent. Text. Res. J. 2019, 89, 4186–4194. [Google Scholar] [CrossRef]
- Moshi, A.A.M.; Ravindran, D.; Bharathi, S.R.S.; Indran, S.; Saravanakumar, S.S.; Liu, Y. Characterization of a New Cellulosic Natural Fiber Extracted from the Root of Ficus religiosa Tree. Int. J. Biol. Macromol. 2020, 142, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Indran, S.; Edwin Raj, R.; Sreenivasan, V.S. Characterization of New Natural Cellulosic Fiber from Cissus quadrangularis Root. Carbohydr. Polym. 2014, 110, 423–429. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Wang, A.; Kiran, S.; Wang, J.; Qureshi, K.; Xu, Y.; Zegaoui, A.; Derradji, M.; Babar, A.A.; Liu, W. Impacts of Hemp Fiber Diameter on Mechanical and Water Uptake Properties of Polybenzoxazine Composites. Ind. Crops Prod. 2018, 111, 277–284. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, X. Novel Modified Distribution Functions of Fiber Length in Fiber Reinforced Thermoplastics. Compos. Sci. Technol. 2019, 182, 107749. [Google Scholar] [CrossRef]
- Karine, C.; Jean-Paul, J.; Moussa, G.; Christophe, B.; Laurent, B.; Joël, B. Morphology And Mechanical Behavior of A Natural Composite: The Flax Fiber. In Proceedings of the 16th International Conference on Composite Materials, Kyoto, Japan, 8–13 July 2007. [Google Scholar]
- John, M.J.; Anandjiwala, R.D. Recent Developments in Chemical Modification and Characterization of Natural Fiber-Reinforced Composites. Polym. Compos. 2008, 29, 187–207. [Google Scholar] [CrossRef]
- Thomas, S.; Paul, S.A.; Pothan, L.A.; Deepa, B. Natural Fibres: Structure, Properties and Applications. In Cellulose Fibers: Bio- and Nano-Polymer Composites: Green Chemistry and Technology; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–42. ISBN 978-3-642-17370-7. [Google Scholar]
- Summerscales, J.; Virk, A.S.; Hall, W. Enhanced Rules-of-Mixture for Natural Fibre Reinforced Polymer Matrix (NFRP) Composites (Comment on Lau et al. in Volume 136). Compos. Part B Eng. 2019, 160, 167–169. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. The Hygroscopic Behavior of Plant Fibers: A Review. Front. Chem. 2014, 1, 43. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M.; Flavius, A.I. General Information about Cellulose. J. Biotechnol. Bioprocess 2022, 3, 43. [Google Scholar]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Kicińska-Jakubowska, A.; Bogacz, E.; Zimniewska, M. Review of Natural Fibers. Part I—Vegetable Fibers. J. Nat. Fibers 2012, 9, 150–167. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Schädel, C.; Richter, A.; Blöchl, A.; Hoch, G. Hemicellulose Concentration and Composition in Plant Cell Walls under Extreme Carbon Source-Sink Imbalances. Physiol. Plant. 2010, 139, 241–255. [Google Scholar] [CrossRef]
- Schädel, C.; Blöchl, A.; Richter, A.; Hoch, G. Quantification and Monosaccharide Composition of Hemicelluloses from Different Plant Functional Types. Plant Physiol. Biochem. 2010, 48, 1–8. [Google Scholar] [CrossRef]
- Shaker, K.; Waseem Ullah Khan, R.M.; Jabbar, M.; Umair, M.; Tariq, A.; Kashif, M.; Nawab, Y. Extraction and Characterization of Novel Fibers from Vernonia Elaeagnifolia as a Potential Textile Fiber. Ind. Crops Prod. 2020, 152, 112518. [Google Scholar] [CrossRef]
- Singam, S.S. Miscanthus× Giganteus Biomass as Feedstock for Cellulose Fibers and Lignin: Extraction, Characterizations, and Potential Applications. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2022. [Google Scholar]
- Hawanis, H.S.N.; Ilyas, R.A.; Jalil, D.R.; Ibrahim, D.R.; Abdul Majid, D.R.; Ab Hamid, D.N.H. Insights into Lignocellulosic Fiber Feedstock and Its Impact on Pulp and Paper Manufacturing: A Comprehensive Review. Sustain. Mater. Technol. 2024, 40, e00922. [Google Scholar] [CrossRef]
- Pickering, K. Properties and Performance of Natural-Fibre Composites; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-1-84569-459-3. [Google Scholar]
- Banik, N.; Dey, V.; Sastry, G.R.K. An Overview of Lignin & Hemicellulose Effect upon Biodegradable Bamboo Fiber Composites Due to Moisture. Mater. Today Proc. 2017, 4, 3222–3232. [Google Scholar]
- George, M.; Mussone, P.G.; Alemaskin, K.; Chae, M.; Wolodko, J.; Bressler, D.C. Enzymatically Treated Natural Fibres as Reinforcing Agents for Biocomposite Material: Mechanical, Thermal, and Moisture Absorption Characterization. J. Mater. Sci. 2016, 51, 2677–2686. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Pu, Y.; Ragauskas, A. Cellulosic Biorefineries—Unleashing Lignin Opportunities. Curr. Opin. Environ. Sustain. 2010, 2, 383–393. [Google Scholar] [CrossRef]
- Henriksson, G. 6. Lignin. In Wood Chemistry and Wood Biotechnology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter: Berlin, Germany, 2009; pp. 121–146. ISBN 978-3-11-021339-3. [Google Scholar]
- Huang, H.; Xu, C.; Zhu, X.; Li, B.; Huang, C. Lignin-Enhanced Wet Strength of Cellulose-Based Materials: A Sustainable Approach. Green Chem. 2023, 25, 4995–5009. [Google Scholar] [CrossRef]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the Potential of Lignin-Containing Cellulose Nanofibrils (LCNFs): A Review on Properties and Applications. Cellulose 2020, 27, 1853–1877. [Google Scholar] [CrossRef]
- Manral, A.; Bajpai, P.K. Analysis of Natural Fiber Constituents: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 455, 012115. [Google Scholar] [CrossRef]
- Elfaleh, I.; Abbassi, F.; Habibi, M.; Ahmad, F.; Guedri, M.; Nasri, M.; Garnier, C. A Comprehensive Review of Natural Fibers and Their Composites: An Eco-Friendly Alternative to Conventional Materials. Results Eng. 2023, 19, 101271. [Google Scholar] [CrossRef]
- Joseleau, J.-P.; Pérez, S. The Plant Cell Walls are Complex Polysaccharide Nano-Composites. 2016. Available online: https://glycopedia.eu/echapter/introduction-8/the-plant-cell-walls-are-complex-nanocomposites-of-polysaccharides/ (accessed on 11 March 2025).
