Electrochemical Impedance Spectroscopy Study of Waterborne Epoxy Coating Film Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Method of Epoxy Paint Preparation
2.2. Electrochemical and Thermo-Mechanical Measurements
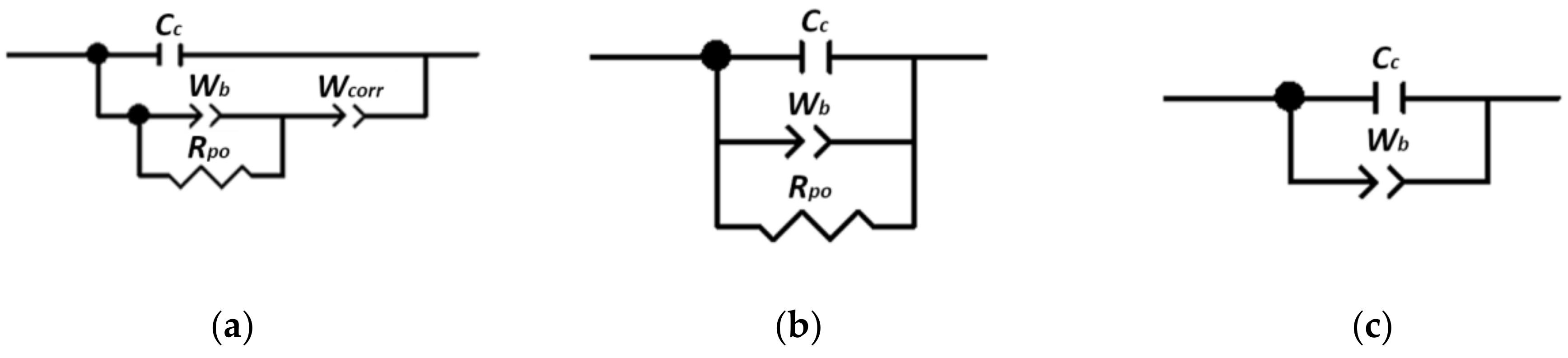
3. Theoretical/Calculation
4. Results and Discussion
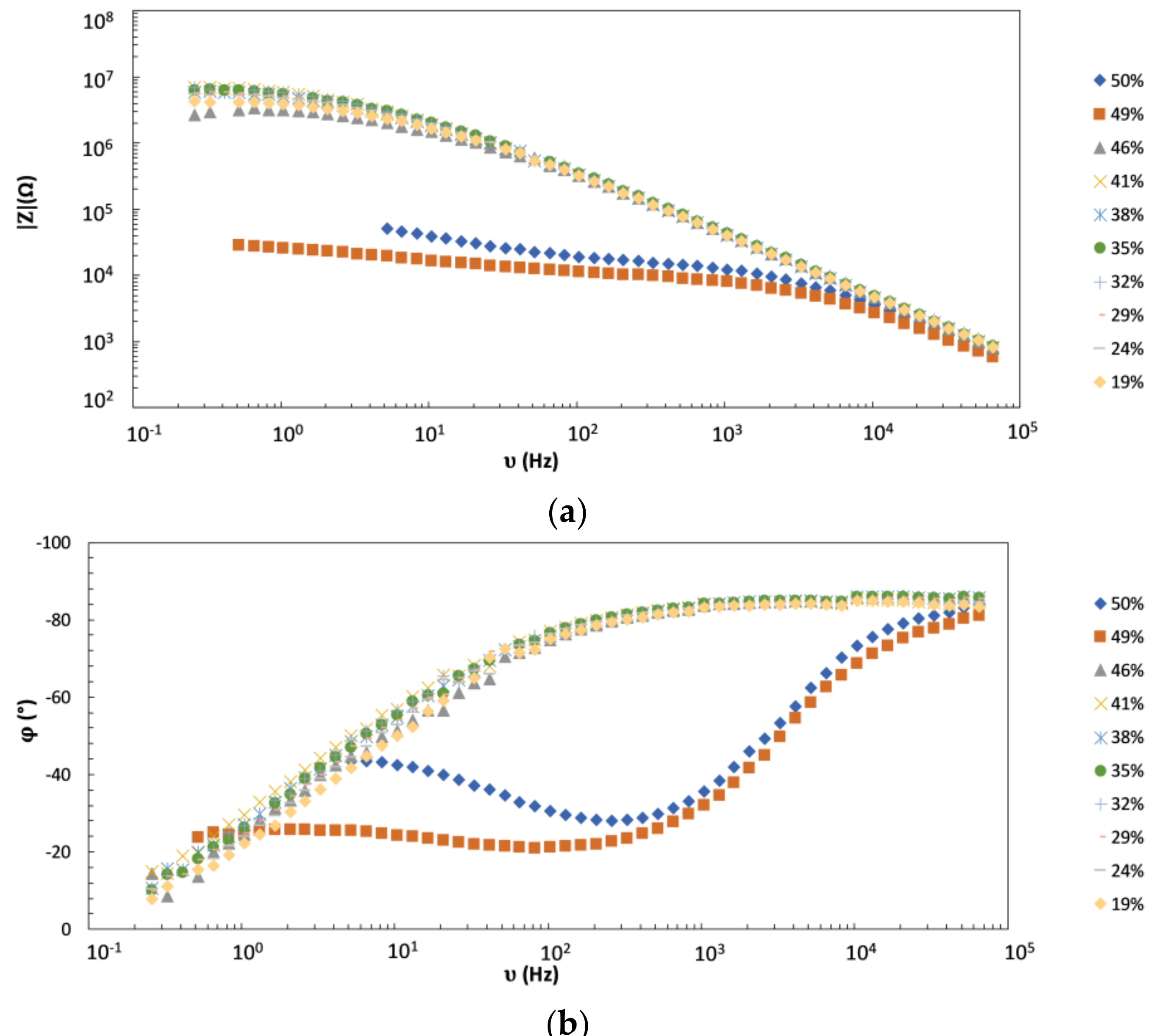
