Influence of Treatment Time on Performance of Cr(III)-Based Conversion Coatings on Hot Dip Zn–55Al–1.6Si Coated Steel Sheet
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation and Cr(III) Treatment Process
2.2. Surface Composition Analysis
2.3. Surface Micromorphology and Roughness Analysis
2.4. Pull-off Adhesion Tests
2.5. Electrochemical Mesurements
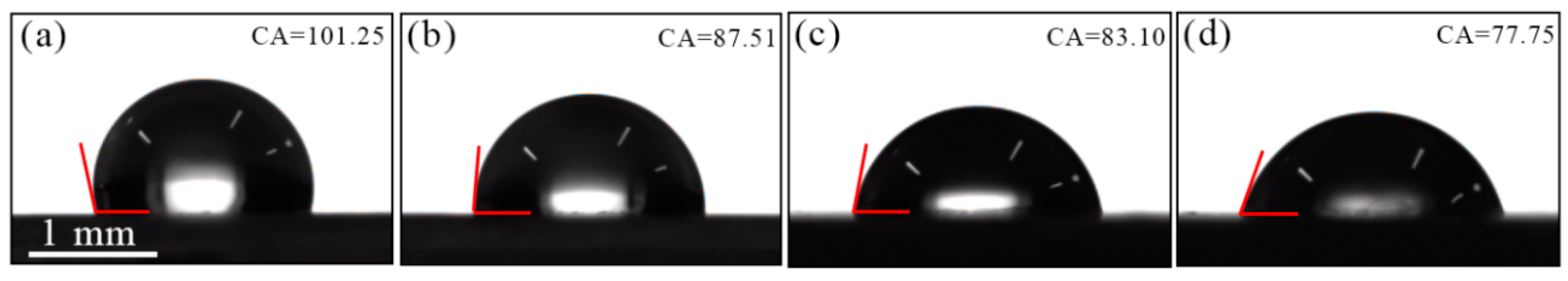
2.6. Contact Angle Mesurements
3. Results
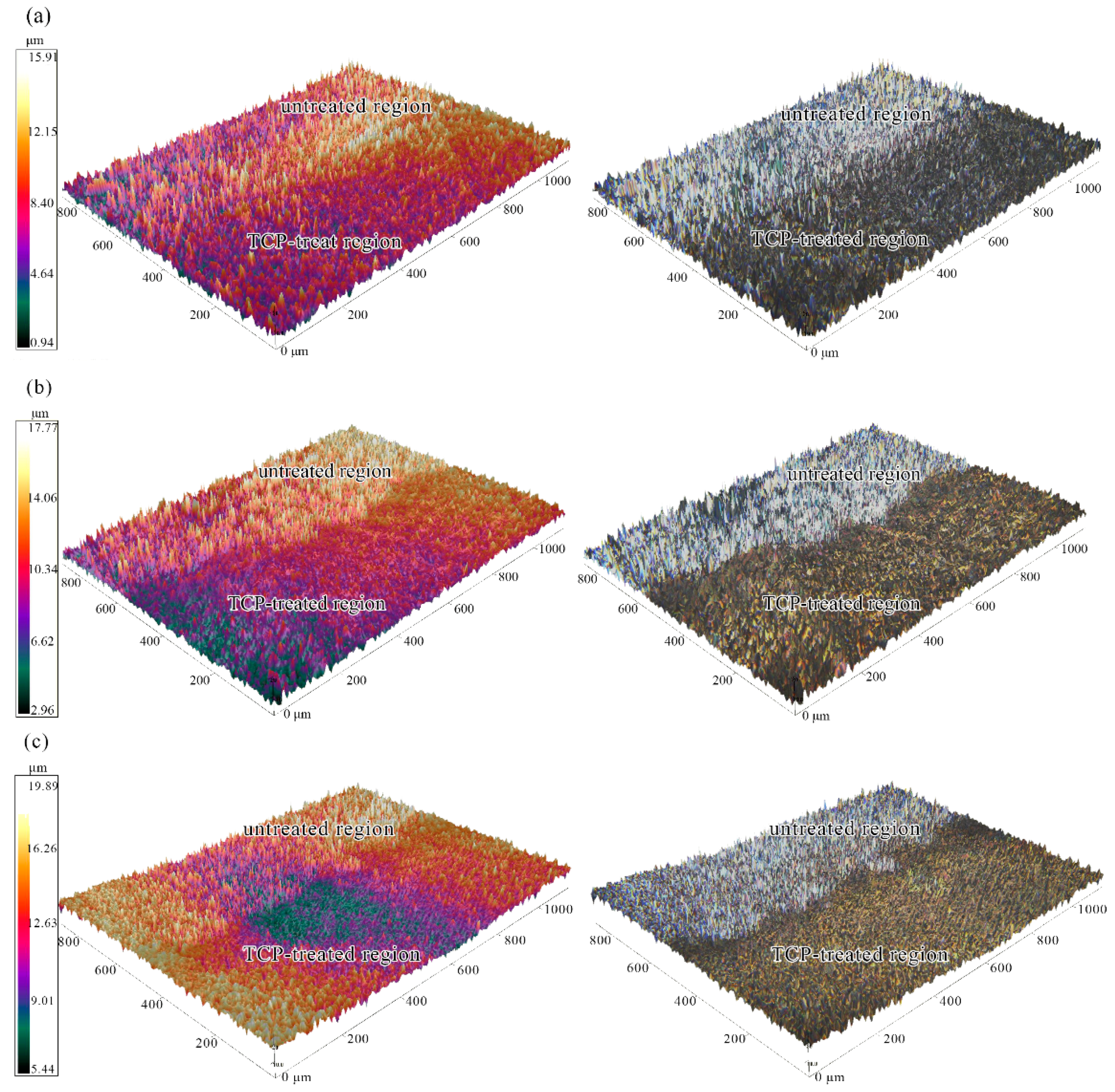
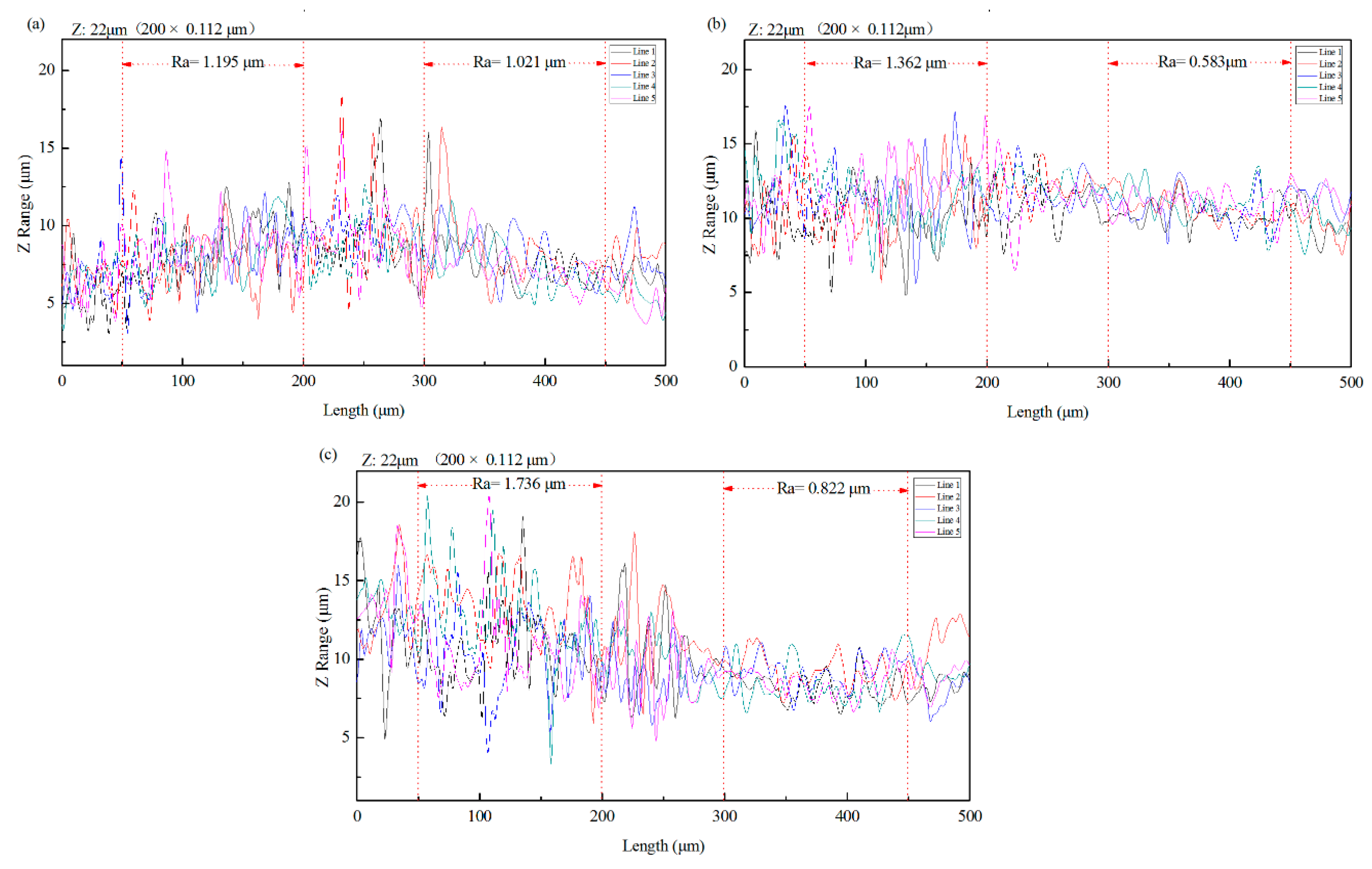
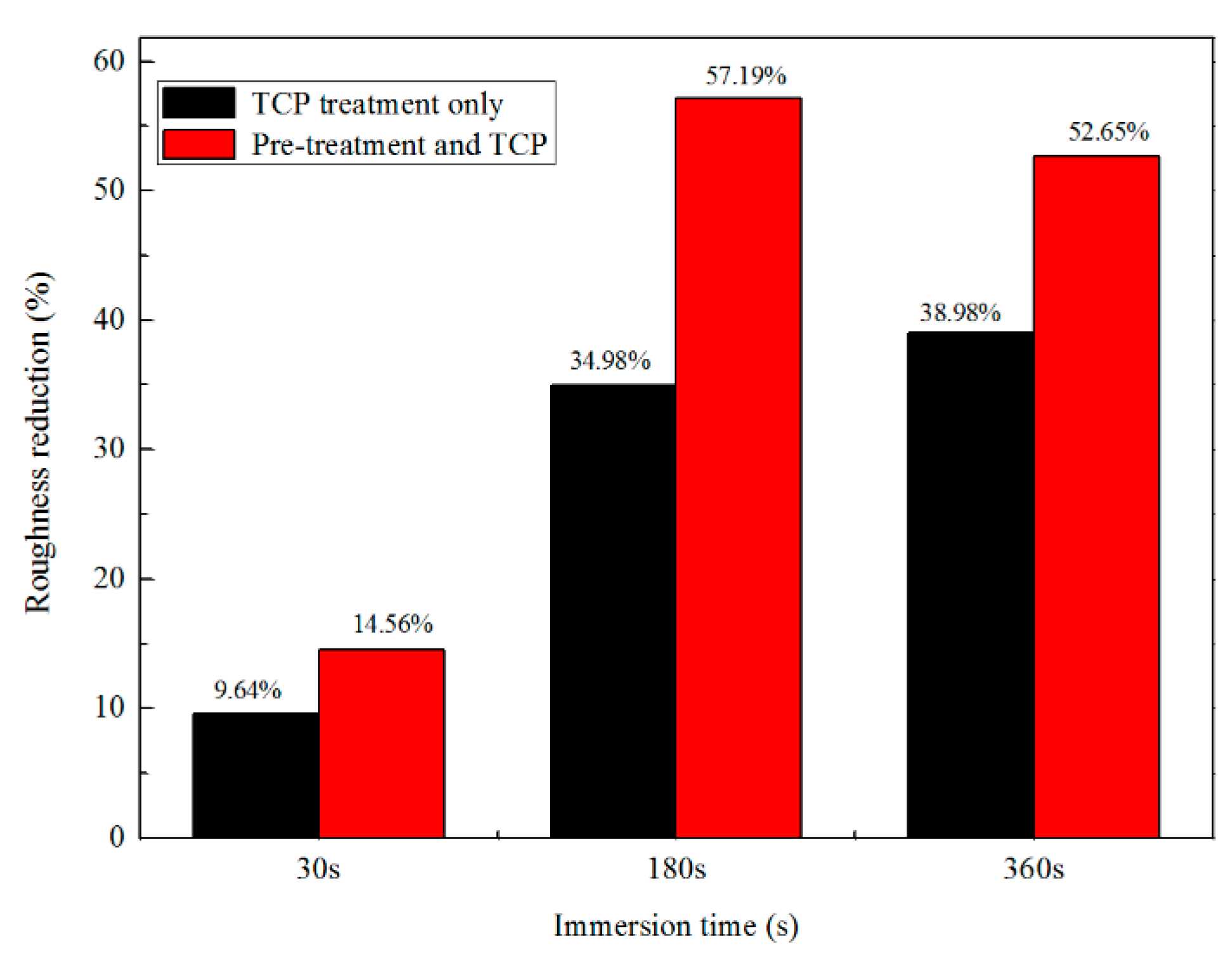
3.1. Roughness and 3D Morphologies Analysis
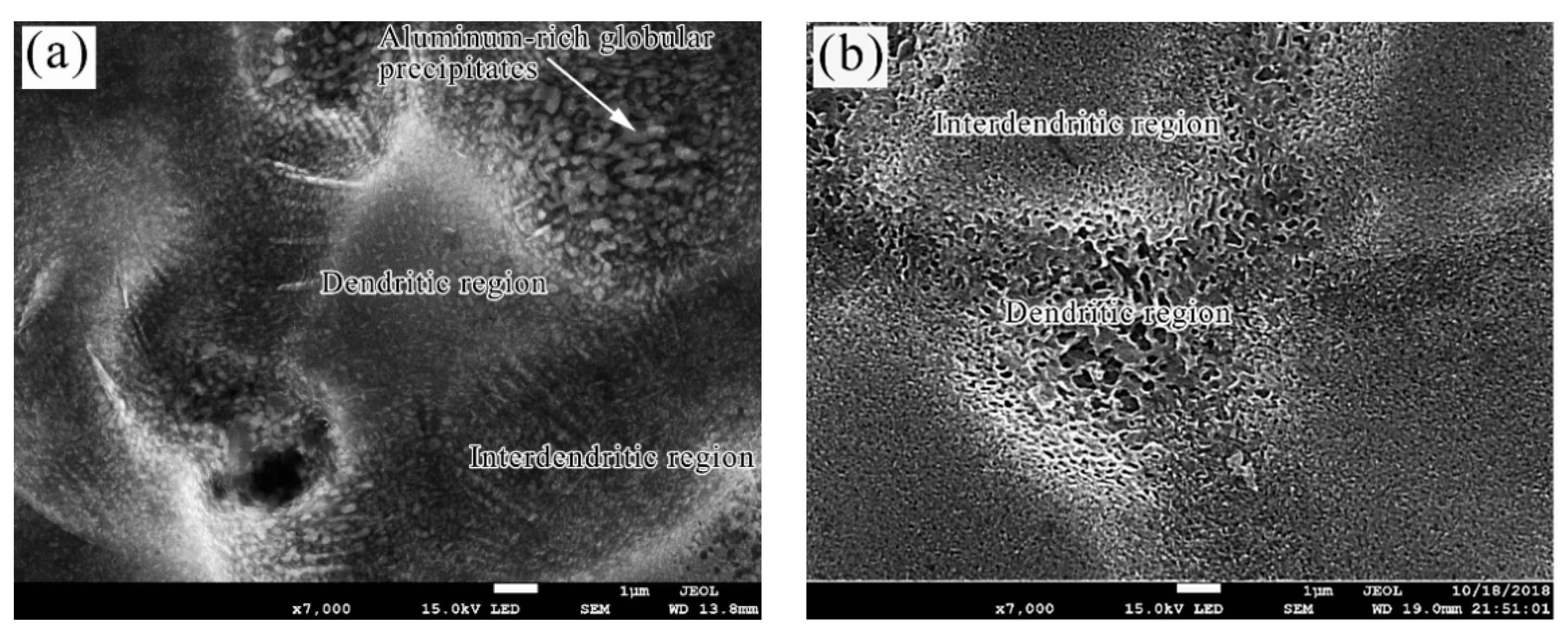
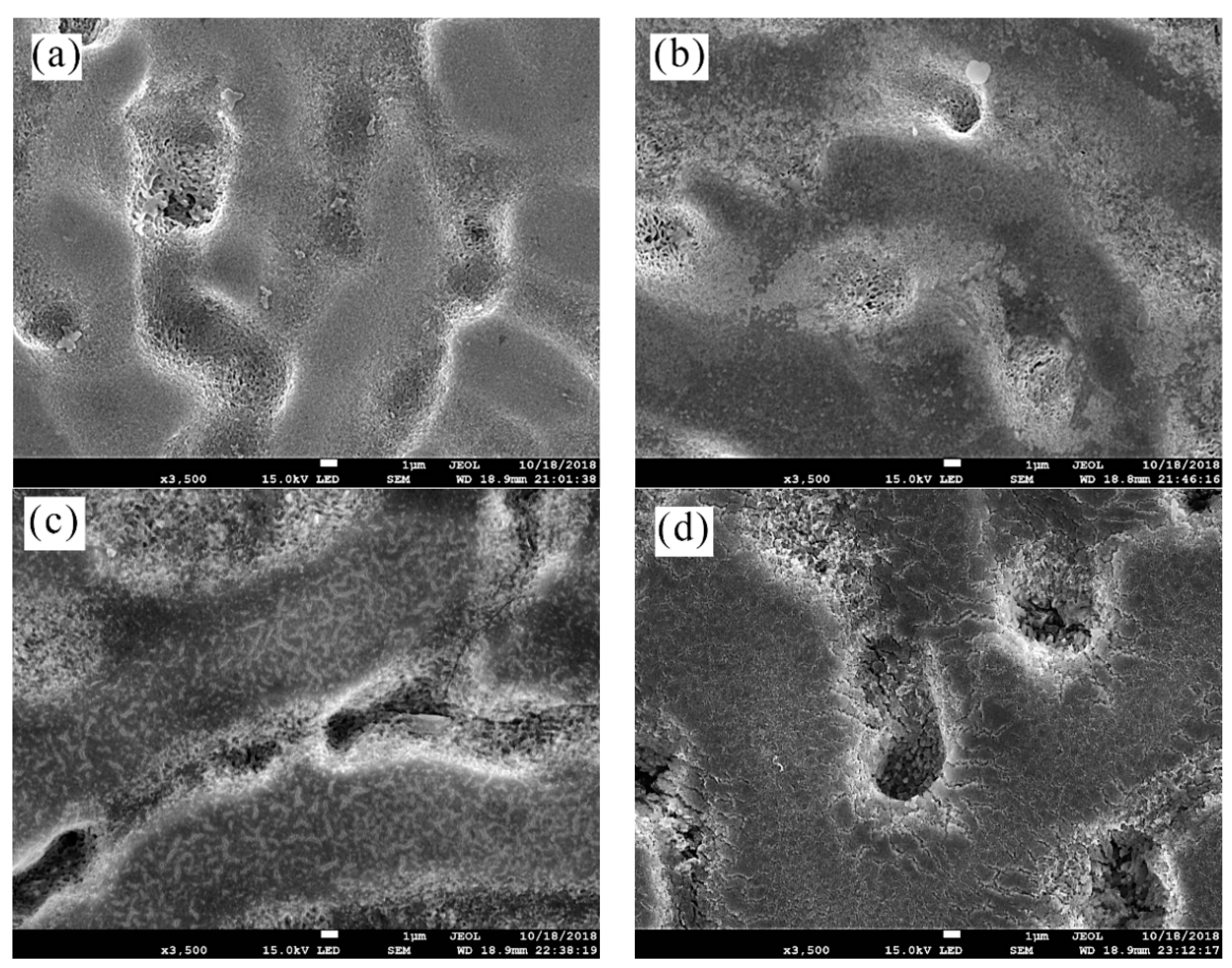
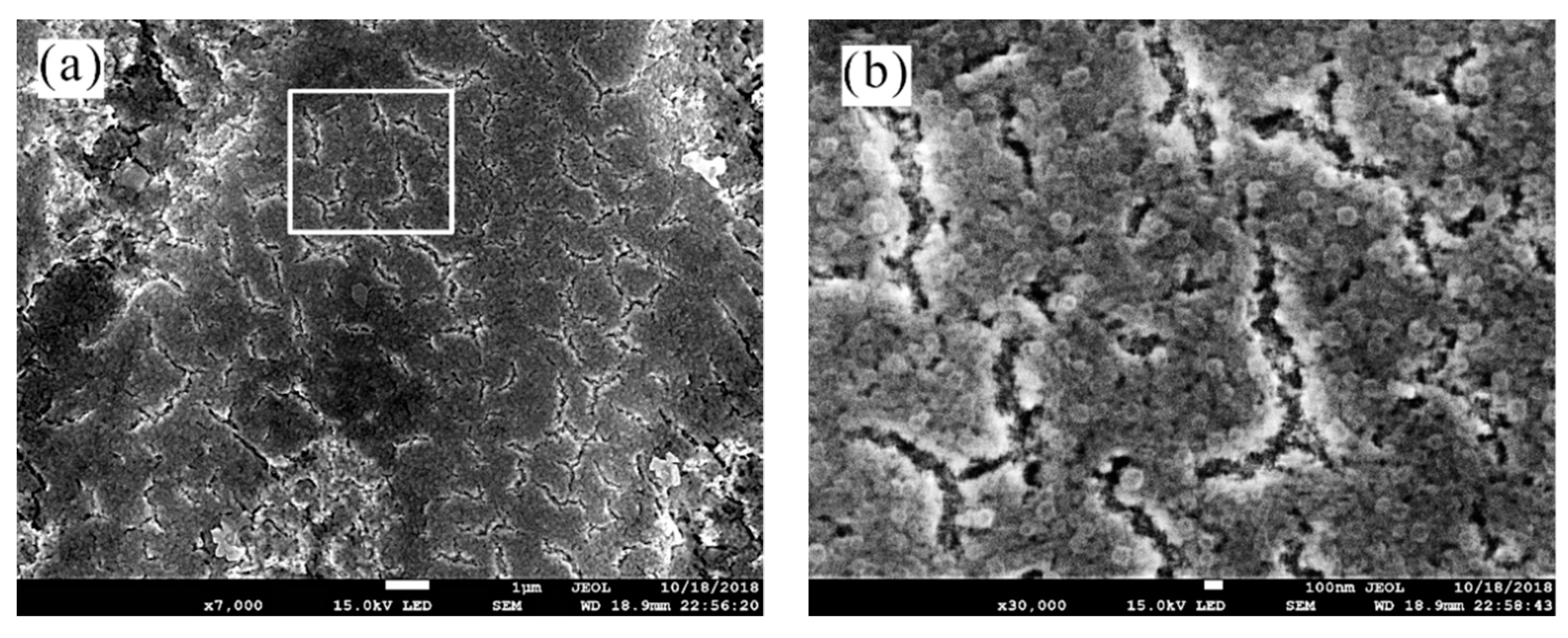
