Coaxial Electrospinning of PCL-PVA Membranes Loaded with N-Heterocyclic Gold Complex for Antitumoral Applications
Highlights
- Coaxial electrospun membranes containing an antitumoral active agent were developed.
- The synthetic antitumoral agent was chosen due to its effective activity against melanoma.
- The antitumoral complex was loaded inside the core section of the coaxial fibers.
- The control of the parameters and modality of the electrospinning process facilitates tailoring of the release of the antitumoral agent.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AuM1 Synthesis and Characterization
2.3. Coaxial Electrospinning Process
2.4. Production and Characterization of the Material
3. Results
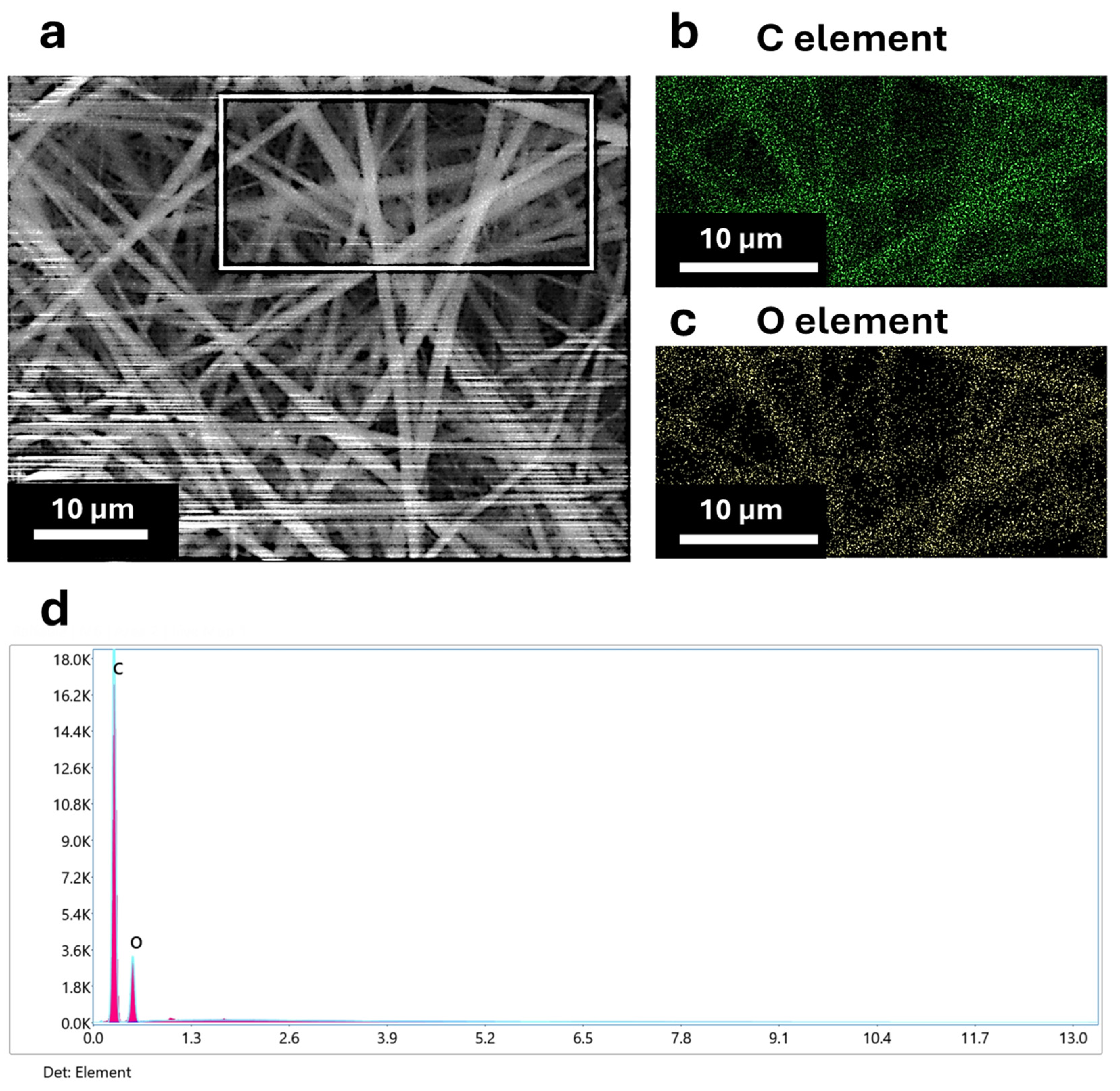
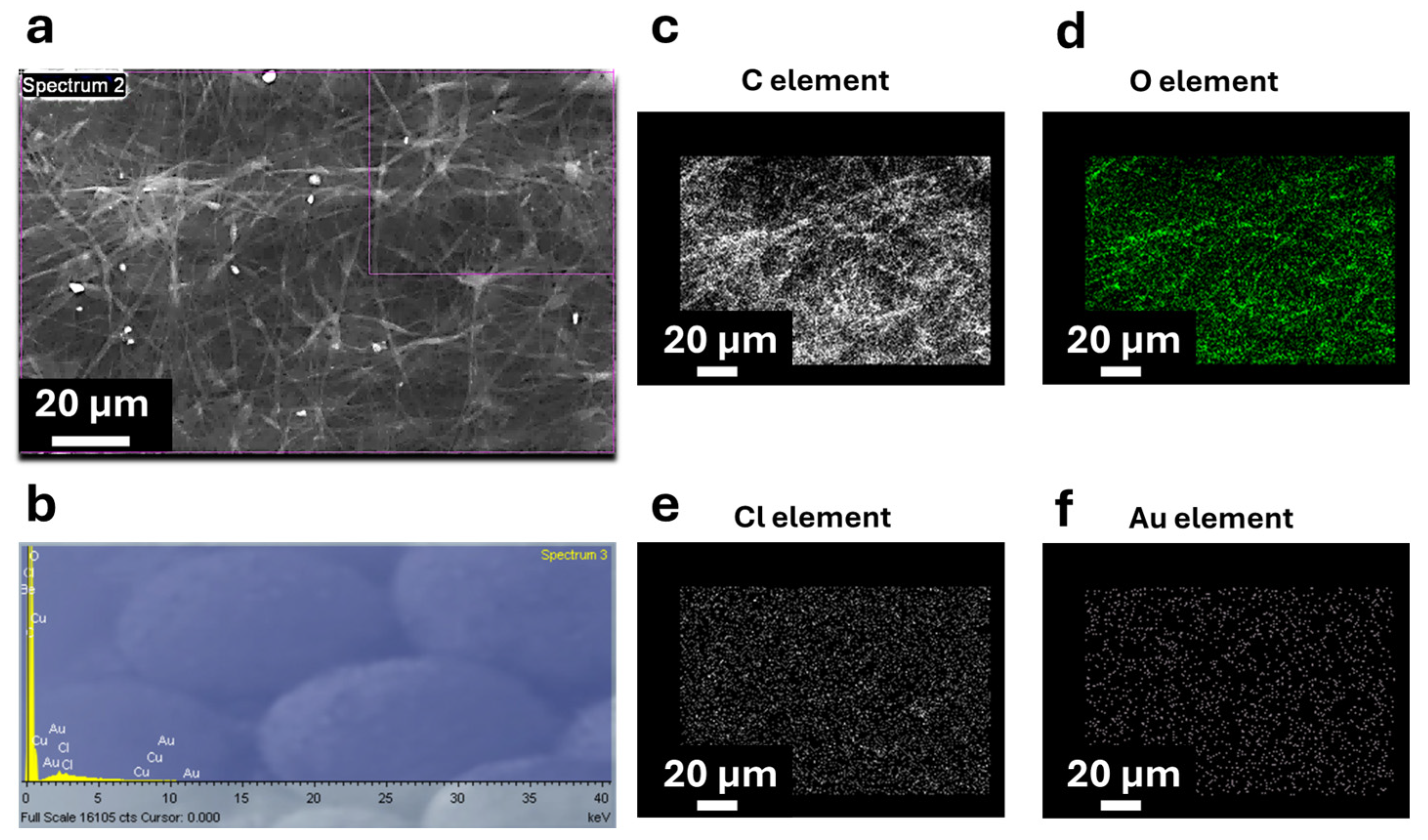
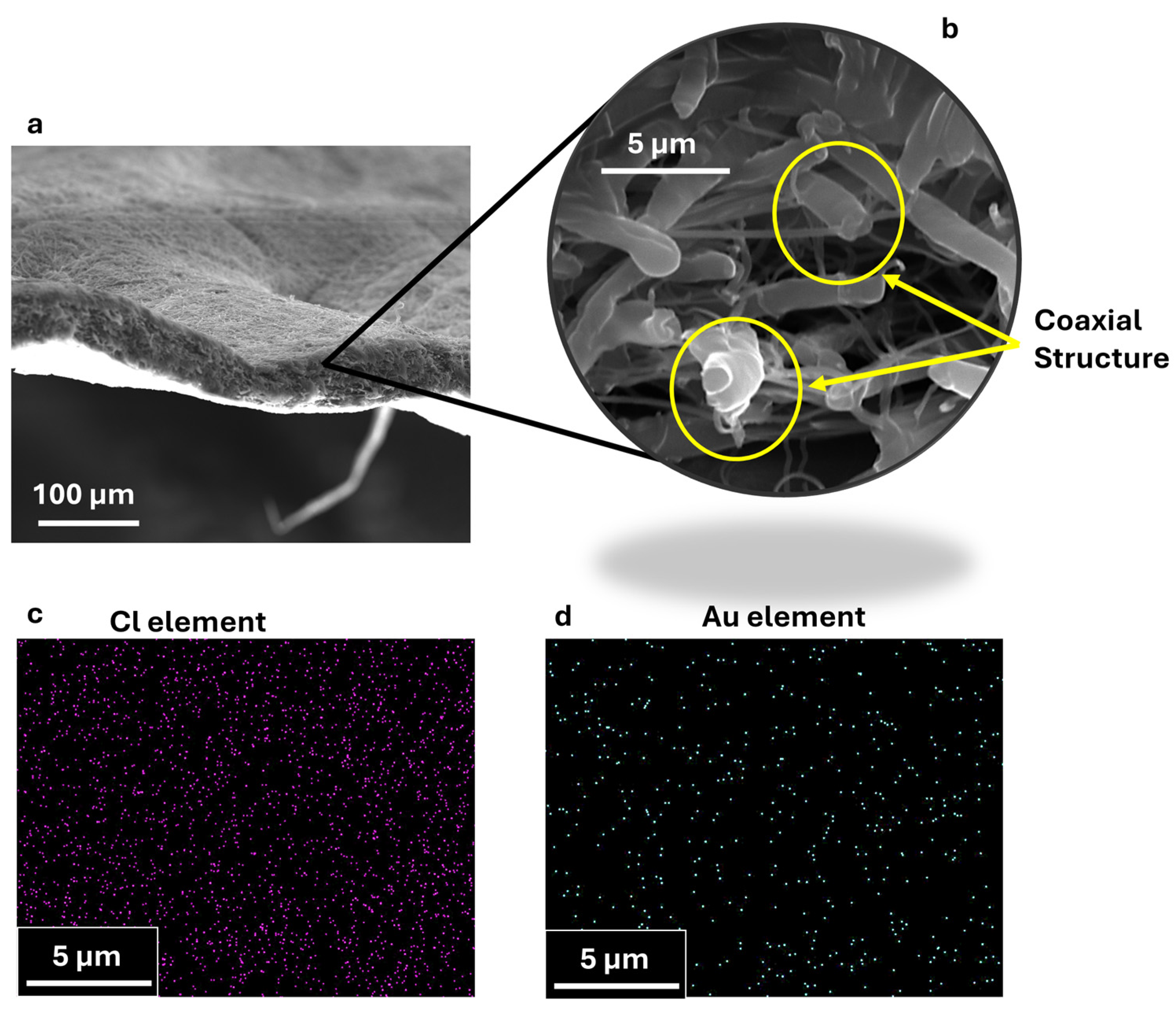
3.1. Morphological and Elemental Characterization of Coaxial Nanofibers
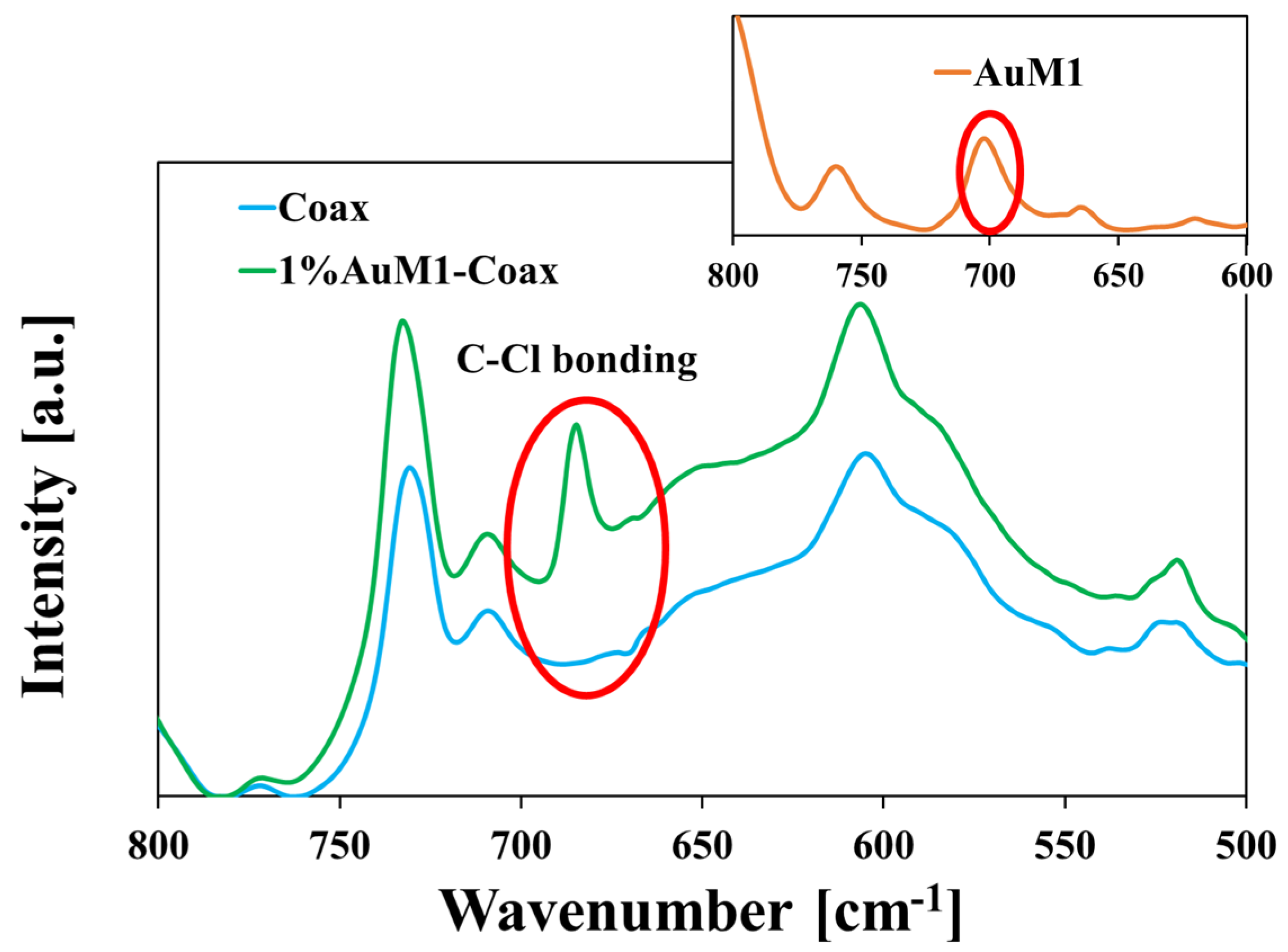
3.2. Fourier-Transform Infrared (FT-IR) Spectroscopy
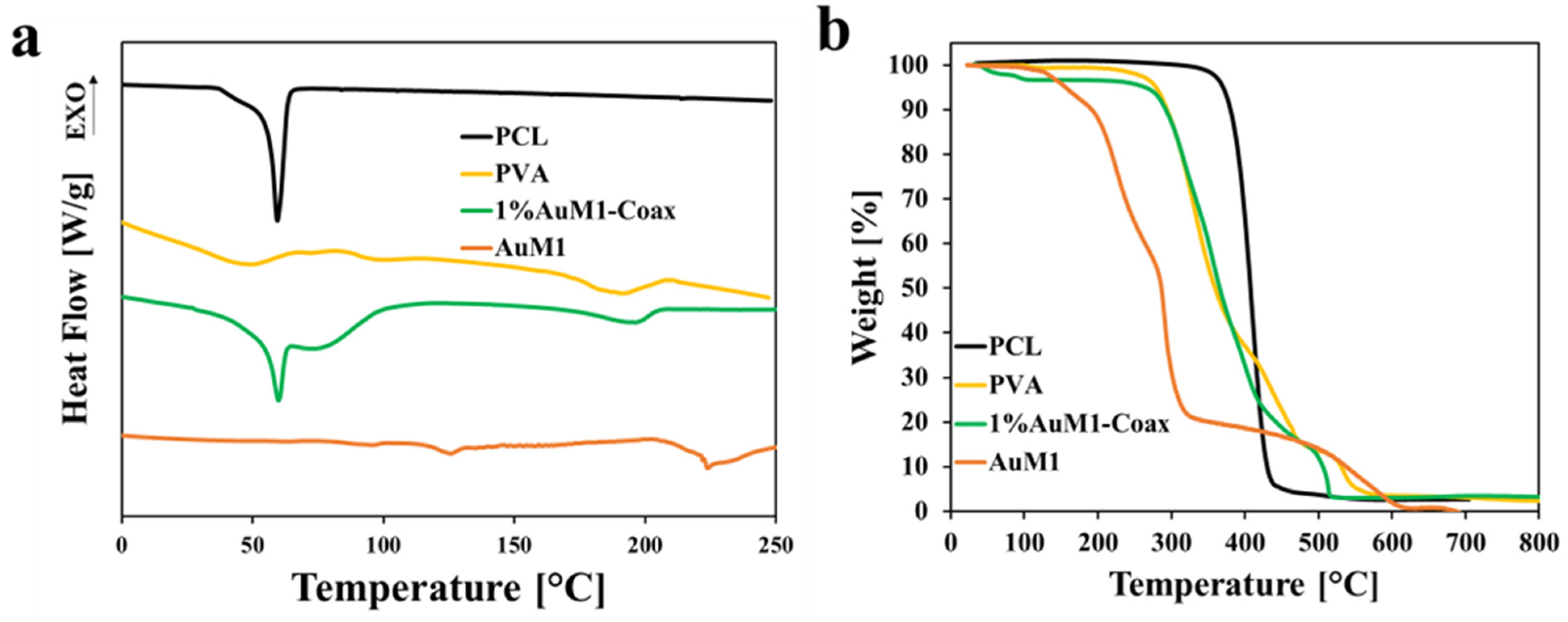
3.3. Thermal Characterization
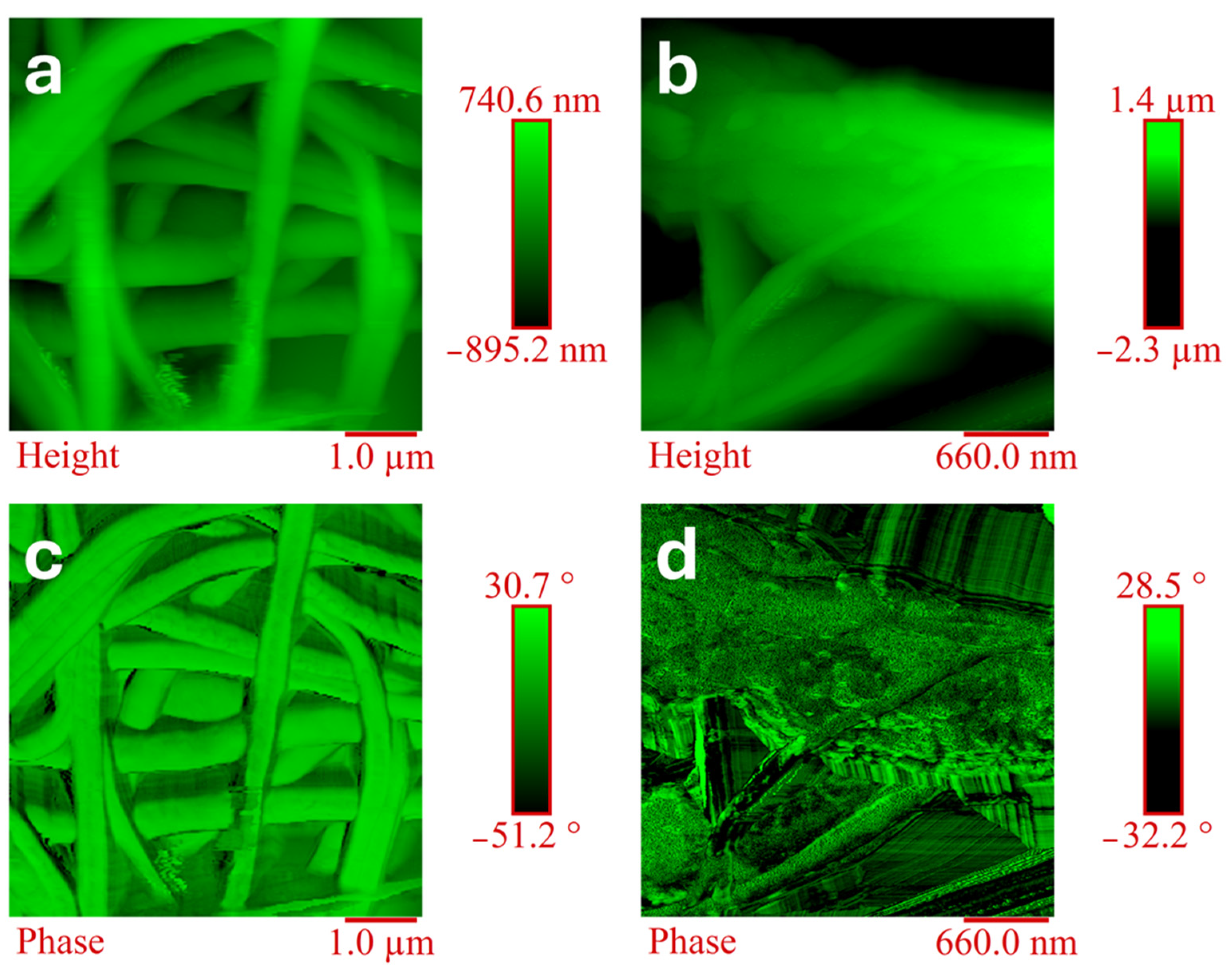
3.4. Morphological Analysis: Atomic Force Microscopy (AFM)
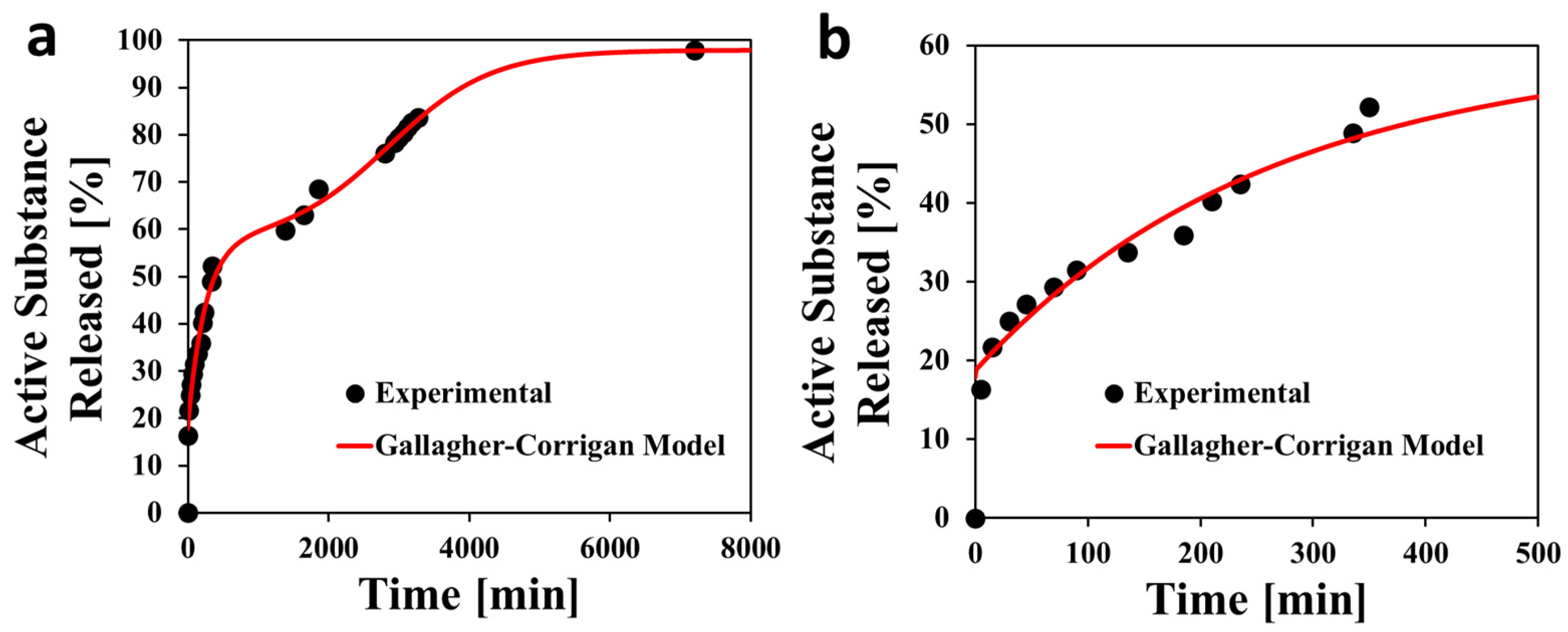
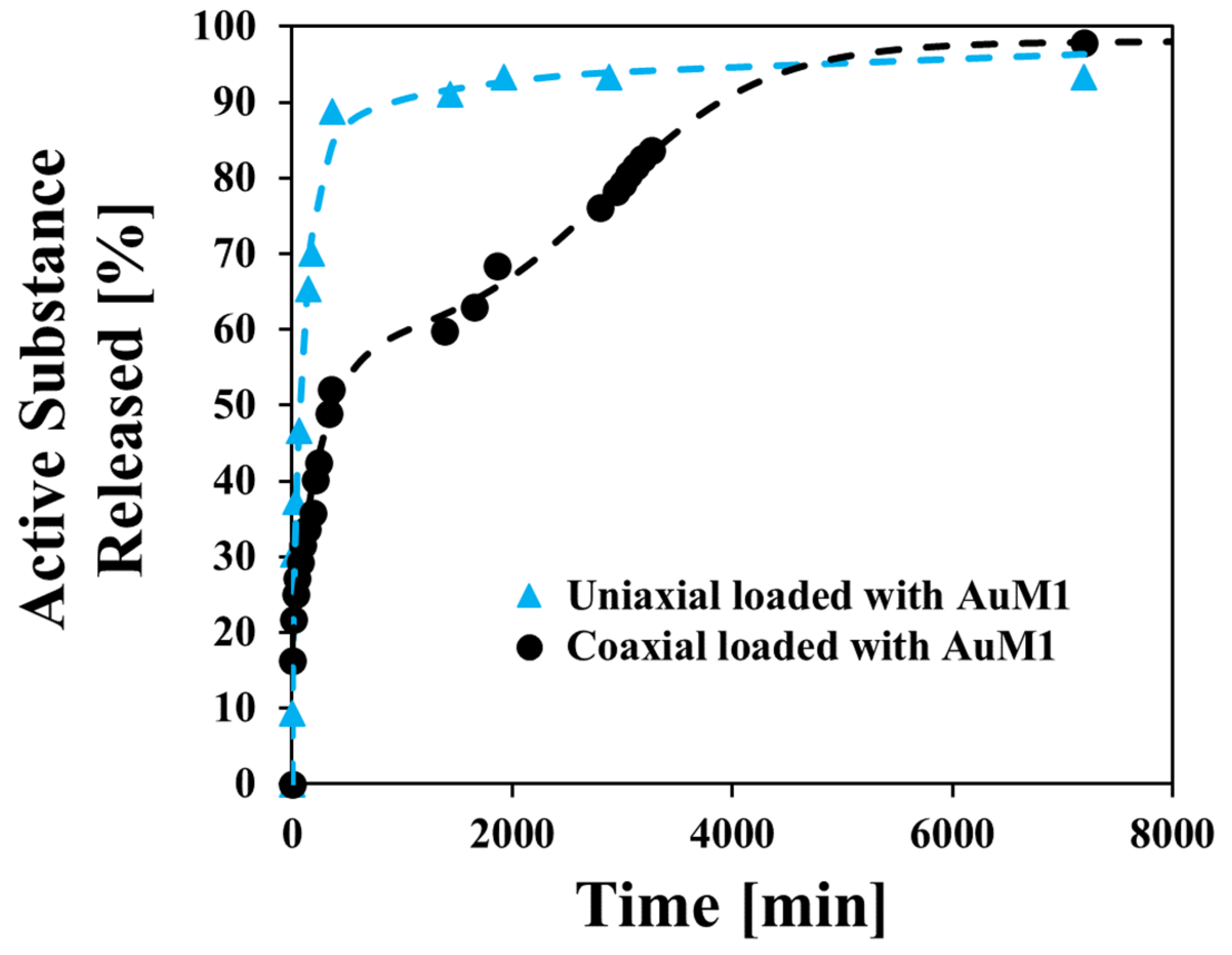
3.5. Release Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.; Ilyas, S.; Mathur, S.; Goya, G.F.; Fuentes-García, J.A. Remote-Controlled Activation of the Release through Drug-Loaded Magnetic Electrospun Fibers. Fibers 2024, 12, 48. [Google Scholar] [CrossRef]
- Catauro, M.; D’Angelo, A.; Fiorentino, M.; Pacifico, S.; Latini, A.; Brutti, S.; Vecchio Ciprioti, S. Thermal, Spectroscopic Characterization and Evaluation of Antibacterial and Cytotoxicity Properties of Quercetin-PEG-Silica Hybrid Materials. Ceram. Int. 2023, 49, 14855–14863. [Google Scholar] [CrossRef]
- Catauro, M.; D’errico, Y.; D’angelo, A.; Clarke, R.J.; Blanco, I. Antibacterial Activity and Iron Release of Organic-inorganic Hybrid Biomaterials Synthesized via the Sol-gel Route. Appl. Sci. 2021, 11, 9311. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics. ACS Pharmacol. Transl. Sci. 2021, 4, 8–54. [Google Scholar] [CrossRef]
