The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Deposition Current Efficiency (CE)
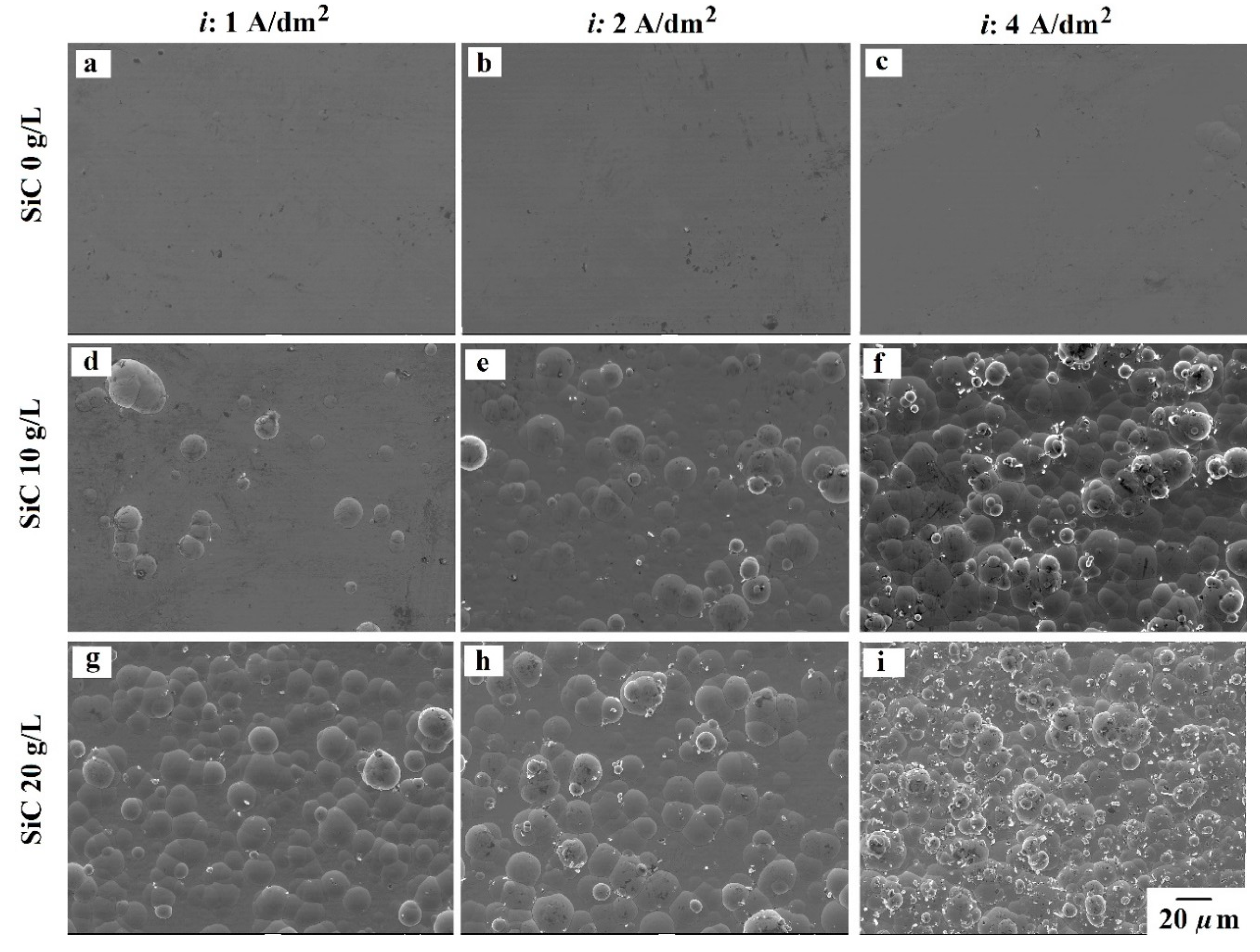
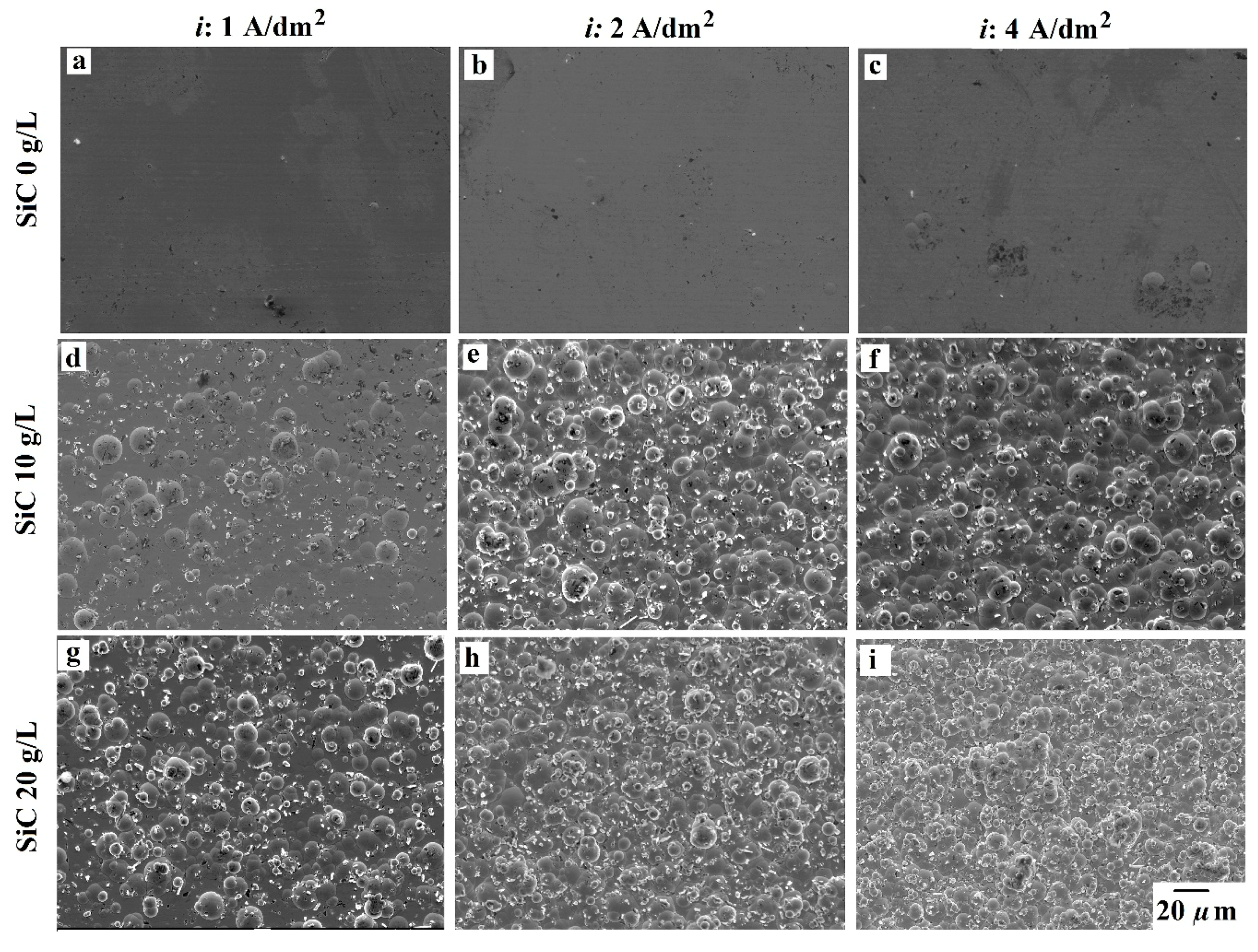