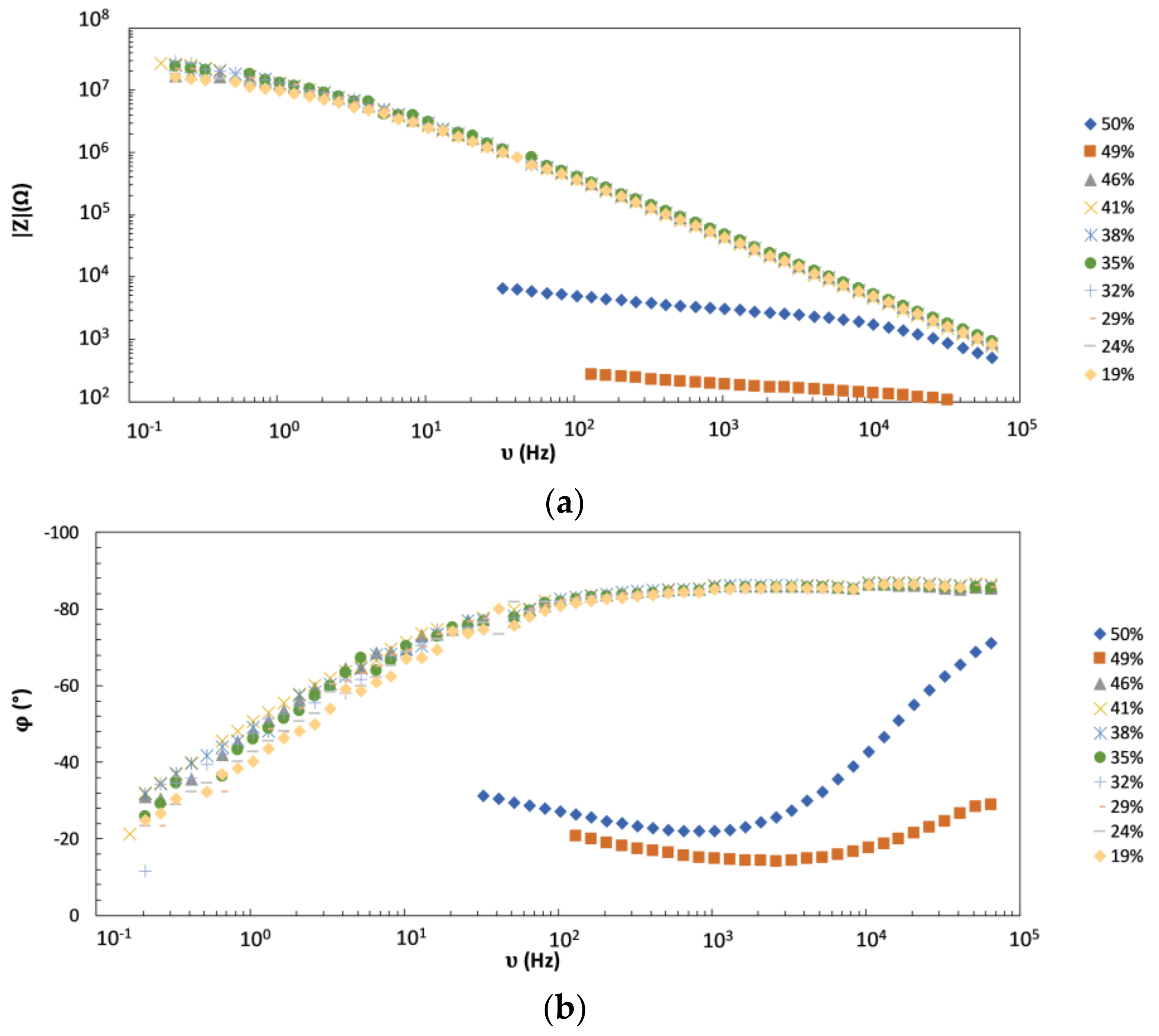
4.1. EIS as a Tool for Experimental Detection of PVC-CPVC Transition
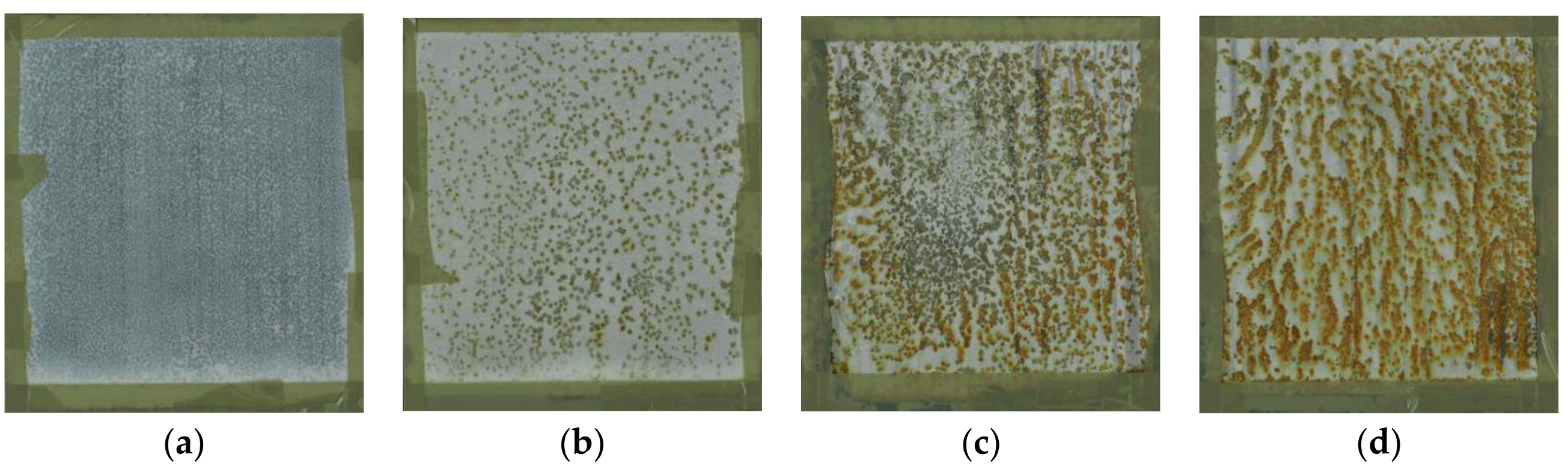
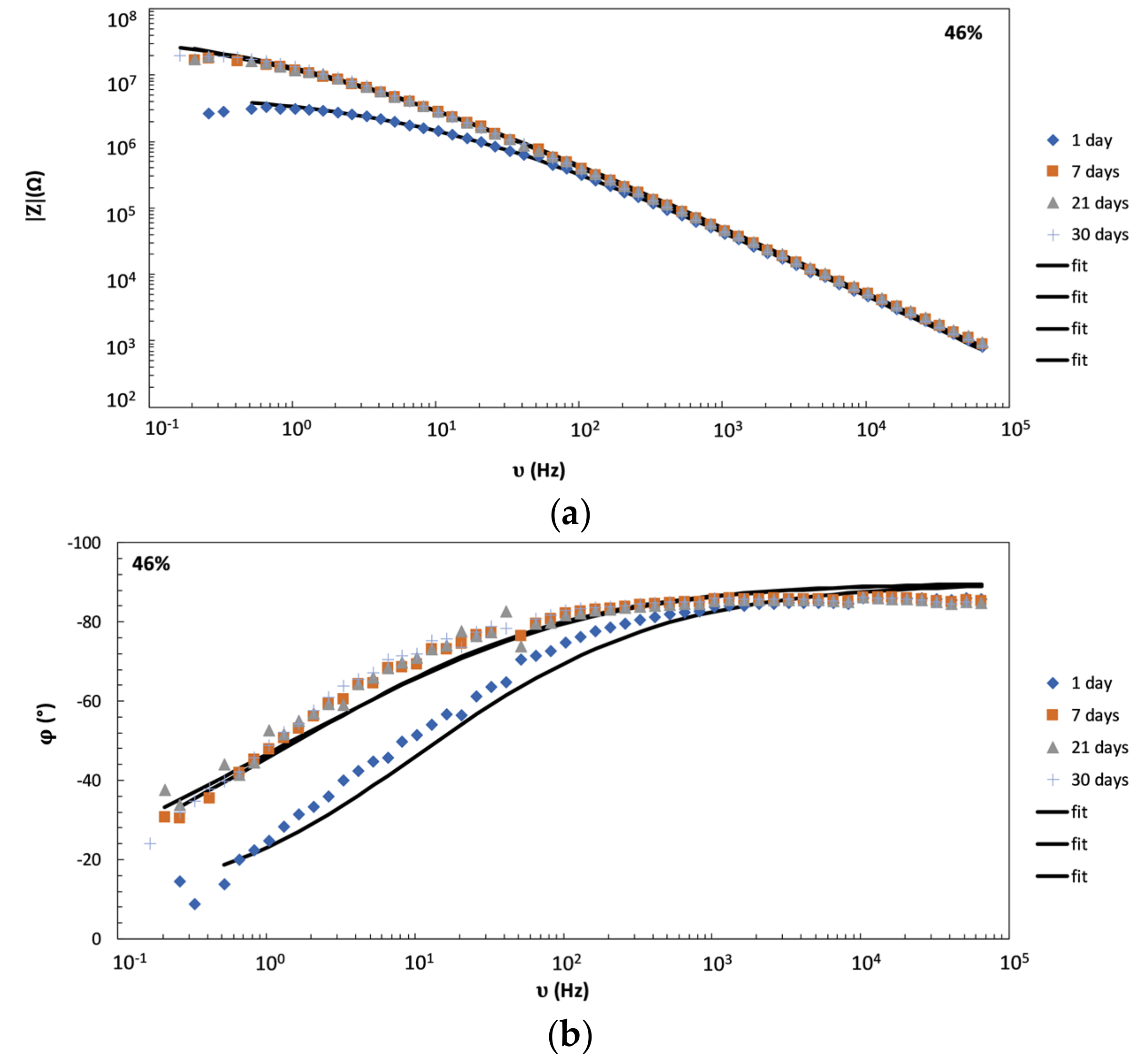
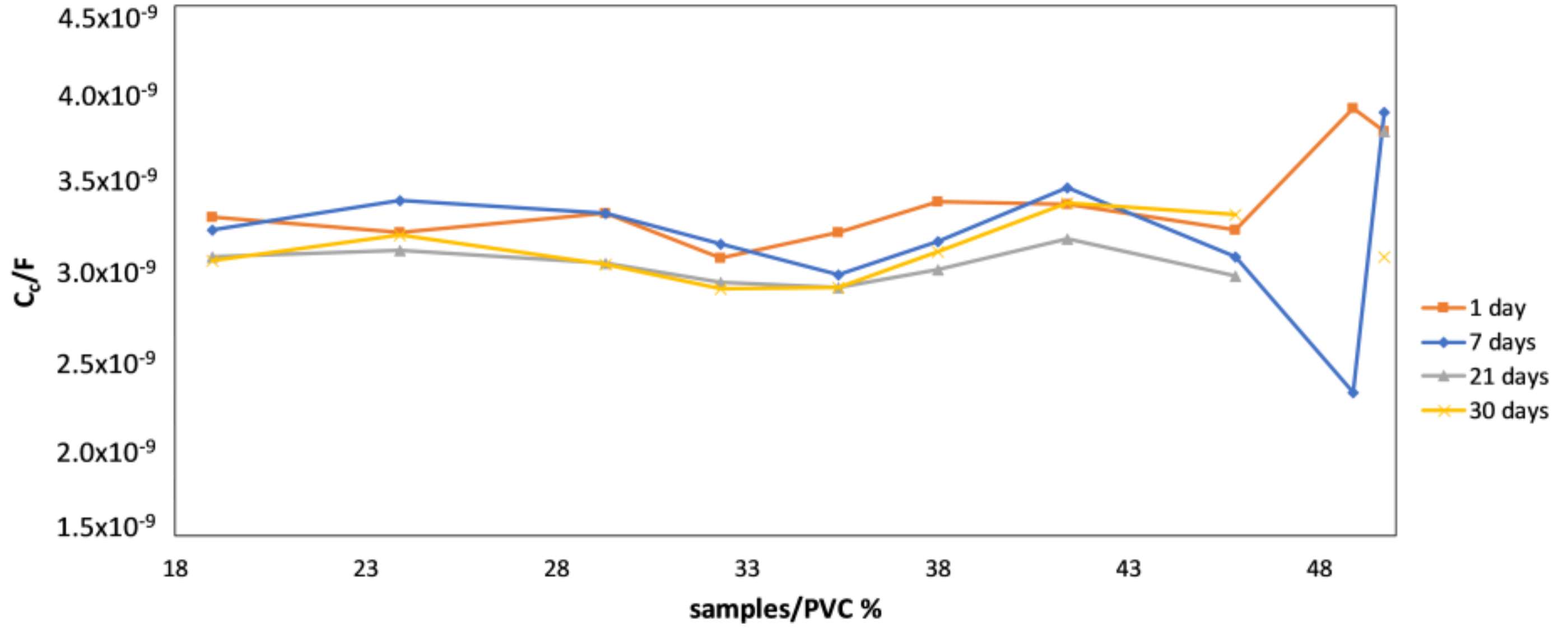
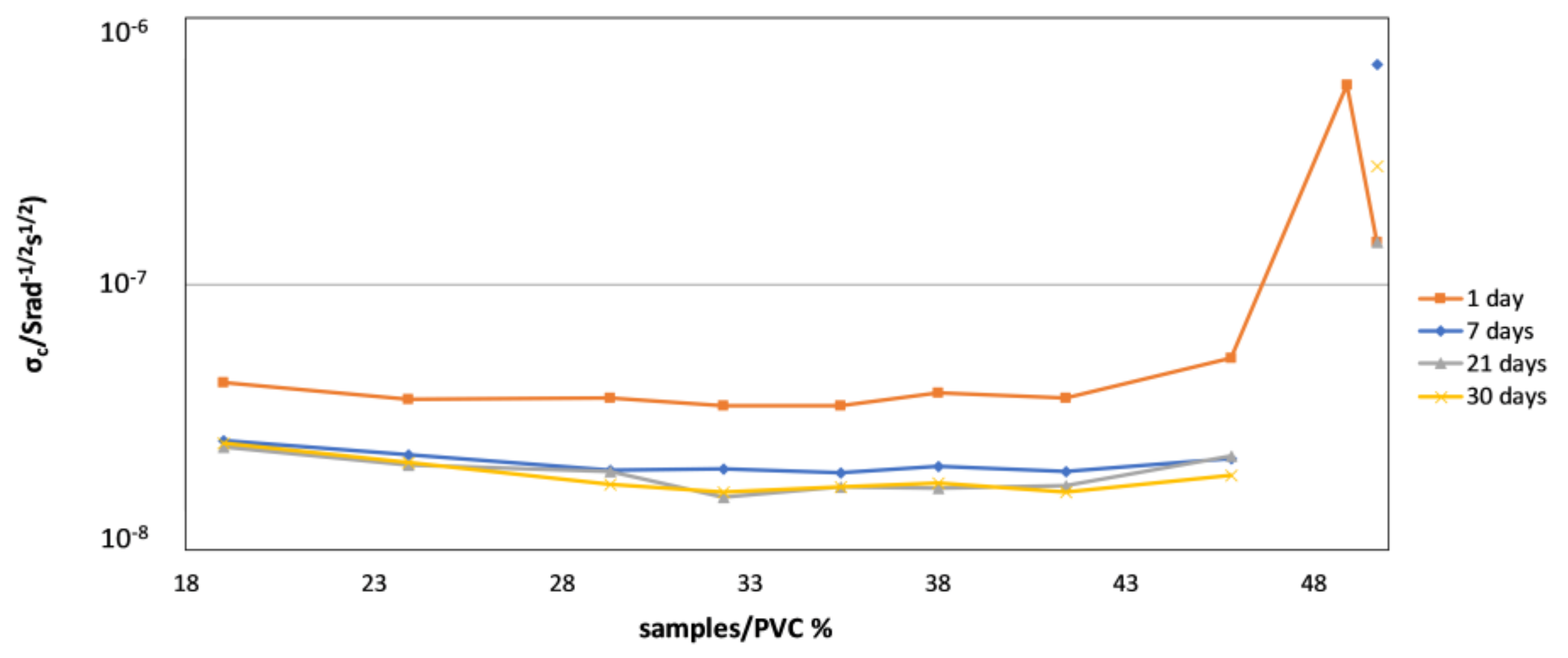
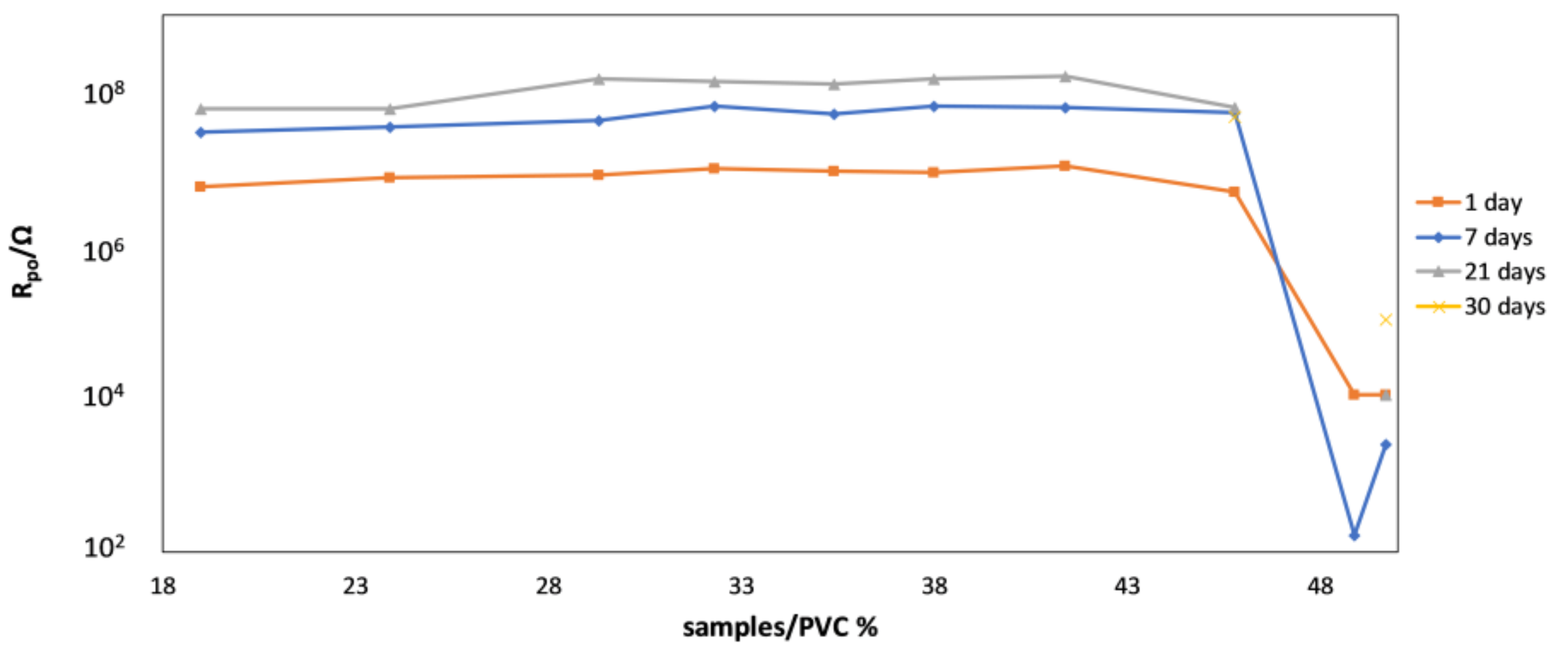