- Longo, R.; Raimondo, M.; Vertuccio, L.; Ciardulli, M.C.; Sirignano, M.; Mariconda, A.; Della Porta, G.; Guadagno, L. Bottom-Up Strategy to Forecast the Drug Location and Release Kinetics in Antitumoral Electrospun Drug Delivery Systems. Int. J. Mol. Sci. 2023, 24, 1507. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Lamparelli, E.P.; Ciardulli, M.C.; Longo, P.; Mariconda, A.; Della Porta, G.; Longo, R. Electrospun Membranes Designed for Burst Release of New Gold-Complexes Inducing Apoptosis of Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 7147. [Google Scholar] [CrossRef]
- Longo, R.; Guadagno, L.; Lamberti, P. Electromagnetic Characterization of Polycaprolactone Electrospun Nanofibers Filled with Fe3O4 Nanoparticles. In Proceedings of the 2020 4th International Symposium on Multidisciplinary Studies and Innovative Technologies (ISMSIT), Istanbul, Turkey, 22 October 2020; pp. 1–5. [Google Scholar]
- Wang, Z.; Zhao, Y.; Shen, M.; Tomás, H.; Zhou, B.; Shi, X. Antitumor Efficacy of Doxorubicin-Loaded Electrospun Attapulgite–Poly(Lactic-co-glycolic Acid) Composite Nanofibers. J. Funct. Biomater. 2022, 13, 55. [Google Scholar] [CrossRef]
- Leppert, W.; Malec-Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef]
- Affaitati, G.; Costantini, R.; Tana, C.; Lapenna, D.; Schiavone, C.; Cipollone, F.; Adele Giamberardino, M. Effects of Topical vs Injection Treatment of Cervical Myofascial Trigger Points on Headache Symptoms in Migraine Patients: A Retrospective Analysis. J. Headache Pain 2018, 19, 104. [Google Scholar] [CrossRef]
- Li, D.; Xu, K.; Niu, Z.; Zhang, C. Annealing and Plasma Effects on the Structural and Photocatalytic Properties of TiO2 Fibers Produced by Electrospinning. Catalysts 2022, 12, 1441. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a Long-Term Controlled Drug Release System Based on Simple Blended Electrospun Fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Li, Y.; Tan, Y.; Liu, Y.; Pan, W.; Tan, G. Stimuli-Responsive Electrospun Nanofibers for Drug Delivery, Cancer Therapy, Wound Dressing, and Tissue Engineering. J. Nanobiotechnol. 2023, 21, 237. