Investigation on Microstructure, Hardness, and Corrosion Resistance of Mo–Ni–B Coatings Prepared by Laser Cladding Technique
Abstract
1. Introduction
2. Preparation and Characterization
2.1. Preparation of the Mo2NiB2 Cermet Coatings
2.2. Characterization of the Mo2NiB2 Cermet Coatings
3. Results
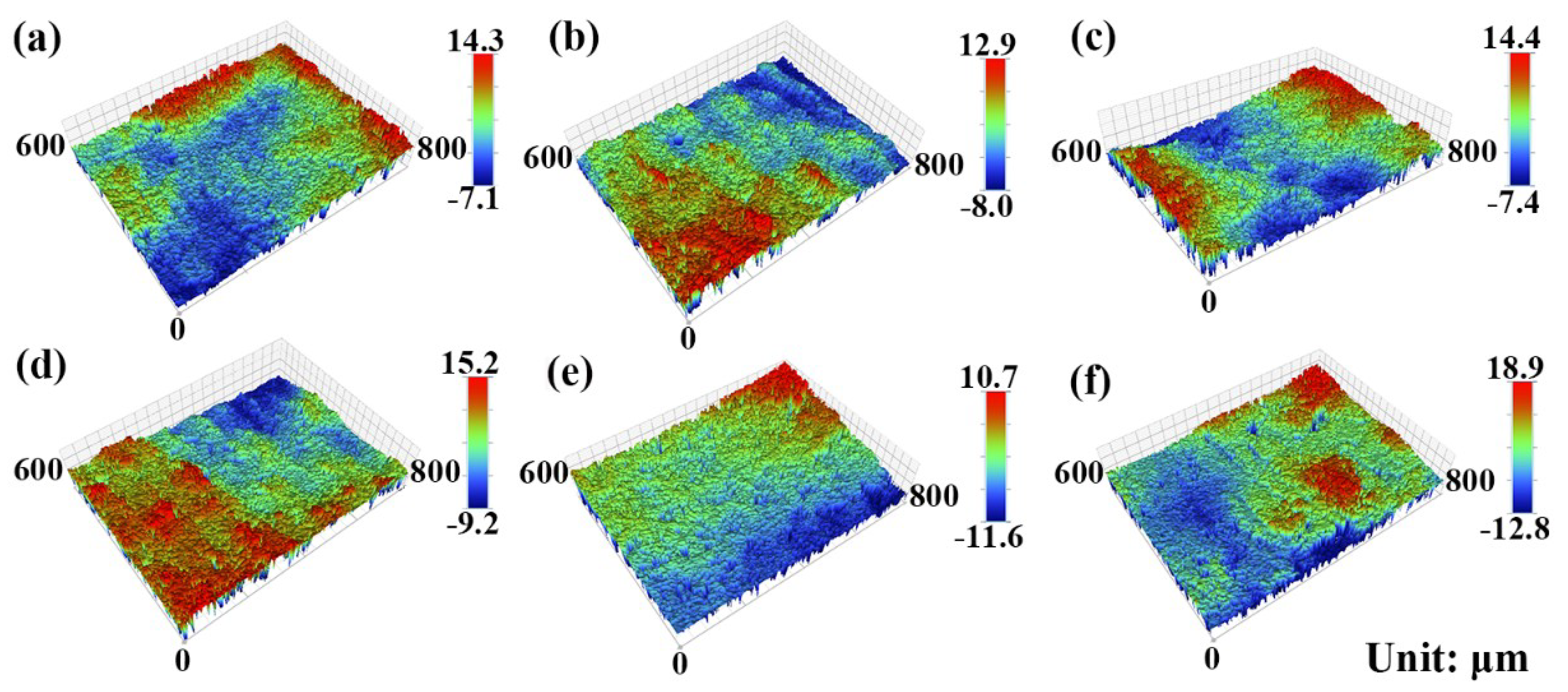
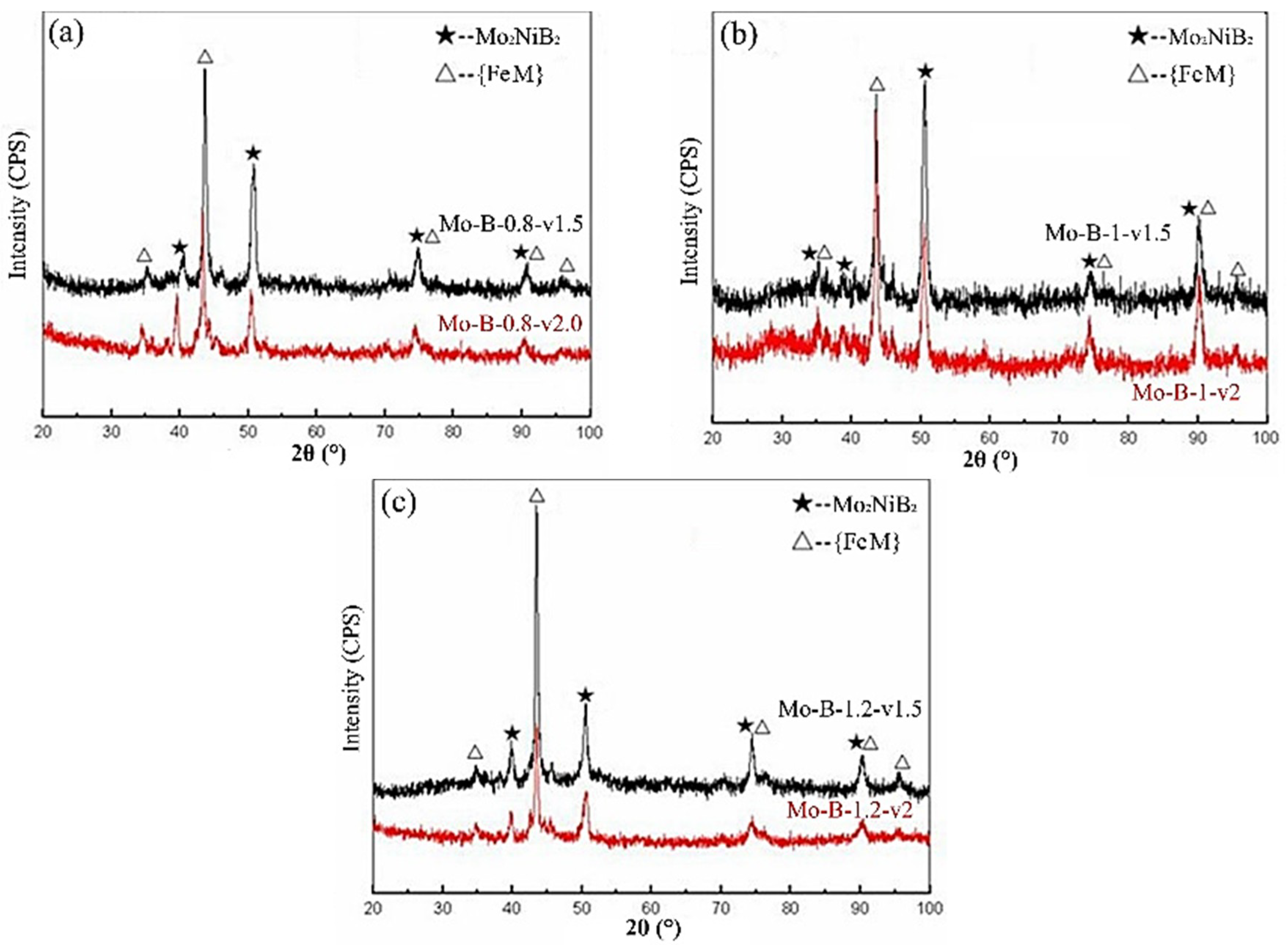
