Copper-Decorated Catalytic Carbon/Ceramic Hollow Fibers for NO Reduction: Enhanced Performance via Tangential Flow Reactor Design and Process Intensification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Hollow Fiber Preparation
2.3. Characterization Techniques
2.4. Catalytic Experiments
3. Results
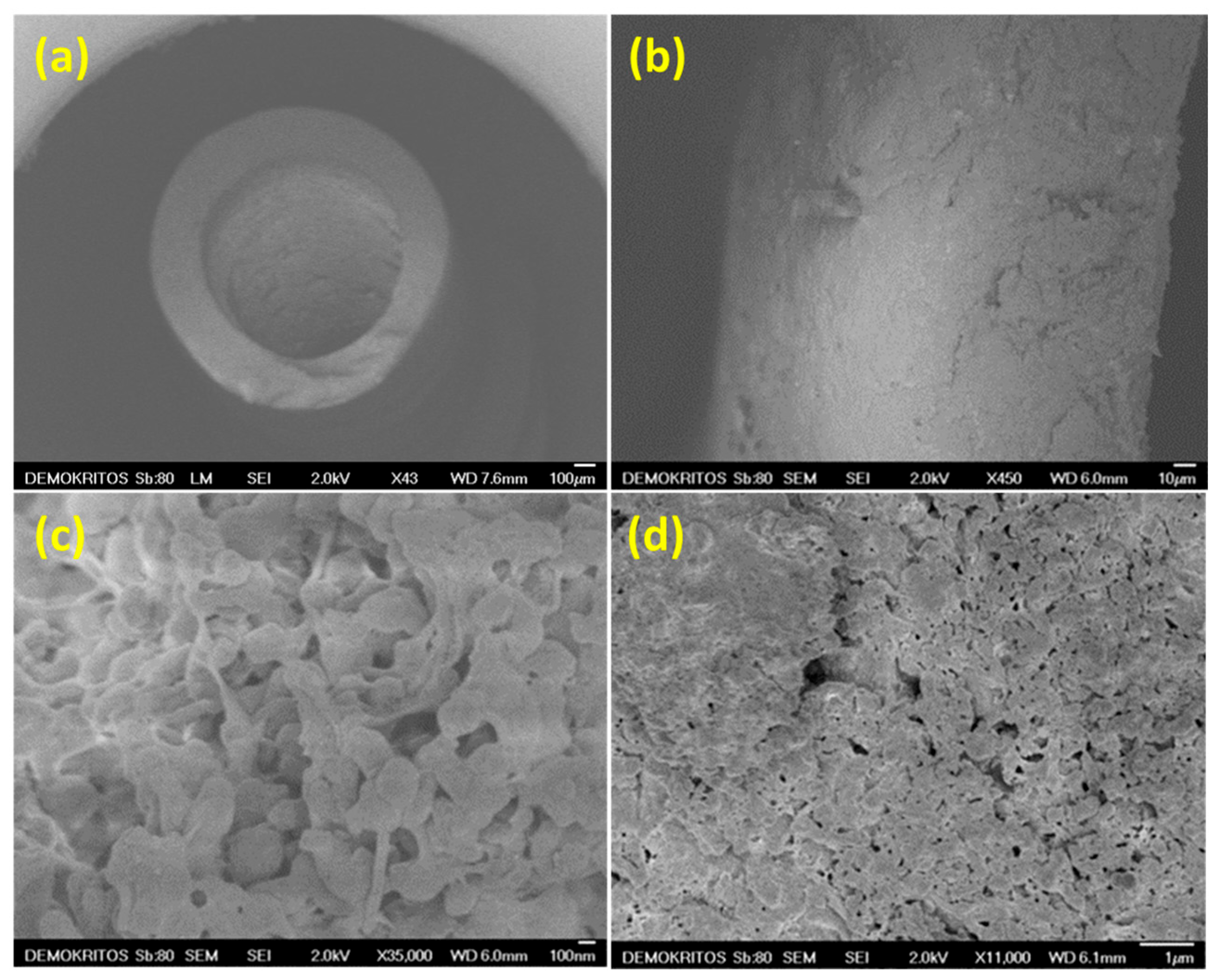
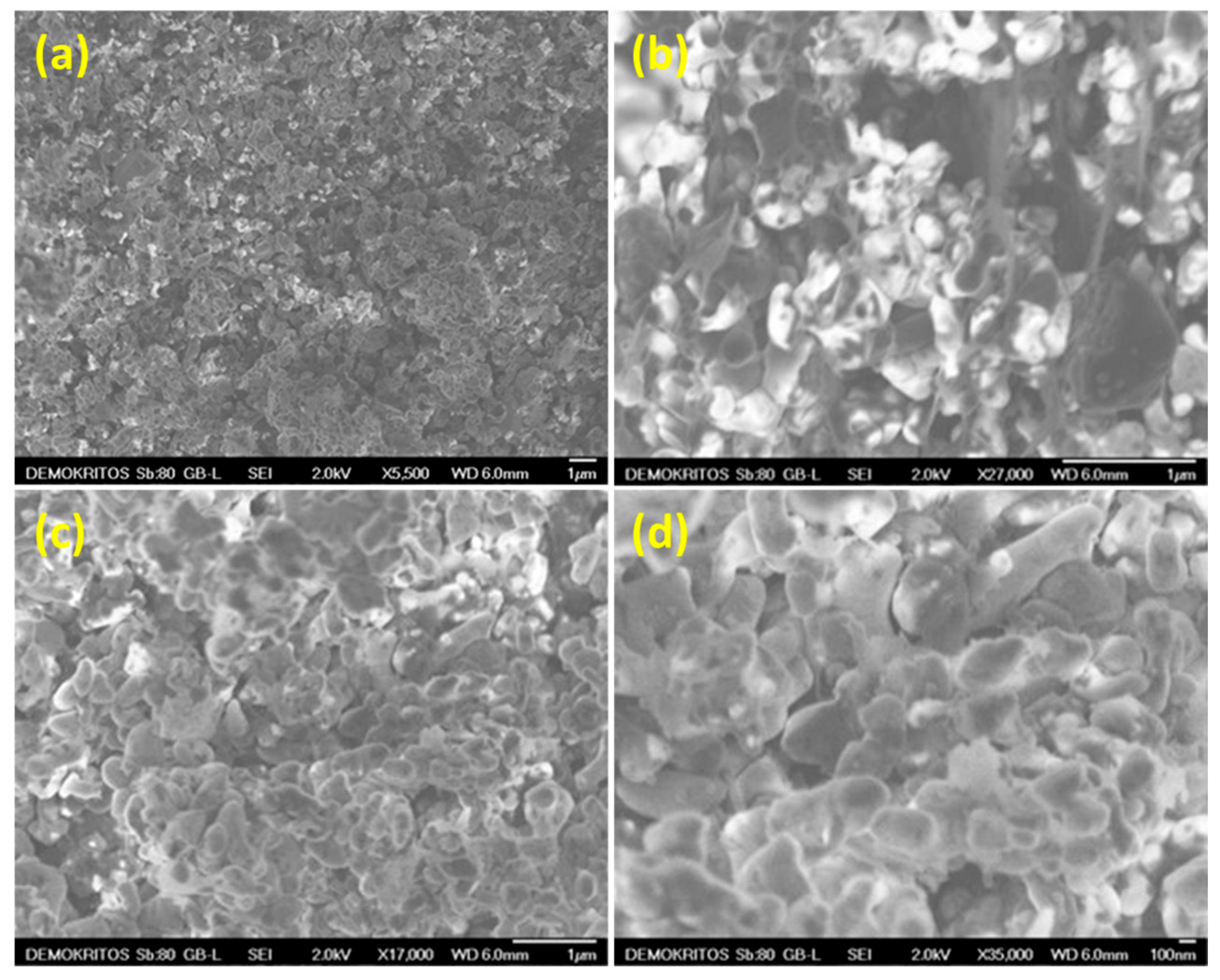
3.1. Morphological and Textural Characterization
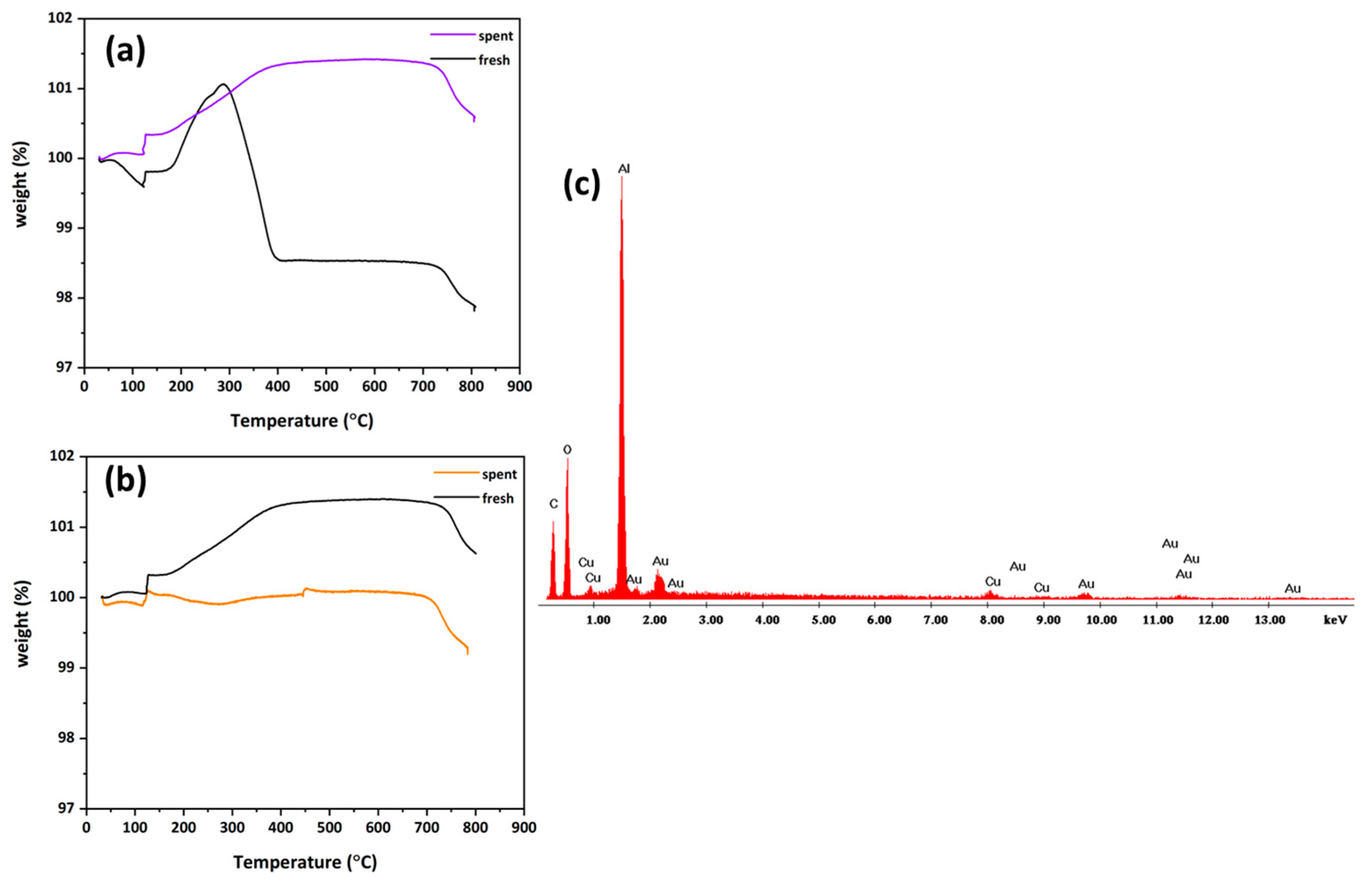
3.2. Thermogravimetric and EDX Analysis
3.3. WAXS Analysis
3.4. Catalytic Performance
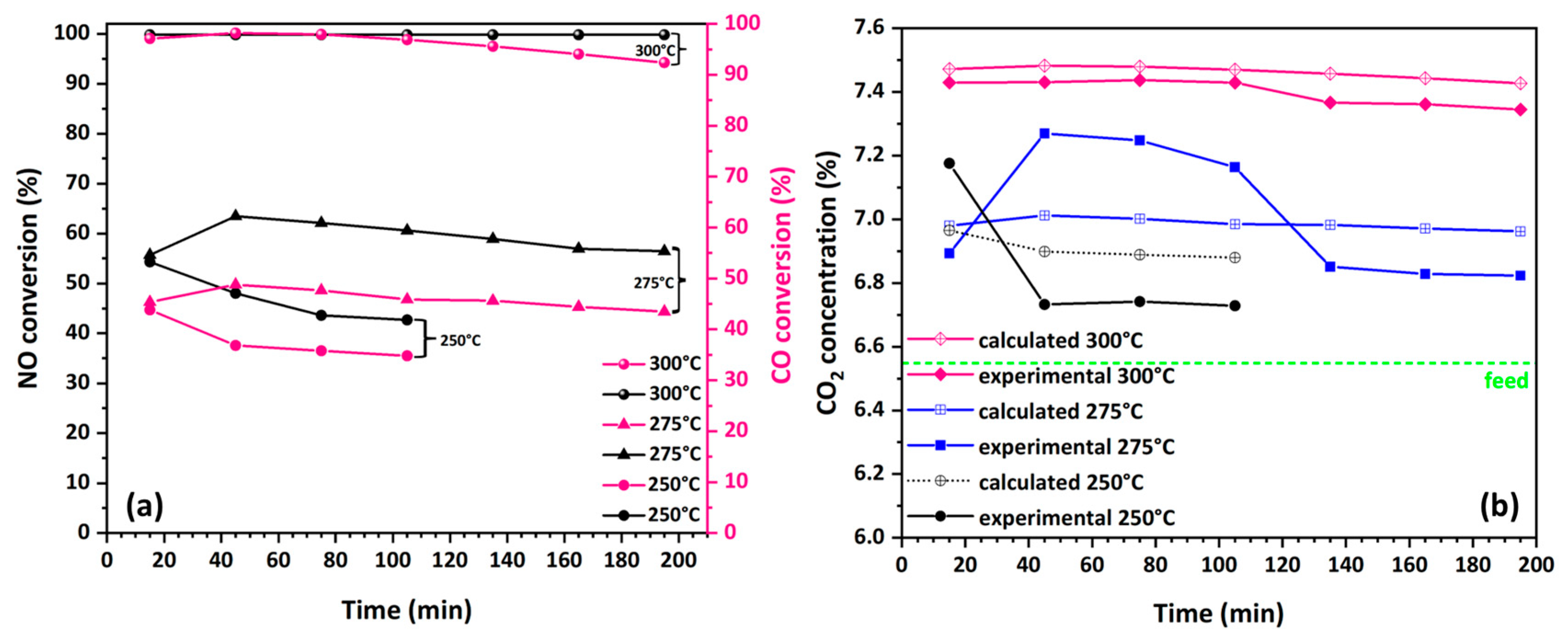
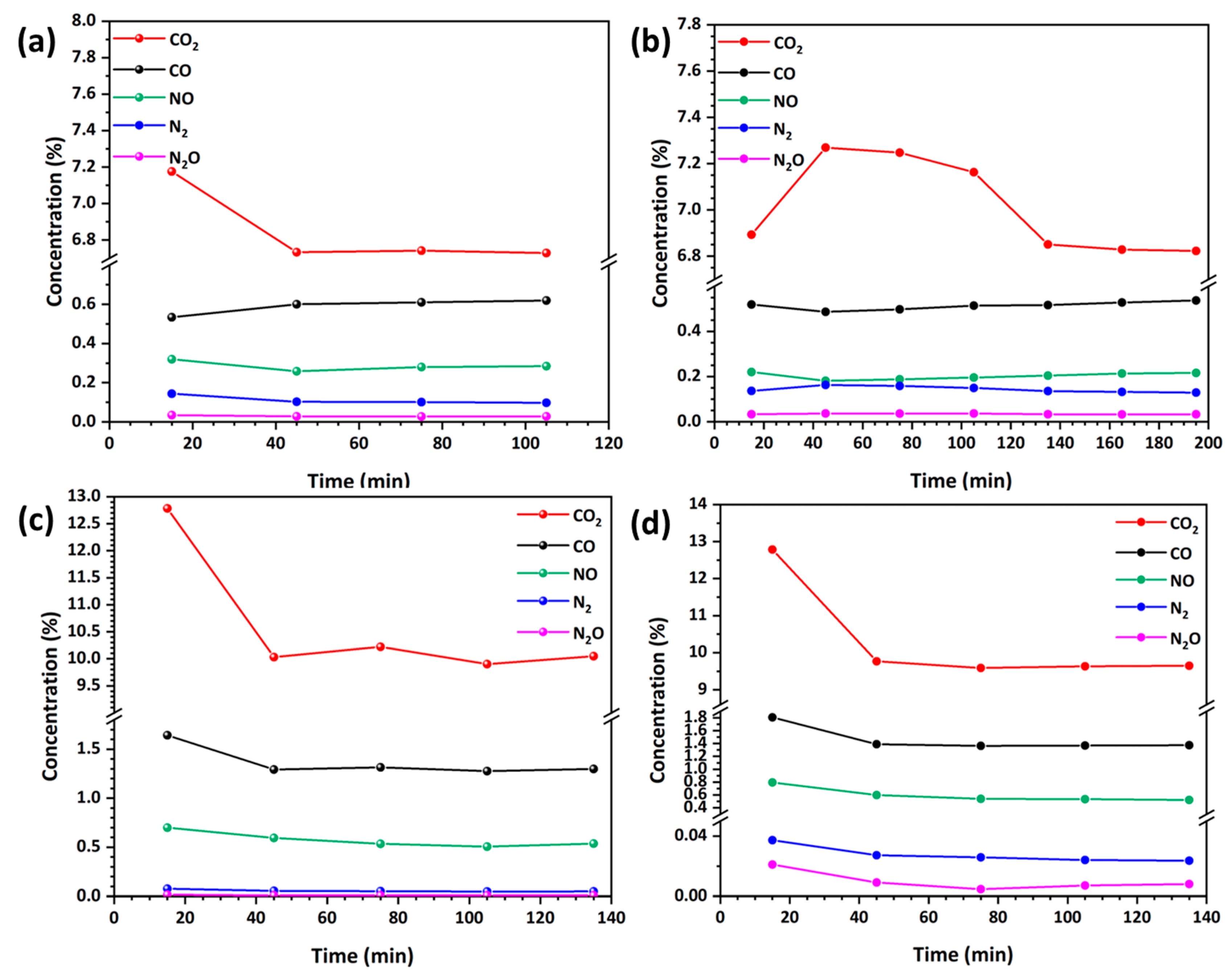
3.4.1. Impact of Carbon Content on the NO Reduction Efficiency by CO
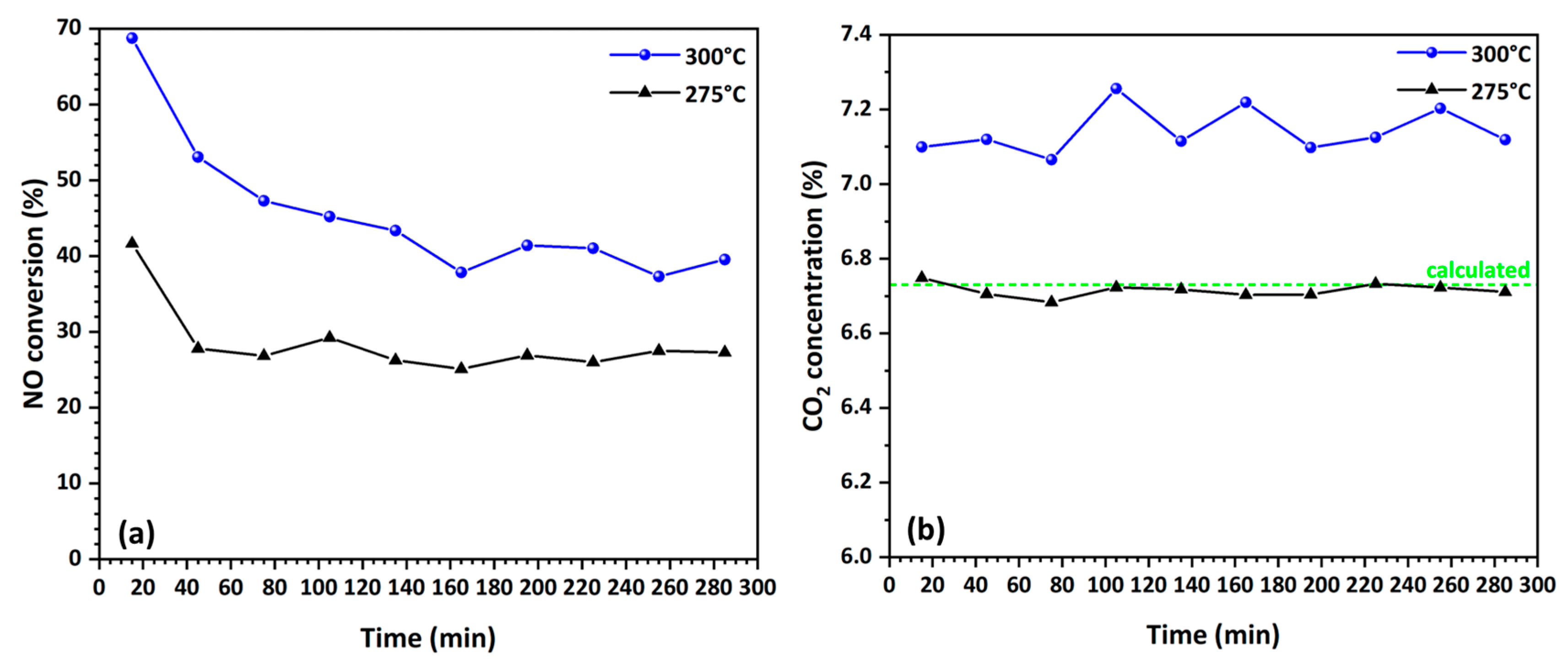
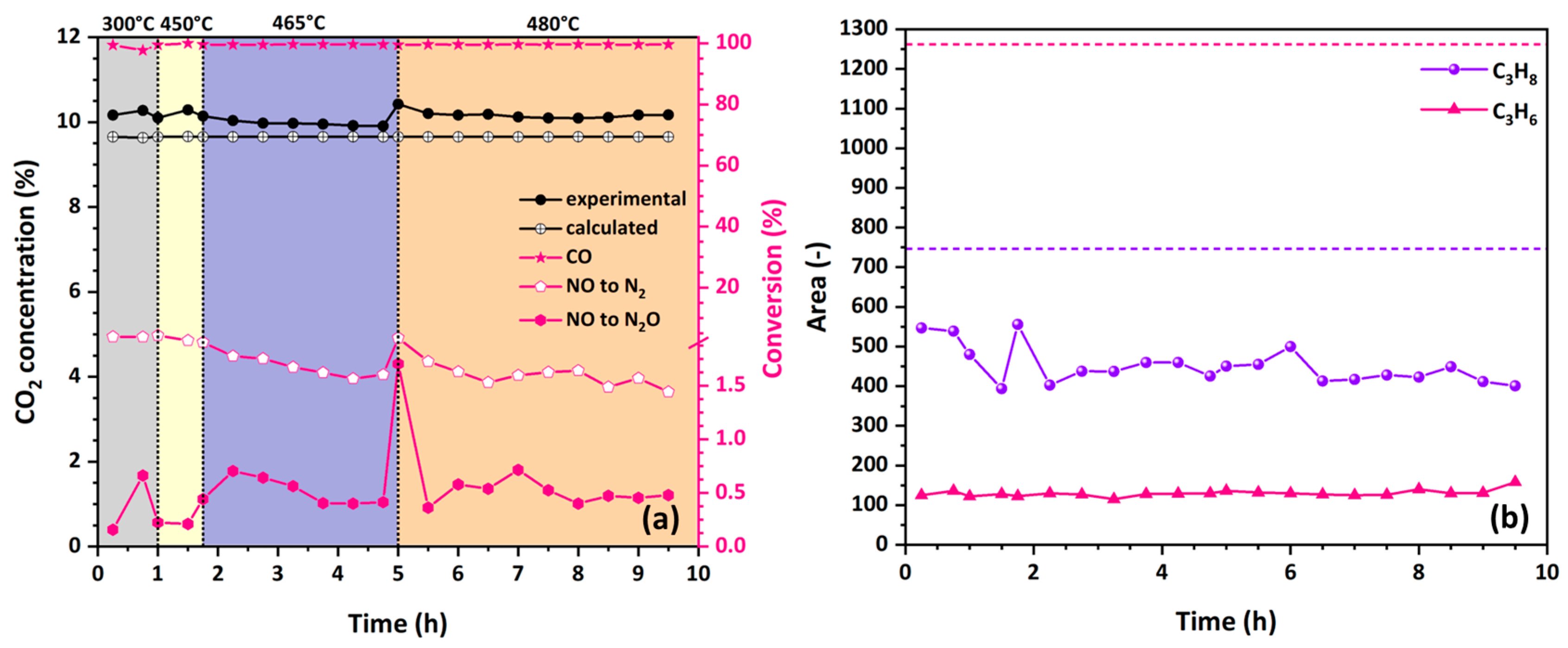
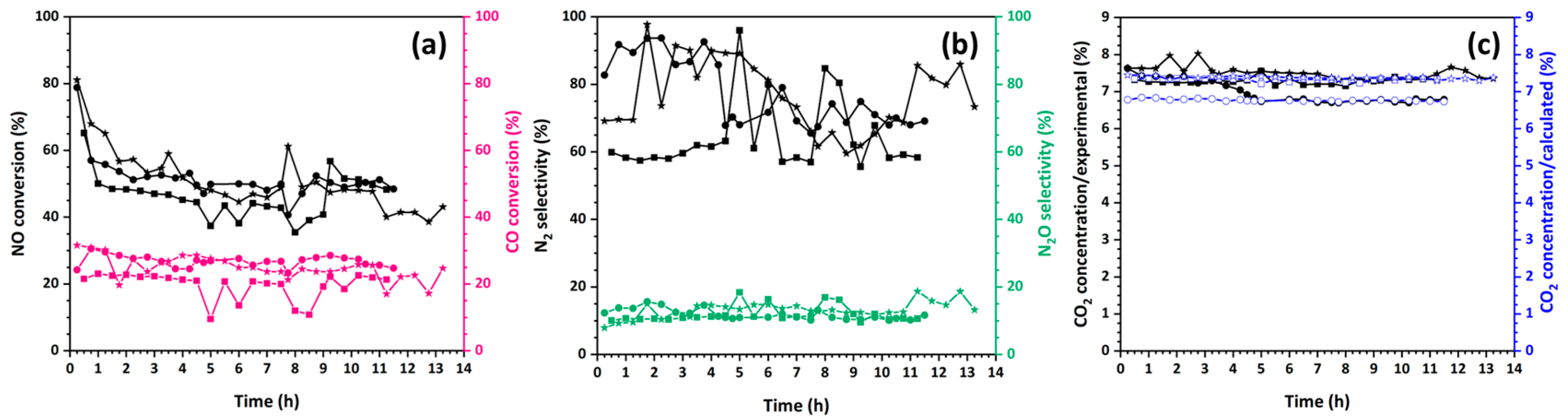
3.4.2. Stability and Selectivity of Catalytic Fibers in NO Reduction by CO
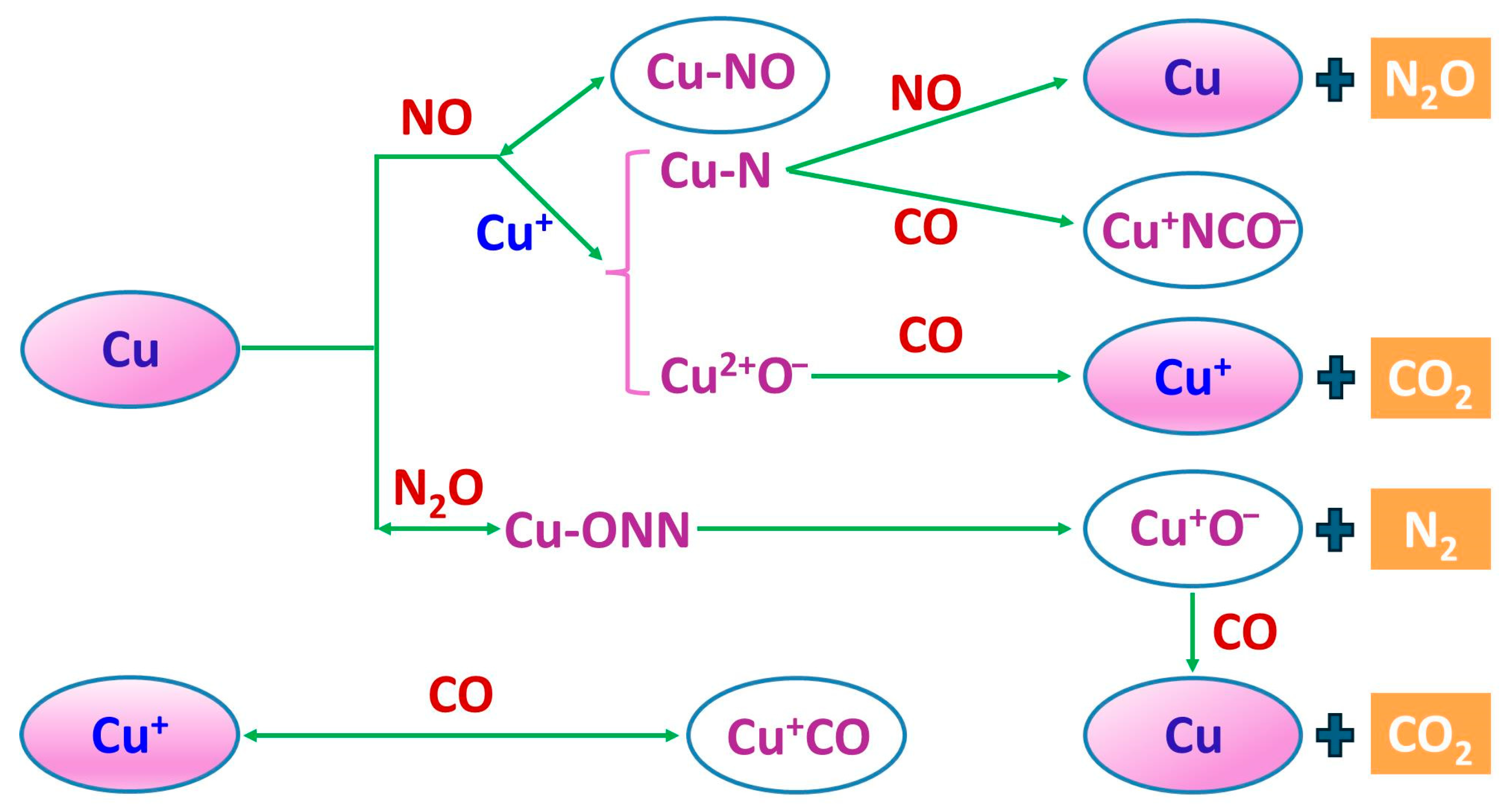
3.4.3. The Role of Oxygen
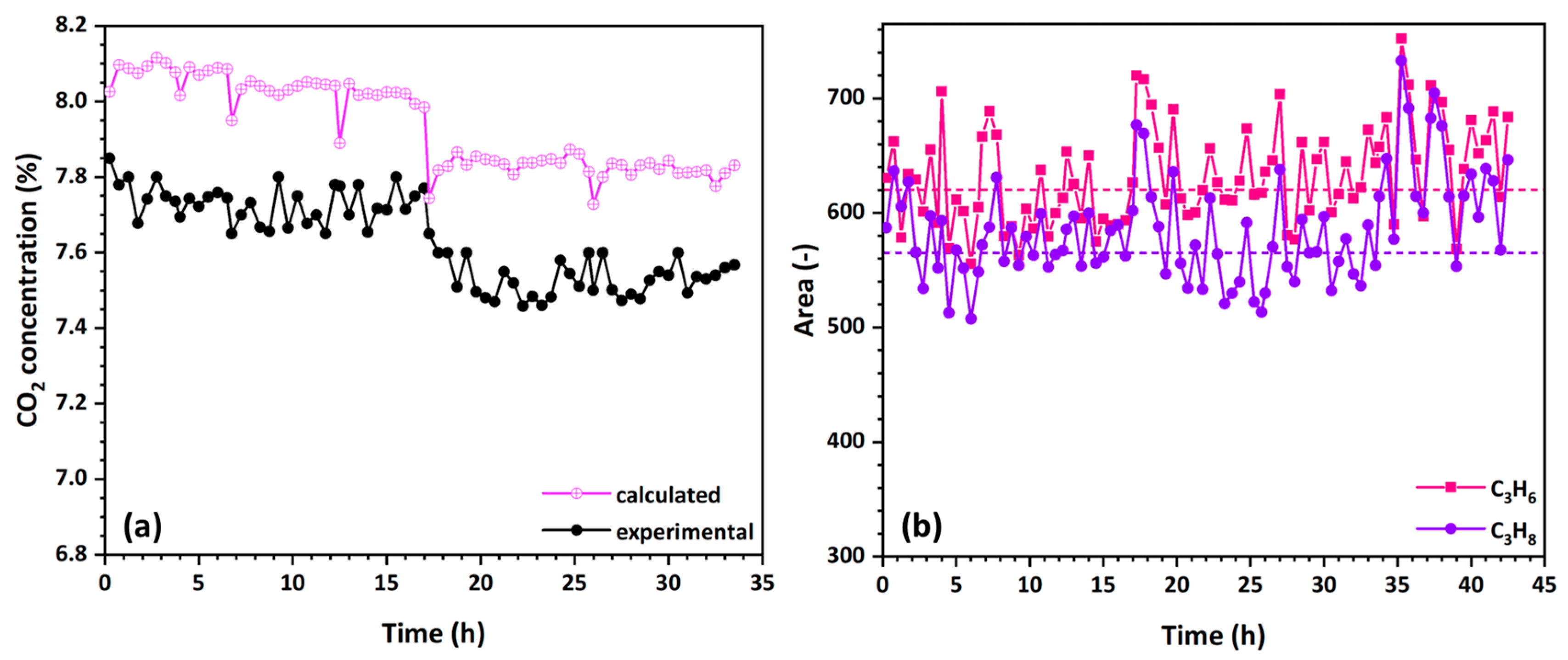
3.4.4. Regeneration Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vermisoglou, E.C.; Romanos, G.E.; Karanikolos, G.N.; Kanellopoulos, N.K. Catalytic NOx removal by single-wall carbon nanotube-supported Rh nanoparticles. J. Hazard. Mater. 2011, 194, 144–155. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Caro, J.; Wang, H. Dense ceramic oxygen permeable membranes and catalytic membrane reactors. Chem. Eng. J. 2013, 220, 185–203. [Google Scholar] [CrossRef]
- Fang, S.; Takagaki, A.; Watanabe, M.; Ishihara, T. The direct decomposition of NO into N2 and O2 over copper doped Ba3Y4O9. Catal. Sci. Technol. 2020, 10, 2513–2522. [Google Scholar] [CrossRef]
- Wang, J.; Ji, S.; Yang, J.; Zhu, Q.; Li, S. Mo2C and Mo2C/Al2O3 catalysts for NO direct decomposition. Catal. Commun. 2005, 6, 389–393. [Google Scholar] [CrossRef]