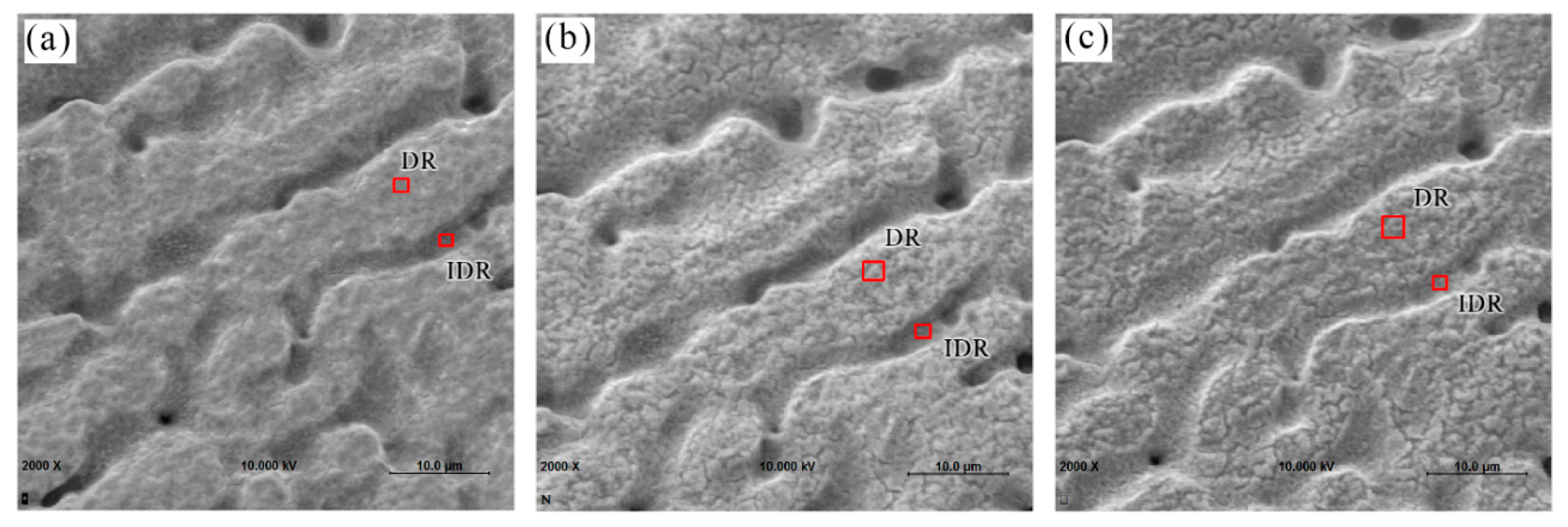
3.2. Surface Morphology Characterization
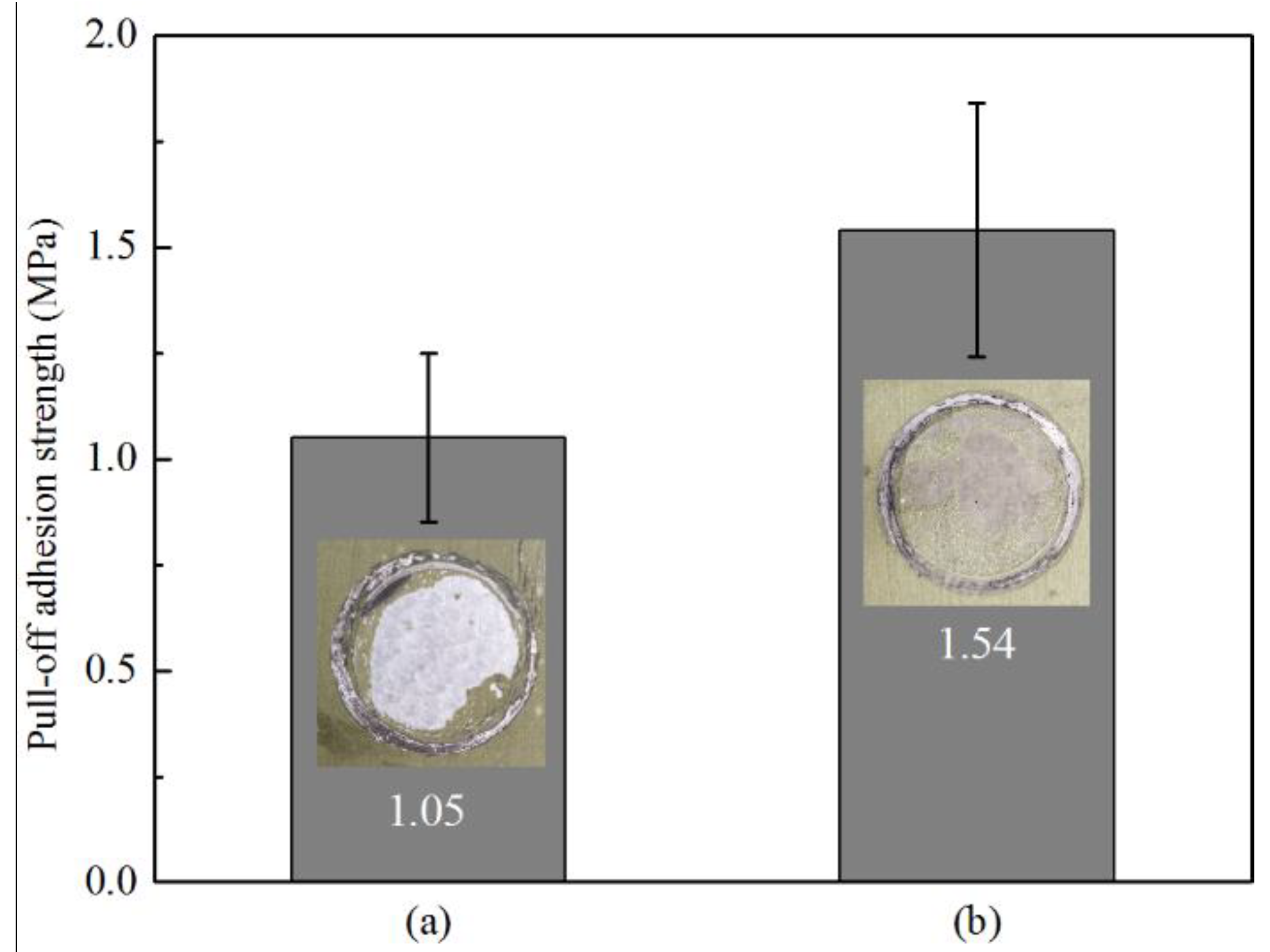
3.3. Pull-off Adhesion Measurements
3.4. Electrochemical Characterization
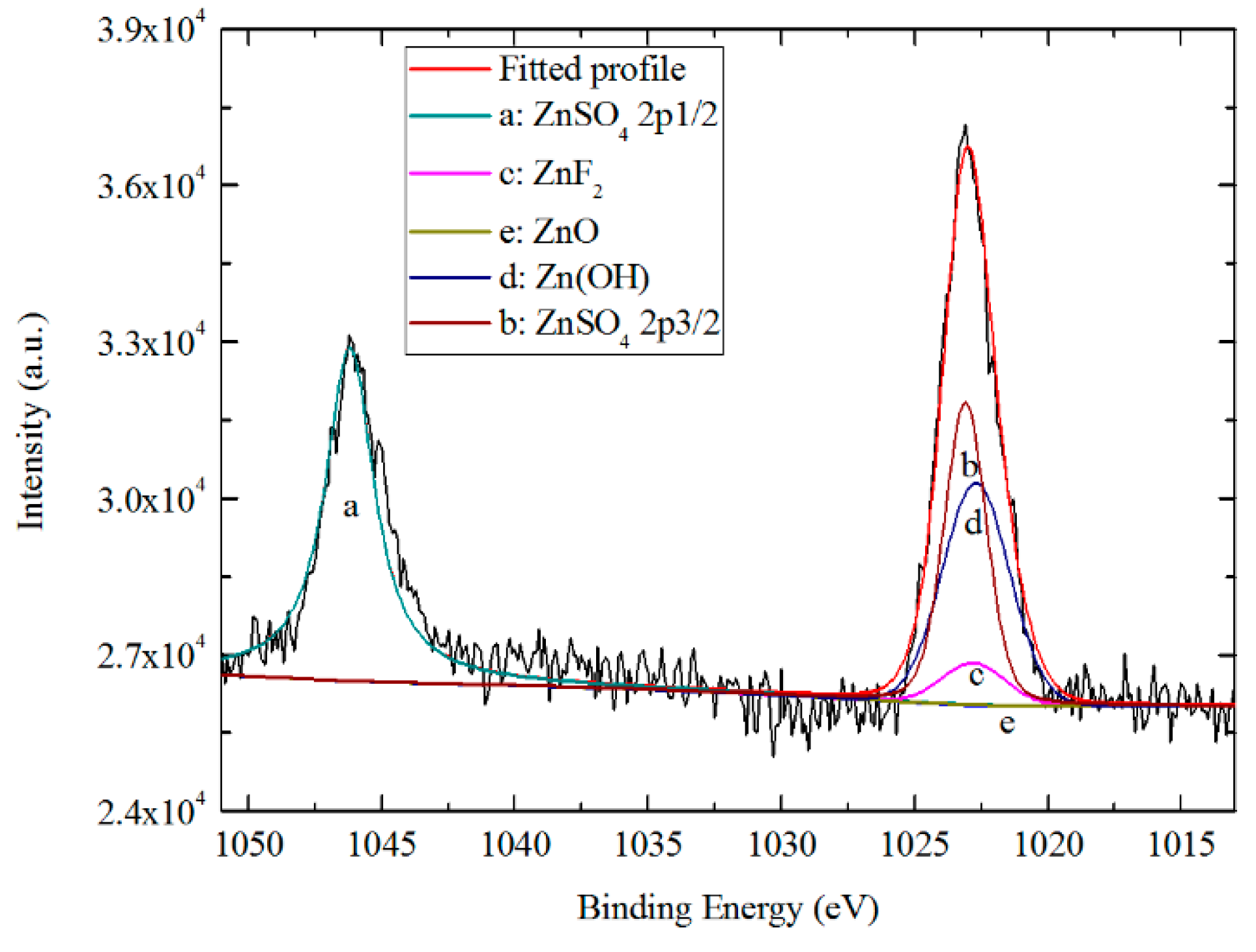
3.5. Surface Composition Analysis
3.5.1. Composition of Coating from EDS/AES
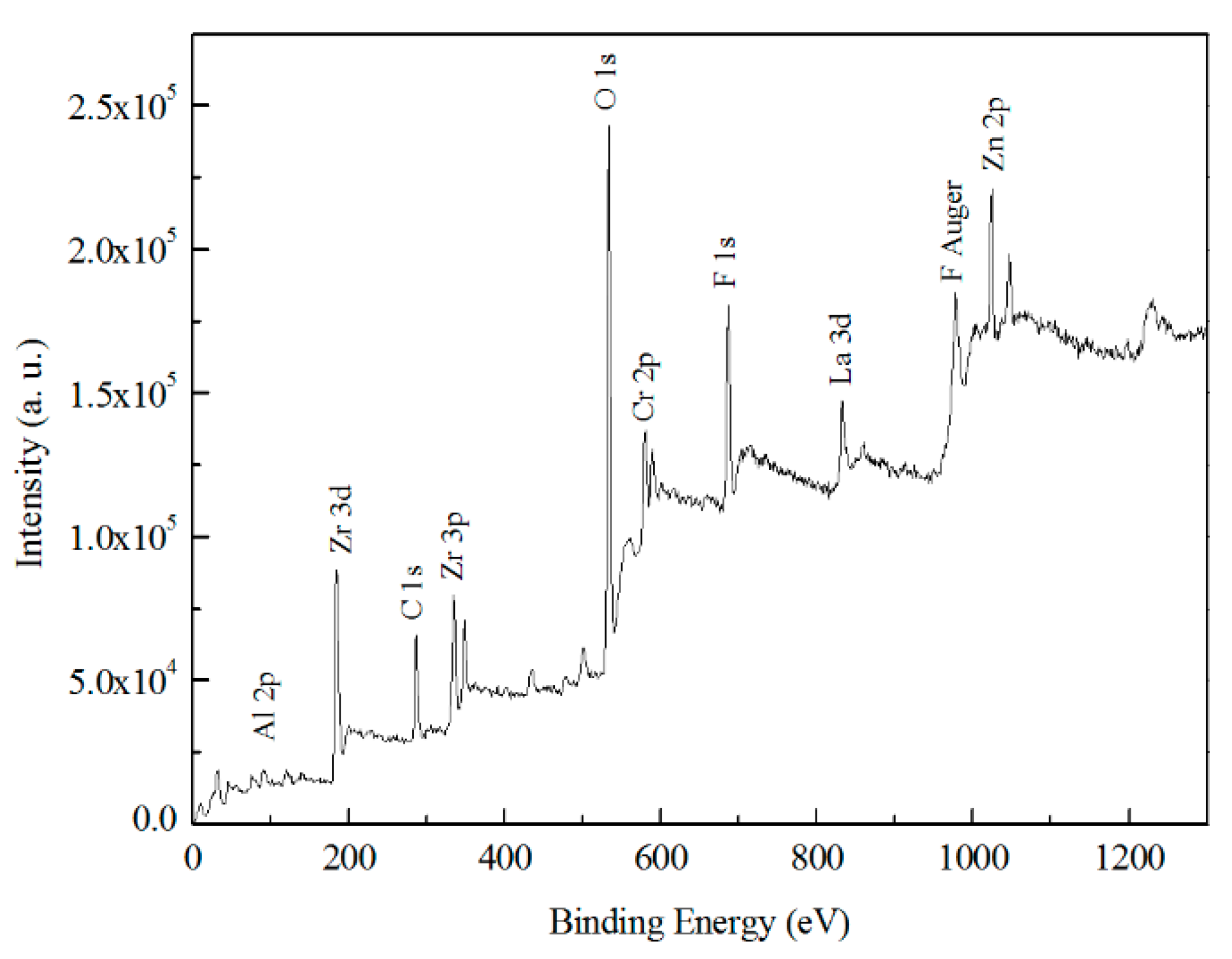