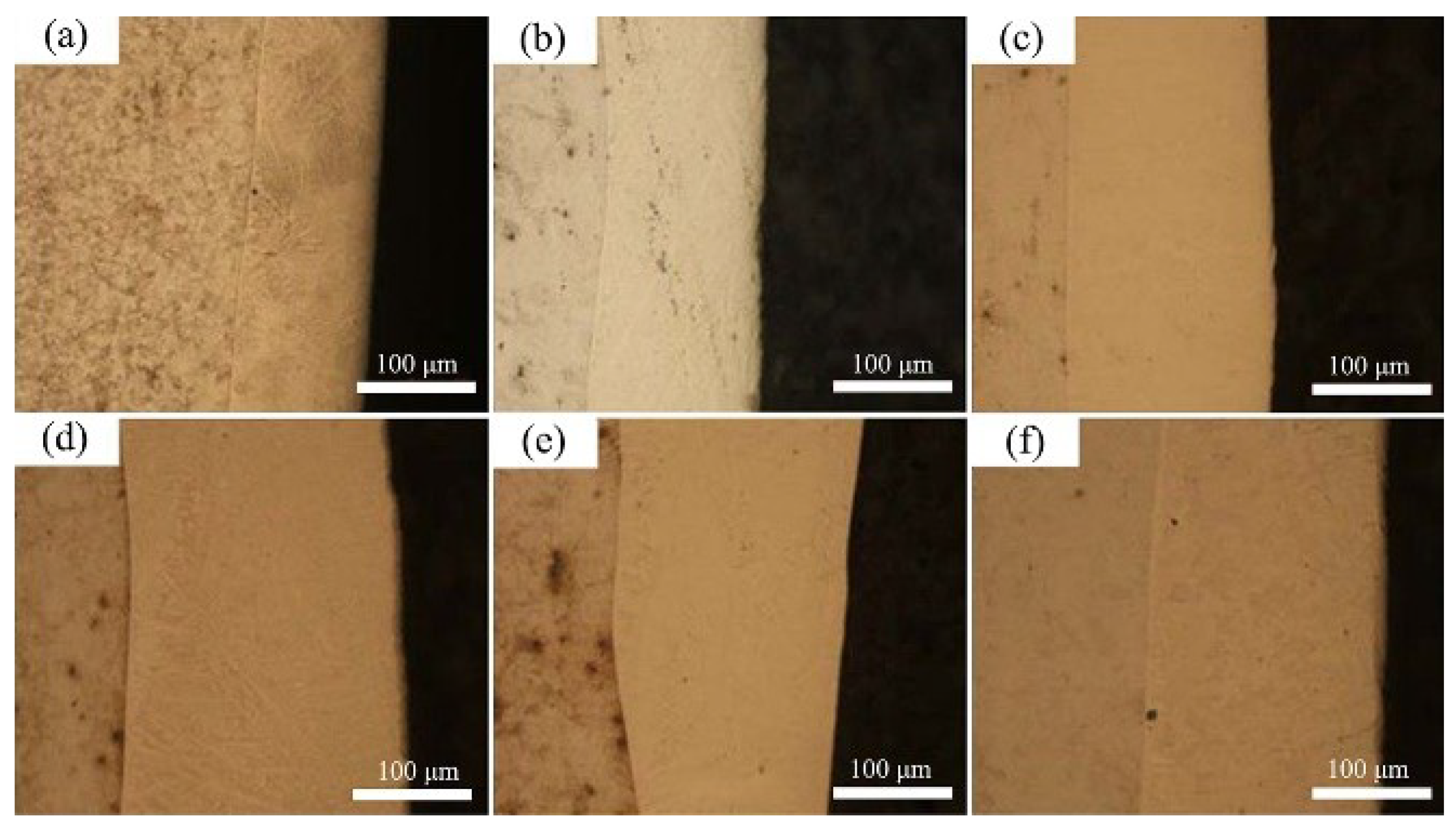
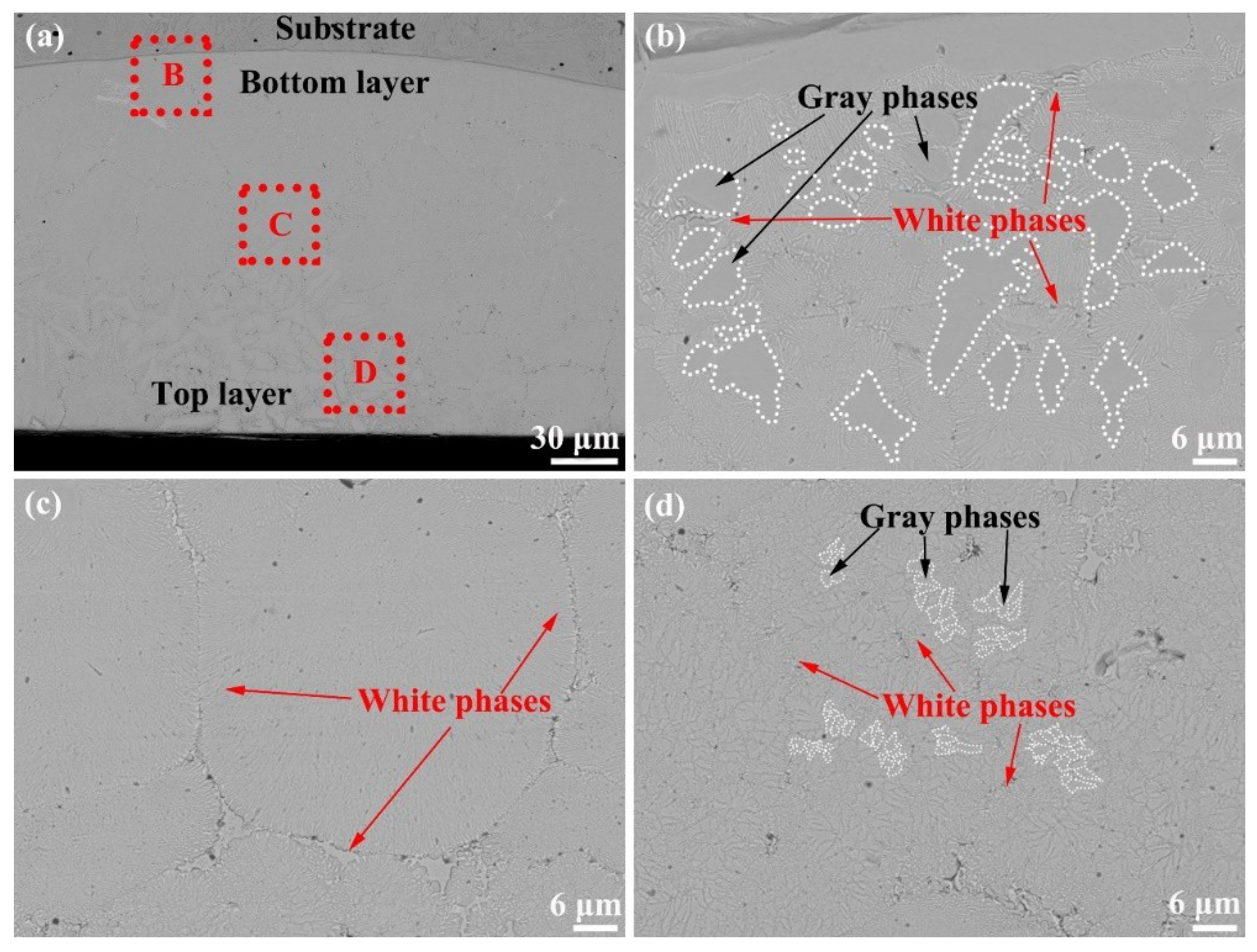