- Alix, S.; Goimard, J.; Morvan, C.; Baley, C. Influence of Pectin Structure on the Mechanical Properties of Flax Fibres: A Comparison between Linseed-Winter Variety (Oliver) and a Fibre-Spring Variety of Flax (Hermes). In Pectins and Pectinases; Wageningen Academic: Wageningen, The Netherlands, 2009; pp. 87–98. [Google Scholar]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Mohammed, M.; Jawad, A.J.M.; Mohammed, A.M.; Oleiwi, J.K.; Adam, T.; Osman, A.F.; Dahham, O.S.; Betar, B.O.; Gopinath, S.C.B.; Jaafar, M. Challenges and Advancement in Water Absorption of Natural Fiber-Reinforced Polymer Composites. Polym. Test. 2023, 124, 108083. [Google Scholar] [CrossRef]
- Karim, A.; Raji, Z.; Habibi, Y.; Khalloufi, S. A Review on the Hydration Properties of Dietary Fibers Derived from Food Waste and Their Interactions with Other Ingredients: Opportunities and Challenges for Their Application in the Food Industry. Crit. Rev. Food Sci. Nutr. 2024, 64, 11722–11756. [Google Scholar] [CrossRef]
- Ntenga, R.; Saidjo, S.; Wakata, A.; Djoda, P.; Tango, M.; Mfoumou, E. Extraction, Applications and Characterization of Plant Fibers. In Natural Fiber; IntechOpen: London, UK, 2022. [Google Scholar]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of Plant Cuticular Waxes; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 23, pp. 145–181. [Google Scholar]
- Ahmad, H.; Rahman, M.; Awan, S. Plant Cuticular Waxes: A Review on Functions, Composition, Biosyntheses Mechanism and Transportation. Life Sci. J. 2015, 12, 60–67. [Google Scholar]
- Franco, P.J.H.; Valadez-González, A. Fiber-Matrix Adhesion in Natural Fiber Composites. In Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005; pp. 196–252. [Google Scholar]
- Lee, C.H.; Khalina, A.; Lee, S.H. Importance of Interfacial Adhesion Condition on Characterization of Plant-Fiber-Reinforced Polymer Composites: A Review. Polymers 2021, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Dittenber, D.B.; GangaRao, H.V. Critical Review of Recent Publications on Use of Natural Composites in Infrastructure. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- Yadav, D.; Yadav, S.; Dwivedi, R.; Anand, G.; Yadav, P.K. Potential of Microbial Enzymes in Retting of Natural Fibers: A Review. Curr. Biochem. Eng. 2016, 3, 89–99. [Google Scholar] [CrossRef]
- Tahir, P.; Ahmed, A.B.; SaifulAzry, S.O.A.; Ahmed, Z. Retting process of some bast plant fibres and its effect on fibre quality: A review. BioResources 2011, 6, 5260–5281. [Google Scholar] [CrossRef]
- Sathish, S.; Karthi, N.; Prabhu, L.; Gokulkumar, S.; Balaji, D.; Vigneshkumar, N.; Ajeem Farhan, T.S.; AkilKumar, A.; Dinesh, V.P. A Review of Natural Fiber Composites: Extraction Methods, Chemical Treatments and Applications. Mater. Today Proc. 2021, 45, 8017–8023. [Google Scholar] [CrossRef]
- Nadlene, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Yusriah, L. A Review on Roselle Fiber and Its Composites. J. Nat. Fibers 2016, 13, 10–41. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Extraction and Characterization of Natural Cellulose Fibers from Common Milkweed Stems. Polym. Eng. Sci. 2009, 49, 2212–2217. [Google Scholar] [CrossRef]
- Ochi, S. Mechanical Properties of Kenaf Fibers and Kenaf/PLA Composites. Mech. Mater. 2008, 40, 446–452. [Google Scholar] [CrossRef]
- Hestiawan, H.; Jamasri, K. Effect of Chemical Treatments on Tensile Properties and Interfacial Shear Strength of Unsaturated Polyester/Fan Palm Fibers. J. Nat. Fibers 2018, 15, 762–775. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Saravanan, R.; Thanikodi, S. Exploring Natural Plant Fiber Choices and Treatment Methods for Contemporary Composites: A Comprehensive Review. Results Eng. 2024, 24, 103270. [Google Scholar] [CrossRef]
- Gümüskaya, E.; Usta, M.; Kirci, H. The Effects of Various Pulping Conditions on Crystalline Structure of Cellulose in Cotton Linters. Polym. Degrad. Stab. 2003, 81, 559–564. [Google Scholar] [CrossRef]
- Caliari, Í.P.; Barbosa, M.H.P.; Ferreira, S.O.; Teófilo, R.F. Estimation of Cellulose Crystallinity of Sugarcane Biomass Using near Infrared Spectroscopy and Multivariate Analysis Methods. Carbohydr. Polym. 2017, 158, 20–28. [Google Scholar] [CrossRef]
- Xu, P.; Liu, H.; Donaldson, L.A.; Zhang, Y. Mechanical Performance and Cellulose Microfibrils in Wood with High S2 Microfibril Angles. J. Mater. Sci. 2011, 46, 534–540. [Google Scholar] [CrossRef]
- Ray, U.; Zhu, S.; Pang, Z.; Li, T. Mechanics Design in Cellulose-Enabled High-Performance Functional Materials. Adv. Mater. 2021, 33, 2002504. [Google Scholar] [CrossRef]
- Fidelis, M.E.A.; Pereira, T.V.C.; Gomes, O.d.F.M.; de Andrade Silva, F.; Toledo Filho, R.D. The Effect of Fiber Morphology on the Tensile Strength of Natural Fibers. J. Mater. Res. Technol. 2013, 2, 149–157. [Google Scholar] [CrossRef]
- Bourahli, M.E.H. Uni- and Bimodal Weibull Distribution for Analyzing the Tensile Strength of Diss Fibers. J. Nat. Fibers 2018, 15, 843–852. [Google Scholar] [CrossRef]
- Constâncio Trindade, A.C.; Sood, S.S.; Silva, D.d.C.; Ozer, A.; Kriven, W.M. Abaca Fiber as an Efficient Reinforcement for High Mechanical Performance in Metakaolin-Based Geopolymers. Int. J. Appl. Ceram. Technol. 2024, 21, 1154–1169. [Google Scholar] [CrossRef]
- Rao, K.M.M.; Rao, K.M. Extraction and Tensile Properties of Natural Fibers: Vakka, Date and Bamboo. Compos. Struct. 2007, 77, 288–295. [Google Scholar] [CrossRef]
- Oyewo, A.T.; Olugbemiga, O.O.; Ajide, O.O.; Emmanuel, T.; Akhter, P.; Hamayun, M.H.; Kang, B.S.; Park, Y.K.; Hussain, M. Physico-Chemical, Thermal and Micro-Structural Characterization of Four Common Banana Pseudo-Stem Fiber Cultivars in Nigeria. J. Nat. Fibers 2023, 20, 2167031. [Google Scholar] [CrossRef]
- Badanayak, P.; Jose, S.; Bose, G. Banana Pseudostem Fiber: A Critical Review on Fiber Extraction, Characterization, and Surface Modification. J. Nat. Fibers 2023, 20, 2168821. [Google Scholar] [CrossRef]
- Chandramohan, D.; Marimuthu, K. A Review on Natural Fibers. Int. J. Res. Rev. Appl. Sci. 2011, 8, 194–206. [Google Scholar]
- Amel, B.A.; Paridah, M.T.; Sudin, R.; Anwar, U.M.K.; Hussein, A.S. Effect of Fiber Extraction Methods on Some Properties of Kenaf Bast Fiber. Ind. Crops Prod. 2013, 46, 117–123. [Google Scholar] [CrossRef]
- Thiruchitrambalam, M.; Athijayamani, A.; Sathiyamurthy, S.; Thaheer, A.S.A. A Review on the Natural Fiber-Reinforced Polymer Composites for the Development of Roselle Fiber-Reinforced Polyester Composite. J. Nat. Fibers 2010, 7, 307–323. [Google Scholar] [CrossRef]
- Betene, A.D.O.; Betene, F.E.; Martoïa, F.; Dumont, P.J.J.; Atangana, A.; Noah, P.M.A. Physico-Chemical and Thermal Characterization of Some Lignocellulosic Fibres: Ananas Comosus (AC), Neuropeltis Acuminatas (NA) and Rhecktophyllum Camerunense (RC). J. Miner. Mater. Charact. Eng. 2020, 8, 205–222. [Google Scholar] [CrossRef]
- Silva, F.d.A.; Chawla, N.; Filho, R.D. de T. Tensile Behavior of High Performance Natural (Sisal) Fibers. Compos. Sci. Technol. 2008, 68, 3438–3443. [Google Scholar] [CrossRef]
- Asim, M.; Saba, N.; Jawaid, M.; Nasir, M. Potential of Natural Fiber/Biomass Filler-Reinforced Polymer Composites in Aerospace Applications. In Sustainable Composites for Aerospace Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 253–268. [Google Scholar]