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and Their Applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Coimbra, P.; Santos, P.; Alves, P.; Miguel, S.P.; Carvalho, M.P.; de Sá, K.D.; Correia, I.J.; Ferreira, P. Coaxial Electrospun PCL/Gelatin-MA Fibers as Scaffolds for Vascular Tissue Engineering. Colloids Surf. B Biointerfaces 2017, 159, 7–15. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial Electrospun PVA/PCL Nanofibers with Dual Release of Tea Polyphenols and ε-Poly (L-Lysine) as Antioxidant and Antibacterial Wound Dressing Materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial Electrospun Fibers: Applications in Drug Delivery and Tissue Engineering. WIREs Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef]
- Duan, G.; Jin, M.; Wang, F.; Greiner, A.; Agarwal, S.; Jiang, S. Core Effect on Mechanical Properties of One Dimensional Electrospun Core-Sheath Composite Fibers. Compos. Commun. 2021, 25, 100773. [Google Scholar] [CrossRef]
- Schulte, V.A.; Díez, M.; Möller, M.; Lensen, M.C. Surface Topography Induces Fibroblast Adhesion on Intrinsically Nonadhesive Poly(Ethylene Glycol) Substrates. Biomacromolecules 2009, 10, 2795–2801. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J. Hydrophilic Polymers. Polymers 2019, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, S.; Guo, R.; Liu, D. CSF1R Inhibitors Are Emerging Immunotherapeutic Drugs for Cancer Treatment. Eur. J. Med. Chem. 2023, 245, 114884. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Xi, J.; Fan, L.; Wang, P.; Zhu, C.; Tang, Y.; Xu, X.; Liang, M.; Jiang, B.; Yan, X.; et al. In Vivo Guiding Nitrogen-Doped Carbon Nanozyme for Tumor Catalytic Therapy. Nat. Commun. 2018, 9, 1440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Liu, T.; Liu, S.; Jing, X. Antitumor Activity of Electrospun Polylactide Nanofibers Loaded with 5-Fluorouracil and Oxaliplatin against Colorectal Cancer. Drug Deliv. 2016, 23, 794–800. [Google Scholar] [CrossRef]
- Mcdonald, M.; Oktaria, S.; Konstantinov, K. Coaxially Electrospun 5-Fluorouracil-Loaded PLGA/PVP Fibrous Membrane for Skin Tumor Treatment. Biomed. Mater. 2021, 16, 065014. [Google Scholar] [CrossRef]