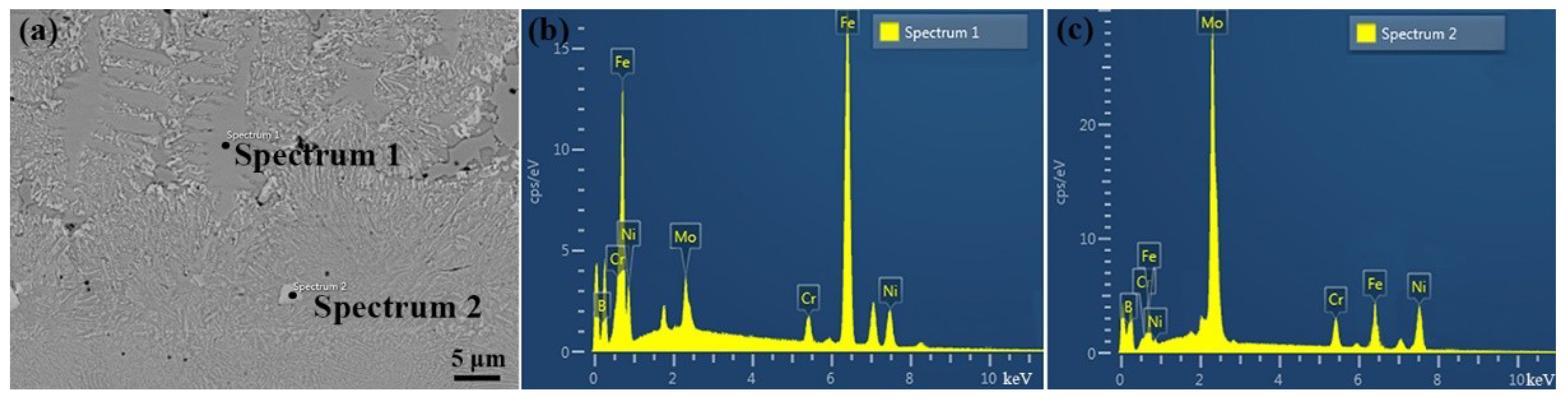
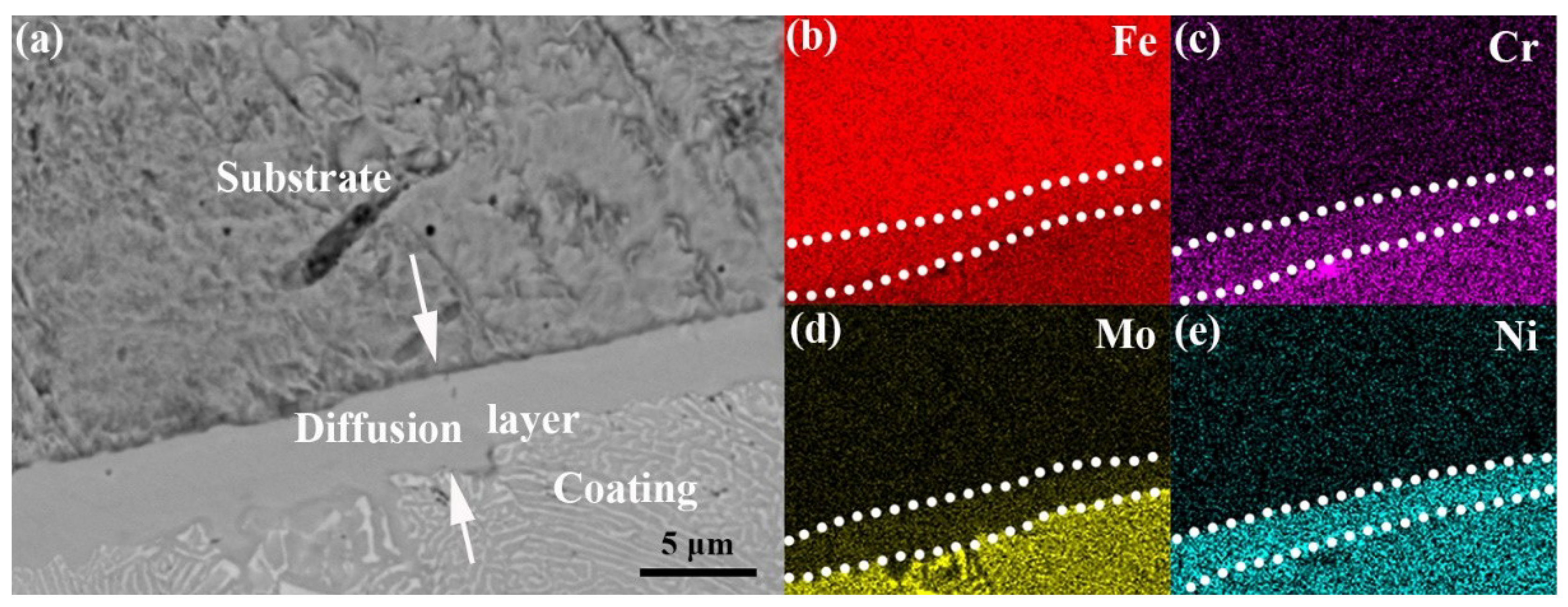