- Asyraf, M.R.M.; Syamsir, A.; Zahari, N.M.; Supian, A.B.M.; Ishak, M.R.; Sapuan, S.M.; Sharma, S.; Rashedi, A.; Razman, M.R.; Zakaria, S.Z.S. Product Development of Natural Fibre-Composites for Various Applications: Design for Sustainability. Polymers 2022, 14, 920. [Google Scholar] [CrossRef]
- Crupi, V.; Epasto, G.; Napolitano, F.; Palomba, G.; Papa, I.; Russo, P. Green Composites for Maritime Engineering: A Review. J. Mar. Sci. Eng. 2023, 11, 599. [Google Scholar] [CrossRef]
- Bavan, D.S.; Kumar, G.M. Potential Use of Natural Fiber Composite Materials in India. J. Reinf. Plast. Compos. 2010, 29, 3600–3613. [Google Scholar] [CrossRef]
- Oliveira Duarte, L.; Kohan, L.; Pinheiro, L.; Fonseca Filho, H.; Baruque-Ramos, J. Textile Natural Fibers Production Regarding the Agroforestry Approach. SN Appl. Sci. 2019, 1, 914. [Google Scholar] [CrossRef]
- Dissanayake, N.P.J.; Summerscales, J.; Grove, S.M.; Singh, M.M. Energy Use in the Production of Flax Fiber for the Reinforcement of Composites. J. Nat. Fibers 2009, 6, 331–346. [Google Scholar] [CrossRef]
- Gandhi, K.; Khan, S.; Patrikar, M.; Markad, A.; Kumar, N.; Choudhari, A.; Sagar, P.; Indurkar, S. Exposure Risk and Environmental Impacts of Glyphosate: Highlights on the Toxicity of Herbicide Co-Formulants. Environ. Chall. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Holbery, J.; Houston, D. Natural-Fiber-Reinforced Polymer Composites in Automotive Applications. JOM 2006, 58, 80–86. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhu, L.; Sun, L.; Zhang, Y.; Li, X.; Wang, L. Carbon Neutrality Potential of Textile Products Made from Plant-Derived Fibers. Sustainability 2023, 15, 7070. [Google Scholar] [CrossRef]
- Joshi, S.V.; Drzal, L.T.; Mohanty, A.K.; Arora, S. Are Natural Fiber Composites Environmentally Superior to Glass Fiber Reinforced Composites? Compos. Part A Appl. Sci. Manuf. 2004, 35, 371–376. [Google Scholar] [CrossRef]
- Tausif, M.; Jabbar, A.; Naeem, M.S.; Basit, A.; Ahmad, F.; Cassidy, T. Cotton in the New Millennium: Advances, Economics, Perceptions and Problems. Text. Prog. 2018, 50, 1–66. [Google Scholar] [CrossRef]
- Dangi, B.M.; Bhise, A.R. Cotton Dust Exposure: Analysis of Pulmonary Function and Respiratory Symptoms. Lung India 2017, 34, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, A.; Shanaz, G.; Bhavana, J.M.; Hussain, M.A.; Raghavendra Prasad, B.N. Severe Byssinosis with Cor Pulmonale and Pulmonary Arterial Hypertension-Textile Industry: A Case Report. Med. Rep. 2025, 13, 100280. [Google Scholar] [CrossRef]
- Sanivada, U.K.; Mármol, G.; Brito, F.P.; Fangueiro, R. PLA Composites Reinforced with Flax and Jute Fibers—A Review of Recent Trends, Processing Parameters and Mechanical Properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef]
- Ahmad, J.; Zhou, Z. Mechanical Properties of Natural as Well as Synthetic Fiber Reinforced Concrete: A Review. Constr. Build. Mater. 2022, 333, 127353. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Arpitha, G.R.; Naik, L.L.; Gopalakrishna, K.; Yogesha, B. Applications of Natural Fibers and Its Composites: An Overview. Nat. Resour. 2016, 7, 108–114. [Google Scholar] [CrossRef]
- Garcia-Chavez, L.; Vural-Gursel, I.; O’Keeffe, S.; Arets, E.J. Understanding the Policies and Carbon Accounting Frameworks Which Are Defining the Potential Role of Biobased Products to Meet Climate Change Targets; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2023. [Google Scholar]
- Soutis, C. Fibre Reinforced Composites in Aircraft Construction. Prog. Aerosp. Sci. 2005, 41, 143–151. [Google Scholar] [CrossRef]
- Subaida, E.A.; Chandrakaran, S.; Sankar, N. Laboratory Performance of Unpaved Roads Reinforced with Woven Coir Geotextiles. Geotext. Geomembr. 2009, 27, 204–210. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Smets, T.; Fullen, M.A.; Poesen, J.; Booth, C.A. Effectiveness of Geotextiles in Reducing Runoff and Soil Loss: A Synthesis. Catena 2010, 81, 184–195. [Google Scholar] [CrossRef]
- Mwasha, A. Using Environmentally Friendly Geotextiles for Soil Reinforcement: A Parametric Study. Mater. Des. 2009, 30, 1798–1803. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in Biomedicine: Current Status and Future Prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- van Dam, J.E. Natural Fibres and the Environment: Environmental Benefits of Natural Fibre Production and Use. In Proceedings of the Symposium on Natural Fibres: Common Fund for Commodities, Rome, Italy, 20 October 2008. [Google Scholar]
- Bala, A.; Singh, B. Development of an Environmental-Benign Process for Efficient Pretreatment and Saccharification of Saccharum Biomasses for Bioethanol Production. Renew. Energy 2019, 130, 12–24. [Google Scholar] [CrossRef]
- Ferraz-Almeida, R.; Ribeiro Ferreira da Silva, O.R. Sisal Fiber (Agave sisalana) Production in the Brazilian Semiarid from 1988 to 2024. J. Nat. Fibers 2025, 22, 2502651. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Z.; Tan, S.; Wu, W.; Chen, T.; Qin, J.; Yi, K.; Chen, H. Investigating the Impact of Soil Properties on the Nutrient Content and Microbial Community of Sisal (Agave sisalana Perrine). Chil. J. Agric. Res. 2025, 85, 215–231. [Google Scholar] [CrossRef]
- Mahir, F.I.; Keya, K.N.; Sarker, B.; Nahiun, K.M.; Khan, R.A. A Brief Review on Natural Fiber Used as a Replacement of Synthetic Fiber in Polymer Composites. Mater. Eng. Res. 2019, 1, 86–97. [Google Scholar] [CrossRef]
- Alves, C.; Silva, A.J.; Reis, L.G.; Freitas, M.; Rodrigues, L.B.; Alves, D.E. Ecodesign of Automotive Components Making Use of Natural Jute Fiber Composites. J. Clean. Prod. 2010, 18, 313–327. [Google Scholar] [CrossRef]
- Arjmandi, R.; Hassan, A.; Majeed, K.; Zakaria, Z. Rice Husk Filled Polymer Composites. Int. J. Polym. Sci. 2015, 2015, 501471. [Google Scholar] [CrossRef]
- Doostkami, H.; Hernández-Figueirido, D.; Albero, V.; Piquer, A.; Serna, P.; Roig-Flores, M. Experimental Study on the Valorization of Rice Straw as Fiber for Concrete. Fibers 2025, 13, 28. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Kuhestani, K. Ethnobotany of Date Palm (Phoenix dactylifera) in Baluch Tribe of Saravan Region, Baluchistan, Iran. J. Agric. Technol. 2014, 10, 1571–1585. [Google Scholar]
- Monteiro, S.N.; Lopes, F.P.D.; Ferreira, A.S.; Nascimento, D.C.O. Natural-Fiber Polymer-Matrix Composites: Cheaper, Tougher, and Environmentally Friendly. JOM 2009, 61, 17–22. [Google Scholar] [CrossRef]
- Terrié, C.; Dobircau, L.; Gopalakrishnan, P.; Galandon, A.; Saiah, R.; Gattin, R.; Leblanc, N.; Saiter, J.M. Biodegradable Materials from Agro-Based By-Products. Macromol. Symp. 2010, 290, 132–136. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Grammatikos, S.A. Comparative Life Cycle Assessment of Cotton and Other Natural Fibers for Textile Applications. Fibers 2019, 7, 101. [Google Scholar] [CrossRef]
- Yu, T.; Li, Y.; Ren, J. Preparation and Properties of Short Natural Fiber Reinforced Poly (Lactic Acid) Composites. Trans. Nonferrous Met. Soc. China 2009, 19, s651–s655. [Google Scholar] [CrossRef]
- Cotton Prices, March, 2025—Data, Chart. Available online: https://www.theglobaleconomy.com/world/cotton_prices/ (accessed on 22 April 2025).