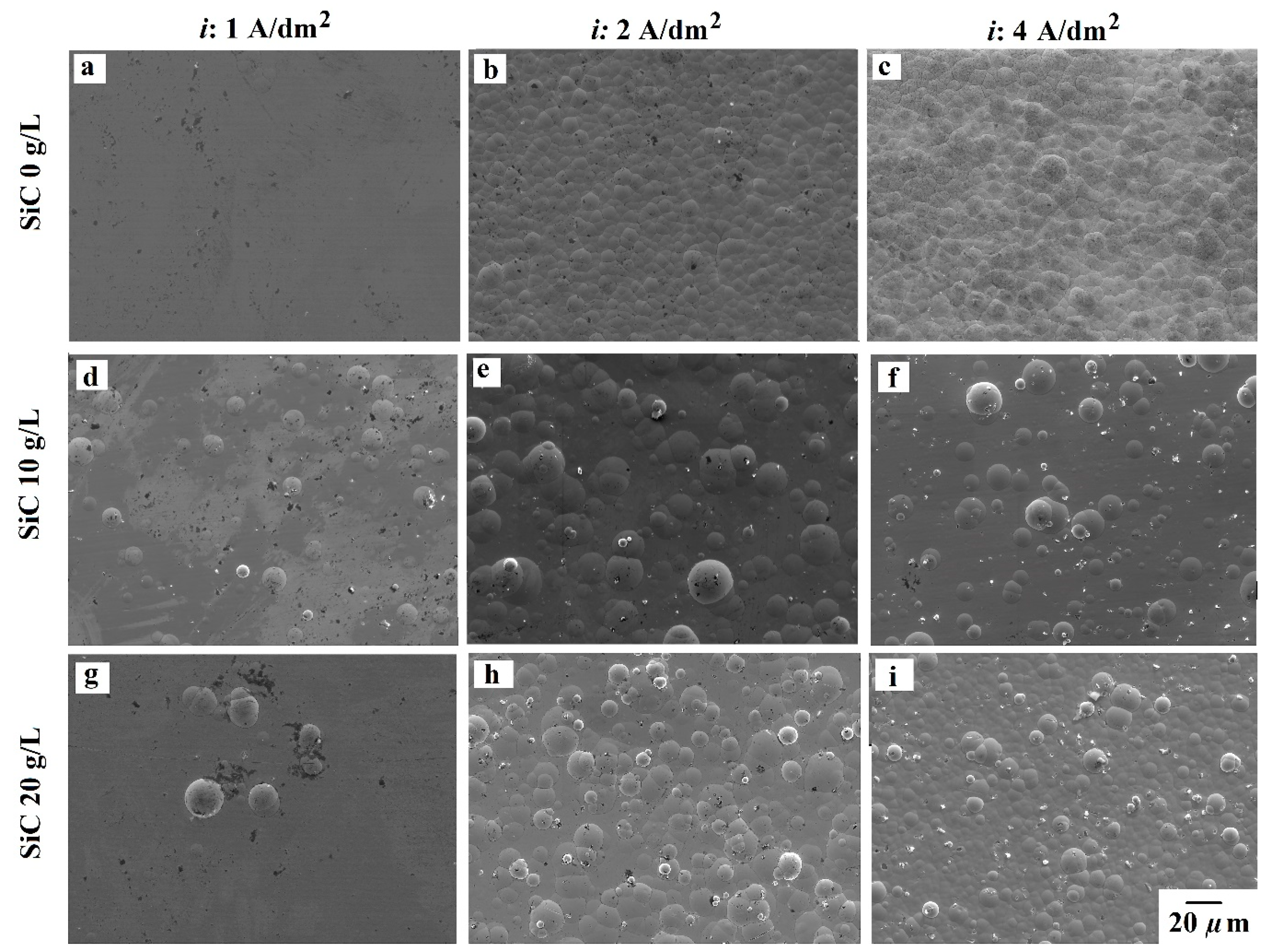
3.2. Coatings Morphology
3.3. Coatings Composition
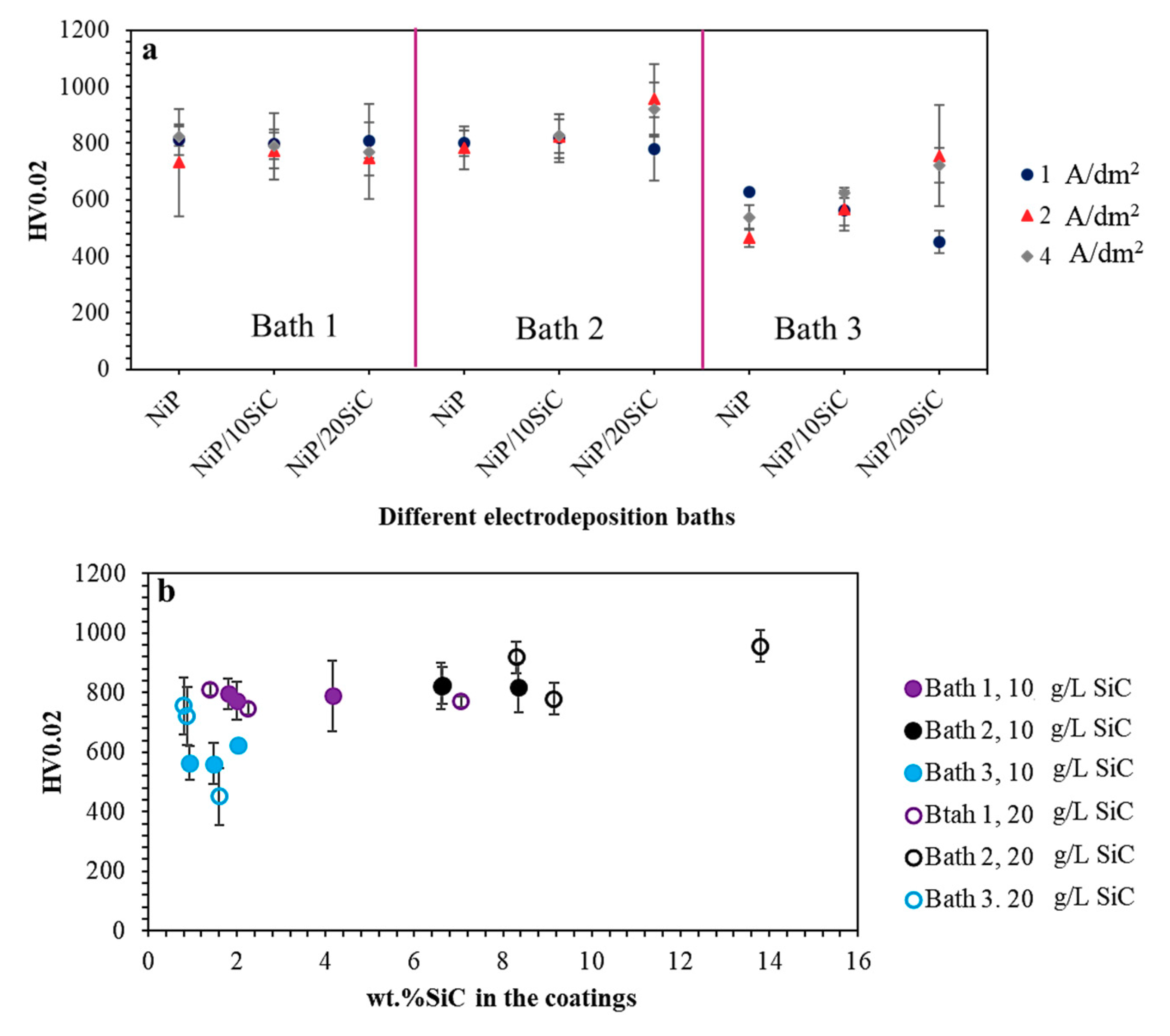
3.4. Coatings Microhardness
4. Conclusions
- Additive-free bath produces smooth surfaces due to the amorphous state of the NiP alloys. However, the presence of the 100 nm SiC powder induced a nodular morphology. Higher current density, as well as SiC loading, resulted in more and finer nodular morphology. Regarding the additives, the addition of SDS promoted the formation of the nodules in the pure NiP coatings and promoted coarser nodules in other cases.
