Novel Bio-Functional Electrospun Membranes by Chios Mastic Gum Encapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Physicochemical Characterization of the Electrospun Mats
2.3.1. Morphological Characterization
2.3.2. Encapsulation Efficiency and Drug Loading
2.3.3. Analysis of Thermal Behavior
2.4. Biological Characterization of the Electrospun Mats
2.4.1. Cell Culture and Seeding
2.4.2. Cell Viability/MTT Assay
3. Results and Discussion
3.1. Physicochemical Properties of the Electrospun Mats
3.1.1. Morphological Characteristics
3.1.2. Encapsulation Efficiency and Drug Loading
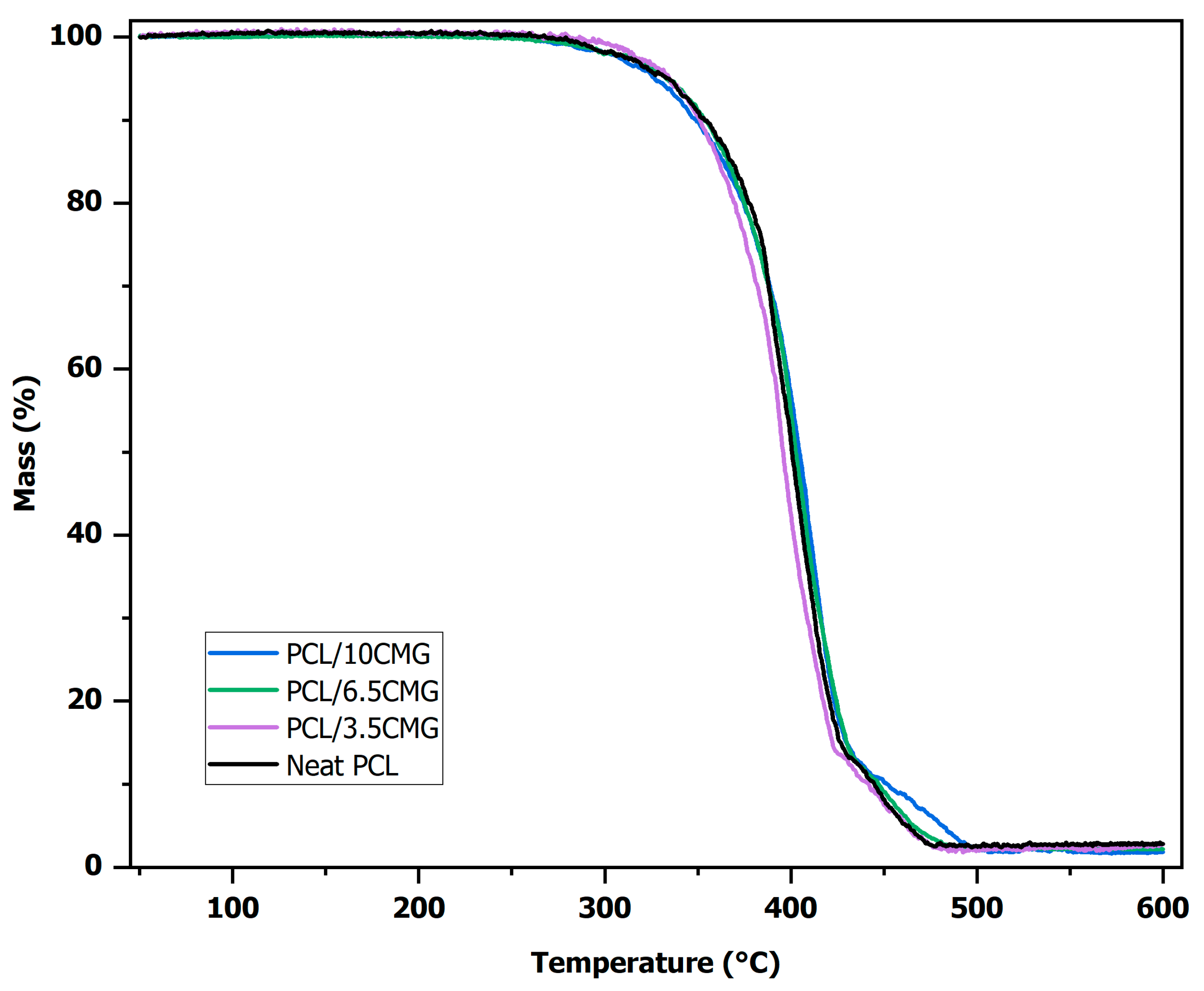
3.1.3. Thermal Behavior
3.2. Biological Proberties of the Electrospun Mats
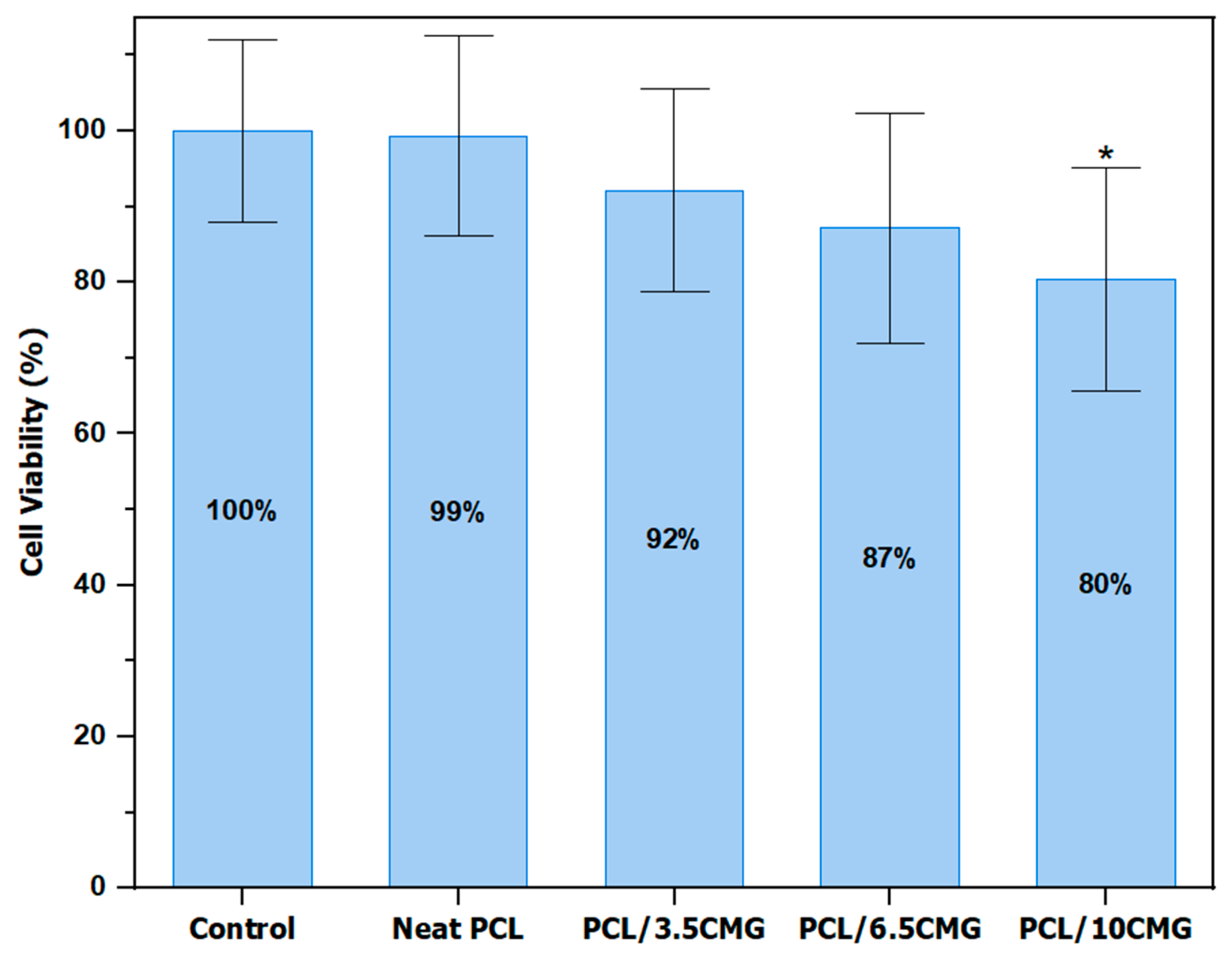