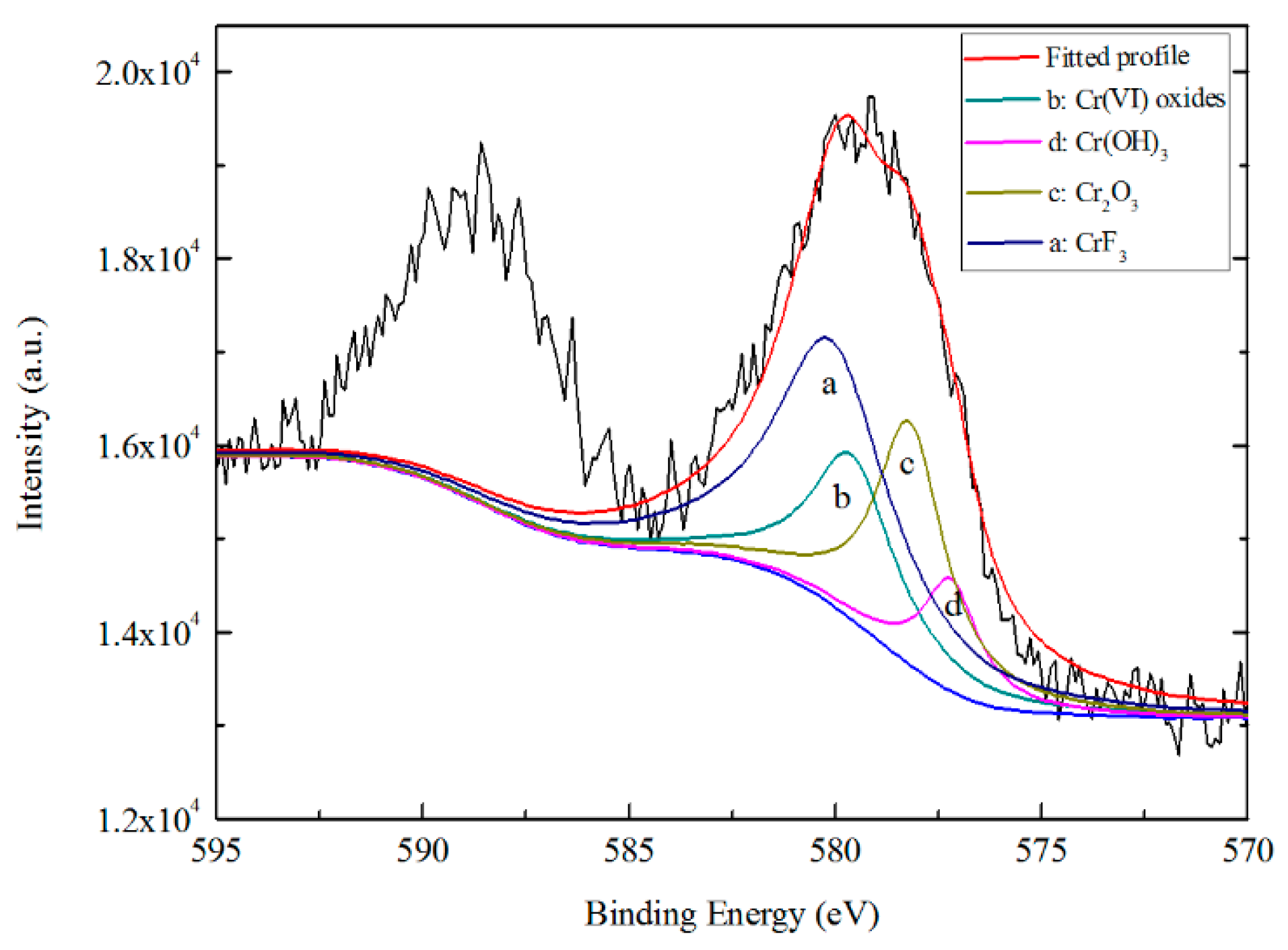
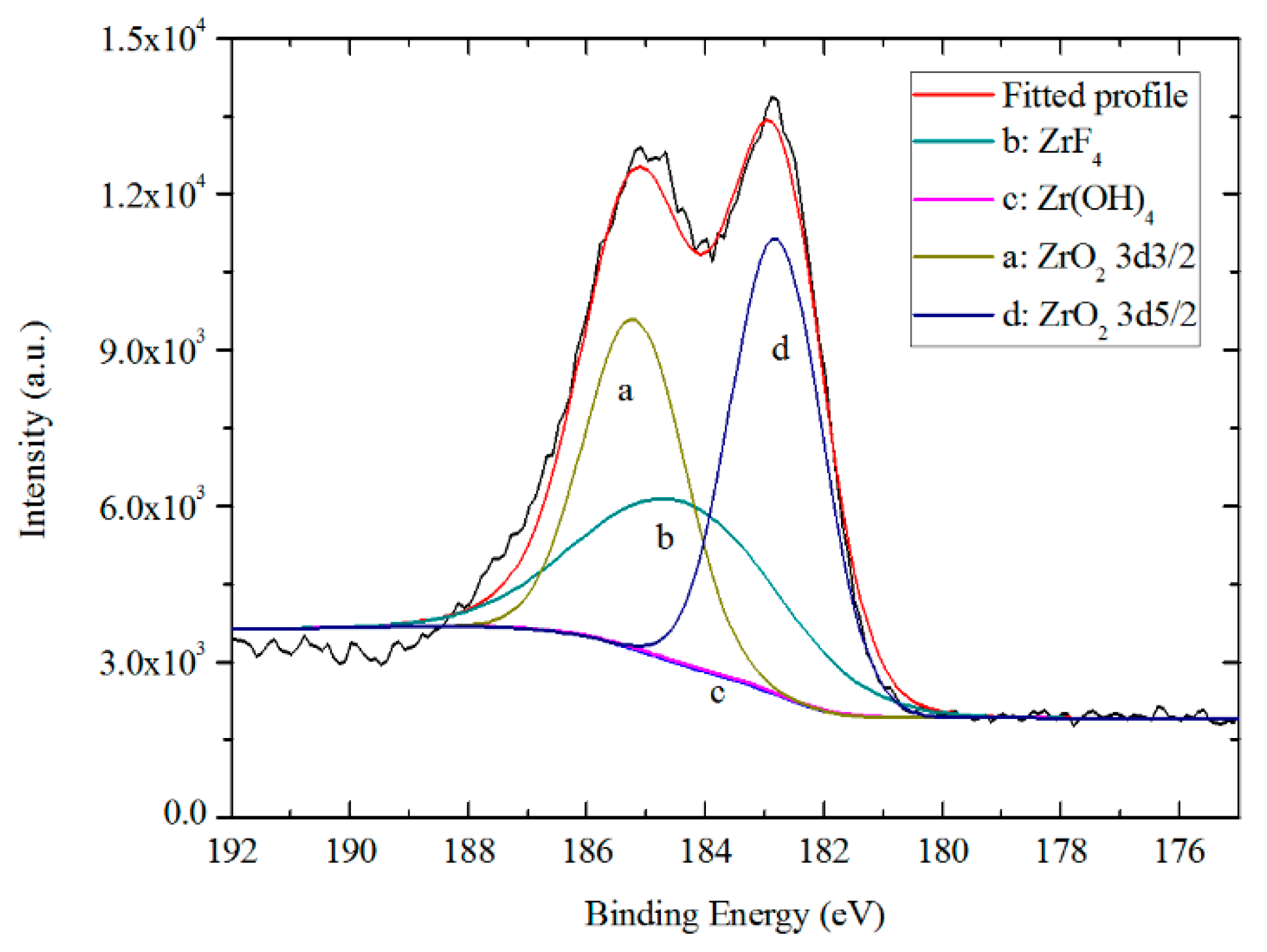
3.5.2. Chemical States of TCC Species from XPS
4. Discussion
5. Conclusions
- The analysis of 3D morphology and roughness shows that the macro-roughness of Zn55Al specimen surface decreases gradually with the prolongation of TCP treatment time. The surface roughness of Zn55Al treated for 180 s in Cr(III)-based dilution commercial solution at 40 °C is ~57% lower than that of the untreated surface. However, the formation of TCC results in the increase of the micro-roughness of the Zn55Al surface in contrast to the untreated specimen, which enhances the adhesion of the coating, and the film formation begins at the transition zone between dendrites and dendrites.