- Xie, P.; Ji, W.; Li, Y.; Zhang, C. NO direct decomposition: Progress, challenges and opportunities. Catal. Sci. Technol. 2021, 11, 374–391. [Google Scholar] [CrossRef]
- Tomašić, V. Application of the monoliths in DeNOx catalysis. Catal. Today 2007, 119, 106–113. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Izquierdo, R.; Rodríguez, L.J.; Añez, R.; Sierraalta, A. Direct catalytic decomposition of NO with Cu–ZSM-5: A DFT–ONIOM study. J. Mol. Catal. A Chem. 2011, 348, 55–62. [Google Scholar] [CrossRef]
- Karásková, K.; Pacultová, K.; Bilková, T.; Fridrichová, D.; Koštejn, M.; Peikertová, P.; Stelmachowski, P.; Kukula, P.; Obalová, L. Effect of zinc on the structure and activity of the cobalt oxide catalysts for NO decomposition. Catalysts 2023, 13, 18. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- De Rosa, M.; Campa, M.C.; Pietrogiacomi, D.; Marpurgo, S. A DFT study on Cu-ZSM-5 as a catalyst for NO decomposition: Possible activity of a Cu(I) pair located at the T3 tetrahedral sites. Mol. Catal. 2024, 559, 114083. [Google Scholar] [CrossRef]
- Houel, V.; James, D.; Millington, P.; Pollington, S.; Poulston, S.; Rajaram, R.; Torbati, R. A comparison of the activity and deactivation of Ag/Al2O3 and Cu/ZSM-5 for HC-SCR under simulated diesel exhaust emission conditions. J. Catal. 2005, 230, 150–157. [Google Scholar] [CrossRef]
- Burch, R.; Breen, J.P.; Meunier, F.C. A review of the selective reduction of NOx with hydrocarbons under lean-burn conditions with non-zeolitic oxide and platinum group metal catalysts. App. Catal. B Environ. 2002, 39, 283–303. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Y.; Zhao, H.; Li, X.; Ma, Y.; Yong, X.; Chen, H.; Li, Y. The promotion effect of nickel and lanthanum on Cu-ZSM-5 catalyst in NO direct decomposition. Catal. Today 2019, 327, 203–209. [Google Scholar] [CrossRef]