- Onen, P.; Muhumuza, R. A Ugandan Business Turns Banana Fiber into Sustainable Handicrafts|AP News. Available online: https://apnews.com/article/uganda-banana-fiber-innovation-sustainable-handicrafts-4bb98b2861a1207770881f97613e53de (accessed on 22 April 2025).
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Progress Report on Natural Fiber Reinforced Composites. Macromol. Mater. Eng. 2014, 299, 9–26. [Google Scholar] [CrossRef]
- Natural Fibres and the World Economy. In RILEM Bookseries; Springer Netherlands: Dordrecht, The Netherlands, 2016; pp. 381–390. ISBN 978-94-017-7513-7.
- Voora, V.; Bermudez, S.; Farrell, J.J.; Larrea, C.; Luna, E. Cotton Prices and Sustainability; International Institute for Sustainable Development: Geneva, Switzerland, 2023; pp. 1–37. [Google Scholar]
- Stelte, W. Steam Explosion for Biomass Pre-Treatment; Danish Technological Institute: Taastrup, Denmark, 2013; pp. 1–15. [Google Scholar]
- Bacci, L.; Baronti, S.; Predieri, S.; di Virgilio, N. Fiber Yield and Quality of Fiber Nettle (Urtica dioica L.) Cultivated in Italy. Ind. Crops Prod. 2009, 29, 480–484. [Google Scholar] [CrossRef]
- Dhanalaxmi, R.K.; Vastrad, J.V. Influence of Retting Methods on Quality of Mesta Fibres. Indian J. Nat. Prod. Resour. 2013, 4, 178–183. [Google Scholar]
- Akin, D.E.; Dodd, R.B.; Perkins, W.; Henriksson, G.; Eriksson, K.-E.L. Spray Enzymatic Retting: A New Method for Processing Flax Fibers. Text. Res. J. 2000, 70, 486–494. [Google Scholar] [CrossRef]
- Bou Orm, E.; Mukherjee, S.; Rifa, E.; Créach, A.; Grec, S.; Bayle, S.; Benezet, J.-C.; Bergeret, A.; Malhautier, L. Enhancing Biodiversity-Function Relationships in Field Retting: Towards Key Microbial Indicators for Retting Control. Environ. Microbiol. Rep. 2025, 17, e70102. [Google Scholar] [CrossRef] [PubMed]
- Garriba, S.; Jailani, H.S.; Pandian, C.K.A.; Diwahar, P. Effect of Water Retting on the Physical and Mechanical Properties of Lignocellulosic Fiber from Mariscus ligularis Plant. Int. J. Biol. Macromol. 2025, 288, 138718. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, A.K.; Chakraborty, A.K. Sustainable In-Situ Jute Retting Technology in Low Volume Water Using Native Microbial Culture to Improve Fibre Quality and Retting Waste Management. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1080–1099. [Google Scholar] [CrossRef]
- Hossain, M.M.; Siddiquee, S.; Kumar, V. Water Sources Derived Bio Retting Effect on Kenaf Fiber Compositions. J. Nat. Fibers 2022, 19, 9396–9409. [Google Scholar] [CrossRef]
- Harsányi, J.; Poraj-Kobielska, M.; Wedwitschka, H.; Tirsch, M.; Kretzschmar, J. Controlled Anaerobic Water Retting of Flax as Part of an Innovative Biorefinery Process. Biomass Conv. Bioref. 2025, 15, 16499–16510. [Google Scholar] [CrossRef]
- Foulk, J.A.; Akin, D.E.; Dodd, R.B. Processing Techniques for Improving Enzyme-Retting of Flax. Ind. Crops Prod. 2001, 13, 239–248. [Google Scholar] [CrossRef]
- Akin, D.E.; Foulk, J.A.; Dodd, R.B. Influence on Flax Fibers of Components in Enzyme Retting Formulations. Text. Res. J. 2002, 72, 510–514. [Google Scholar] [CrossRef]
- Ventorino, V.; Chouyia, F.E.; Romano, I.; Mori, M.; Pepe, O. Water Retting Process with Hemp Pre-Treatment: Effect on the Enzymatic Activities and Microbial Populations Dynamic. Appl. Microbiol. Biotechnol. 2024, 108, 464. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Peng, J.; Tan, M.; Fang, J.; Li, K. An Efficient Preparation Process of Sisal Fibers via the Specialized Retting Microorganisms: Based on the Ideal Combination of Degumming-Related Enzymes for the Effective Removal of Non-Cellulosic Macromolecules. Int. J. Biol. Macromol. 2024, 274, 133416. [Google Scholar] [CrossRef]
- Nassar, M.M.A.; Alzebdeh, K.I.; Al-Hinai, N.; Pervez, T. A New Multistep Chemical Treatment Method for High Performance Natural Fibers Extraction. J. Nat. Fibers 2023, 20, 2150922. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Cosentino, S.L.; Danalatos, D.; Picco, D.; Lips, S.; van den Berg, D.; Fernando, A.L.; Monti, A.; Tenorio, J.L.; Kipriotis, E.; et al. New Insights from the Biokenaf Project. In Kenaf: A Multi-Purpose Crop for Several Industrial Applications; Monti, A., Alexopoulou, E., Eds.; Green Energy and Technology; Springer: London, UK, 2013; ISBN 978-1-4471-5066-4. [Google Scholar]
- Akin, D.E. Linen Most Useful: Perspectives on Structure, Chemistry, and Enzymes for Retting Flax. ISRN Biotechnol. 2013, 2013, 186534. [Google Scholar] [CrossRef]
- Ramesh, M. Flax (Linum usitatissimum L.) Fibre Reinforced Polymer Composite Materials: A Review on Preparation, Properties and Prospects. Prog. Mater. Sci. 2019, 102, 109–166. [Google Scholar] [CrossRef]
- Eleutério, T.; Pereira, M.J.; Vasconcelos, H.C. Effect of Extraction Method on Physicochemical Characteristics of Kahili Ginger (Hedychium gardnerianum) Fibres. Mater. Sci. Nanotechnol. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Puttegowda, M.; Mavinkere Rangappa, S.; Siengchin, S. A Review on Extraction, Chemical Treatment, Characterization of Natural Fibers and Its Composites for Potential Applications. Polym. Compos. 2021, 42, 6239–6264. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Chemical Treatments on Plant-Based Natural Fibre Reinforced Polymer Composites: An Overview. Compos. Part B Eng. 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Renouard, S.; Hano, C.; Doussot, J.; Blondeau, J.-P.; Lainé, E. Characterization of Ultrasonic Impact on Coir, Flax and Hemp Fibers. Mater. Lett. 2014, 129, 137–141. [Google Scholar] [CrossRef]
- Easson, M.; Condon, B.; Villalpando, A.; Chang, S. The Application of Ultrasound and Enzymes in Textile Processing of Greige Cotton. Ultrasonics 2018, 84, 223–233. [Google Scholar] [CrossRef]
- Lecoublet, M.; Khennache, M.; Leblanc, N.; Ragoubi, M.; Poilâne, C. Physico-Mechanical Performances of Flax Fiber Biobased Composites: Retting and Process Effects. Ind. Crops Prod. 2021, 173, 114110. [Google Scholar] [CrossRef]
- Lyu, P.; Zhang, Y.; Wang, X.; Hurren, C. Degumming Methods for Bast Fibers—A Mini Review. Ind. Crops Prod. 2021, 174, 114158. [Google Scholar] [CrossRef]
- Konczewicz, W.; Zimniewska, M.; Valera, M.A. The Selection of a Retting Method for the Extraction of Bast Fibers as Response to Challenges in Composite Reinforcement. Text. Res. J. 2018, 88, 2104–2119. [Google Scholar] [CrossRef]