- Ignatova, M.; Yossifova, L.; Gardeva, E.; Manolova, N.; Toshkova, R.; Rashkov, I.; Alexandrov, M. Bioactive and Comaptible Polymers Antiproliferative Activity of Nanofibers Containing Quaternized Chitosan and/or Doxorubicin against MCF-7 Human Breast Carcinoma Cell Line by Apoptosis. J. Bioact. Compat. Polym. 2011, 26, 539–551. [Google Scholar] [CrossRef]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the Way to Fight Cancer Paved with Gold? Metal-Based Carbene Complexes with Multiple and Fascinating Biological Features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Mariconda, A.; Sirignano, M.; Costabile, C.; Longo, P. New NHC-Silver and Gold Complexes Active in A3-Coupling (Aldehyde-Alkyne-Amine) Reaction. Mol. Catal. 2020, 480, 110570. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, Z.; Wan, X.; Fang, H.; Yu, D.G.; Chen, X.; Liu, P. The Relationships between Process Parameters and Polymeric Nanofibers Fabricated Using a Modified Coaxial Electrospinning. Nanomaterials 2019, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun Nanofibers: Exploring Process Parameters, Polymer Selection, and Recent Applications in Pharmaceuticals and Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Zargham, S.; Bazgir, S.; Tavakoli, A.; Rashidi, A.S.; Damerchely, R. The Effect of Flow Rate on Morphology and Deposition Area of Electrospun Nylon 6 Nanofiber. J. Eng. Fiber Fabr. 2012, 7, 42–49. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The History of Electrospinning: Past, Present, and Future Developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Guadagno, L.; Sorrentino, A.; Longo, R.; Raimondo, M. Multifunctional Properties of Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Epoxy Nanocomposites. Polymers 2023, 15, 2297. [Google Scholar] [CrossRef]
- Hopkins, E.; Sanvictores, T.; Sharma, S. Physiology, Acid Base Balance. Urolithiasis 2022, 19–22. [Google Scholar] [CrossRef]
- Phillipson, K.; Hay, J.N.; Jenkins, M.J. Thermal Analysis FTIR Spectroscopy of Poly(ε-Caprolactone). Thermochim. Acta 2014, 595, 74–82. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, Optical, Opto-Thermal and Thermal Properties of ZnS-PVA Nanofluids Synthesized through a Radiolytic Approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Şen, P.; Hirel, C.; Andraud, C.; Aronica, C.; Bretonnière, Y.; Mohammed, A.; Ågren, H.; Minaev, B.; Minaeva, V.; Baryshnikov, G.; et al. Fluorescence and FTIR Spectra Analysis of Trans-A2B2-Substituted Di- and Tetra-Phenyl Porphyrins. Materials 2010, 3, 4446–4475. [Google Scholar] [CrossRef]