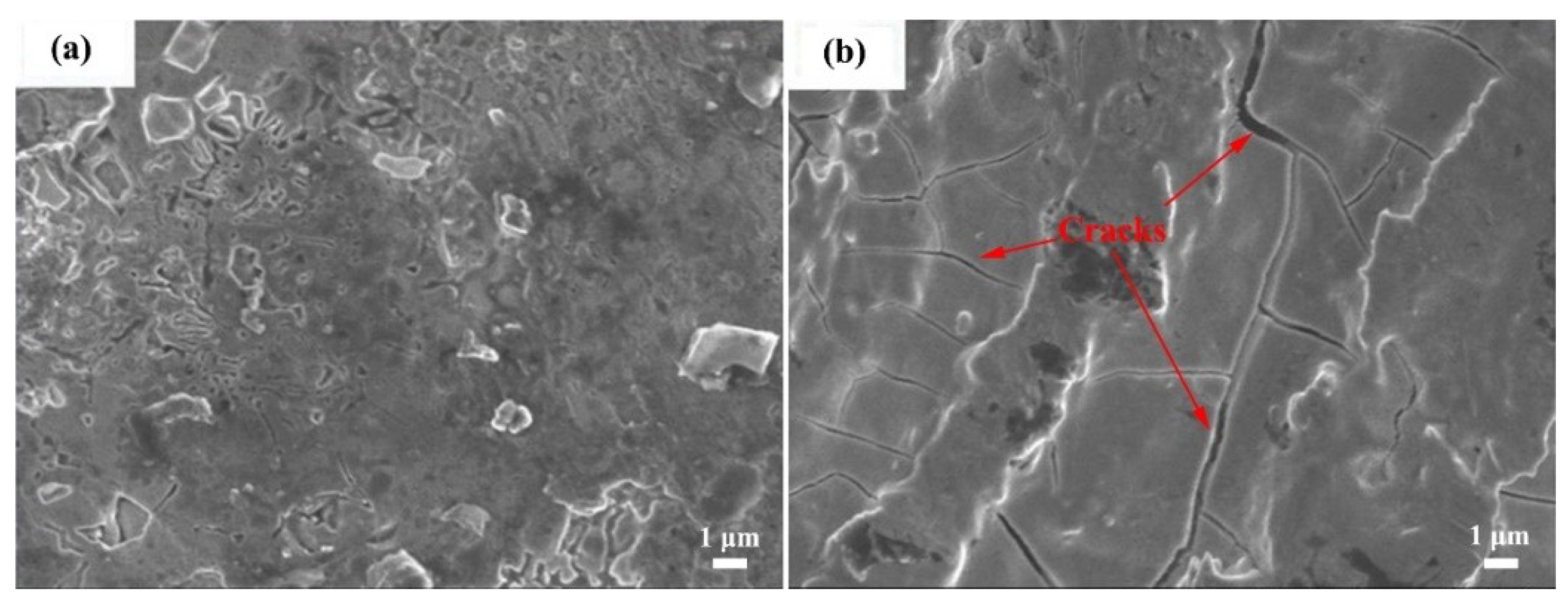
3.1. Composition and Microstructure of the Mo2NiB2 Cermet Coatings