- Patel, A.; Shukla, P.; Chen, J.; Rufford, T.E.; Wang, S.; Rudolph, V.; Zhu, Z. Structural sensitivity of mesoporous alumina for copper catalyst loading used for NO reduction in presence of CO. Chem. Eng. Res. Des. 2015, 101, 27–43. [Google Scholar] [CrossRef]
- Rico, M.J.O.; Moreno-Tost, R.; Jiménez-López, A.; Rodríguez-Castellón, E.; Pereñíguez, R.; Caballero, A.; Holgado, J.P. Study of nanoporous catalysts in the selective catalytic reduction of NOx. Catal. Today 2010, 158, 78–88. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, M.; Gao, F.; Kong, Y.; Dong, L.; Chen, Y.; Jian, C.; Liu, Z. A study of CuO/CeO2/Al–Zr–O in “NO + CO”. Catal. Commun. 2004, 5, 453–456. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Nishimura, C.; Masui, T.; Imanaka, N. Direct decomposition of nitrogen monoxide on (Ho, Zr, Pr)2O3+δ catalysts. Catal. Commun. 2014, 43, 84–87. [Google Scholar] [CrossRef]
- Iwakuni, H.; Shinmyou, Y.; Yano, H.; Matsumoto, H.; Ishihara, T. Direct decomposition of NO into N2 and O2 on BaMnO3-based perovskite oxides. Appl. Catal. B Environ. 2007, 74, 299–306. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Nakamura, I.; Fujitani, T.; Hamada, H. Comprehensive study combining surface science and real catalyst for NO direct decomposition. Chem. Commun. 2002, 2816–2817. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, Y.; Torigoshi, K.-I.; Yamaguchi, H.; Ikeda, T.; Kagawa, S. Direct decomposition of nitric oxide over stannate pyrochlore oxides: Relationship between solid-state chemistry and catalytic activity. J. Mol. Catal. A Chem. 2000, 155, 73–80. [Google Scholar] [CrossRef]
- Masui, T.; Uejima, S.; Tsujimoto, S.; Imanaka, N. Direct decomposition of NO into N2 and O2 over C-Type Cubic Y2O3-Tb4O7-ZrO2. Mat. Sci. Appl. 2012, 3, 733–738. [Google Scholar]