- De Lorenzi, A.; Giorgianni, S.; Bini, R. High-Resolution FTIR Spectroscopy of the C—Cl Stretching Mode of Vinyl Chloride. Mol. Phys. 1999, 96, 101–108. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X. A Thermal Degradation Mechanism of Polyvinyl Alcohol/Silica Nanocomposites. Polym. Degrad. Stab. 2007, 92, 1061–1071. [Google Scholar] [CrossRef]
- Luo, H.; Huang, Y.; Wang, D.; Shi, J. Coaxial-Electrospinning as a New Method to Study Confined Crystallization of Polymer. J. Polym. Sci. B Polym. Phys. 2013, 51, 376–383. [Google Scholar] [CrossRef]
- Ero-Phillips, O.; Jenkins, M.; Stamboulis, A. Tailoring Crystallinity of Electrospun Plla Fibres by Control of Electrospinning Parameters. Polymers 2012, 4, 1331–1348. [Google Scholar] [CrossRef]
- Longo, R.; Vertuccio, L.; Speranza, V.; Pantani, R.; Raimondo, M.; Calabrese, E.; Guadagno, L. Nanometric Mechanical Behavior of Electrospun Membranes Loaded with Magnetic Nanoparticles. Nanomaterials 2023, 13, 1252. [Google Scholar] [CrossRef]
- Reguieg, F.; Ricci, L.; Bouyacoub, N.; Belbachir, M.; Bertoldo, M. Thermal Characterization by DSC and TGA Analyses of PVA Hydrogels with Organic and Sodium MMT. Polym. Bull. 2020, 77, 929–948. [Google Scholar] [CrossRef]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Łojkowski, W.; Pahlevanneshan, Z.; Ahmadi, M. Crystallinity Study of Electrospun Poly (Vinyl Alcohol) Nanofibers: Effect of Electrospinning, Filler Incorporation, and Heat Treatment. Iran. Polym. J. 2016, 25, 647–659. [Google Scholar] [CrossRef]
- Longo, R.; Catauro, M.; Sorrentino, A.; Guadagno, L. Thermal and Mechanical Characterization of Complex Electrospun Systems Based on Polycaprolactone and Gelatin. J. Therm. Anal. Calorim. 2022, 147, 5391–5399. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Corrigan, O.I. Mechanistic Aspects of the Release of Levamisole Hydrochloride from Biodegradable Polymers. J. Control. Release 2000, 69, 261–272. [Google Scholar] [CrossRef]