- The particles slightly influenced the current efficiency of the process, and therefore, pH changed a bit (From 2.15 to 2.32) during the deposition which led to a slight reduction in wt.% P of the coatings by increasing SiC particles codeposition. Additives did not influence the current efficiency noticeably, while SDS increased the P content of pure NiP coatings.
- The addition of saccharin increased the SiC codeposited fraction from 7 wt.% (maximum content of SiC in the coatings without additives) to the maximum 13 wt.%, while SDS, reduced it to 1 wt.%.
- There is no direct relation between codeposited wt.% SiC and the coating´s microhardness values since SiC codeposition rate is not the only factor determining the hardness of these coatings, as the particles might interact with the microstructure as well.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lekka, M.; Offoiach, R.; Lanzutti, A.; Mughal, M.Z.; Sebastiani, M.; Bemporad, E.; Fedrizzi, L. Ni-B electrodeposits with low B content: Effect of DMAB concentration on the internal stresses and the electrochemical behavior. Surf. Coat. Technol. 2018, 344, 190–196. [Google Scholar] [CrossRef]
- Bonin, L.; Vitry, V.; Delaunois, F. Corrosion behaviour of electroless high boron-mid phosphorous nickel duplex coatings in the as-plated and heat-treated states in NaCl, H2SO4, NaOH and Na2SO4 media. Mater. Chem. Phys. 2018, 208, 77–84. [Google Scholar] [CrossRef]
- Vitry, V.; Sens, A.; Kanta, A.F.; Delaunois, F. Wear and corrosion resistance of heat treated and as-plated Duplex NiP/NiB coatings on 2024 aluminum alloys. Surf. Coat. Technol. 2012, 206, 3421–3427. [Google Scholar] [CrossRef]
- Vitry, V.; Bonin, L.; Malet, L. Chemical, morphological and structural characterisation of electroless duplex NiP/NiB coatings on steel. Surf. Eng. 2017, 36, 475–484. [Google Scholar] [CrossRef]
- Shakoor, R.A.; Kahraman, R.; Waware, U.; Wang, Y.; Gao, W. Properties of electrodeposited Ni–B–Al2O3 composite coatings. Mater. Des. 2014, 64, 127–135. [Google Scholar] [CrossRef]
- Vitry, V.; Sens, A.; Delaunois, F. Comparison of various electroless nickel coatings on steel: Structure, hardness and abrasion resistance. Mater. Sci. 2014, 786, 1405–1413. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Eriksson, F.; Leisner, P.; Zanella, C. Effect of SiC particle size and heat-treatment on microhardness and corrosion resistance of NiP electrodeposited coatings. J. Alloy. Compd. 2018, 769, 1080–1087. [Google Scholar] [CrossRef]
- Mirak, A.; Akbari, M. Microstructural characterization of electrodeposited and heat treated Ni–B coatings. Surf. Coat. Technol. 2018, 349, 442–451. [Google Scholar] [CrossRef]
- Bekish, Y.N.; Poznyak, S.K.; Tsybulskaya, L.S.; Gaevskaya, T.V. Electrodeposited Ni–B alloy coatings: Structure, corrosion resistance and mechanical properties. Electrochim. Acta 2010, 55, 2223–2231. [Google Scholar] [CrossRef]
- Kim, D.; Kim, M.J.; Kim, J.S.; Kim, H.P. Material characteristics of Ni–P–B electrodeposits obtained from a sulfamate solution. Surf. Coat. Technol. 2008, 202, 2519–2526. [Google Scholar] [CrossRef]