- Pull-off adhesion strength tests revealed that the Cr(III) conversion coating improved the adhesion of the epoxy primer on Zn55Al by ~31.8% after TCP treatment of 180 s, which may be attributed to the increase of micro-roughness and the physicochemical properties of TCC.
- AES depth profiling indicates the trivalent conversion coating on Zn55Al treated by TCP for 180 s consists of a bilayer structure in the dendritic and inter-dendritic regions. The total thickness of TCC in the inter-dendritic region (~410 nm) is larger than that in the dendritic region (~310 nm), which is up to the main film-forming element of zirconium and chromium. The maximum Cr/Zr atomic concentration ratio is ~5.92 at the inter-dendritic region. The chemical states results from XPS revealed that the main chemical components on the TCC surface are oxides and fluorides of Al, Zn, Cr, and Zr, with a small number of hydroxides.
- Results of the electrochemical tests show that in an aggressive 0.5 M NaCl solution, the corrosion resistance of Zn55Al can be greatly enhanced by TCP treatment. Zn55Al specimens after 180 s of TCC film formation have the lowest corrosion current density and the largest polarization resistance. The cathodic polarization curves measured in a less aggressive 0.05 M NaCl solutions indicated that the corrosion of Zn55Al is cathodic control and the Cr(III) can act as a barrier for corrosion mass transfer. In addition, the corrosion resistance of Zn55Al surface treated by TCP is sensitive to surface micro-cracks.
Author Contributions
Funding
Conflicts of Interest
References
- Vu, T.N.; Volovitch, P.; Ogle, K. The effect of pH on the selective dissolution of Zn and Al from Zn–Al coatings on steel. Corros. Sci. 2013, 67, 42–49. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Peña, J.J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O.T.; et al. Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Tachibana, K.; Morinaga, Y.; Mayuzumi, M. Hot dip fine Zn and Zn–Al alloy double coating for corrosion resistance at coastal area. Corros. Sci. 2007, 49, 149–157. [Google Scholar] [CrossRef]
- Moreira, A.R.; Panossian, Z.; Camargo, P.L.; Moreira, M.F.; da Silva, I.C.; de Carvalho, J.E.R. Zn/55Al coating microstructure and corrosion mechanism. Corros. Sci. 2006, 48, 564–576. [Google Scholar] [CrossRef]
- Persson, D.; Thierry, D.; Karlsson, O. Corrosion and corrosion products of hot dipped galvanized steel during long term atmospheric exposure at different sites world-wide. Corros. Sci. 2017, 126, 152–165. [Google Scholar] [CrossRef]
- Li, Y. Formation of nano-crystalline corrosion products on Zn–Al alloy coating exposed to seawater. Corros. Sci. 2001, 43, 1793–1800. [Google Scholar] [CrossRef]
- Vakili, H.; Ramezanzadeh, B.; Amini, R. The corrosion performance and adhesion properties of the epoxy coating applied on the steel substrates treated by cerium-based conversion coatings. Corros. Sci. 2015, 94, 466–475. [Google Scholar] [CrossRef]
- Mahidashti, Z.; Shahrabi, T.; Ramezanzadeh, B. The role of post-treatment of an ecofriendly cerium nanostructure conversion coating by green corrosion inhibitor on the adhesion and corrosion protection properties of the epoxy coating. Prog. Org. Coat. 2018, 114, 19–32. [Google Scholar] [CrossRef]
- Eivaz Mohammadloo, H.; Sarabi, A.A.; Asemani, H.R.; Ahmadi, P. A comparative study of eco-friendly hybrid thin films: With and without organic coating application. Prog. Org. Coat. 2018, 125, 432–442. [Google Scholar] [CrossRef]
- Sababi, M.; Terryn, H.; Mol, J.M.C. The influence of a Zr-based conversion treatment on interfacial bonding strength and stability of epoxy coated carbon steel. Prog. Org. Coat. 2017, 105, 29–36. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. An evaluation of the corrosion resistance and adhesion properties of an epoxy-nanocomposite on a hot-dip galvanized steel (HDG) treated by different kinds of conversion coatings. Surf. Coat. Technol. 2011, 205, 4649–4657. [Google Scholar] [CrossRef]
- Asemani, H.R.; Ahmadi, P.; Sarabi, A.A.; Eivaz Mohammadloo, H. Effect of zirconium conversion coating: Adhesion and anti-corrosion properties of epoxy organic coating containing zinc aluminum polyphosphate (ZAPP) pigment on carbon mild steel. Prog. Org. Coat. 2016, 94, 18–27. [Google Scholar] [CrossRef]
- Mohammad Hosseini, R.; Sarabi, A.A.; Eivaz Mohammadloo, H.; Sarayloo, M. The performance improvement of Zr conversion coating through Mn incorporation: With and without organic coating. Surf. Coat. Technol. 2014, 258, 437–446. [Google Scholar] [CrossRef]
- Sharifi Golru, S.; Attar, M.M.; Ramezanzadeh, B. Effects of surface treatment of aluminium alloy 1050 on the adhesion and anticorrosion properties of the epoxy coating. Appl. Surf. Sci. 2015, 345, 360–368. [Google Scholar] [CrossRef]
- Golru, S.S.; Attar, M.M.; Ramezanzadeh, B. Morphological analysis and corrosion performance of zirconium based conversion coating on the aluminum alloy 1050. J. Ind. Eng. Chem. 2015, 24, 233–244. [Google Scholar] [CrossRef]
- Ghanbari, A.; Attar, M.M. Surface free energy characterization and adhesion performance of mild steel treated based on zirconium conversion coating: A comparative study. Surf. Coat. Technol. 2014, 246, 26–33. [Google Scholar] [CrossRef]
- Kendig, M.W.; Buchheit, R.G. Corrosion inhibition of aluminum and aluminum alloys by soluble chromates, chromate coatings, and chromate-free coatings. Corrosion 2003, 59, 379–400. [Google Scholar] [CrossRef]
- Tomachuk, C.R.; Elsner, C.I.; Di Sarli, A.R.; Ferraz, O.B. Corrosion resistance of Cr(III) conversion treatments applied on electrogalvanised steel and subjected to chloride containing media. Mater. Chem. Phys. 2010, 119, 19–29. [Google Scholar] [CrossRef]
- Sheu, H.H.; Lin, C.H.; Jian, S.Y.; Lee, H.B.; Yang, B.R.; Ger, M.D. Effects of complexing agents and current density on carbon content of trivalent chromium carbon coating and its properties. Int. J. Electrochem. Sci. 2016, 11, 7099–7110. [Google Scholar] [CrossRef]
- Sheu, H.H.; Lee, H.B.; Jian, S.Y.; Hsu, C.Y.; Lee, C.Y. Investigation on the corrosion resistance of trivalent chromium conversion passivate on electroplated Zn–Ni alloy. Surf. Coat. Technol. 2016, 305, 241–248. [Google Scholar] [CrossRef]