- Potseleev, V.; Uspenskii, S.; Trofimchuk, E.; Bolshakova, A.; Kasatova, A.; Kasatov, D.; Taskaev, S. Nanocomposite Materials Based on Polylactide and Gold Complex Compounds for Absorbed Dose Diagnostics in BNCT. Int. J. Mol. Sci. 2023, 24, 16492. [Google Scholar] [CrossRef] [PubMed]
- Lamarra, J.; Calienni, M.N.; Rivero, S.; Pinotti, A. Electrospun Nanofibers of Poly(Vinyl Alcohol) and Chitosan-Based Emulsions Functionalized with Cabreuva Essential Oil. Int. J. Biol. Macromol. 2020, 160, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, F.; Pellerin, C.; Omidfar, K.; Bagheri, R. Engineered Robust Hydrophobic/Hydrophilic Nanofibrous Scaffolds with Drug-Eluting, Antioxidant, and Antimicrobial Capacity. ACS Appl. Bio Mater. 2024, 7, 3687–3700. [Google Scholar] [CrossRef] [PubMed]
- Balcerzak, J.; Mucha, M. Analysis of model drug release kinetics from complex matrices of polylactide-chitosan. Prog. Chem. Appl. Chitin Deriv. 2010, 15, 117–126. [Google Scholar]

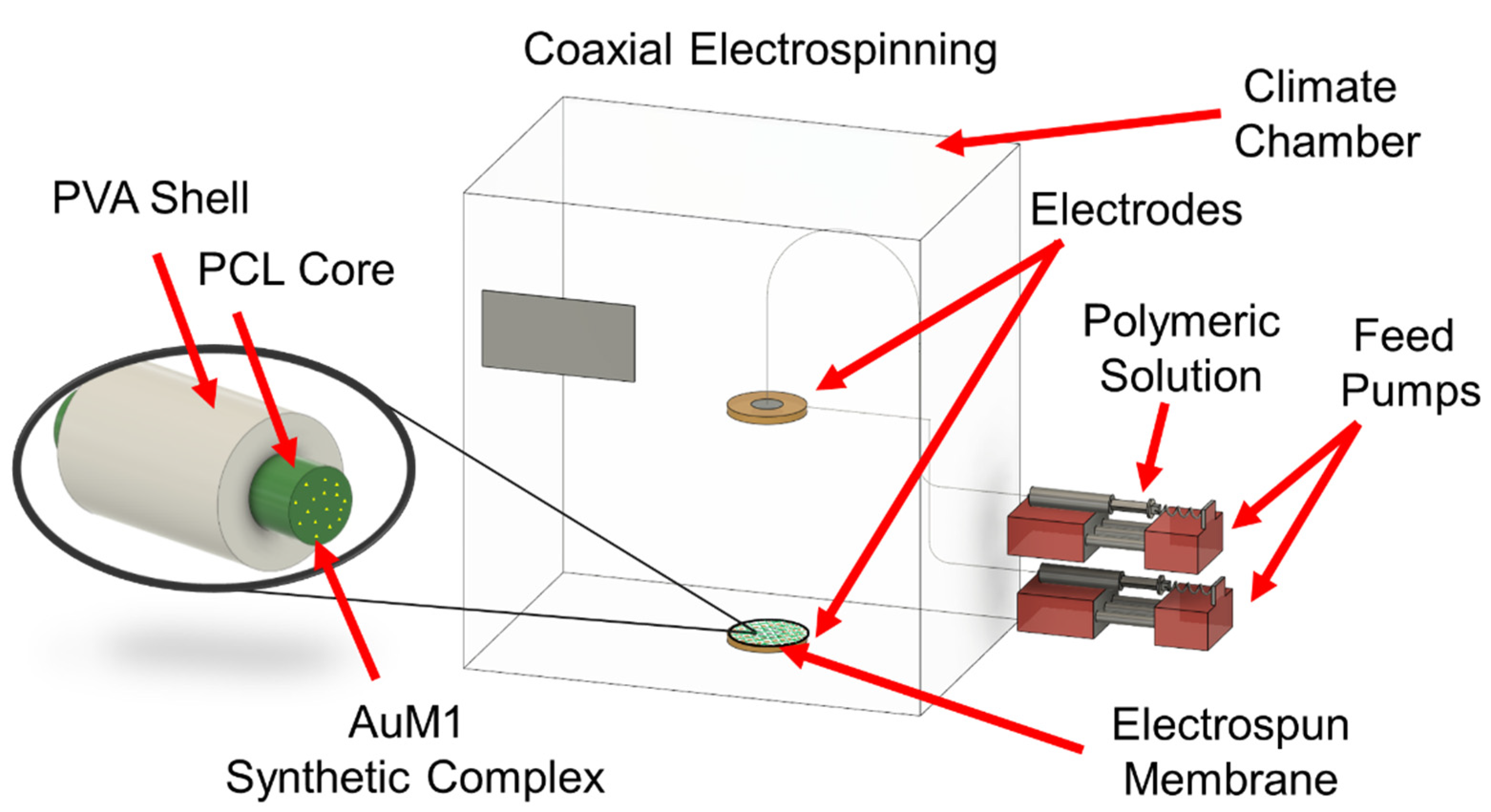

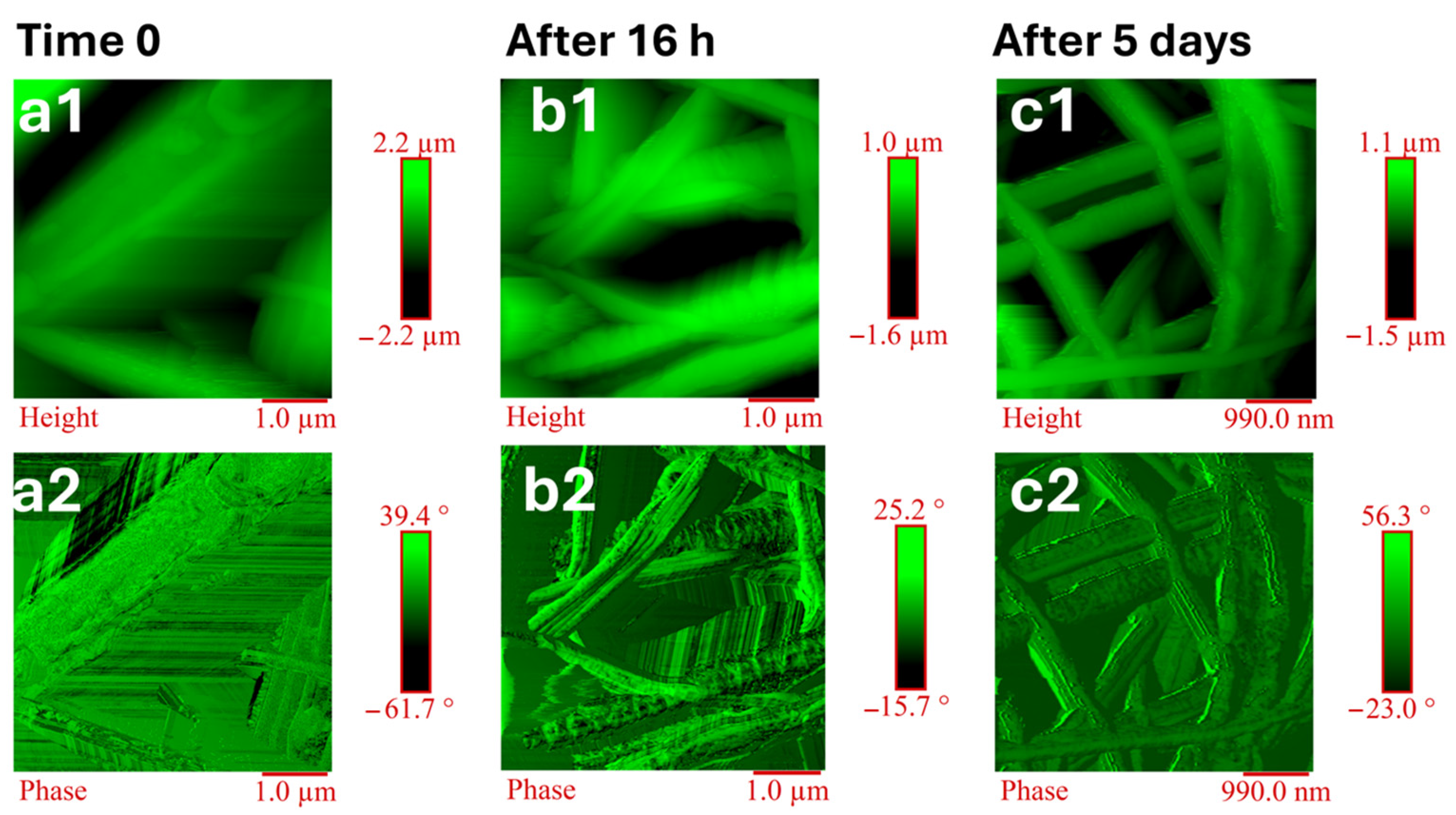
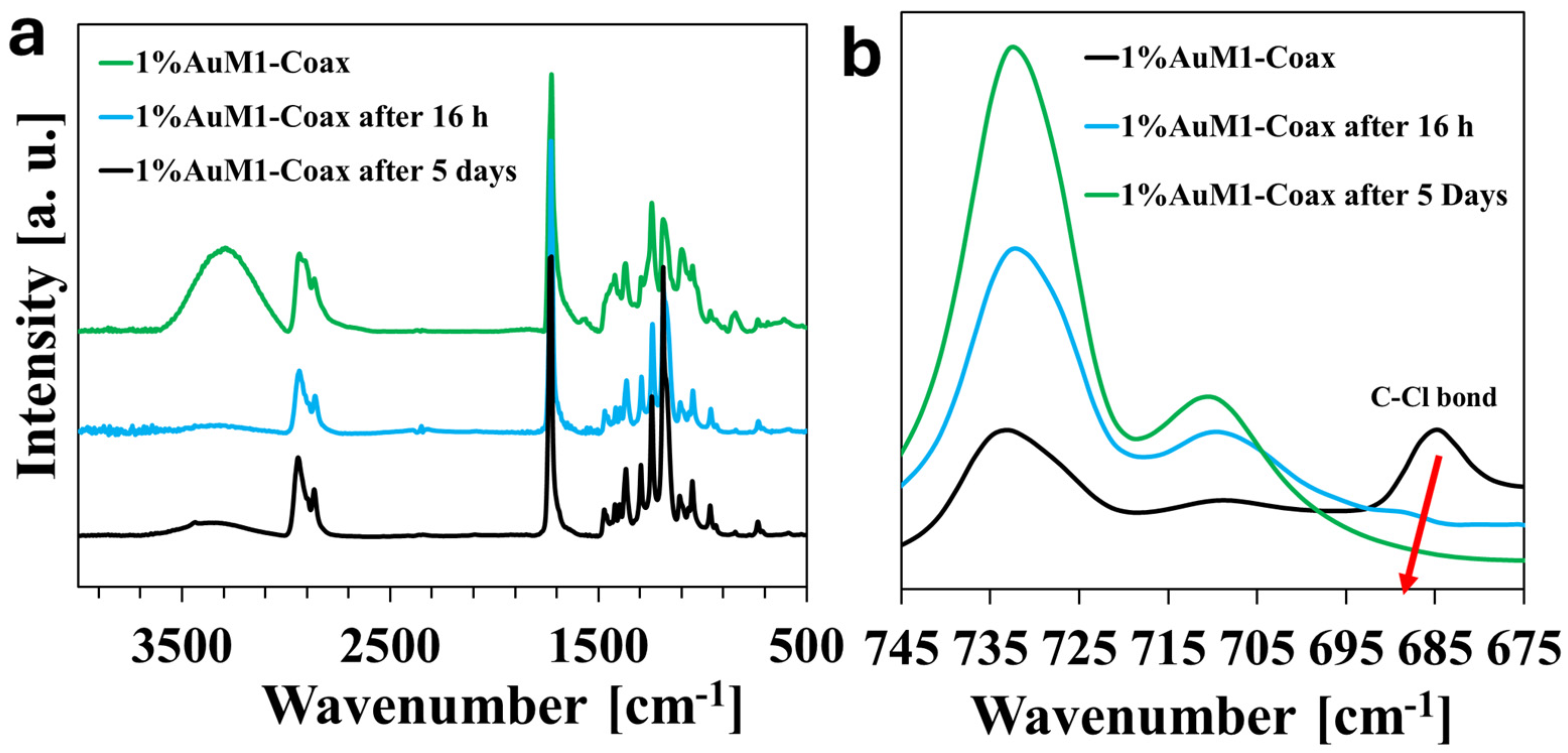
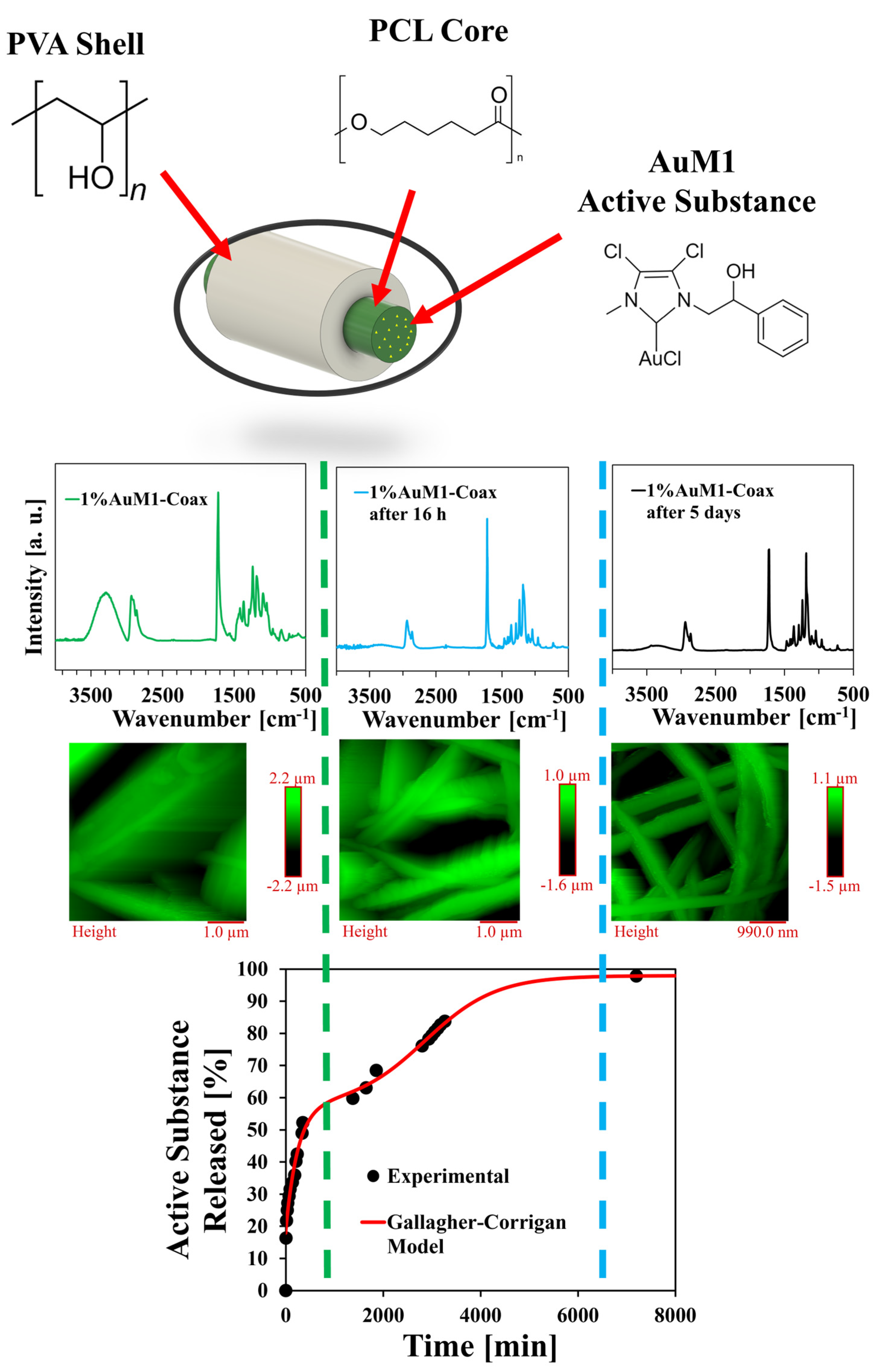
| Polymeric Materials [Matrix] | Solvents | Active Agent [Filler] |
|---|---|---|
| PCL (CAS N° 24980-41-4) with an average molecular weight of 80 kDa is provided from Perstorp (Malmö, Sweden) | Ethanol (EtOH) provided by Carlo Erba Reagents (Cornaredo, MI, Italy) with a purity ≥ 99.9%. | AuM1 was synthesized according to [30]. |
| PVA (CAS N° 9002-89-5) with an average molecular weight of 30–70 kDa provided by Sigma-Aldrich (Saint Louis, MO, USA) | Hexafluoro-isopropanol (HFIP) provided by Apollo Scientific Limited (Whitefield Rd Bredbury, Stockport, Cheshire, UK) with a purity ≥ 99%. |
| # | Material | Concentration [%] | Solvent | Flow Rate [mL/h] | Injector–Collector Distance [cm] | Applied Potential Difference [kV] | Loading |
|---|---|---|---|---|---|---|---|
| PVA | PVA | 16.5 | H2O-EtOH (1:1) | 0.5 | 15 | 16 | - |
| PCL | PCL | 6 | HFIP | 1 | 22.5 | 13 | - |
| 1%AuM1-Coax | Core: PCL | 5 | HFIP | 1 | 17.5 | 25 | 1%AuM1 |
| Shell: PVA | 6 | HFIP | 1 | - |
| Characteristics of the Instrumentation | |
|---|---|