- Ünal, E.; Yaşar, A.; Karahan, İ.H. A review of electrodeposited composite coatings with Ni–B alloy matrix. Mater. Research Exp. 2019, 6, 1–18. [Google Scholar] [CrossRef]
- Torabinejad, V.; Rouhaghdam, A.S.; Aliofkhazraei, M.; Allahyarzadeh, M.H. Ni–Fe–Al2O3 electrodeposited nanocomposite coating with functionally graded microstructure. Bull. Mater. Sci. 2016, 39, 857–864. [Google Scholar] [CrossRef]
- Garcia, I.; Fransaer, J.; Celis, J.P. Electrodeposition and sliding wear resistance of nickel composite coatings containing micron and submicron SiC particles. Surf. Coat. Technol. 2001, 148, 171–178. [Google Scholar] [CrossRef]
- Zanella, C.; Lekka, M.; Bonora, P.L. Influence of the particle size on the mechanical and electrochemical behaviour of micro-and nano-nickel matrix composite coatings. J. Appl. Electrochem. 2009, 39, 31–38. [Google Scholar] [CrossRef]
- Papavasilopoulou, P.; Pavlatou, E.; Spyrellis, N. Effect of plating parameters on NiP-SiC electrodeposition. In Proceedings of the 7th International Conference—The Coatings in Manufacturing Engineering, Kallithe, Greece, 1–3 October 2008; pp. 417–425. [Google Scholar]
- Gül, H.; Kılıc, F.; Uysal, M.; Aslan, S.; Alp, A.; Akbulut, H. Effect of particle concentration on the structure and tribological properties of submicron particle SiC reinforced Ni metal matrix composite (MMC) coatings produced by electrodeposition. Appl. Surf. Sci. 2012, 258, 4260–4267. [Google Scholar] [CrossRef]
- Sheu, H.H.; Huang, P.C.; Tsai, L.C.; Hou, K.H. Effects of plating parameters on the Ni–P–Al2O3 composite coatings prepared by pulse and direct current plating. Surf. Coat. Technol. 2013, 235, 529–535. [Google Scholar] [CrossRef]
- Thiemig, D.; Bund, A.; Talbot, J.B. Influence of hydrodynamics and pulse plating parameters on the electrocodeposition of nickel-alumina nanocomposite films. Electrochim. Acta 2009, 54, 2491–2498. [Google Scholar] [CrossRef]
- Alirezaei, S.; Monirvaghefi, S.M.; Salehi, M.; Saatchi, A.; Kargosha, M. Effect of alumina content on wear behaviour of Ni–P–Al2O3(α). Surf. Eng. 2005, 21, 60–66. [Google Scholar] [CrossRef]
- Malfatti, C.F.; Veit, H.M.; Menezes, T.L.; Ferreira, J.Z.; Rodriguês, J.S.; Bonino, J. The surfactant addition effect in the elaboration of electrodepositated NiP–SiC composite coatings. Surf. Coat. Technol. 2007, 201, 6318–6324. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Zeng, Z.; Zhang, J. Effect of surfactant on the electrodeposition and wear resistance of Ni–Al2O3 composite coatings. Mater. Sci. Eng. A 2006, 434, 319–325. [Google Scholar] [CrossRef]
- Rezaei-Sameti, M.; Nadali, S.; Falahatpisheh, A.; Rakhshi, M. The effects of sodium dodecyl sulfate and sodium saccharin on morphology, hardness and wear behavior of Cr–WC nano composite coatings. Solid State Commun. 2013, 159, 18–21. [Google Scholar] [CrossRef]
- Ünal, E.; Karahan, İ.H. Effects of ultrasonic agitation prior to deposition and additives in the bath on electrodeposited Ni–B/hBN composite coatings. J. Alloy. Compd. 2018, 763, 329–341. [Google Scholar] [CrossRef]