4.2. Monitoring of the Coating Film Formation by EIS Measurements (Versus PVC)
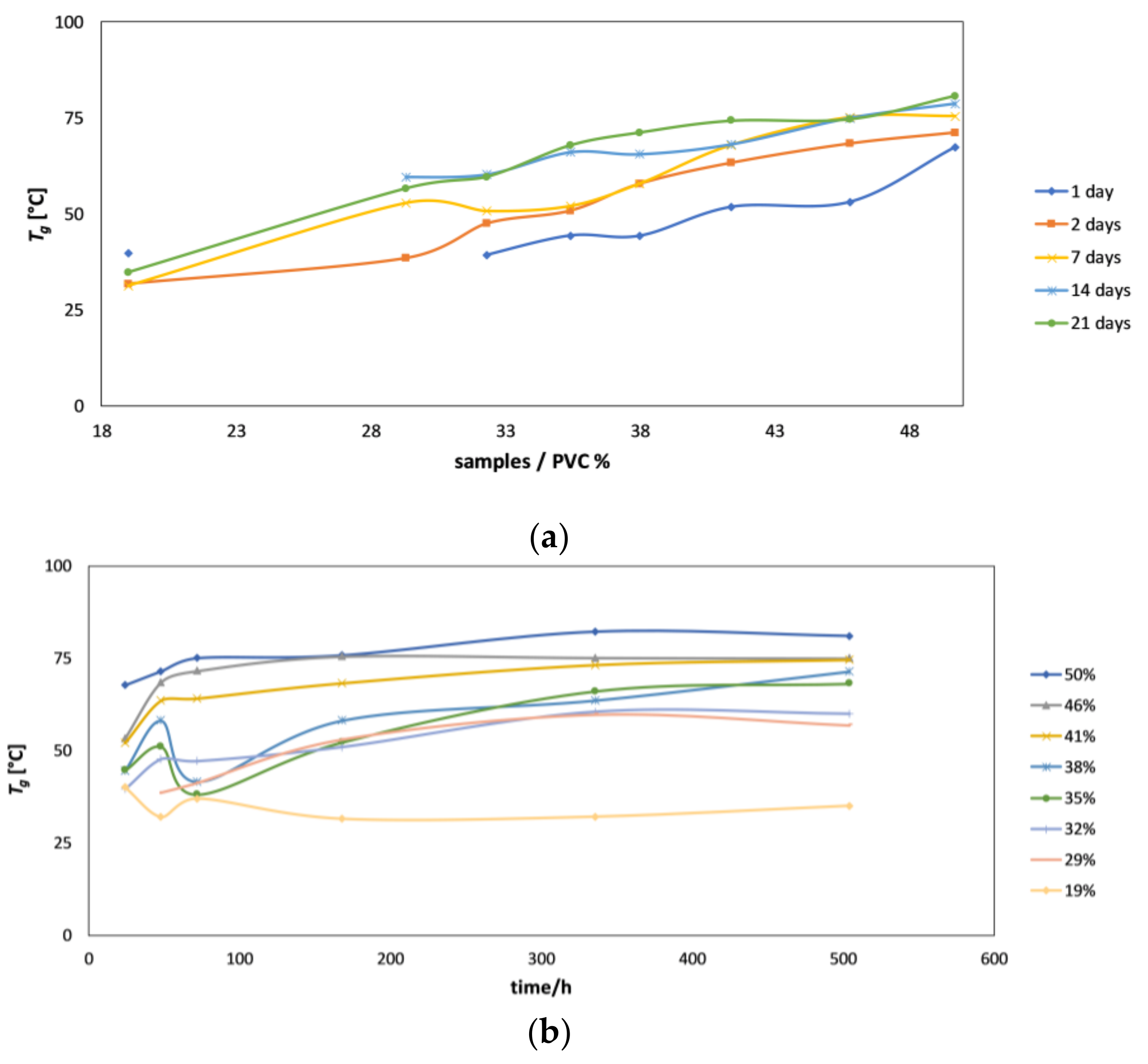
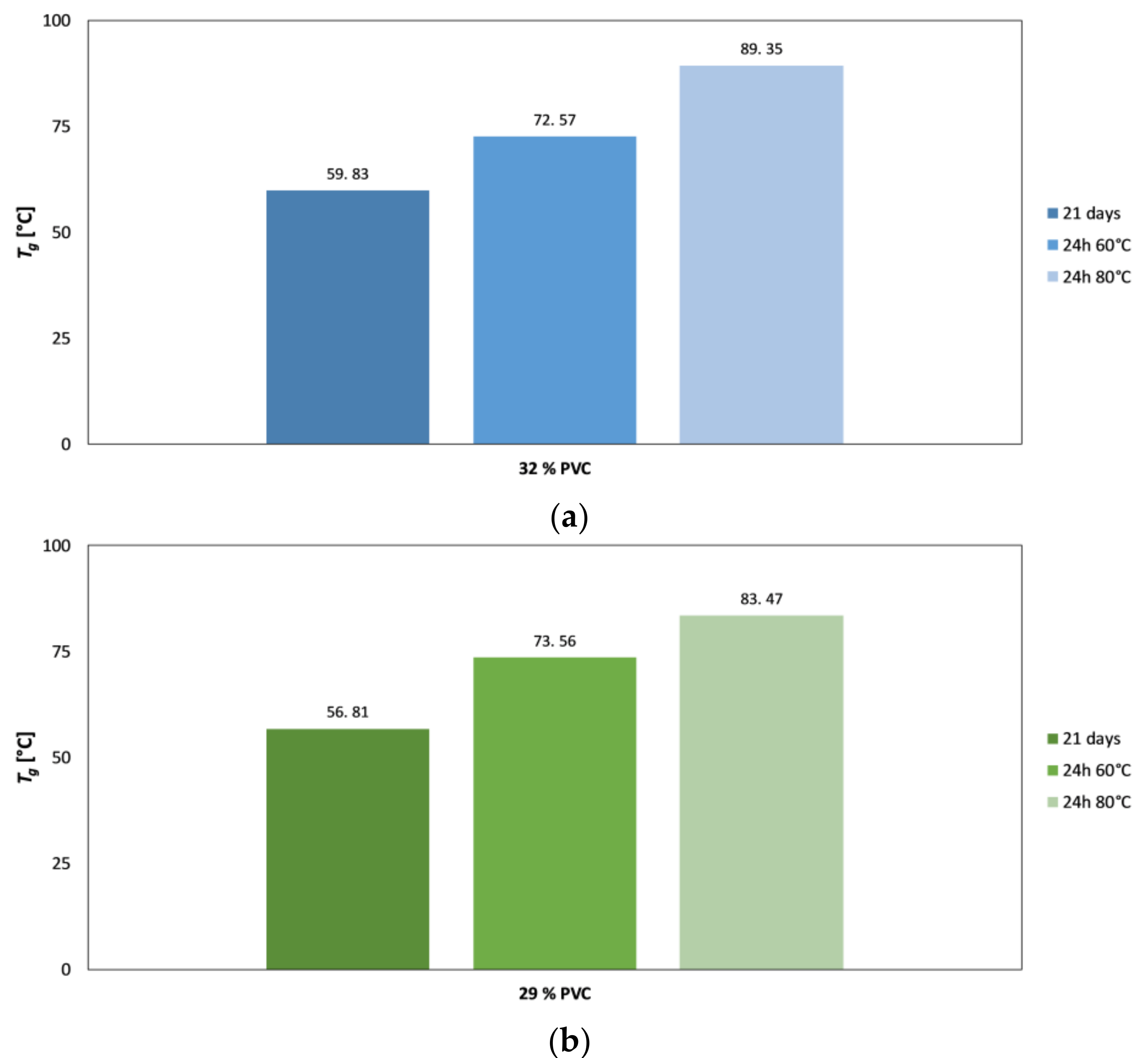
4.3. DMTA as a Supporting Technique for Explanation of EIS Results
5. Conclusions
- We showed that the magnitude (modulus) of impedance at frequencies below circa 100 Hz changes dramatically (for several orders of magnitude) when the pigment volume concentration (PVC) is increased from slightly below to slightly above its critical value (CPVC). The reason is the transformation of coating from a continuous to a discontinuous layer that greatly enhances the penetration of electrolyte to the metallic substrate. Thus, EIS seems a very appropriate technique for accurate detection of practical CPVC value of a given coating, a crucial parameter related to coating protection efficiency.
- Due to reasons similar to those stated above, impedance spectroscopy was also found to be a suitable technique for monitoring the coating film formation in samples with different PVC values. It was shown that—throughout the entire time of film formation—the most indicative impedance parameters of conventional corrosion equivalent circuits (pore resistance, coating capacity, Warburg coefficient) have distinctly different values below and above CPVC. Additionally, temporal evolution of impedance parameters was shown to be different in coatings with PVC above and below the critical value.
