Effect of Mesoporous Silica Nanoparticles on Glycerol-Plasticized Anionic and Cationic Polysaccharide Edible Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Film Thickness and Opacity
2.4. Film Mechanical Properties
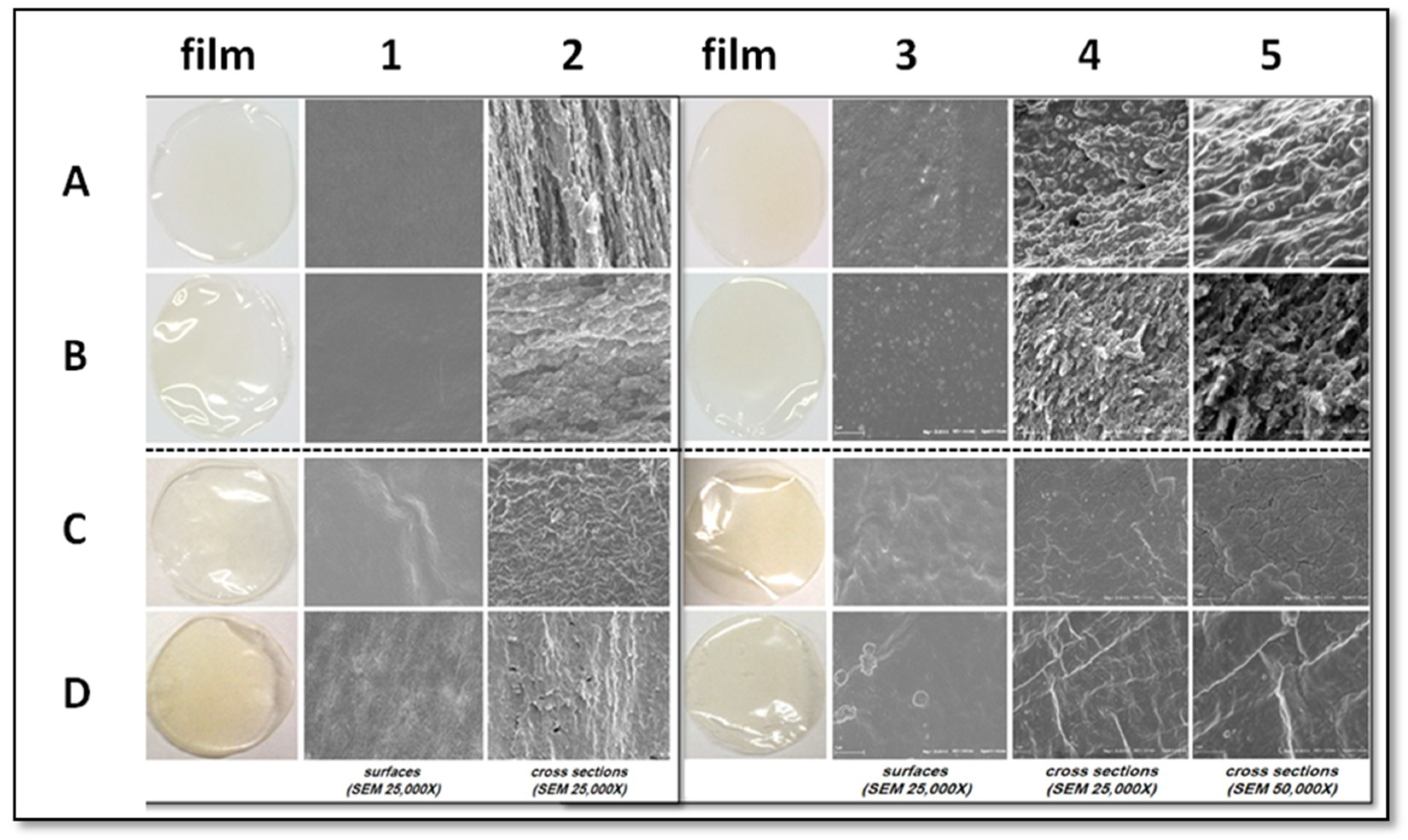
2.5. Morphology Analysis (SEM)
2.6. Fourier Transform Infrared (FT-IR) Spectra
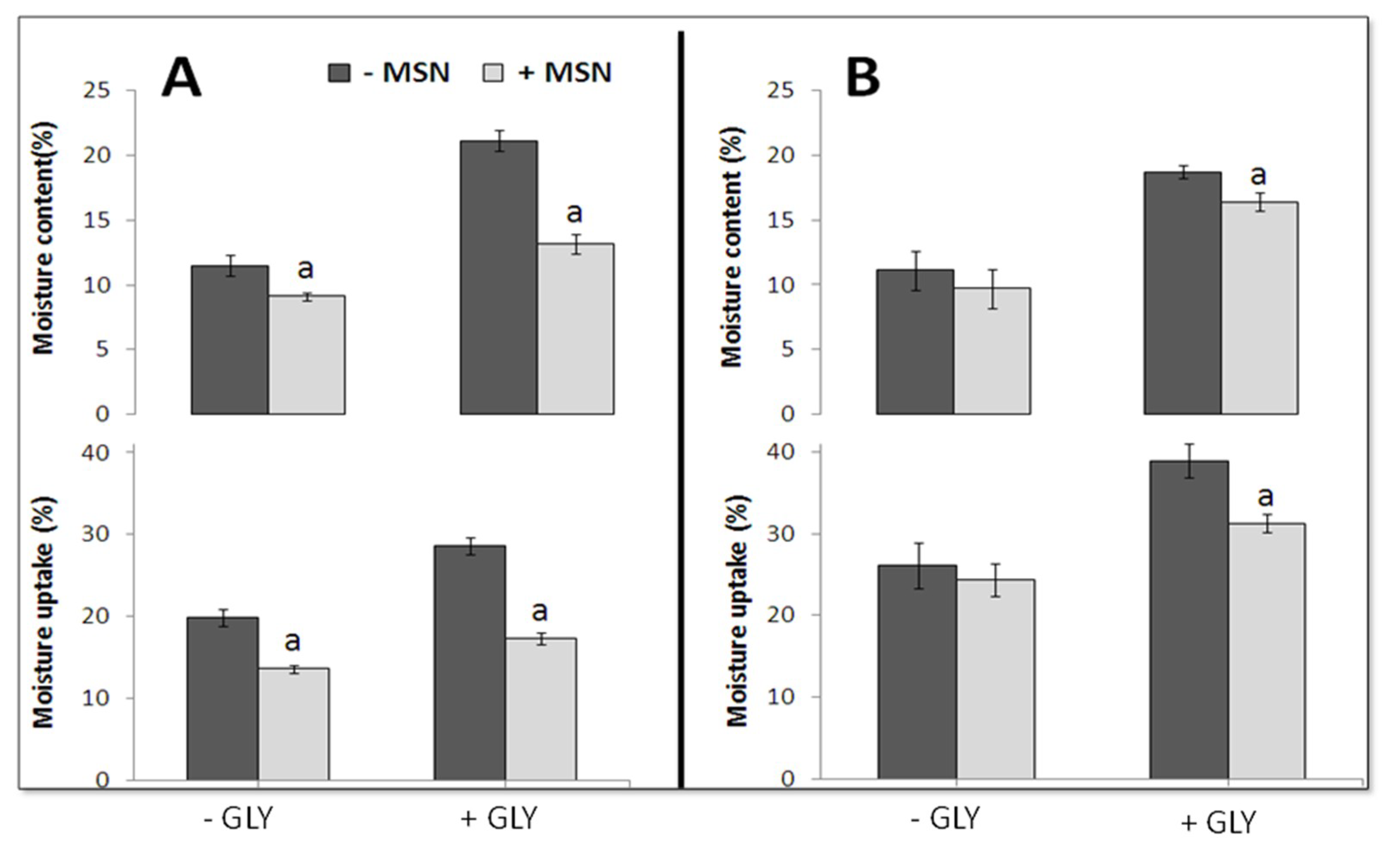
2.7. Film Moisture Content
2.8. Film Moisture Uptake
2.9. Statistical Analysis
3. Results and Discussion
3.1. Film Thickness and Opacity
3.2. Film Mechanical Properties
3.3. Film Morphology
3.4. Film FT-IR Analyses
3.5. Film Moisture Content and Uptake
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rossi Marquez, G.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT Food Sci. Technol. 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Sabbah, M.; Porta, R. Plastic pollution and the challenge of bioplastics. J. Appl. Biotechnol. Bioeng. 2017, 2, 00033. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, A.P.; Mackie, A.; Porta, R.; Mariniello, L. Trehalose-containing hydrocolloid edible films prepared in the presence of transglutaminase. Biopolymers 2014, 101, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and characterization of bioplastics from grass pea flour cast in the presence of microbial transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef]
- Krochta, J.M. Proteins as raw materials for films and coatings: Definitions, current status, and opportunities. In Protein-Based Films and Coatings, 1st ed.; Gennadios, A., Ed.; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 2002; pp. 1–41. [Google Scholar]
- Vodnar, D.C.; Pop, O.L.; Dulf, F.V.; Socaciu, C. Antimicrobial efficiency of edible films in food industry. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2015, 43, 302–312. [Google Scholar] [CrossRef]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res. Int. 2012, 49, 588–595. [Google Scholar] [CrossRef]
- Fernandez-Bats, I.; Di Pierro, P.; Villalonga-Santana, R.; Garcia-Almendarez, B.; Porta, R. Bioactive mesoporous silica nanocomposite films obtained from native and transglutaminase-crosslinked bitter vetch proteins. Food Hydrocolloid 2018, 82, 106–115. [Google Scholar] [CrossRef]