3.2.1. Determination of Cell Viability on HEK293 Cells
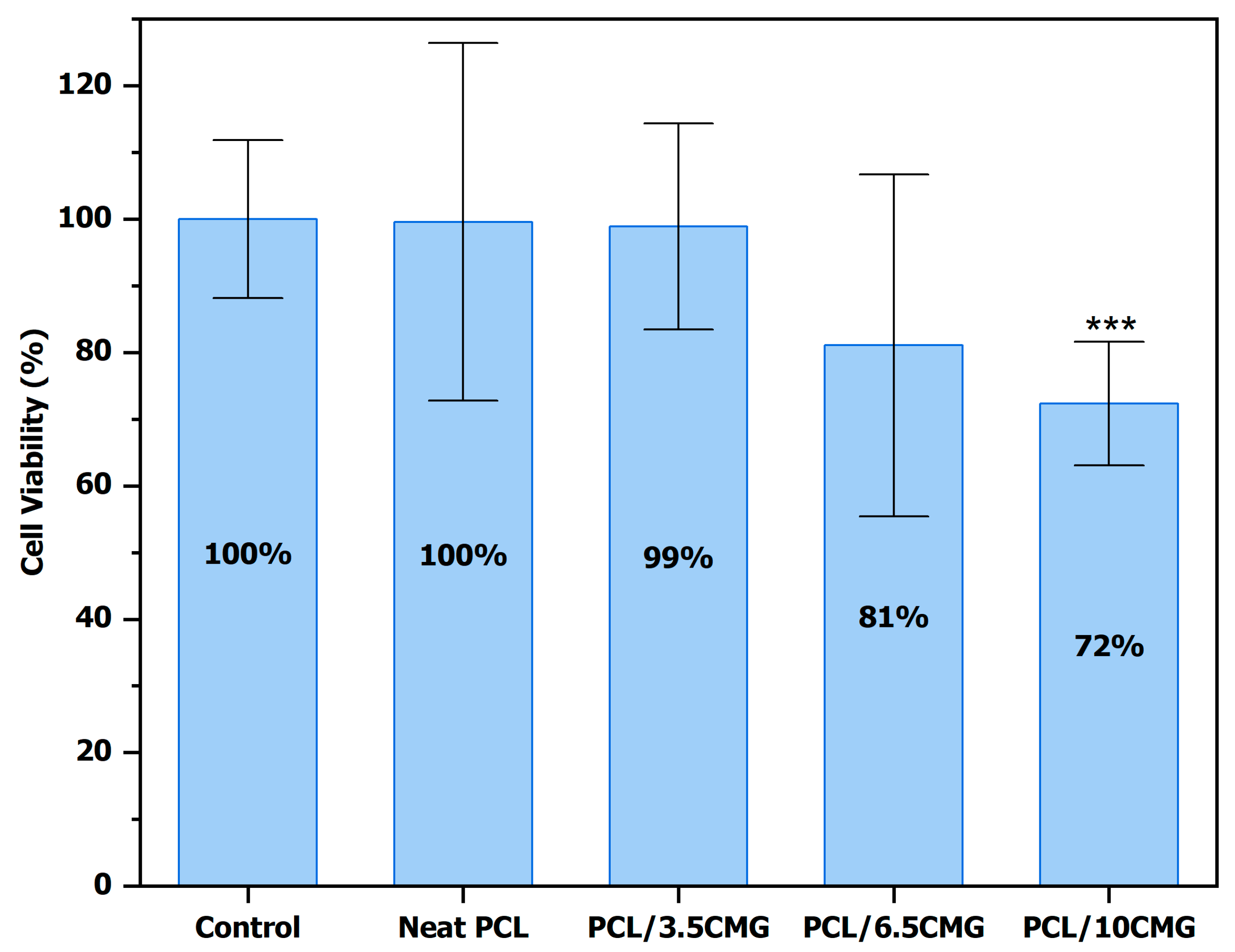
3.2.2. Determination of Cell Viability on HepG2 Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMG | Chios Mastic Gum |
| PCL | Poly-ε-caprolactone |
| SEM | Scanning electron microscopy |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| PDO | Protected designation of origin |
| GR | Glucocorticoid receptor |
| AMPK | AMP-activated protein kinase |
| PPARα | Peroxisome proliferator-activated receptor α |
| NF-κB | Nuclear factor-kappa B |
| PKC | Protein kinase C |
| oxLDL | Oxidized, low-density lipoprotein |
| NO | Nitric oxide |
| PEG2 | Prostaglandin E2 |
| EMA | European Medicines Agency |
| TMEWP | Total mastic extract without polymer |
| ECM | Extracellular matrix |
| UV | Ultraviolet |
| DCM | Dichloromethane |
| DMF | Dimethylformamide |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | Fetal bovine serum |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | Dimethyl sulfonyl chloride |
| ATCC | American Type Culture Collection |
References
- European Commission. Commission Regulation (EC) No 123/97 of 23 January 1997 Supplementing the Annex to Commission Regulation (EC) No 1107/96 on the Registration of Geographical Indications and Designations of Origin Under the Procedure Laid Down in Article 17 of Regulation (EEC) No 2081/92 (Text with EEA Relevance). 1997, pp. 19–25. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31997R0123 (accessed on 20 August 2025).