- Sisti, L.; Totaro, G.; Vannini, M.; Celli, A. Retting Process as a Pretreatment of Natural Fibers for the Development of Polymer Composites. In Lignocellulosic Composite Materials; Kalia, S., Ed.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Germany, 2018; pp. 97–135. ISBN 978-3-319-68695-0. [Google Scholar]
- Nair, G.R. Role of Microwave Pre Treatment in the Extraction of High Quality Natural Fibers. J. Text. Eng. Fash. Technol. 2017, 2, 333–335. [Google Scholar] [CrossRef]
- Romero-Zúñiga, G.Y.; González-Morones, P.; Sánchez-Valdés, S.; Yáñez-Macías, R.; Sifuentes-Nieves, I.; García-Hernández, Z.; Hernández-Hernández, E. Microwave Radiation as Alternative to Modify Natural Fibers: Recent Trends and Opportunities—A Review. J. Nat. Fibers 2022, 19, 7594–7610. [Google Scholar] [CrossRef]
- Patra, A.; Bisoyi, D.K.; Manda, P.K.; Singh, A.K. Effect of Microwave Radiation on the Macromolecular, Morphological and Crystallographic Structures of Sisal Fiber. Appl. Phys. A 2013, 112, 1063–1071. [Google Scholar] [CrossRef]
- Ruan, P.; Du, J.; Gariepy, Y.; Raghavan, V. Characterization of Radio Frequency Assisted Water Retting and Flax Fibers Obtained. Ind. Crops Prod. 2015, 69, 228–237. [Google Scholar] [CrossRef]
- Zhao, D.; Ji, H.; Du, R.; Wang, Q.; Ping, W.; Ge, J. Optimization of Process Conditions for Microwave-Assisted Flax Water Retting by Response Surface Methodology and Evaluation of Its Fiber Properties. BioResources 2020, 15, 5859. [Google Scholar] [CrossRef]
- Bismarck, A.; Aranberri-Askargorta, I.; Springer, J.; Lampke, T.; Wielage, B.; Stamboulis, A.; Shenderovich, I.; Limbach, H. Surface Characterization of Flax, Hemp and Cellulose Fibers; Surface Properties and the Water Uptake Behavior. Polym. Compos. 2002, 23, 872–894. [Google Scholar] [CrossRef]
- Pott, G.T. Natural Fibers with Low Moisture Sensitivity. In Natural Fibers, Plastics and Composites; Wallenberger, F.T., Weston, N.E., Eds.; Springer: Boston, MA, USA, 2004; pp. 105–122. ISBN 978-1-4419-9050-1. [Google Scholar]
- Mohammed, M.; Rahman, R.; Mohammed, A.M.; Adam, T.; Betar, B.O.; Osman, A.F.; Dahham, O.S. Surface Treatment to Improve Water Repellence and Compatibility of Natural Fiber with Polymer Matrix: Recent Advancement. Polym. Test. 2022, 115, 107707. [Google Scholar] [CrossRef]
- Godara, S.S. Effect of Chemical Modification of Fiber Surface on Natural Fiber Composites: A Review. Mater. Today Proc. 2019, 18, 3428–3434. [Google Scholar]
- Shukla, N. Surface Treatment of Natural Fibers (Chemical Treatment). In Natural Fiber Composites; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-003-20172-4. [Google Scholar]
- Kalia, S.; Kumar, A. Surface Modification of Sunn Hemp Fibers Using Acrylation, Peroxide and Permanganate Treatments: A Study of Morphology, Thermal Stability and Crystallinity. Polym.-Plast. Technol. Eng. 2013, 52, 24–29. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of Natural Fibers and Their Application as Reinforcing Material in Polymer Composites—A Review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Han, G.; Aita, G.M.; Wu, Q. Cellulose Fibers Isolated from Energycane Bagasse Using Alkaline and Sodium Chlorite Treatments: Structural, Chemical and Thermal Properties. Ind. Crops Prod. 2015, 76, 355–363. [Google Scholar] [CrossRef]
- Kaur, V.; Chattopadhyay, D.P.; Kaur, S.; Kaur, K. Study on the Performance of Bamboo Fibre Modified with Different Concentrations of Sodium Hydroxide and Chlorine Containing Agents. J. Text. Sci. Eng. 2018, 8, 2. [Google Scholar]
- Maqsood, H.S.; Bashir, U.; Wiener, J.; Puchalski, M.; Sztajnowski, S.; Militky, J. Ozone Treatment of Jute Fibers. Cellulose 2017, 24, 1543–1553. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, C.; Fu, J.; Lou, J.; Zhang, X.; Gao, W.; Fan, X. Effect of Ozone Treatment on the Chemical and Mechanical Properties of Flax Fibers. Ind. Crops Prod. 2022, 189, 115694. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Vishnu, V.; Kothakota, A.; Pandiselvam, R.; Venkatesh, T.; Pillai, S.; Manikantan, M.R. Impact of Integrated Ultra Violet-Ozone Treatment on Textural and Structural Properties of Dough Made of Natural Fiber Based Agro Residues. J. Nat. Fibers 2023, 20, 2161690. [Google Scholar] [CrossRef]
- Yang, F.; Meng, C.; Shang, S.; Lin, R.; Yu, C. Effect of Ozone Pretreatment of Flax Fiber Processing Waste under Less Water Environment: Physicochemical Property Evaluation and Digestibility Metrics. Ind. Crops Prod. 2023, 205, 117464. [Google Scholar] [CrossRef]
- Seki, Y.; Sever, K.; Sarikanat, M.; Güleç, H.A.; Tavman, I.H. The Influence of Oxygen Plasma Treatment of Jute Fibers on Mechanical Properties of Jute Fiber Reinforced Thermoplastic Composites. In Proceedings of the 5th International Advanced Technologies Symposium, Karabuk, Turkey, 13–15 May 2009; pp. 1–4. [Google Scholar]
- Goda, K.; Cao, Y. Research and Development of Fully Green Composites Reinforced with Natural Fibers. J. Solid Mech. Mater. Eng. 2007, 1, 1073–1084. [Google Scholar] [CrossRef]
- Gulati, D.; Sain, M. Fungal-Modification of Natural Fibers: A Novel Method of Treating Natural Fibers for Composite Reinforcement. J. Polym. Environ. 2006, 14, 347–352. [Google Scholar] [CrossRef]
- Lee, C.H.; Khalina, A.; Lee, S.H.; Padzil, F.N.M.; Ainun, Z.M.A. Physical, Morphological, Structural, Thermal and Mechanical Properties of Pineapple Leaf Fibers. In Pineapple Leaf Fibers; Jawaid, M., Asim, M., Tahir, P.M., Nasir, M., Eds.; Green Energy and Technology; Springer: Singapore, 2020; pp. 91–121. ISBN 978-981-15-1415-9. [Google Scholar]
- Özdil, N.; Dalbaşı, E.S.; Özgüney, A.T.; Atiker, L. Multifunctional Modification with TiO2, SiO2, and Flame Retardant Agent on Upholstery Fabrics Produced From Recycled Cotton Fibers. AATCC J. Res. 2023, 10, 223–231. [Google Scholar] [CrossRef]
- Cireli, A.; Onar, N.; Ebeoglugil, M.F.; Kayatekin, I.; Kutlu, B.; Culha, O.; Celik, E. Development of Flame Retardancy Properties of New Halogen-Free Phosphorous Doped SiO2 Thin Films on Fabrics. J. Appl. Polym. Sci. 2007, 105, 3748–3756. [Google Scholar] [CrossRef]
- Zhang, F.-Q.; Wang, B.; Xu, Y.-J.; Li, P.; Liu, Y.; Zhu, P. Convenient Blending of Alginate Fibers with Polyamide Fibers for Flame-Retardant Non-Woven Fabrics. Cellulose 2020, 27, 8341–8349. [Google Scholar] [CrossRef]