- Beltowska-Lehman, E.; Indyka, P.; Bigos, A.; Szczerba, M.J.; Kot, M. Ni–W/ZrO2 nanocomposites obtained by ultrasonic DC electrodeposition. Mater. Des. 2015, 80, 1–11. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Bello, J.O. Influence of surfactants on electrodeposition of a Ni-nanoparticulate SiC composite coating. Int. J. Surf. Eng. Coat. 2015, 2967. [Google Scholar] [CrossRef]
- Gül, H.; Kılıc, F.; Aslan, S.; Alp, A.; Akbulut, H. Characteristics of electro-co-deposited Ni–Al2O3 nano-particle reinforced metal matrix composite (MMC) coatings. Wear 2009, 267, 976–990. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A. A comparative study of the effects of saccharin and β-SiC nano-particles on the properties of Ni and Ni–Co alloy coatings. Surf. Coat. Technol. 2014, 253, 76–82. [Google Scholar] [CrossRef]
- Chen, F.J.; Pan, Y.N.; Lee, C.Y.; Lin, C.S. Internal stress control of Nickel-Phosphorus electrodeposits using Pulse currents. J. Electrochem. Soc. 2010, 157, D154. [Google Scholar] [CrossRef]
- Hansal, W.E.G.; Sandulache, G.; Mann, R.; Leisner, P. Pulse-electrodeposited NiP–SiC composite coatings. Electrochim. Acta 2013, 114, 851–858. [Google Scholar] [CrossRef]
- Berkh, O.; Bodnevas, A.; Zahavi, J. Electrodeposited Ni–P–SiC composite coatings. Plat. Surf. Finish. 1995, 82, 62–66. [Google Scholar]
- Tamilarasan, T.R.; Rajendran, R.; Rajagopal, G.; Sudagar, J. Effect of surfactants on the coating properties and corrosion behaviour of Ni–P-nano-TiO2 coatings. Surf. Coat. Technol. 2015, 276, 320–326. [Google Scholar] [CrossRef]
- Sen, R.; Bhattacharya, S.; Das, S.; Das, K. Effect of surfactant on the co-electrodeposition of the nano-sized ceria particle in the nickel matrix. J. Alloy. Compd. 2010, 489, 650–658. [Google Scholar] [CrossRef]
- Benea, L.; Bonora, L.; Borello, A.; Martelli, S. Composite electrodeposition to obtain nanostructured coatings. J. Elec. Soc. 2001, 148, 461–465. [Google Scholar] [CrossRef]
- Rudnik, E.; Burzy, L.; Dolasi, Ł.; Misiak, M. Electrodeposition of nickel/SiC composites in the presence of cetyltrimethylammonium bromide. Appl. Surf. Sci. 2010, 256, 7414–7420. [Google Scholar] [CrossRef]
- Kan, H.; Feng, X.; Wei, X.; Zhang, N.; Wang, X.; Long, H. Effects of surfactants SDS and CTAB on Ni–SiC deposition. J. Wuhan Univ. Technol. Mater. Sci. 1901, 33, 836–842. [Google Scholar] [CrossRef]
- Hou, K.H.; Ger, M.D.; Wang, L.M.; Ke, S.T. The wear behaviour of electro-codeposited Ni–SiC composites. Wear 2002, 253, 994–1003. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni–SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Spyrells, N.; Pavlatou, E.A.; Spanou, S.; Zoikis-Karathansis, A. Nickel and nickel-phosphorous matrix composite electrocoatings. Trans. Nonferrous Met. Soc. China 2009, 19, 800–804. [Google Scholar] [CrossRef]
- Chou, M.C.; Der Ger, M.; Ke, S.T.; Huang, Y.R.; Wu, S.T. The Ni–P–SiC composite produced by electro-codeposition. Mater. Chem. Phys. 2005, 92, 146–151. [Google Scholar] [CrossRef]
- Sarret, M.; Müller, C.; Amell, A. Electroless NiP micro- and nano-composite coatings. Surf. Coat. Technol. 2006, 201, 389–395. [Google Scholar] [CrossRef]