- UNESCO. Decision of the Intergovernmental Committee: 9.COM 10.18. In Inscription of “Traditional Know-How of Cultivating Mastic” on the Representative List of the Intangible Cultural Heritage of Humanity on the Island of Chios, Proceedings of the Intergovernmental Committee for the Safeguarding of the Intangible Cultural Heritage, Paris, France, 24–28 November 2014; Ninth Session: Paris, France, 2014. [Google Scholar]
- Georgantopoulos, A.; Kalousi, F.D.; Pollastro, F.; Tsialtas, I.; Kalogiouri, N.P.; Psarra, A.-M.G. Chemical Analysis and Antioxidant Activities of Resin Fractions from Pistacia lentiscus L. var. Chia in Neuroblastoma SH-SY5Y Cells. Molecules 2025, 30, 997. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.L. In vitro and in vivo activities of Chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007, 51, 551–559. [Google Scholar] [CrossRef]
- Sharif Sharifi, M.; Hazell, S.L. Fractionation of Mastic Gum in Relation to Antimicrobial Activity. Pharmaceuticals 2009, 2, 2–10. [Google Scholar] [CrossRef]
- Ottria, R.; Xynomilakis, O.; Casati, S.; Abbiati, E.; Maconi, G.; Ciuffreda, P. Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future. Int. J. Mol. Sci. 2023, 24, 12038. [Google Scholar] [CrossRef] [PubMed]
- Dabos, K.J.; Sfika, E.; Vlatta, L.J.; Frantzi, D.; Amygdalos, G.I.; Giannikopoulos, G. Is Chios mastic gum effective in the treatment of functional dyspepsia? A prospective randomised double-blind placebo controlled trial. J. Ethnopharmacol. 2010, 127, 205–209. [Google Scholar] [CrossRef]
- Koychev, S.; Dommisch, H.; Chen, H.; Pischon, N. Antimicrobial Effects of Mastic Extract Against Oral and Periodontal Pathogens. J. Periodontol. 2017, 88, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Faruque, M.R.J.; Potocka, W.; Nazmi, K.; Ligtenberg, A.J.; Bikker, F.J.; Laine, M.L. Scent of relief: Mastic resin scent recovers salivation in chronic dry mouth patients. Biomed. Pharmacother. 2024, 178, 117245. [Google Scholar] [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999, 65, 749–752. [Google Scholar] [CrossRef]
- Gortzi, O.; Rovoli, M.; Katsoulis, K.; Graikou, K.; Karagkini, D.-A.; Stagos, D.; Kouretas, D.; Tsaknis, J.; Chinou, I. Study of Stability, Cytotoxic and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) after Encapsulation in Liposomes. Foods 2022, 11, 271. [Google Scholar] [CrossRef]
- Kalousi, F.D.; Pollastro, F.; Karra, A.G.; Tsialtas, I.; Georgantopoulos, A.; Salamone, S.; Psarra, A.-M.G. Regulation of Energy Metabolism and Anti-Inflammatory Activities of Mastiha Fractions from Pistacia lentiscus L. var. chia. Foods 2023, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, X.; An, X.-R.; Liu, W.; Yuan, T. Masticadienonic acid from Chios mastic gum mitigates colitis in mice via modulating inflammatory response, gut barrier integrity and microbiota. Phytomedicine 2023, 108, 154518. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, A.; Bikineyeva, A.; Dikalova, A.; Nazarewicz, R.; Lerakis, S.; Dikalov, S. Anti-inflammatory activity of Chios mastic gum is associated with inhibition of TNF-alpha induced oxidative stress. Nutr. J. 2011, 10, 64. [Google Scholar] [CrossRef]