- Cruz, J.; Fangueiro, R. Surface Modification of Natural Fibers: A Review. Proced. Eng. 2016, 155, 285–288. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Pinheiro, I.F.; de Souza, S.F.; Mei, L.H.; Lona, L.M. Polymer Composites Reinforced with Natural Fibers and Nanocellulose in the Automotive Industry: A Short Review. J. Compos. Sci. 2019, 3, 51. [Google Scholar] [CrossRef]
- Murugu Nachippan, N.; Alphonse, M.; Bupesh Raja, V.K.; Shasidhar, S.; Varun Teja, G.; Harinath Reddy, R. Experimental Investigation of Hemp Fiber Hybrid Composite Material for Automotive Application. Mater. Today Proc. 2021, 44, 3666–3672. [Google Scholar] [CrossRef]
- Chauhan, V.; Kärki, T.; Varis, J. Review of Natural Fiber-Reinforced Engineering Plastic Composites, Their Applications in the Transportation Sector and Processing Techniques. J. Thermoplast. Compos. Mater. 2022, 35, 1169–1209. [Google Scholar] [CrossRef]
- Al-Oqla, F.M.; Sapuan, S.M. Natural Fiber Reinforced Polymer Composites in Industrial Applications: Feasibility of Date Palm Fibers for Sustainable Automotive Industry. J. Clean. Prod. 2014, 66, 347–354. [Google Scholar] [CrossRef]
- Ghosh, G.; Bhattacharyya, R.; Penumadu, D. Advances in Multi-Functional Composite Materials: Applications and Opportunities in Automotive Industry. 2024. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5081906 (accessed on 7 April 2025).
- Bajwa, D.S.; Bhattacharjee, S. Current Progress, Trends and Challenges in the Application of Biofiber Composites by Automotive Industry. J. Nat. Fibers 2016, 13, 660–669. [Google Scholar]
- Carvalho, D.; Ferreira, N.; França, B.; Marques, R.; Silva, M.; Silva, S.; Silva, E.; Macário, D.; Barroso, L.; Silva, C.J. Advancing Sustainability in the Automotive Industry: Bioprepregs and Fully Bio-Based Composites. Compos. Part C Open Access 2024, 14, 100459. [Google Scholar] [CrossRef]
- Sripaiboonkij, P.; Sripaiboonkij, N.; Phanprasit, W.; Jaakkola, M.S. Respiratory and Skin Health among Glass Microfiber Production Workers: A Cross-Sectional Study. Env. Health 2009, 8, 36. [Google Scholar] [CrossRef]
- Hardcastle, A.; Waterman-Hoey, S. Advanced Materials Manufacturing, Sustainability and Workforce Development; Washington State University Extension Energy Program: Spokane, WA, USA, 2010; pp. 1–37. [Google Scholar]
- Arockiam, N.J.; Jawaid, M.; Saba, N. Sustainable Bio Composites for Aircraft Components. In Sustainable Composites for Aerospace Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 109–123. [Google Scholar]
- Mansor, M.R.; Nurfaizey, A.H.; Tamaldin, N.; Nordin, M.N.A. Natural Fiber Polymer Composites: Utilization in Aerospace Engineering. In Biomass, Biopolymer-Based Materials, and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 203–224. [Google Scholar]
- Skoczylas, J.; Samborski, S.; Kłonica, M. The Application of Composite Materials in the Aerospace Industry. J. Technol. Exploit. Mech. Eng. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Bharath, K.N.; Basavarajappa, S. Applications of Biocomposite Materials Based on Natural Fibers from Renewable Resources: A Review. Sci. Eng. Compos. Mater. 2016, 23, 123–133. [Google Scholar] [CrossRef]
- Dweib, M.A.; Hu, B.; Shenton Iii, H.W.; Wool, R.P. Bio-Based Composite Roof Structure: Manufacturing and Processing Issues. Compos. Struct. 2006, 74, 379–388. [Google Scholar] [CrossRef]
- Lertwattanaruk, P.; Suntijitto, A. Properties of Natural Fiber Cement Materials Containing Coconut Coir and Oil Palm Fibers for Residential Building Applications. Constr. Build. Mater. 2015, 94, 664–669. [Google Scholar] [CrossRef]
- Swamy, R.P.; Kumar, G.C.M.; Vrushabhendrappa, Y.; Joseph, V. Study of Areca-Reinforced Phenol Formaldehyde Composites. J. Reinf. Plast. Compos. 2004, 23, 1373–1382. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Saba, N.; Rajini, N.; Chandrasekar, M.; Jawaid, M.; Siengchin, S.; Alotman, O.Y. Mechanical Properties Evaluation of Sisal Fibre Reinforced Polymer Composites: A Review. Constr. Build. Mater. 2018, 174, 713–729. [Google Scholar] [CrossRef]
- Vijaya Ramnath, B.; Manickavasagam, V.M.; Elanchezhian, C.; Vinodh Krishna, C.; Karthik, S.; Saravanan, K. Determination of Mechanical Properties of Intra-Layer Abaca–Jute–Glass Fiber Reinforced Composite. Mater. Des. 2014, 60, 643–652. [Google Scholar] [CrossRef]
- Zimniewska, M. Functionalization of Natural Fibres Textiles by Improvement of Nanoparticles Fixation on Their Surface. J. Fiber Bioeng. Inf. 2012, 5, 321–339. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Cheng, L.; Qian, J.; Yu, J. Modification of Natural Bamboo Fibers for Textile Applications. Fibers Polym. 2011, 12, 95–103. [Google Scholar] [CrossRef]
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the Technical Performance of Leather, Artificial Leather, and Trendy Alternatives. Coatings 2021, 11, 226. [Google Scholar] [CrossRef]
- Jawaid, M.; Abdul Khalil, H.P.S. Cellulosic/Synthetic Fibre Reinforced Polymer Hybrid Composites: A Review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Hernandez, C.C.; dos Santos Rosa, D. Extraction of Cellulose Nanowhiskers: Natural Fibers Source, Methodology and Application. Matrix 2016, 3, 16. [Google Scholar]
- Khan, A.; Huq, T.; Khan, R.A.; Riedl, B.; Lacroix, M. Nanocellulose-Based Composites and Bioactive Agents for Food Packaging. Crit. Rev. Food Sci. Nutr. 2014, 54, 163–174. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Ferreira, F.; Felgueiras, H.P. Biofunctionalization of Natural Fiber-Reinforced Biocomposites for Biomedical Applications. Biomolecules 2020, 10, 148. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Saurabh, C.K.; Hossain, M.S.; Adnan, A.S.; Dungani, R.; Paridah, M.T.; Sarker, M.Z.I.; Fazita, M.N.; Syakir, M.I. A Review on Nanocellulosic Fibres as New Material for Sustainable Packaging: Process and Applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Simon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C. Renewable Fibers and Bio-Based Materials for Packaging Applications-a Review of Recent Developments. BioResources 2012, 7, 2506–2552. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Mane, R.; Lee, Y.S. Sustainable Food Packaging. In Future Crops and Processing Technologies for Sustainability and Nutritional Security; CRC Press: Boca Raton, FL, USA, 2024; pp. 174–199. [Google Scholar]