- Mangiacapra, P.; Gorras, G.; Sorrentino, A.; Vittoria, V. Biodegradable nanocomposites obtained by ball milling of pectin and montmorillonites. Carbohydr. Polym. 2006, 64, 516–523. [Google Scholar] [CrossRef]
- Mariniello, L.; Giosafatto, C.V.L.; Di Pierro, P.; Sorrentino, A.; Porta, R. Swelling, mechanical and barrier properties of albedo-based films prepared in the presence of phaseolin crosslinked or not by transglutaminase. Biomacromolecules 2010, 11, 2394–2398. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Izumi, R.; Osaki, T.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Minami, S.; Okamoto, Y. Chitin, chitosan, and its derivatives for wound healing: Old and new materials. J. Funct. Biomater. 2015, 6, 104–142. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, M.; Di Pierro, P.; Cammarota, M.; Dell’Olmo, E.; Arciello, A.; Porta, R. Development and properties of new chitosan-based films plasticized with spermidine and/or glycerol. Food Hydrocolloid 2019, 87, 245–252. [Google Scholar] [CrossRef]
- Esposito, M.; Di Pierro, P.; Regalado-Gonzales, C.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Polyamines as new cationic plasticizers for pectin-based edible films. Carbohydr. Polym. 2016, 153, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Tonyali, B.; Cikrikci, S.; Oztop, M.H. Physicochemical and microstructural characterization of gum tragacanth added whey protein based films. Food Res. Int. 2018, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-97. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM: Philadelphia, PA, USA, 1997. [Google Scholar]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocolloid 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of chitosan based edible films: Process optimization using response surface methodology. J. Food Sci. Technol. 2015, 52, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Manrich, A.; Moreira, F.K.V.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H.C. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, N.; Naal-Ek, M.G.; Ayora-Talavera, T.; Shirai, K.; Román-Guerrero, A.; Fabela-Morón, M.F.; Cuevas-Bernardino, J.C. Effect of bio-chemical chitosan and gallic acid into rheology andphysicochemical properties of ternary edible films. Int. J. Biol. Macromol. 