- Papada, E.; Forbes, A.; Amerikanou, C.; Torović, L.; Kalogeropoulos, N.; Tzavara, C.; Triantafillidis, J.K.; Kaliora, A.C. Antioxidative Efficacy of a Pistacia Lentiscus Supplement and Its Effect on the Plasma Amino Acid Profile in Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1779. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K.; Kaliora, A.C.; Assimopoulou, A.N.; Papapeorgiou, V.P. Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytother. Res. 2003, 17, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Satoh, K.; Takahashi, K.; Watanabe, S.; Nakamura, W.; Maki, J.; Hatano, H.; Takekawa, F.; Shimada, C.; Sakagami, H. Re-evaluation of anti-inflammatory activity of mastic using activated macrophages. In Vivo 2009, 23, 583–589. [Google Scholar]
- Georgantopoulos, A.; Vougioukas, A.; Kalousi, F.D.; Tsialtas, I.; Psarra, A.-M.G. Comparative Studies on the Anti-Inflammatory and Apoptotic Activities of Four Greek Essential Oils: Involvement in the Regulation of NF-κΒ and Steroid Receptor Signaling. Life 2023, 13, 1534. [Google Scholar] [CrossRef]
- Loutrari, H.; Magkouta, S.; Papapetropoulos, A.; Roussos, C. Mastic Oil Inhibits the Metastatic Phenotype of Mouse Lung Adenocarcinoma Cells. Cancers 2011, 3, 789–801. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, J.-H.; Jung, Y.-S.; Cho, M.-J.; Lee, Y.-E.; Park, D.-O.; Song, K.-B. Anticancer effect of mastic on human oral cancer cells. J. Korean Acad. Oral Health 2016, 40, 143–148. [Google Scholar] [CrossRef][Green Version]
- Balan, K.V.; Demetzos, C.; Prince, J.; Dimas, K.; Cladaras, M.; Han, Z.; Wyche, J.H.; Pantazis, P. Induction of Apoptosis in Human Colon Cancer HCT116 Cells Treated with an Extract of the Plant Product, Chios Mastic Gum. In Vivo 2005, 19, 93–102. [Google Scholar][Green Version]
- Sawidis, T.; Yurukova, L.; Askitis, T. Chios mastic, a natural supplement for zinc to enhance male sexuality and prostate function. Pharm. Biol. 2010, 48, 48–54. [Google Scholar] [CrossRef][Green Version]
- Triantafyllou, A.; Chaviaras, N.; Sergentanis, T.N.; Protopapa, E.; Tsaknis, J. Chios mastic gum modulates serum biochemical parameters in a human population. J. Ethnopharmacol. 2007, 111, 43–49. [Google Scholar] [CrossRef]
- Kartalis, A.; Didagelos, M.; Georgiadis, I.; Benetos, G.; Smyrnioudis, N.; Marmaras, H.; Voutas, P.; Zotika, C.; Garoufalis, S.; Andrikopoulos, G. Effects of Chios mastic gum on cholesterol and glucose levels of healthy volunteers: A prospective, randomized, placebo-controlled, pilot study (CHIOS-MASTIHA). Eur. J. Prev. Cardiol. 2016, 23, 722–729. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Pistacia Lentiscus L., Resin (Mastix); Committee on Herbal Medicinal Products (HMPC): London, UK, 2016. [Google Scholar]
- Bebb, J.R.; Bailey-Flitter, N.; Ala’Aldeen, D.; Atherton, J.C. Mastic gum has no effect on Helicobacter pylori load in vivo. J. Antimicrob. Chemother. 2003, 52, 522–523. [Google Scholar] [CrossRef]
- Loughlin, M.F.; Ala’Aldeen, D.A.; Jenks, P.J. Monotherapy with mastic does not eradicate Helicobacter pylori infection from mice. J. Antimicrob. Chemother. 2003, 51, 367–371. [Google Scholar] [CrossRef]
- Andreadou, I.; Mitakou, S.; Paraschos, S.; Efentakis, P.; Magiatis, P.; Kaklamanis, L.; Halabalaki, M.; Skaltsounis, L.; Iliodromitis, E.K. “Pistacia lentiscus L.” reduces the infarct size in normal fed anesthetized rabbits and possess antiatheromatic and hypolipidemic activity in cholesterol fed rabbits. Phytomedicine 2016, 23, 1220–1226. [Google Scholar] [CrossRef]