- Additional insight into the differences in film formation mechanisms of coatings with different PVC values was obtained using dynamic mechanical thermal analysis (DTMA). The effects of PVC on water evaporation and polymer cross-linking process during film formation were discussed in considerable detail. The effect of curing temperature on these processes was elucidated as well.
- The results indicate that, in order to further increase the coating protection of waterborne epoxy coatings, one needs to find the best compromise between the so-called “barrier effect” and the degree of epoxy-amino cross-linking. Both are decisively affected by the PVC level, however, in different ways. In order to achieve a fine tuning of PVC near the CVPC value, one might want to exploit the very high sensitivity of impedance spectroscopy, as demonstrated in this paper.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wicks, Z.W.; Jones, F.N.; Pappas, S.P. Organic Coatings: Science and Technology, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2007; pp. 466–468. [Google Scholar]
- Perera, D.Y. Effect of pigmentation on organic coating characteristics. Prog. Org. Coat. 2004, 50, 247–262. [Google Scholar] [CrossRef]
- Lobnig, R.E.; Villalba, W.; Goll, K.; Vogelsang, J.; Winkels, I.; Schmidt, R.; Zanger, P.; Soetemann, J. Development of a new experimental method to determine critical pigment–volume–concentrations using impedance spectroscopy. Prog. Org. Coat. 2006, 55, 363–374. [Google Scholar] [CrossRef]
- Lobnig, R.E.; Bonitz, V.; Goll, K.; Sinlge, M.; Villalba, W.; Vogelsang, J.; Winkels, I.; Schmidt, R.; Zanger, P. Development of a new experimental method to determine critical pigment-volume-concentrations using impedance spectroscopy: Part II: Solvent based coatings with components typical for commercial organic anticorrosion coatings or with nanoparticles. Prog. Org. Coat. 2007, 60, 1–10. [Google Scholar] [CrossRef]
- Lobnig, R.E.; Bonitz, V.; Goll, K.; Villalba, W.; Schmidt, R.; Zanger, P.; Vogelsang, J.; Winkels, I. Development of a new experimental method to determine critical pigment-volume-concentrations using impedance spectroscopy: Part III: Water-based coatings with components typical for commercial organic anticorrosion coatings. Prog. Org. Coat. 2007, 60, 77–89. [Google Scholar] [CrossRef]
- Le Pen, C.; Lacabanne, C.; Pebere, N. Structure of waterborne coatings by electrochemical impedance spectroscopy and a thermostimulated current method: influence of fillers. Prog. Org. Coat. 2000, 39, 167–175. [Google Scholar] [CrossRef]
- Rodriguez, M.T.; Gracenea, J.J.; Saura, J.J.; Suay, J.J. The influence of the critical pigment volume concentration (CPVC) on the properties of an epoxy coating: Part II. Anticorrosion and economic properties. Prog. Org. Coat. 2004, 50, 68–74. [Google Scholar] [CrossRef]
- Del Grosso Destreri, M.; Vogelsang, J.; Fedrizzi, L.; Deflorian, F. Water up-take evaluation of new waterborne and high solid epoxy coatings. Part II: Electrochemical impedance spectroscopy. Prog. Org. Coat. 1999, 37, 69–81. [Google Scholar] [CrossRef]
- Denton, D.D.; Day, D.R.; Priore, D.F.; Senuria, S.D. Moisture diffusion in poltimide films in integrated circuits. J. Electron. Mater. 1985, 14, 119–136. [Google Scholar] [CrossRef]
- Downey, S.J.; Devereux, O.F. The Use of impedance spectroscopy in evaluating moisture-caused failure of adhesives and paints. Corrosion 1989, 45, 675–684. [Google Scholar] [CrossRef]
- Pebere, N.; Picaud, T.; Duprat, M.; Dabosi, F. Evaluation of corrosion performance of coated steel by the impedance technique. Corros. Sci. 1989, 29, 1073–1086. [Google Scholar] [CrossRef]
- Bellucci, F.; Nicodemo, L. Water transport in organic coatings. Corrosion 1993, 49, 235–247. [Google Scholar] [CrossRef]
- Wind, M.M.; Lenderink, H.J.W. A capacitance study of pseudo-fickian diffusion in glassy polymer coatings. Prog. Org. Coat. 1996, 28, 239–250. [Google Scholar] [CrossRef]
- Perez, C.; Collazo, A.; Izquierdo, M.; Merino, P.; Novoa, X.R. Characterisation of the barrier properties of different paint systems: Part II. Non-ideal diffusion and water uptake kinetics. Prog. Org. Coat. 1999, 37, 169–177. [Google Scholar] [CrossRef]
- Shiller, C.A.; Strunz, W. The evaluation of experimental dielectric data of barrier coatings by means of different models. Electrochim. Acta 2001, 46, 3619–3625. [Google Scholar] [CrossRef]
- Skerry, B.S.; Chen, C.T.; Ray, C.J. Pigment volume concentration and its effect on the corrosion resistance properties of organic paint films. J. Coat. Technol. 1992, 64, 77–86. [Google Scholar]
- Gowri, S.; Balakrishnank, K. The effect of the PVC/CPVC ratio on the corrosion resistance properties of organic coatings. Prog. Org. Coat. 1994, 23, 363–377. [Google Scholar] [CrossRef]
- Gervasi, C.A.; DiSarli, A.R.; Cavalcanti, E.; Ferraz, O.; Bucharsky, E.C.; Real, S.G.; Vilche, J.R. The corrosion protection of steel in sea water using zinc-rich alkyd paints. An assessment of the pigment-content effect by EIS. Corros. Sci. 1994, 36, 1963–1972. [Google Scholar] [CrossRef]
- Shao, J.; Wan, P.; Liu, X.; Lu, H.; Zhang, S. The effect of Pigment volume concentration on corrosion protection of new sacrificial coating. Mater. Corros. 1995, 46, 33–38. [Google Scholar] [CrossRef]
- Barbbucci, A.; Delucchia, M.; Goretta, L.; Cerisola, G. Electrochemical and physico-chemical characterization of fluorinated organic coatings for steel and concrete protection: Influence of the pigment volume concentration. Prog. Org. Coat. 1998, 33, 139–148. [Google Scholar] [CrossRef]
- Hernandez, L.S.; Garcia, G.; Lopez, C.; delAmo, B.; Romagnoli, R. Evaluation, using EIS, of anticorrosive paints pigmented with zinc phosphate. Surf. Coat. Int. 1998, 81, 19–25. [Google Scholar]
- Liu, B.; Li, Y.; Lin, H.; Cao, C. Effect of PVC on the diffusion behaviour of water through alkyd coatings. Corros. Sci. 2002, 44, 2657–2664. [Google Scholar] [CrossRef]
- Berce, P.; Skale, S.; Slemnik, M. Electrochemical impedance spectroscopy study of waterborne coatings film formation. Prog. Org. Coat. 2015, 82, 1–6. [Google Scholar] [CrossRef]
- Bianchini, G.; Fream, A.; McOwan, S.P.; Roystron, I. Waterborne and Solvent Based Surface Coatings and Their Applications, 2nd ed.; John Wiley & Sons Ltd: London, US, 1996. [Google Scholar]
- Pruskowski, S.J. Coating Fundamentals: Waterborne Coating Technology; Federation of Societies for Coatings Technology: Washington, DC, USA, 2004. [Google Scholar]
- ISO 8501-1, Preparation of Steel Substrates before Application of Paints and Related Products—Visual Assessment of Surface Cleanliness—Part 1: Rust Grades and Preparation Grades of Uncoated Steel Substrates and of Steel Substrates after Overall Removal of Previous Coatings; ISO: Geneva, Switzerland, 2007.