- Cónsul, J.M.D.; Peralta, C.A.; Benvenutti, E.V.; Ruiz, J.A.C.; Pastore, H.O.; Baibich, I.M. Direct decomposition of nitric oxide on alumina-modified amorphous and mesoporous silica-supported palladium catalysts. J. Mol. Catal. A Chem. 2006, 246, 33–38. [Google Scholar] [CrossRef]
- Gómez-García, M.A.; Pitchon, V.; Kiennemann, A. Pollution by nitrogen oxides: An approach to NOx abatement by using sorbing catalytic materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef]
- Ramalho, P.S.F.; Soares, O.S.G.P.; Figueiredo, J.L.; Pereira, M.F.R. Catalytic reduction of NO over copper supported on activated carbon. Catal. Today 2023, 418, 114044. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, H.; Li, L.; Guo, Y. Cost-effective activated carbon (AC) production from partial substitution of coal with red mud (RM) as additive for SO2 and NOx abatement at low temperature. Fuel 2021, 293, 120448. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Song, Q. Deactivation of activated carbon during low-temperature NO adsorption-regeneration cycles. Sep. Purif. Technol. 2025, 377, 134380. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Li, T.; Mahmood, N.; Ahmad, M.; Xu, J.; Mahmood, A.; Djellabi, R.; Yang, B. Thermally activated epoxy-functionalized carbon as an electrocatalyst for efficient NOx reduction. Carbon 2021, 182, 516–524. [Google Scholar] [CrossRef]
- Wan, J.; Xu, W.; Zhang, X.; Jia, X.; Zhang, C. Study and application of nitric-acid-modified activated carbon fiber on nitrogen oxide adsorption performance. Processes 2025, 13, 760. [Google Scholar] [CrossRef]