| Atomic Force Microscopy (AFM) | The materials’ morphology was examined using Bruker NanoScope V multimode AFM (Digital Instruments, Santa Barbara, CA, USA) in room conditions. The characteristics of the nominal spring constant, resonance frequencies, and tip radius were used in previous research [7]. The software used for image processing is Nanoscope Analysis 1.80 (Bruker Corporation, Billerica, MA, USA), which is widely used for these types of analysis [36]. |
| Scanning Electron Microscopy (SEM) | SEM analysis (TESCAN-VEGA LMH, Brno, Czech Republic) coupled with an energy dispersive X-ray spectroscopy (EDX) probe has been performed to evaluate the morphology of the coaxial membranes and the elemental distribution, both over the plane surface (so-called top view) and into the cross section. In this last case, membrane samples have been cut in liquid nitrogen to have a fragile fracture and preserve the internal structure of the nanofibers. To ensure that the penetration factor of the X-ray could track the presence of AuM1 characteristic elements (Au and Cl) in EDX analysis, the nanofiber morphologies were studied on non-metalized samples. The samples analyzed in the cross section were metalized using a sputter coater (QUORUM 150 T, Judges House, Lewes Road, Laughton, UK) with a thin layer of chromium (≈50 Å). |
| Thermogravimetric Analysis (TGA) | The thermal stability of the obtained membranes was evaluated via TGA by using Mettler Toledo TC-10 thermobalance (Mettler-Toledo, Columbus, OH, USA) with an airflow rate of 50 mL/min and a heating rate of 10 °C/min heating rate from 30 to 800 °C. |
| Differential Scanning Calorimetry (DSC) | The thermal transitions of the obtained membranes were evaluated using Mettler Toledo DSC 822e (Mettler-Toledo, Columbus, OH, USA) with an N2 flow rate of 50 mL/min and a heating rate of 10 °C /min heating rate from 0 to 250 °C. |
| Fourier Transform Infrared spectrophotometry (FT-IR) | The chemical interactions of the obtained materials were evaluated using a Bruker Vertex 70 FTIR-spectrophotometer (Bruker Optics Inc., Billerica, MA, USA). All the spectroscopic analyses were performed in absorbance by setting a resolution of 4 cm−1 and 16 scans per point in the range 4000–400 cm−1 on thin membranes (thickness around ≈ 40 μm) to avoid the saturation of the signals. |