- El-Chaghaby, G.; Ahmad, A. Biosynthesis of silver nanoparticles using pistacia lentiscus leaves extract and investigation of their antimicrobial effect. Orient. J. Chem. 2011, 27, 929–936. [Google Scholar]
- Zintle, M.; Siwaphiwe, P.; Marthe Carine, F.; Thierry Youmbi, F.; Derek Tantoh, N.; Suprakas Sinha, R.; Blessing Atim, A. Antibacterial study of carbopol-mastic gum/silver nanoparticle-based topical gels with carvacrol/neem bark extract in vitro. J. Wound Care 2023, 32, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Vrouvaki, I.; Koutra, E.; Kornaros, M.; Avgoustakis, K.; Lamari, F.N.; Hatziantoniou, S. Polymeric Nanoparticles of Pistacia lentiscus var. chia Essential Oil for Cutaneous Applications. Pharmaceutics 2020, 12, 353. [Google Scholar] [CrossRef]
- Park, J.-H.; Yang, J.-Y.; Jeon, Y.; Kim, J.; Baek, J.-H. Evaluating the efficacy of curcumin and mastic gum enclosed in phospholipid-based lipid nanoparticles on Helicobacter pylori treatment using urea breath test delta values: A single-arm prospective pilot study. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Selvaraj, K.; Lee, S.-J.; Song, K.-B.; Yoo, B.-K.; Karuppaiah, A. Fabrication of Mastic Gum Resin Tethered Phospholipid Nanocarriers for the Evaluation and Enhancement of Anti-inflammatory and Anti-bacterial Effects. Curr. Pharm. Des. 2025, 31, 1866–1876. [Google Scholar] [CrossRef]
- Ao, F.; Yin, C.; Luo, X.; Shen, W.; Ge, X.; Zheng, Y. Controlled dual drug delivery system based on gelatin electrospinning membranes for wound healing promotion. Int. J. Biol. Macromol. 2025, 289, 138720. [Google Scholar] [CrossRef]
- Eldem, A.; Tekintaş, Y.; Ucuncu, M.; Horzum, N. Electrospun Nanofiber Platforms for Photodynamic Therapy: Role and Efficacy in Cancer, Antimicrobial, and Wound Healing Applications. Macromol. Mater. Eng. 2025, 310, 2500014. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, H.; Yang, J.; Liu, X.; Chen, L.; Li, W.; Mi, S.; Zhou, H.; Zheng, W.; Xue, W.; et al. Recent advances in structural and functional design of electrospun nanofibers for wound healing. J. Mater. Chem. B 2025, 13, 5226–5263. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun Nanofibrous Materials for Wound Healing. Adv. Fiber Mater. 2020, 2, 212–227. [Google Scholar] [CrossRef]
- Han, D.; Gouma, P.I. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomedicine 2006, 2, 37–41. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Polycaprolactone Composites/Blends and Their Applications Especially in Water Treatment. ChemEngineering 2023, 7, 104. [Google Scholar] [CrossRef]
- Spartali, C.; Psarra, A.-M.G.; Marras, S.I.; Tsioptsias, C.; Georgantopoulos, A.; Kalousi, F.D.; Tsakalof, A.; Tsivintzelis, I. Silybin-Functionalized PCL Electrospun Fibrous Membranes for Potential Pharmaceutical and Biomedical Applications. Polymers 2024, 16, 2346. [Google Scholar] [CrossRef]
- Marras, S.I.; Kladi, K.P.; Tsivintzelis, I.; Zuburtikudis, I.; Panayiotou, C. Biodegradable polymer nanocomposites: The role of nanoclays on the thermomechanical characteristics and the electrospun fibrous structure. Acta Biomater. 2008, 4, 756–765. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Guo, Y.; Han, Z.; Zhang, J.; Lu, Y.; Li, C.; Liu, G. Development of a high-speed and ultrasensitive UV/Vis-CM for detecting total triterpenes in traditional Chinese medicine and its application. Heliyon 2024, 10, e32239. [Google Scholar] [CrossRef]