2019, 125, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Situ, A.; Kang, Y.; Villabroza, K.R.; Liao, Y.; Chang, C.H.; Donahue, T.; Nel, A.E.; Meng, H. Irinotecan delivery by lipid-coated mesoporous silica nanoparticles shows improved efficacy and safety over liposomes for pancreatic cancer. ACS Nano 2016, 10, 2702–2715. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Zahraee, Z. Controlled release of metformin from chitosan-based nanocomposite films containing mesoporous MCM-41 nanoparticles as novel drug delivery systems. J. Colloid Interface Sci. 2017, 501, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Rangelova, N.; Aleksandrov, L.; Nenkova, S. Synthesis and characterization of pectin/SiO2 hybrid materials. J. Sol-Gel Sci. Technol. 2018, 85, 330–339. [Google Scholar] [CrossRef]
- Nesic, R.A.; Kokunesoski, J.M.; Ilic, M.S.; Gordic, V.M.; Ostojic, B.S.; Micic, M.D.; Velickovic, J.S. Biocomposite membranes of highly methylated pectin and mesoporous silica SBA-15. Compos. Part B. 2014, 64, 162–167. [Google Scholar] [CrossRef]
- Efimov, M.A.; Pogareva, G.V. IR absorption spectra of vitreous silica and silicate glasses: The nature of bands in the 1300 to 5000 cm−1 region. Chem. Geol. 2006, 229, 198–217. [Google Scholar] [CrossRef]
- Benmouhoub, N.; Simmonet, N.; Agoudjil, N.; Coradin, T. Aqueous sol-gel routes to bio-composite capsules and gels. Green Chem. 2008, 10, 957–964. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Fazli, M. Mechanical properties and antibacterial activities of novel nanobiocomposite films of chitosan and starch. Food Hydrocolloid 2015, 46, 112–124. [Google Scholar] [CrossRef]
- Vejdan, A.; Mahdi Ojagh, S.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Nasri-Nasrabadi, B.; Mehrasa, M.; Rafienia, M.; Bonakdar, S.; Behzad, T.; Gavanji, S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr. Polym. 2014, 108, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tunç, S.; Duman, O. Preparation of active antimicrobial methyl cellulose/carvacrol/montmorillonite nanocomposite films and investigation of carvacrol release. LWT Food Sci. Technol. 2011, 44, 465–472. [Google Scholar] [CrossRef]