| UV-Vis Spectrophotometry | The release kinetics of the active substance from the membranes were obtained by monitoring the presence of the phenyl group of AuM1 in the phosphate buffer solution (PBS, pH = 7.3) that was used to simulate the physiological environment [37] following previous research studies [6,7]. The solutions in which the membranes were kept were analyzed by using the Spectrometer UV-2401 PC (Shimadzu, Kyoto, Japan). The physical characteristics of the vessels (exposed area, lightpath) and the calibration curves of AuM1 in PBS are reported in previous research papers of the research group [6]. |
| Sample | Crystallinity [%] |
|---|---|
| PCL | 50.7 |
| PVA | 22.7 |
| 1%AuM1-Coax | PCL: 62.1 |
| PVA: 33.3 |
| A [-] | X1 [-] | C1 [h−1] | X2 [-] | C2 [h−1] | tmax [h−1] | R2 |
|---|---|---|---|---|---|---|
| 0.179 | 0.394 | 0.238 | 0.407 | 0.082 | 47.6 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, R.; Vertuccio, L.; Aliberti, F.; Mariconda, A.; Raimondo, M.; Longo, P.; Guadagno, L. Coaxial Electrospinning of PCL-PVA Membranes Loaded with N-Heterocyclic Gold Complex for Antitumoral Applications. Fibers 2024, 12, 101. https://doi.org/10.3390/fib12120101
Longo R, Vertuccio L, Aliberti F, Mariconda A, Raimondo M, Longo P, Guadagno L. Coaxial Electrospinning of PCL-PVA Membranes Loaded with N-Heterocyclic Gold Complex for Antitumoral Applications. Fibers. 2024; 12(12):101. https://doi.org/10.3390/fib12120101
Chicago/Turabian StyleLongo, Raffaele, Luigi Vertuccio, Francesca Aliberti, Annaluisa Mariconda, Marialuigia Raimondo, Pasquale Longo, and Liberata Guadagno. 2024. "Coaxial Electrospinning of PCL-PVA Membranes Loaded with N-Heterocyclic Gold Complex for Antitumoral Applications" Fibers 12, no. 12: 101. https://doi.org/10.3390/fib12120101
APA StyleLongo, R., Vertuccio, L., Aliberti, F., Mariconda, A., Raimondo, M., Longo, P., & Guadagno, L. (2024). Coaxial Electrospinning of PCL-PVA Membranes Loaded with N-Heterocyclic Gold Complex for Antitumoral Applications. Fibers, 12(12), 101. https://doi.org/10.3390/fib12120101