- Santillan-Jimenez, E.; Miljković-Kocić, V.; Crocker, M.; Wilson, K. Carbon nanotube-supported metal catalysts for NOx reduction using hydrocarbon reductants. Part: 1 catalyst preparation, characterization and NOx reduction characteristics. Appl. Catal. B Environ. 2011, 102, 1–8. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Qiu, F.; Zhang, X. Promotional effects of carbon nanotubes on V2O5/TiO2 for NOx removal. J. Hazard. Mater. 2011, 192, 915–921. [Google Scholar] [CrossRef]
- Dasireddy, V.D.B.C.; Likozar, B. Selective catalytic reduction of NOx by CO over bimetallic transition metals supported by multi-walled carbon nanotubes (MWCNT). Chem. Eng. J. 2017, 326, 886–900. [Google Scholar] [CrossRef]
- Lu, X.; Song, C.; Jia, S.; Tong, Z.; Tang, X.; Teng, Y. Low-temperature selective catalytic reduction of NOx with NH3 over cerium and manganese oxides supported on TiO2–graphene. Chem. Eng. J. 2015, 260, 776–784. [Google Scholar] [CrossRef]
- Sousa, J.P.S.; Pereira, M.F.R.; Figueiredo, J.L. NO oxidation over nitrogen doped carbon xerogels. Appl. Catal. B Environ. 2012, 125, 398–408. [Google Scholar] [CrossRef]
- García-García, F.R.; Li, K. New catalytic reactors prepared from symmetric and asymmetric ceramic hollow fibres. Appl. Catal. A Gen. 2013, 456, 1–10. [Google Scholar] [CrossRef]
- Yakoumis, I.; Theodorakopoulos, G.; Papageorgiou, S.K.; Romanos, G.; Veziri, C.; Panias, D. Tubular C/Cu decorated γ-alumina membranes for NO abatement. J. Membr. Sci. 2016, 515, 134–143. [Google Scholar] [CrossRef]
- Tan, X.; Li, K. Inorganic hollow fibre membranes in catalytic processing. Curr. Opin. Chem. Eng. 2011, 1, 69–76. [Google Scholar] [CrossRef]
- García-Vázquez, M.; Marín, P.; Ordóñez, S.; Li, K.; Tan, J.; Zhang, G.; García-García, F.R. Scaling up a hollow fibre reactor: A study on non-PGM hollow fibre after-treatments for methane emission control under extreme conditions. J. Environ. Chem. Eng. 2021, 9, 106880. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Schiestel, T.; Werth, S.; Caro, J. Partial oxidation of methane to syngas in a perovskite hollow fiber membrane reactor. Catal. Commun. 2006, 7, 907–912. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.V.; Romanos, G.E.; Katsaros, F.K.; Papageorgiou, S.K.; Kontos, A.G.; Spyrou, K.; Beazi-Katsioti, M.; Falaras, P. Structuring efficient photocatalysts into bespoke fiber shaped systems for applied water treatment. Chemosphere 2021, 277, 130253. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.V.; Papageorgiou, S.K.; Katsaros, F.K.; Romanos, G.E.; Beazi-Katsioti, M. Investigation of MO adsorption kinetics and photocatalytic degradation utilizing hollow fibers of Cu-CuO/TiO2 nanocomposite. Materials 2024, 17, 4663. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Boddenberg, B. Copper exchanged zeolites studied with 13C and 129Xe NMR of adsorbed carbon monoxide and xenon. Stud. Surf. Sci. Catal. 1994, 84, 509–517. [Google Scholar] [CrossRef]
- Halasz, I.; Brenner, A.; Shelef, M.; Ng, K.Y.S. Decomposition of nitric oxide and its reduction by CO over superconducting and related cuprate catalysts. Catal. Lett. 1991, 11, 327–334. [Google Scholar] [CrossRef]
- Shelef, M.; Gandhi, H.S. Ammonia formation in catalytic reduction of nitric oxide by molecular hydrogen. I. Base metal oxide catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1972, 11, 2–11. [Google Scholar] [CrossRef]
- London, J.W.; Bell, A.T. A simultaneous infrared and kinetic study of the reduction of nitric oxide by carbon monoxide over copper oxide. J. Catal. 1973, 31, 96–109. [Google Scholar] [CrossRef]
- Buccuzzi, F.; Guglielminotti, E.; Martra, G.; Cerrato, G. Nitric oxide reduction by CO on Cu/TiO2 catalysts. J. Catal. 1994, 146, 449–459. [Google Scholar] [CrossRef]