| Film | Thickness (µm) | Opacity (mm−1) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| PEC-based | |||||
| Control | 37.5 ± 1.3 | 1.91 ± 0.16 | 33.4 ± 7.8 | 0.8 ± 0.2 | 5750.2 ± 612.2 |
| + MSN | 40.1 ± 1.2 | 3.63 ± 0.72 * | 40.8 ± 2.5 | 2.2 ± 0.7 | 3732.5 ± 264.0 * |
| + GLY | 56.8 ± 2.5 * | 2.05 ± 0.28 | 27.7 ± 4.4 | 9.4 ± 2.5 * | 1067.6 ± 49.3 * |
| + MSN + GLY | 58.2 ± 2.1 * | 1.88 ± 0.29 | 16.6 ± 2.4 * | 15.2 ± 1.4 * | 712.3 ± 52.4 * |
| CH-based | |||||
| Control | 30.1 ± 3.1 | 2.20 ± 0.28 | 56.2 ± 4.2 | 7.7 ± 1.8 | 3438.1 ± 501.3 |
| + MSN | 51.3 ± 1.2 * | 6.62 ± 1.88 * | 42.4 ± 8.3 | 13.0 ± 5.6 | 2088.8 ± 137.3 * |
| + GLY | 61.2 ± 2.3 * | 1.44 ± 0.89 | 12.5 ± 2.3 * | 82.5 ± 2.0 * | 130.5 ± 52.2 * |
| + MSN + GLY | 81.7 ± 3.7 * | 1.87 ± 0.35 | 28.1 ± 2.2 * | 54.9 ± 4.2 * | 427.3 ± 64.2 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giosafatto, C.V.L.; Sabbah, M.; Al-Asmar, A.; Esposito, M.; Sanchez, A.; Villalonga Santana, R.; Cammarota, M.; Mariniello, L.; Di Pierro, P.; Porta, R. Effect of Mesoporous Silica Nanoparticles on Glycerol-Plasticized Anionic and Cationic Polysaccharide Edible Films. Coatings 2019, 9, 172. https://doi.org/10.3390/coatings9030172
Giosafatto CVL, Sabbah M, Al-Asmar A, Esposito M, Sanchez A, Villalonga Santana R, Cammarota M, Mariniello L, Di Pierro P, Porta R. Effect of Mesoporous Silica Nanoparticles on Glycerol-Plasticized Anionic and Cationic Polysaccharide Edible Films. Coatings. 2019; 9(3):172. https://doi.org/10.3390/coatings9030172
Chicago/Turabian StyleGiosafatto, Concetta Valeria Lucia, Mohammed Sabbah, Asmaa Al-Asmar, Marilena Esposito, Alfredo Sanchez, Reynaldo Villalonga Santana, Marcella Cammarota, Loredana Mariniello, Prospero Di Pierro, and Raffaele Porta. 2019. "Effect of Mesoporous Silica Nanoparticles on Glycerol-Plasticized Anionic and Cationic Polysaccharide Edible Films" Coatings 9, no. 3: 172. https://doi.org/10.3390/coatings9030172
APA StyleGiosafatto, C. V. L., Sabbah, M., Al-Asmar, A., Esposito, M., Sanchez, A., Villalonga Santana, R., Cammarota, M., Mariniello, L., Di Pierro, P., & Porta, R. (2019). Effect of Mesoporous Silica Nanoparticles on Glycerol-Plasticized Anionic and Cationic Polysaccharide Edible Films. Coatings, 9(3), 172. https://doi.org/10.3390/coatings9030172