- Yang, M.; Xiang, G.; Li, D.; Zhang, Y.; Xu, W.; Tao, L. The insecticide spinosad induces DNA damage and apoptosis in HEK293 and HepG2 cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 812, 12–19. [Google Scholar] [CrossRef]
- Kehinde, I.; Khan, R.; Nlooto, M.; Gordon, M. Modulatory influences of antiviral bioactive compounds on cell viability, mRNA and protein expression of cytochrome P450 3A4 and P-glycoprotein in HepG2 and HEK293 cells. Bioorg. Chem. 2021, 107, 104573. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Shabani, A.; Al, G.A.; Berri, N.; Castro-Dominguez, B.; Leese, H.S.; Martinez-Hernandez, U. Electrospinning Technology, Machine Learning, and Control Approaches: A Review. Adv. Eng. Mater. 2025, 27, 2401353. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Huang, J.; Yang, X.; Wang, W.; Yu, D.-G.; He, H.; Liu, P.; Du, K. Electrospun nanofibers and their application as sensors for healthcare. Front. Bioeng. Biotechnol. 2025, 13, 1533367. [Google Scholar] [CrossRef] [PubMed]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Yuan, Y.; Choi, K.; Choi, S.-O.; Kim, J. Early stage release control of an anticancer drug by drug-polymer miscibility in a hydrophobic fiber-based drug delivery system. RSC Adv. 2018, 8, 19791–19803. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Jhurry, D. Drug Loading and Release from Electrospun Biodegradable Nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2173–2199. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Eren Böncü, T.; Özdemir, N. Effects of drug concentration and PLGA addition on the properties of electrospun ampicillin trihydrate-loaded PLA nanofibers. Beilstein J. Nanotechnol. 2022, 13, 245–254. [Google Scholar] [CrossRef]
- Blakney, A.K.; Krogstad, E.A.; Jiang, Y.H.; Woodrow, K.A. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int. J. Nanomed. 2014, 9, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Krogstad, E.A.; Woodrow, K.A. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int. J. Pharm. 2014, 475, 282–291. [Google Scholar] [CrossRef]
- Aydogdu Emir, A.; Akgun, M.; Kirtil, E. Effect of mastic gum integration on improvement of polylactic acid biodegradable films. Polym. Eng. Sci. 2023, 63, 1539–1550. [Google Scholar] [CrossRef]
- Kalousi, F.D.; Pollastro, F.; Christodoulou, E.C.; Karra, A.G.; Tsialtas, I.; Georgantopoulos, A.; Salamone, S.; Psarra, A.-M.G. Apoptotic, Anti-Inflammatory Activities and Interference with the Glucocorticoid Receptor Signaling of Fractions from Pistacia lentiscus L. var. chia Leaves. Plants 2022, 11, 934. [Google Scholar] [CrossRef]
- Laskari, V.; Katsanou, E.; Kyriakopoulou, K.; Termentzi, A.; Skaltsounis, A.L.; Fokialakis, N.; Machera, K. In vitro protection and toxicity assessment of Chios Mastic Gum extracts and isolated triterpenic acids using human hepatocarcinoma cells. Planta Med. 2015, 81, PM_68. [Google Scholar] [CrossRef]
- He, M.-L.; Yuan, H.-Q.; Jiang, A.-L.; Gong, A.Y.; Chen, W.-W.; Zhang, P.-J.; Young, C.Y.F.; Zhang, J.-Y. Gum mastic inhibits the expression and function of the androgen receptor in prostate cancer cells. Cancer 2006, 106, 2547–2555. [Google Scholar] [CrossRef]