- Taheri, P.; Laha, P.; Terryn, H.; Mol, J.M.C. An in situ study of zirconium-based conversion treatment on zinc surfaces. Appl. Surf. Sci. 2015, 356, 837–843. [Google Scholar] [CrossRef]
- Li, L.; Swain, G.M. Effects of aging temperature and time on the corrosion protection provided by trivalent chromium process coatings on AA2024-T3. ACS Appl. Mater. Interfaces 2013, 5, 7923–7930. [Google Scholar] [CrossRef]
- Qi, J.T.; Hashimoto, T.; Walton, J.R.; Zhou, X.; Skeldon, P.; Thompson, G.E. Trivalent chromium conversion coating formation on aluminium. Surf. Coat. Technol. 2015, 280, 317–329. [Google Scholar] [CrossRef]
- Guo, Y.; Frankel, G.S. Characterization of trivalent chromium process coating on AA2024-T3. Surf. Coat. Technol. 2012, 206, 3895–3902. [Google Scholar] [CrossRef]
- Viroulaud, R.; Światowska, J.; Seyeux, A.; Zanna, S.; Tardelli, J.; Marcus, P. Influence of surface pretreatments on the quality of trivalent chromium process coatings on aluminum alloy. Appl. Surf. Sci. 2017, 423, 927–938. [Google Scholar] [CrossRef]
- Verdalet-Guardiola, X.; Bonino, J.-P.; Duluard, S.; Fori, B.; Blanc, C. Influence of the alloy microstructure and surface state on the protective properties of trivalent chromium coatings grown on a 2024 aluminium alloy. Surf. Coat. Technol. 2018, 344, 276–287. [Google Scholar] [CrossRef]
- Chen, W.-K.; Bai, C.-Y.; Liu, C.-M.; Lin, C.-S.; Ger, M.-D. The effect of chromic sulfate concentration and immersion time on the structures and anticorrosive performance of the Cr(III) conversion coatings on aluminum alloys. Appl. Surf. Sci. 2010, 256, 4924–4929. [Google Scholar] [CrossRef]
- Cerezo, J.; Vandendael, I.; Posner, R.; de Wit, J.H.W.; Mol, J.M.C.; Terryn, H. Initiation and growth of modified Zr-based conversion coatings on multi-metal surfaces. Surf. Coat. Technol. 2013, 236, 284–289. [Google Scholar] [CrossRef]
- Cerezo, J.; Taheri, P.; Vandendael, I.; Posner, R.; Lill, K.; de Wit, J.H.W.; Mol, J.M.C.; Terryn, H. Influence of surface hydroxyls on the formation of Zr-based conversion coatings on AA6014 aluminum alloy. Surf. Coat. Technol. 2014, 254, 277–283. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Mu, S.; Yang, Y.; Zuo, X. The adhesion performance of epoxy coating on AA6063 treated in Ti/Zr/V based solution. Appl. Surf. Sci. 2016, 384, 333–340. [Google Scholar] [CrossRef]
- Cavanaugh, M.K.; Li, J.C.; Birbilis, N.; Buchheit, R.G. Electrochemical characterization of intermetallic phases common to aluminum alloys as a function of solution temperature. J. Electrochem. Soc. 2014, 161, C535–C543. [Google Scholar] [CrossRef]
- Munson, C.A.; Swain, G.M. Structure and chemical composition of different variants of a commercial trivalent chromium process (TCP) coating on aluminum alloy 7075-T6. Surf. Coat. Technol. 2017, 315, 150–162. [Google Scholar] [CrossRef]
- Munson, C.A.; McFall-Boegeman, S.A.; Swain, G.M. Cross comparison of TCP conversion coating performance on aluminum alloys during neutral salt-spray and thin-layer mist accelerated degradation testing. Electrochim. Acta 2018, 282, 171–184. [Google Scholar] [CrossRef]
- Li, L.; Doran, K.P.; Swain, G.M. Electrochemical characterization of trivalent chromium process (TCP) coatings on aluminum alloys 6061 and 7075. J. Electrochem. Soc. 2013, 160, C396–C401. [Google Scholar] [CrossRef]
- Fockaert, L.I.; Taheri, P.; Abrahami, S.T.; Boelen, B.; Terryn, H.; Mol, J.M.C. Zirconium-based conversion film formation on zinc, aluminium and magnesium oxides and their interactions with functionalized molecules. Appl. Surf. Sci. 2017, 423, 817–828. [Google Scholar] [CrossRef]
- Selverian, J.H.; Notis, M.R.; Marder, A.R. The microstructure of 55 w/o Al-Zn-Si (Galvalume) hot dip coatings. J. Mater. Eng. 1987, 9, 133–140. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Hou, B.; Feng, F.; Wei, X. Chromate passivation of hot dipped Zn25Al alloy coatings. Br. Corros. J. 2001, 36, 56–58. [Google Scholar] [CrossRef]
- ISO 12085: 1996 Geometrical Product Specification (GPS)-Surface Texture: Profile Method-Motif Parameters; International Organization for Standardization: Geneva, Switzerland, 1996.
- ASTM D4541-02 Standard Test Method for Pull-off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2002.
- Campestrini, P.; van Westing, E.P.M.; de Wit, J.H.W. Influence of surface preparation on performance of chromate conversion coatings on Alclad 2024 aluminium alloy Part I: Nucleation and growth. Electrochim. Acta 2001, 46, 2553–2571. [Google Scholar] [CrossRef]
- Cerezo, J.; Vandendael, I.; Posner, R.; de Wit, J.H.W.; Mol, J.M.C.; Terryn, H. The effect of surface pre-conditioning treatments on the local composition of Zr-based conversion coatings formed on aluminium alloys. Appl. Surf. Sci. 2016, 366, 339–347. [Google Scholar] [CrossRef]
- Qi, J.; Němcová, A.; Walton, J.R.; Zhou, X.; Skeldon, P.; Thompson, G.E. Influence of pre- and post-treatments on formation of a trivalent chromium conversion coating on AA2024 alloy. Thin Solid Films 2016, 616, 270–278. [Google Scholar] [CrossRef]
- Treverton, J.A.; Amor, M.P. High-resolution SEM studies of chromate conversion coatings. J. Mater. Sci. 1988, 23, 3706–3710. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Wang, F.; Kang, W.; Jing, H. Principle, Method and Application of Corrosion Electrochemistry, 1st ed.; Chemical Industry Press: Beijing, China, 2008; p. 64. [Google Scholar]
- Flitt, H.J.; Schweinsberg, D.P. Evaluation of corrosion rate from polarisation curves not exhibiting a Tafel region. Corros. Sci. 2005, 47, 3034–3052. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.-C.; Luo, Q.; Fan, H.-Q.; Zhang, J.-Y.; Lu, H.-S.; Chou, K.-C.; Wang, X.-L.; Li, Q. Influence of alloyed magnesium on the microstructure and long-term corrosion behavior of hot-dip Al–Zn–Si coating in NaCl solution. Corros. Sci. 2016, 104, 217–226. [Google Scholar] [CrossRef]
- Qi, J.; Hashimoto, T.; Walton, J.; Zhou, X.; Skeldon, P.; Thompson, G.E. Formation of a trivalent chromium conversion coating on AA2024-T351 Alloy. J. Electrochem. Soc. 2016, 163, C25–C35. [Google Scholar] [CrossRef]