- ISO 2178, Non-Magnetic Coatings on Magnetic Substrates–Measurement of Coating Thickness by the Magnetic Method; ISO: Geneva, Switzerland, 2016.
- Skale, S.; Doleček, V.; Slemnik, M. Substitution of the constant phase element by Warburg impedance for protective coatings. Corros. Sci. 2007, 49, 1045–1055. [Google Scholar] [CrossRef]
- Skale, S.; Doleček, V.; Slemnik, M. Electrochemical impedance studies of corrosion protected surfaces covered by epoxy polyamide coating systems. Pro. Org. Coat. 2008, 62, 387–392. [Google Scholar] [CrossRef]
- Murray, J.N. Electrochemical test methods for evaluating organic coatings on metals: an update. Part III: Multiple test parameter measurements. Prog. Org. Coat. 1997, 31, 375–391. [Google Scholar] [CrossRef]
- Kendig, M.; Scully, J.R. Basic aspects of electrochemical impedance application for the life prediction of organic coatings on metals. Corrosion 1990, 46, 22–29. [Google Scholar] [CrossRef]
- Castela, A.S.; Simões, A.M. An impedance model for the estimation of water absorption in organic coatings. Part I: A linear dielectric mixture equation. Corros. Sci. 2003, 45, 1631–1646. [Google Scholar] [CrossRef]
| Sample PVC [%] (Theoretical) | Pigment:Binder (p/b) Ratio | Λ (PVC/CPVC) (Theoretical) | Λ (PVC/CPVC) (EIS Detection) |
|---|---|---|---|
| 50 | 2.65 | 1.12 | – |
| 49 | 2.57 | 1.09 | – |
| 46 | 2.26 | 1.03 | ≥1 |
| 41 | 1.88 | 0.92 | <1 |
| 38 | 1.62 | 0.85 | – |
| 35 | 1.45 | 0.78 | – |
| 32 | 1.26 | 0.72 | – |
| 29 | 1.09 | 0.65 | – |
| 24 | 0.82 | 0.54 | – |
| 19 | 0.61 | 0.42 | – |
| Sample PVC [%] | p/b Ratio | Dry Film Thickness on Steel Substrates (µm) after 1 Day of Coating Application | Standard Deviation of Dry Film Thickness (µm) |
|---|---|---|---|
| 50 | 2.65 | 63.7 | ±8.8 |
| 49 | 2.57 | 72.3 | ±5.2 |
| 46 | 2.26 | 55.8 | ±1.9 |
| 41 | 1.88 | 69.1 | ±2.2 |
| 38 | 1.62 | 73.7 | ±1.2 |
| 35 | 1.45 | 75.6 | ±1.5 |
| 32 | 1.26 | 72.2 | ±1.7 |
| 29 | 1.09 | 69.5 | ±1.1 |
| 24 | 0.82 | 68.7 | ±0.8 |
| 19 | 0.61 | 70.2 | ±1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Končan Volmajer, N.; Steinbücher, M.; Berce, P.; Venturini, P.; Gaberšček, M. Electrochemical Impedance Spectroscopy Study of Waterborne Epoxy Coating Film Formation. Coatings 2019, 9, 254. https://doi.org/10.3390/coatings9040254
Končan Volmajer N, Steinbücher M, Berce P, Venturini P, Gaberšček M. Electrochemical Impedance Spectroscopy Study of Waterborne Epoxy Coating Film Formation. Coatings. 2019; 9(4):254. https://doi.org/10.3390/coatings9040254
Chicago/Turabian StyleKončan Volmajer, Natalija, Miha Steinbücher, Peter Berce, Peter Venturini, and Miran Gaberšček. 2019. "Electrochemical Impedance Spectroscopy Study of Waterborne Epoxy Coating Film Formation" Coatings 9, no. 4: 254. https://doi.org/10.3390/coatings9040254
APA StyleKončan Volmajer, N., Steinbücher, M., Berce, P., Venturini, P., & Gaberšček, M. (2019). Electrochemical Impedance Spectroscopy Study of Waterborne Epoxy Coating Film Formation. Coatings, 9(4), 254. https://doi.org/10.3390/coatings9040254