- Rewick, R.T.; Wise, H. Reduction of nitric oxide by carbon monoxide on copper catalysts. J. Catal. 1975, 40, 301–311. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Nickolov, R.; Mehandjiev, D. Effect of chromium on copper containing activated carbon catalysts for methanol decomposition. React. Kinet. Catal. Lett. 2001, 72, 383–390. [Google Scholar] [CrossRef]
- Li, D.; Yu, Q.; Li, S.-S.; Wan, H.-Q.; Liu, L.-J.; Qi, L.; Liu, B.; Gao, F.; Dong, L.; Chen, Y. The remarkable enhancement of CO-pretreated CuO-Mn2O3/γ-Al2O3 supported catalyst for the reduction of NO with CO: The formation of surface synergetic oxygen vacancy. Chem. Eur. J. 2011, 17, 5668–5679. [Google Scholar] [CrossRef]
- Zecchina, A.; Cerruti, L.; Borrello, E. An infrared study of nitrous oxide adsorption on α-chromia. J. Catal. 1972, 25, 55–64. [Google Scholar] [CrossRef]
- Selef, M.; Otto, K. Appearance of N2O in the catalytic reduction of NO by CO. J. Catal. 1968, 10, 408–412. [Google Scholar] [CrossRef]
- Petunchi, J.O.; Hall, W.K. Effects of selective reduction of nitric oxide on zeolite structure. Appl. Catal. B Environ. 1994, 3, 239–257. [Google Scholar] [CrossRef]
- Ansell, G.P.; Diwell, A.F.; Golunski, S.E.; Hayes, J.W.; Rajaram, R.R.; Truex, T.J.; Walker, A.P. Mechanism of the lean NOx reaction over Cu/ZSM-5. Appl. Catal. B Environ. 1993, 2, 81–100. [Google Scholar] [CrossRef]
- Lukyanov, D.B.; Sill, G.; Ditri, J.L.; Hall, W.K. Comparison of catalyzed and homogeneous reactions of hydrocarbons for selective catalytic reduction (SCR) of NOx. J. Catal. 1995, 153, 265–274. [Google Scholar] [CrossRef]
- Ditri, J.L.; Sachtler, W.M.H. Reduction of NO over impregnated Cu/ZSM-5 in the presence of O2. Catal. Lett. 1992, 15, 289–295. [Google Scholar] [CrossRef]