- Zhao, X.; Copenhaver, K.; Wang, L.; Korey, M.; Gardner, D.J.; Li, K.; Lamm, M.E.; Kishore, V.; Bhagia, S.; Tajvidi, M. Recycling of Natural Fiber Composites: Challenges and Opportunities. Resour. Conserv. Recycl. 2022, 177, 105962. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Bobade, R.G.; Doong, R.; Pandit, B.; Ky, N.M.; Ambare, R.C.; Hoang, T.-D.; Kumar, K.J. Materials Today Chemistry. Mater. Today 2025, 46, 8. [Google Scholar]
- Promdontree, P.; Ummartyotin, S.; Kheolamai, P. Development of Composite Hydrogels from Hemp-Derived Cellulose Nanofibers for Biomedical Application. Ph.D. Thesis, Thammasat University, Bangkok, Thailand, 2023. [Google Scholar]
- Tudoroiu, E.-E.; Dinu-Pîrvu, C.-E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Felgueiras, H.P. Emerging Antimicrobial and Immunomodulatory Fiber-Based Scaffolding Systems for Treating Diabetic Foot Ulcers. Pharmaceutics 2023, 15, 258. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem Cell-Based Tissue Engineering with Silk Biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef]
- Asadpour, S.; Kargozar, S.; Moradi, L.; Ai, A.; Nosrati, H.; Ai, J. Natural Biomacromolecule Based Composite Scaffolds from Silk Fibroin, Gelatin and Chitosan toward Tissue Engineering Applications. Int. J. Biol. Macromol. 2020, 154, 1285–1294. [Google Scholar] [CrossRef]
- Petrovic, S.; Bita, B.; Barbinta-Patrascu, M.-E. Nanoformulations in Pharmaceutical and Biomedical Applications: Green Perspectives. Int. J. Mol. Sci. 2024, 25, 5842. [Google Scholar] [CrossRef] [PubMed]
- Jaya Prakash, N.; Wang, X.; Kandasubramanian, B. Regenerated Silk Fibroin Loaded with Natural Additives: A Sustainable Approach towards Health Care. J. Biomater. Sci. Polym. Ed. 2023, 34, 1453–1490. [Google Scholar] [CrossRef] [PubMed]
- Theivasanthi, T.; Anne Christma, F.L.; Toyin, A.J.; Gopinath, S.C.B.; Ravichandran, R. Synthesis and Characterization of Cotton Fiber-Based Nanocellulose. Int. J. Biol. Macromol. 2018, 109, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Viner, D.; Morison, J.I.L.; Wallace, C. Recent and Future Climate Change and their Implications for Plant Growth. In Plant Growth and Climate Change, 1st ed.; Morison, J.I.L., Morecroft, M.D., Eds.; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-1-4051-3192-6. [Google Scholar]
- Hossain, M.D.; Hanafi, M.M.; Saleh, G.; Foroughi, M.; Behmaram, R.; Noori, Z. Growth, Photosynthesis and Biomass Allocation of Different Kenaf (“Hibiscus cannabinus” L.) Accessions Grown on Sandy Soil. Aust. J. Crop Sci. 2012, 6, 480–487. [Google Scholar]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Bousfield, G.; Morin, S.; Jacquet, N.; Richel, A. Extraction and Refinement of Agricultural Plant Fibers for Composites Manufacturing. Comptes Rendus Chim. 2018, 21, 897–906. [Google Scholar] [CrossRef]
- Kazemi, M.; Faisal Kabir, S.; Fini, E.H. State of the Art in Recycling Waste Thermoplastics and Thermosets and Their Applications in Construction. Resour. Conserv. Recycl. 2021, 174, 105776. [Google Scholar] [CrossRef]
- Muthu, S.S. Green Composites: Sustainable Raw Materials; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Khan, U.A.; Srivastava, A.; Asthana, N. Harnessing Green Composites for Energy Efficiency in the Era of Industry 4.0. In Engineering Materials for Efficient Energy Storage and Conversion; IGI Global Scientific Publishing: Hershey, PA, USA, 2024; pp. 195–222. [Google Scholar]
| Fiber Type | Species Name | Fiber Origin |
|---|---|---|
| Abaca | Musa textilis | Leaf Fiber |
| Alfa Grass | Stipa tenacissima | Grass Fiber |
| Bamboo | Bambusoideae spp. | Grass Fiber |
| Banana | Musa spp. | Leaf Fiber |
| Barley Straw | Hordeum vulgare | Stalk Fiber |
| Betel Nut | Areca catechu | Fruit Fiber |
| Buriti | Mauritia flexuosa | Fruit Fiber |
| Coir | Cocos nucifera | Fruit Fiber |
| Cotton | Gossypium spp. | Seed Fiber |
| Curaua | Ananas erectifolius | Leaf Fiber |
| Date palm | Phoenix dactylifera | Leaf Fiber |
| Elephant Grass | Pennisetum purpureum | Grass Fiber |
| Fig Tree | Ficus religiosa L | Root Fiber |
| Flax | Linum usitatissimum | Bast Fiber |
| Harakeke | Phormium tenax | Leaf Fiber |
| Hemp | Cannabis sativa | Bast Fiber |
| Henequen | Agave fourcroydes | Leaf Fiber |
| Isora | Helicteres isora | Bast Fiber |
| Jute | Corchorus capsularis/C. olitorius | Bast Fiber |
| Loofah | Luffa cylindrica | Fruit Fiber |
| Kahili ginger | Hedychium gardnerianum | Leaf Fiber |
| Kapok | Ceiba pentandra | Seed Fiber |
| Kenaf | Hibiscus cannabinus | Bast Fiber |
| Milkweed Fiber | Asclepias spp. | Seed Fiber |
| Miscanthus | Miscanthus giganteus | Grass Fiber |
| Nettle | Urtica spp. | Bast Fiber |
| Oil palm | Elaeis guineensis | Fruit Fiber |
| Piassava | Attalea funifera | Leaf Fiber |
| Pineapple | Ananas comosus | Leaf Fiber |
| Ramie | Boehmeria nivea | Bast Fiber |
| Reed Canary Grass | Phalaris arundinacea | Grass Fiber |
| Rice Straw and Husk | Oryza sativa | Stalk Fiber |
| Roselle | Hibiscus sabdariffa | Bast Fiber |
| Sisal | Agave sisalana | Leaf Fiber |
| Sorghum | Sorghum bicolor | Stalk Fiber |
| Sponge gourd | Luffa aegyptiaca | Fruit Fiber |
| Sugarcane | Saccharum officinarum | Stalk Fiber |
| Sunn hemp | Crotalaria juncea | Bast Fiber |
| Switchgrass | Panicum virgatum | Grass Fiber |
| Vakka | Calotropis gigantea | Seed Fiber |
| Wheat Straw | Triticum aestivum | Stalk Fiber |
| Windmill Palm | Trachycarpus fortunei | Leaf Fiber |
| Properties | Natural Fibers | Synthetic Fibers |
|---|---|---|
| Abundance | Infinite | Finite |
| Recyclability | Good | Moderate |
| Carbon Footprint | Neutral | High |
| Environmental Impact | No | Yes |
| Durability | Moderate | High |
| Biodegradability | High | Low |
| Weight | Low | Moderate |
| Cost | Low | High |
| Toxicity | Non-toxic | Toxic |
| Mechanical Properties | Moderate | High |
| Humidity Sensitivity | High | Low |
| Thermal Properties | Moderate | High |
| Acoustic Properties | Moderate | Moderate |
| Interfacial Adhesion | Low | Moderate |
| Method | Type | Description | Advantages | Disadvantages |
|---|---|---|---|---|
| Dew Retting | Biological | Fibers are left in the field to be decomposed by natural microbial activity. | No chemicals or water required; very low cost; low environmental impact | Weather-dependent; long processing time; inconsistent fiber quality, low scalability and efficiency |
| Water Retting | Biological | Stalks submerged in water to facilitate microbial breakdown of pectins. | Traditional method; low cost; moderate efficiency; medium scalability; good fiber quality | Time-consuming; large water use; moderate–high environmental impact due to wastewater |