- Mafi, I.R.; Dehghanian, C. Comparison of the coating properties and corrosion rates in electroless Ni–P/PTFE composites prepared by different types of surfactants. Appl. Surf. Sci. 2011, 257, 8653–8658. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.S.; Jiang, Q.; Jiang, Z.H.; Li, G.Y.; Elansezhian, R. The performance of surfactant on the surface characteristics of electroless nickel coating on magnesium alloy. Prog. Org. Coat. 2012, 74, 788–793. [Google Scholar] [CrossRef]
- Grosjean, A.; Rezrazi, M.U.; Berc, P. Some morphological characteristics of the incorporation of silicon carbide SiC/particles into electroless nickel deposits. Surf. Coat. Technol. 2000, 130, 252–256. [Google Scholar] [CrossRef]
- Kalantary, M.R.; Holbrook, K.A.; Wells, P.B. Optimisation of a bath for electroless plating and its use for the production of nickel-phosphorus-silicon carbide coatings. Int. J. Surf. Eng. Coat. 2017, 55–61. [Google Scholar] [CrossRef]
- Amell, A.; Muller, C.; Sarret, M. Influence of fluorosurfactants on the codeposition of ceramic nanoparticles and the morphology of electroless NiP coatings. Surf. Coat. Technol. 2010, 205, 356–362. [Google Scholar] [CrossRef]
- Sohrabi, A.; Dolati, A.; Ghorbani, M.; Monfared, A.; Stroeve, P. Nanomechanical properties of functionally graded composite coatings: Electrodeposited nickel dispersions containing silicon micro-and nanoparticles. Mater. Chem. Phys. 2010, 121, 497–505. [Google Scholar] [CrossRef]
- Ger, M. Electrochemical deposition of nickel/SiC composites in the presence of surfactants. Mater. Chem. Phys. 2004, 87, 67–74. [Google Scholar] [CrossRef]
- Alexis, J.; Etcheverry, B.; Beguin, J.D.; Bonino, J.P. Structure, morphology and mechanical properties of electrodeposited composite coatings Ni–P/SiC. Mater. Chem. Phys. 2010, 120, 244–250. [Google Scholar] [CrossRef]



| Chemical Compositions | g/L | Bath 1 | Bath 2 | Bath 3 | Parameters |
|---|---|---|---|---|---|
| NiSO4·7H2O | 100–150 | ✓ | ✓ | ✓ | i: 1, 2, 4 A/dm2 |
| NiCl2·6H2O | 15–30 | ✓ | ✓ | ✓ | T: 70 °C |
| H3BO3 | 15–30 | ✓ | ✓ | ✓ | Agitation: 250 rpm |
| H3PO3 | 100–130 | ✓ | ✓ | ✓ | Anode: Pure Ni sheet |
| SiC | 10, 20 | ✓ | ✓ | ✓ | pH: 2.15 |
| Sodium dodecyl sulfate | 0–1 | – | – | ✓ | – |
| Saccharin | 0–3 | – | ✓ | ✓ | – |
| Areas | Area 1 | Area 2 | Area 3 |
|---|---|---|---|
| wt.% P | 14.35 | 8.02 | 14.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadkhaniha, D.; Zanella, C. The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings. Coatings 2019, 9, 554. https://doi.org/10.3390/coatings9090554
Ahmadkhaniha D, Zanella C. The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings. Coatings. 2019; 9(9):554. https://doi.org/10.3390/coatings9090554
Chicago/Turabian StyleAhmadkhaniha, Donya, and Caterina Zanella. 2019. "The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings" Coatings 9, no. 9: 554. https://doi.org/10.3390/coatings9090554
APA StyleAhmadkhaniha, D., & Zanella, C. (2019). The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings. Coatings, 9(9), 554. https://doi.org/10.3390/coatings9090554