- He, M.-L.; Li, A.; Xu, C.-S.; Wang, S.-L.; Zhang, M.-J.; Gu, H.; Yang, Y.-Q.; Tao, H.-H. Mechanisms of antiprostate cancer by gum mastic: NF-κB signal as target. Acta Pharmacol. Sin. 2007, 28, 446–452. [Google Scholar] [CrossRef]
- Koo, B.-C.; Kim, D.-H.; Kim, I.-R.; Kim, G.-C.; Kwak, H.-H.; Park, B.-S. A natural product, chios gum mastic, induces the death of HL-60 cells via apoptosis and cell cycle arrest. Int. J. Oral Biol. 2011, 36, 13–21. [Google Scholar]
- Huang, X.-Y.; Wang, H.-C.; Yuan, Z.; Li, A.; He, M.-L.; Ai, K.-X.; Zheng, Q.; Qin, H.-L. Gemcitabine combined with gum mastic causes potent growth inhibition and apoptosis of pancreatic cancer cells. Acta Pharmacol. Sin. 2010, 31, 741–745. [Google Scholar] [CrossRef]
- Li, S.; Cha, I.-H.; Nam, W. Chios mastic gum extracts as a potent antitumor agent that inhibits growth and induces apoptosis of oral cancer cells. Asian Pac. J. Cancer Prev. 2011, 12, 1877–1880. [Google Scholar]
- Lee, S.-E.; Hur, Y.-J.; Kim, I.-R.; Kwak, H.-H.; Kim, G.-C.; Shin, S.-H.; Kim, C.-H.; Park, B.-S. Mechanism underlying Chios gum mastic-induced apoptosis on SCC25 human tongue squamous cell carcinoma cell line. Int. J. Oral Biol. 2009, 34, 61–72. [Google Scholar]
- Espinoza, S.M.; Indrabhan, P.H.; Eduardo, S.M.M.; Rocio, C.P.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Mitxelena-Iribarren, O.; Riera-Pons, M.; Pereira, S.; Calero-Castro, F.J.; Castillo Tuñón, J.M.; Padillo-Ruiz, J.; Mujika, M.; Arana, S. Drug-loaded PCL electrospun nanofibers as anti-pancreatic cancer drug delivery systems. Polym. Bull. 2023, 80, 7763–7778. [Google Scholar] [CrossRef]
- Rasekh, M.; Pisapia, F.; Nokhodchi, A. Innovative applications of electrospun nanofibers in cancer research. J. Drug Deliv. Sci. Technol. 2024, 91, 105255. [Google Scholar] [CrossRef]
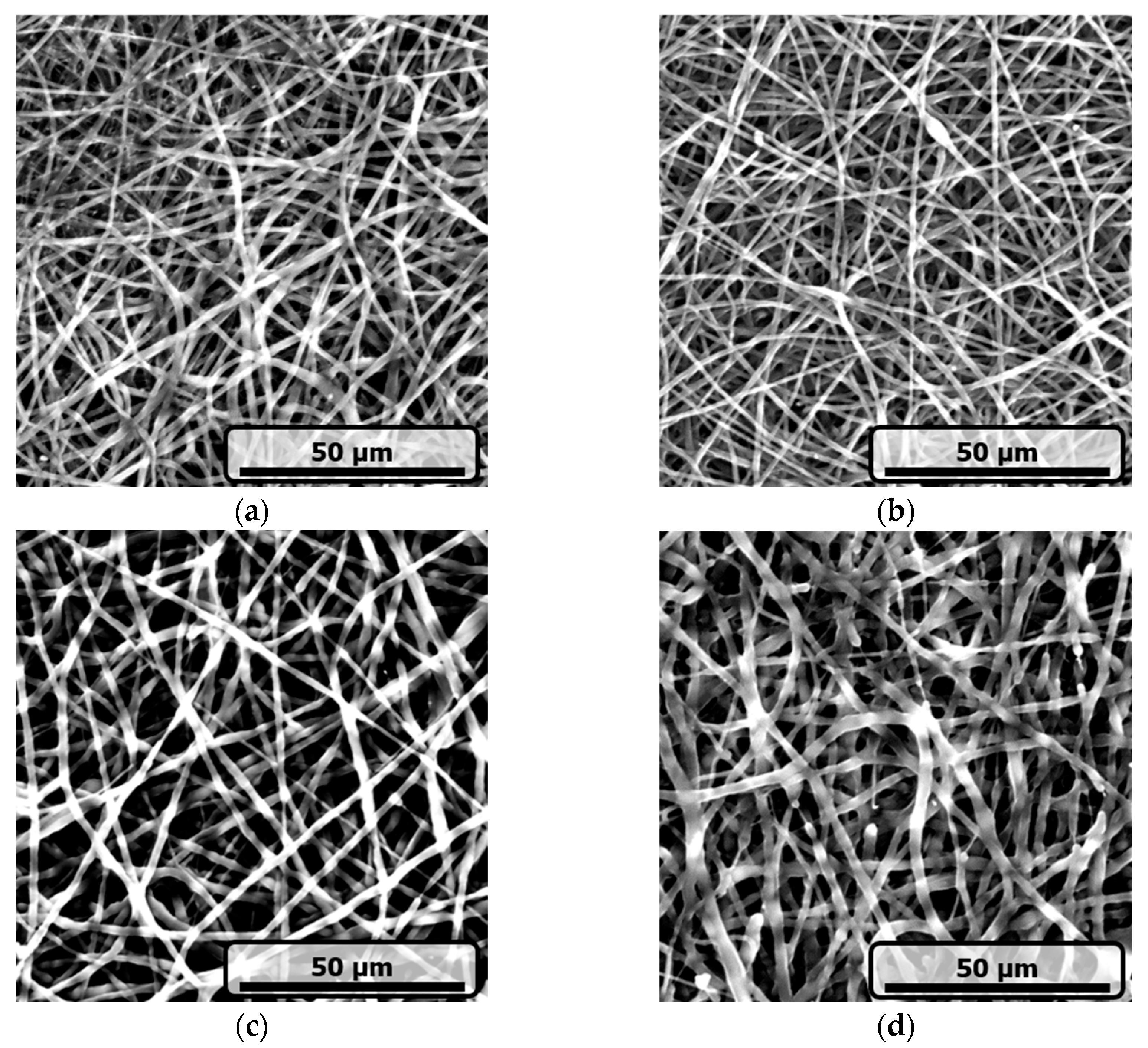




| Samples | Encapsulation Efficiency (%) | CMG Loading (wt%) |
|---|---|---|
| PCL/3.5CMG | 87 ± 6 | 3.0 ± 0.2 |
| PCL/6.5CMG | 88 ± 3 | 5.7 ± 0.2 |
| PCL/10CMG | 87 ± 5 | 8.7 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastorakis, P.M.; Marras, S.I.; Tsioptsias, C.; Zaoutsos, S.P.; Leonidas, D.D.; Tsivintzelis, I.; Psarra, A.-M.G. Novel Bio-Functional Electrospun Membranes by Chios Mastic Gum Encapsulation. Fibers 2025, 13, 116. https://doi.org/10.3390/fib13090116
Mastorakis PM, Marras SI, Tsioptsias C, Zaoutsos SP, Leonidas DD, Tsivintzelis I, Psarra A-MG. Novel Bio-Functional Electrospun Membranes by Chios Mastic Gum Encapsulation. Fibers. 2025; 13(9):116. https://doi.org/10.3390/fib13090116
Chicago/Turabian StyleMastorakis, Panagiotis M., Sotirios I. Marras, Costas Tsioptsias, Stephanos P. Zaoutsos, Demetres D. Leonidas, Ioannis Tsivintzelis, and Anna-Maria G. Psarra. 2025. "Novel Bio-Functional Electrospun Membranes by Chios Mastic Gum Encapsulation" Fibers 13, no. 9: 116. https://doi.org/10.3390/fib13090116
APA StyleMastorakis, P. M., Marras, S. I., Tsioptsias, C., Zaoutsos, S. P., Leonidas, D. D., Tsivintzelis, I., & Psarra, A.-M. G. (2025). Novel Bio-Functional Electrospun Membranes by Chios Mastic Gum Encapsulation. Fibers, 13(9), 116. https://doi.org/10.3390/fib13090116