- Kucherov, A.V.; Kucherova, T.N.; Slinkin, A.A. Alkane oxidation on isolated Cu2+ ions in zeolitic matrix: A relation between catalytic activity and Cu2+-site local topography. Catal. Lett. 1991, 10, 289–296. [Google Scholar] [CrossRef]






| Sample | Condition | TPV 1 | SBET | Porosity ε | dmean 2 |
|---|---|---|---|---|---|
| (mL/g) | (m2/g) | (%) | (nm) | ||
| HF800 | fresh | 0.196 | 8.4 | 43.6 | 92.7 |
| spent | 0.093 | 15.8 | 26.8 | 23.5 | |
| HF1300 | fresh | 0.140 | 4.5 | 38.5 | 124.4 |
| spent | 0.088 | 5.7 | 25.9 | 62.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorakopoulos, G.V.; Papageorgiou, S.K.; Katsaros, F.K.; Beltsios, K.G.; Romanos, G.E. Copper-Decorated Catalytic Carbon/Ceramic Hollow Fibers for NO Reduction: Enhanced Performance via Tangential Flow Reactor Design and Process Intensification. Fibers 2025, 13, 112. https://doi.org/10.3390/fib13090112
Theodorakopoulos GV, Papageorgiou SK, Katsaros FK, Beltsios KG, Romanos GE. Copper-Decorated Catalytic Carbon/Ceramic Hollow Fibers for NO Reduction: Enhanced Performance via Tangential Flow Reactor Design and Process Intensification. Fibers. 2025; 13(9):112. https://doi.org/10.3390/fib13090112
Chicago/Turabian StyleTheodorakopoulos, George V., Sergios K. Papageorgiou, Fotios K. Katsaros, Konstantinos G. Beltsios, and George Em. Romanos. 2025. "Copper-Decorated Catalytic Carbon/Ceramic Hollow Fibers for NO Reduction: Enhanced Performance via Tangential Flow Reactor Design and Process Intensification" Fibers 13, no. 9: 112. https://doi.org/10.3390/fib13090112
APA StyleTheodorakopoulos, G. V., Papageorgiou, S. K., Katsaros, F. K., Beltsios, K. G., & Romanos, G. E. (2025). Copper-Decorated Catalytic Carbon/Ceramic Hollow Fibers for NO Reduction: Enhanced Performance via Tangential Flow Reactor Design and Process Intensification. Fibers, 13(9), 112. https://doi.org/10.3390/fib13090112