| Enzymatic Retting | Biological | Uses specific enzymes (e.g., pectinases) to break down binding materials. | Controlled process; consistent fiber quality; environmentally friendly | High cost; requires precise conditions |
| Chemical Retting | Chemical | Use various types of chemicals, like alkalis or acids to dissolve pectin and lignin. | Fast process; high efficiency; high scalability; decent fiber quality | High environmental hazards; moderate cost; fiber damage if not controlled |
| Steam Explosion | Hybrid | High-pressure steam treatment followed by rapid decompression. | Fast and effective; high efficiency; retains fiber strength; moderate scalability | Requires pressure systems; high cost; moderate environmental impact due to energy use; possible fiber damage |
| Ultrasound-Assisted Retting | Hybrid | Use ultrasonic waves in liquid medium to accelerate cell wall disruption. | Accelerates retting; high efficiency; preserves fiber quality; low environmental impact | Expensive equipment; high cost; limited scalability |
| Microwave-Assisted Retting | Hybrid | Applies microwave energy to heat plant material internally and aid pectin breakdown. | Fast, energy-efficient; high efficiency; good fiber preservation; low environmental impact | Expensive equipment; scaling-up challenges |
| Stand Retting | Hybrid | Field retting with modifications like pre-harvest chemical application or thermal treatments. | Reduces weather dependency; can improve fiber quality | Use of chemicals or energy may raise costs or environmental concerns |
| Duralin Process | Hybrid | Alkali pretreatment followed by thermal compression and drying for stiffer fibers. | Produces high-stiffness fibers; dimensionally stable; high efficiency; moderate scalability | Involves heat and chemicals; moderate–high environmental impact; medium–high cost |
| Combined Mechanical and Chemical | Hybrid | Sequential mechanical extraction followed by chemical treatment. | Improved fiber purity and yield; high efficiency; medium scalability | Requires multiple steps; moderate–high environmental impact; moderate cost |
| Mechanical Extraction | Mechanical | Physical decortication or beating to separate fibers. | No chemicals needed; rapid processing; moderate cost; high scalability | Produces coarse fibers; possible fiber damage; moderate efficiency; moderate environmental impact from energy use |
| Manual Extraction | Mechanical | Manual physical separation of fibers using scrapers, knives scuffing. | No chemicals needed; straightforward; low cost; low environmental impact | Extremely low efficiency, low scalability; labor-intensive; inconsistent fiber quality |
| Treatment Method | Purpose/Effect | Fiber Quality Impact | Advantages | Limitations |
|---|---|---|---|---|
| Alkali | Removes lignin, hemicellulose, waxes; increases surface roughness | Enhances adhesion and fibrillation | Widely used; improves mechanical properties; low cost, high efficiency, and high scalability | Excessive exposure may degrade cellulose; moderate–high environmental impact due to chemical effluents |
| Acrylation | Grafts polymer chains to fiber surface | Increases hydrophobicity and interfacial bonding | Improves compatibility with hydrophobic matrices; high efficiency, moderate cost | Requires initiators and controlled conditions; moderate environmental impact; not highly scalable |
| Acetylation | Replaces hydroxyl groups with acetyl groups | Reduces hydrophilicity; improves dimensional stability | Enhances moisture resistance and durability; moderate scalability and cost | Uses acetic anhydride; moderate environmental impact; may reduce biodegradability |
| Silane | Introduces silane coupling agents for bonding | Enhances fiber–matrix bonding | Excellent fiber–matrix adhesion; high efficiency and scalability | Medium–high cost; requires safe handling of chemicals; moderate environmental impact |
| Peroxide | Initiates free radical grafting or cleaning | Increases surface energy and bonding | Fast surface activation; high efficiency, moderate cost | Risk of fiber degradation; moderate–high environmental impact from oxidizing agents |
| Sodium chlorite | Delignification through oxidation | Increases cellulose purity | Effective lignin removal; low cost, moderate scalability | Generates chlorine-containing waste; high environmental impact |
| Ozone | Oxidative surface modification | Enhances bonding; reduces impurities | Dry method; no chemical waste; low environmental impact | Requires specialized equipment; moderate cost; limited industrial use |
| Plasma | Physical surface activation via ionized gas | Increases surface energy | Clean, dry method; no chemicals; low environmental impact | High cost, low scalability; requires advanced equipment |
| Corona | Surface oxidation through electrical discharge | Improves wettability and bonding | No chemicals needed; fast; moderate scalability and low environmental impact | Surface effect is shallow; uniformity may vary; moderate cost |
| Fungal | Biologically removes lignin and hemicellulose | Preserves cellulose integrity | Sustainable and safe; low cost and environmental impact | Long processing time; sensitive to contamination; low–moderate scalability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleutério, T.; Trota, M.J.; Meirelles, M.G.; Vasconcelos, H.C. A Review of Natural Fibers: Classification, Composition, Extraction, Treatments, and Applications. Fibers 2025, 13, 119. https://doi.org/10.3390/fib13090119
Eleutério T, Trota MJ, Meirelles MG, Vasconcelos HC. A Review of Natural Fibers: Classification, Composition, Extraction, Treatments, and Applications. Fibers. 2025; 13(9):119. https://doi.org/10.3390/fib13090119
Chicago/Turabian StyleEleutério, Telmo, Maria João Trota, Maria Gabriela Meirelles, and Helena Cristina Vasconcelos. 2025. "A Review of Natural Fibers: Classification, Composition, Extraction, Treatments, and Applications" Fibers 13, no. 9: 119. https://doi.org/10.3390/fib13090119
APA StyleEleutério, T., Trota, M. J., Meirelles, M. G., & Vasconcelos, H. C. (2025). A Review of Natural Fibers: Classification, Composition, Extraction, Treatments, and Applications. Fibers, 13(9), 119. https://doi.org/10.3390/fib13090119