- Li, L.; Swain, G.P.; Howell, A.; Woodbury, D.; Swain, G.M. The formation, structure, electrochemical properties and stability of trivalent chrome process (TCP) coatings on AA2024. J. Electrochem. Soc. 2011, 158, C274–C283. [Google Scholar] [CrossRef]
- Sinha, S.; Badrinarayanan, S.; Sinha, A. Interaction of oxygen with Zr76Fe24 metglass: An X-ray photoelectron spectroscopy study. J. Less Common Met. 1986, 125, 85–95. [Google Scholar] [CrossRef]
- Sleigh, C.; Pijpers, A.P.; Jaspers, A.; Coussens, B.; Meier, R.J. On the determination of atomic charge via ESCA including application to organometallics. J. Electron Spectrosc. Relat. Phenom. 1996, 77, 41–57. [Google Scholar] [CrossRef]
- Qi, J.; Gao, L.; Liu, Y.; Liu, B.; Hashimoto, T.; Wang, Z.; Thompson, G.E. Chromate formed in a trivalent chromium conversion coating on aluminum. J. Electrochem. Soc. 2017, 164, C442–C449. [Google Scholar] [CrossRef]
- Chidambaram, D.; Clayton, C.R.; Halada, G.P. The role of hexafluorozirconate in the formation of chromate conversion coatings on aluminum alloys. Electrochim. Acta 2006, 51, 2862–2871. [Google Scholar] [CrossRef]
- Bolt, P.H.; Ten Grotenhuis, E.; Geus, J.W.; Habraken, F.H.P.M. The interaction of thin NiO layers with single crystalline α-Al2O3 (1120) substrates. Surf. Sci. 1995, 329, 227–240. [Google Scholar] [CrossRef]
- McGuire, G.E.; Schweitzer, G.K.; Carlson, T.A. Core electron binding energies in some group IIIA, VB, and VIB compounds. Inorg. Chem. 1973, 12, 2450–2453. [Google Scholar] [CrossRef]
- Lindsay, J.R.; Rose, H.J.; Swartz, W.E.; Watts, P.H.; Rayburn, K.A. X-ray photoelectron spectra of aluminum oxides: structural effects on the “chemical shift”. Appl. Spectrosc. 1973, 27, 1–5. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Gaarenstroom, S.W.; Winograd, N. Initial and final state effects in the ESCA spectra of cadmium and silver oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Klein, J.C.; Hercules, D.M. Surface characterization of model Urushibara catalysts. J. Catal. 1983, 82, 424–441. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Shen, Y.; Liu, S.; Wang, W.; Tao, J. Improvement of adhesion performance between aluminum alloy sheet and epoxy based on anodizing technique. Int. J. Adhes. Adhes. 2016, 70, 74–80. [Google Scholar] [CrossRef]
- Niknahad, M.; Moradian, S.; Mirabedini, S.M. The adhesion properties and corrosion performance of differently pretreated epoxy coatings on an aluminium alloy. Corros. Sci. 2010, 52, 1948–1957. [Google Scholar] [CrossRef]
- Qi, J.; Gao, L.; Li, Y.; Wang, Z.; Thompson, G.E.; Skeldon, P. An optimized trivalent chromium conversion coating process for AA2024-T351 alloy. J. Electrochem. Soc. 2017, 164, C390–C395. [Google Scholar] [CrossRef]
- Zhang, X.; Sloof, W.G.; Hovestad, A.; van Westing, E.P.M.; Terryn, H.; de Wit, J.H.W. Characterization of chromate conversion coatings on zinc using XPS and SKPFM. Surf. Coat. Technol. 2005, 197, 168–176. [Google Scholar] [CrossRef]
- Kong, G.; Lingyan, L.; Lu, J.; Che, C.; Zhong, Z. Corrosion behavior of lanthanum-based conversion coating modified with citric acid on hot dip galvanized steel in aerated 1 M NaCl solution. Corros. Sci. 2011, 53, 1621–1626. [Google Scholar] [CrossRef]
- Chang, Y.T.; Wen, N.T.; Chen, W.K.; Ger, M.D.; Pan, G.T.; Yang, T.C.K. The effects of immersion time on morphology and electrochemical properties of the Cr(III)-based conversion coatings on zinc coated steel surface. Corros. Sci. 2008, 50, 3494–3499. [Google Scholar] [CrossRef]
- Li, L.L.; Desouza, A.L.; Swain, G.M. In situ pH measurement during the formation of conversion coatings on an aluminum alloy (AA2024). Analyst 2013, 138, 4398–4402. [Google Scholar] [CrossRef]
- Li, L.; Whitman, B.W.; Munson, C.A.; Estrada, R.; Matzdorf, C.A.; Swain, G.M. Structure and corrosion performance of a non-chromium process (NCP) Zr/Zn pretreatment conversion coating on aluminum alloys. J. Electrochem. Soc. 2016, 163, C718–C728. [Google Scholar] [CrossRef]
- Lostak, T.; Timma, C.; Krebs, S.; Flock, J.; Schulz, S. Organosilane modified Zr-based conversion layer on Zn-Al alloy coated steel sheets. Surf. Coat. Technol. 2016, 305, 223–230. [Google Scholar] [CrossRef]
- Taheri, P.; Lill, K.; de Wit, J.H.W.; Mol, J.M.C.; Terryn, H. Effects of zinc surface acid-based properties on formation mechanisms and interfacial bonding properties of zirconium-based conversion layers. J. Phys. Chem. C 2012, 116, 8426–8436. [Google Scholar] [CrossRef]
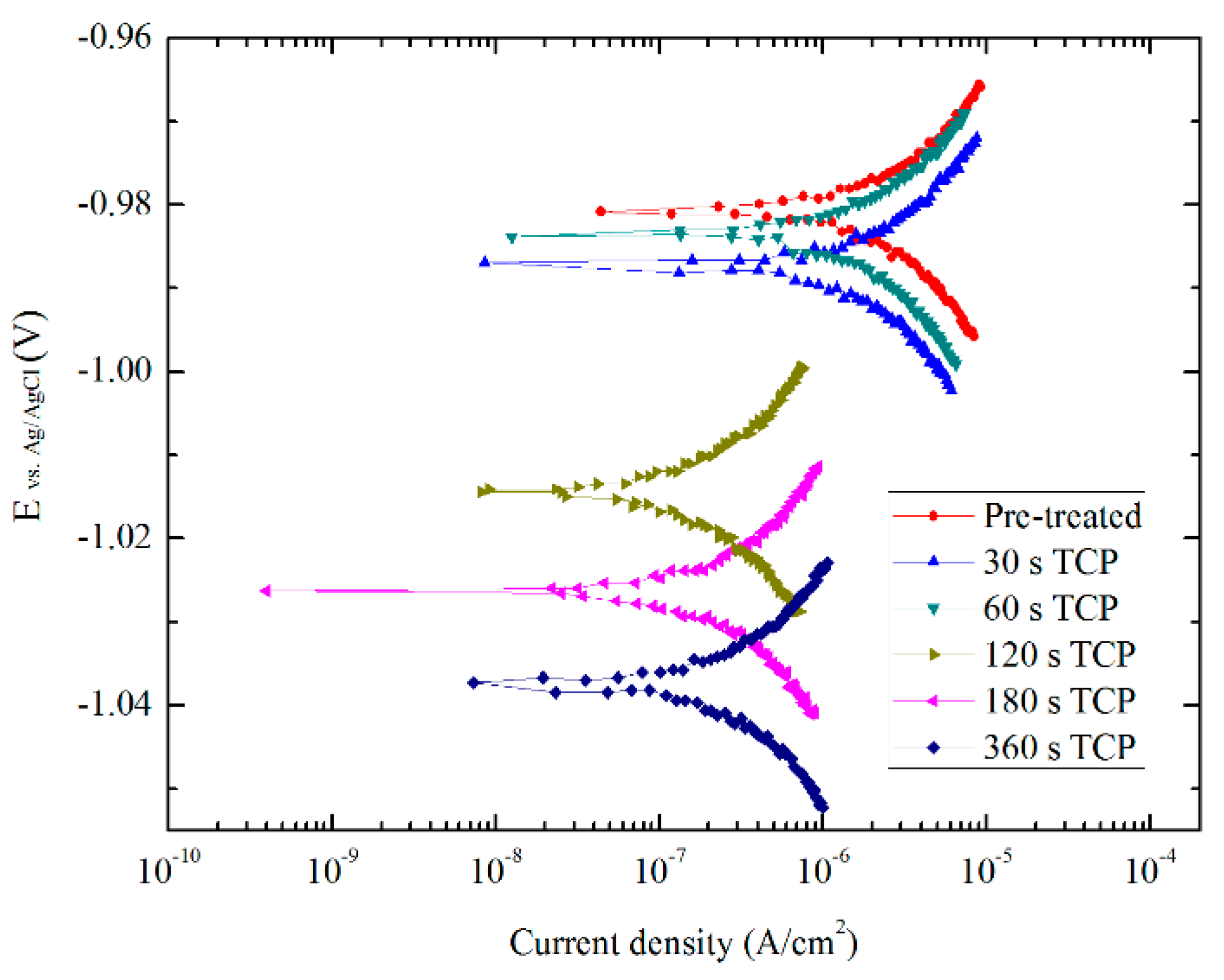




| Specimens | Rp (Ω) | icorr (A/cm2) | Ecorr (V) |
|---|---|---|---|
| Pre-treated | 1.7675 × 103 | 1.0750 × 105 | −0.981 |
| TCP for 30 s | 2.1773 × 103 | 1.0104 × 105 | −0.987 |
| TCP for 60 s | 2.2851 × 103 | 9.6276 × 106 | −0.984 |
| TCP for 120 s | 1.7180 × 104 | 1.2806 × 106 | −1.014 |
| TCP for 180 s | 2.2487 × 104 | 9.7834 × 107 | −1.026 |
| TCP for 360 s | 1.4671 × 104 | 1.4996 × 106 | −1.037 |
| Specimens | Curve | Ecorr (V) | icorr (A/cm2) |
|---|---|---|---|
| Uncoated Zn55Al in aerated 0.05 M NaCl | CP | −0.8936 | 2.13 × 10−7 |
| Uncoated Zn55Al in de-aerated 0.05 M NaCl | CP | −0.9305 | 5.21 × 10−8 |
| Cr(III) treated Zn55Al in aerated 0.05 M NaCl | CP | −1.0598 | 4.81 × 10−8 |
| Cr(III) treated Zn55Al in de-aerated 0.05 M NaCl | CP | −1.0432 | 7.72 × 10−8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Tang, X.; Li, Y. Influence of Treatment Time on Performance of Cr(III)-Based Conversion Coatings on Hot Dip Zn–55Al–1.6Si Coated Steel Sheet. Coatings 2019, 9, 297. https://doi.org/10.3390/coatings9050297
Pan J, Tang X, Li Y. Influence of Treatment Time on Performance of Cr(III)-Based Conversion Coatings on Hot Dip Zn–55Al–1.6Si Coated Steel Sheet. Coatings. 2019; 9(5):297. https://doi.org/10.3390/coatings9050297
Chicago/Turabian StylePan, Jie, Xiao Tang, and Yan Li. 2019. "Influence of Treatment Time on Performance of Cr(III)-Based Conversion Coatings on Hot Dip Zn–55Al–1.6Si Coated Steel Sheet" Coatings 9, no. 5: 297. https://doi.org/10.3390/coatings9050297
APA StylePan, J., Tang, X., & Li, Y. (2019). Influence of Treatment Time on Performance of Cr(III)-Based Conversion Coatings on Hot Dip Zn–55Al–1.6Si Coated Steel Sheet. Coatings, 9(5), 297. https://doi.org/10.3390/coatings9050297