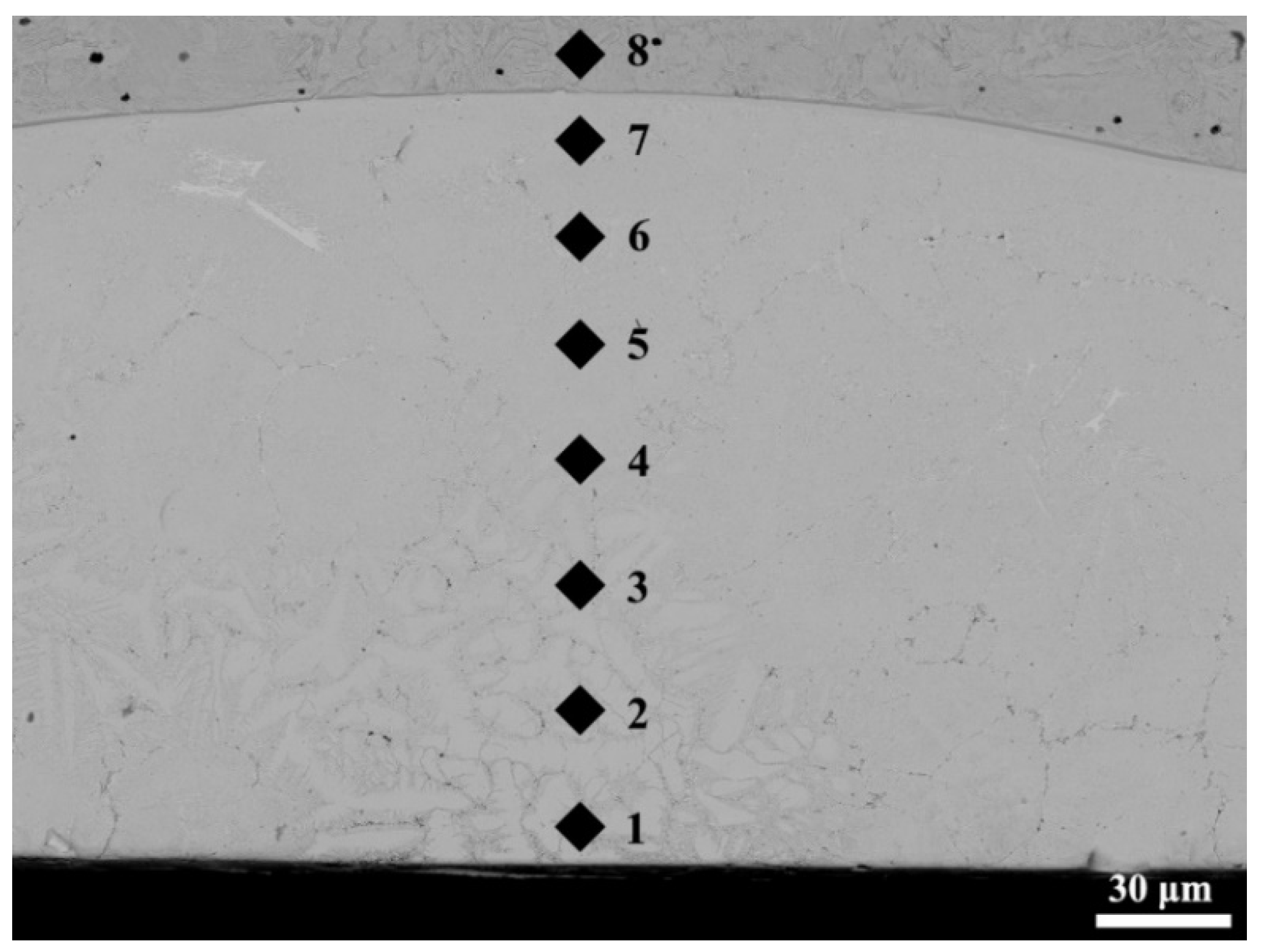
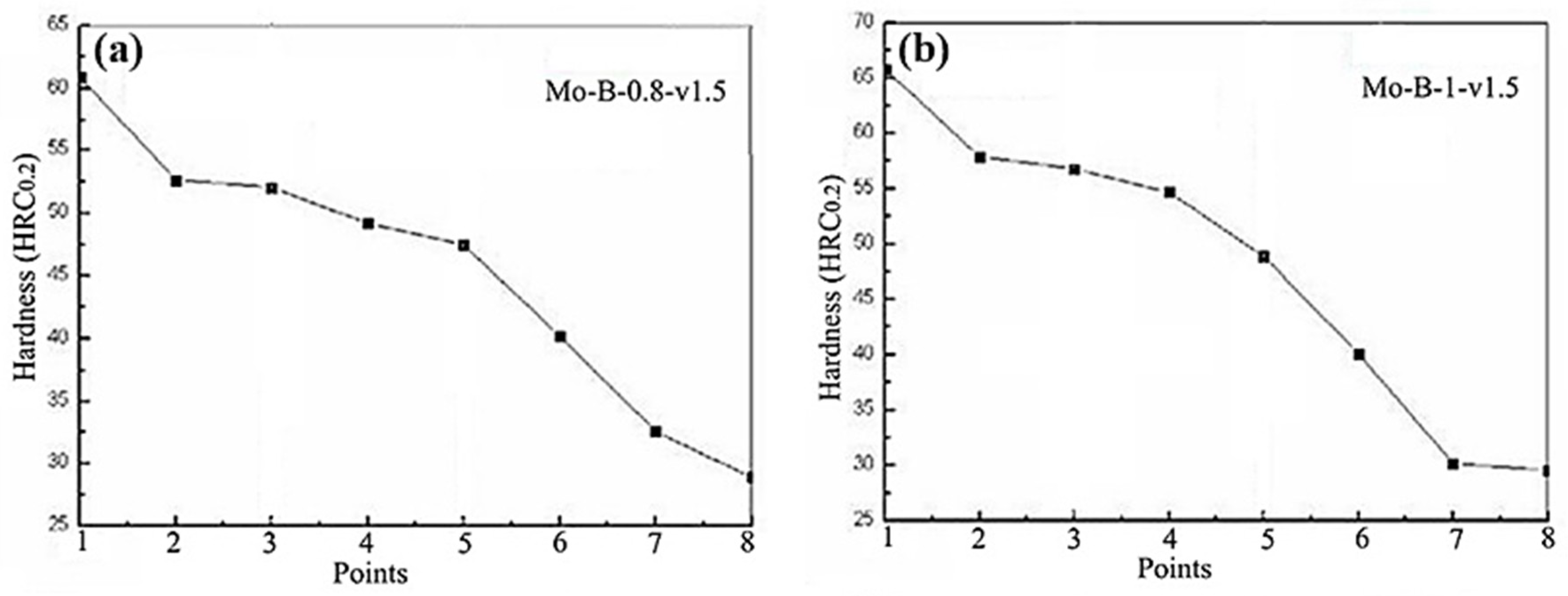
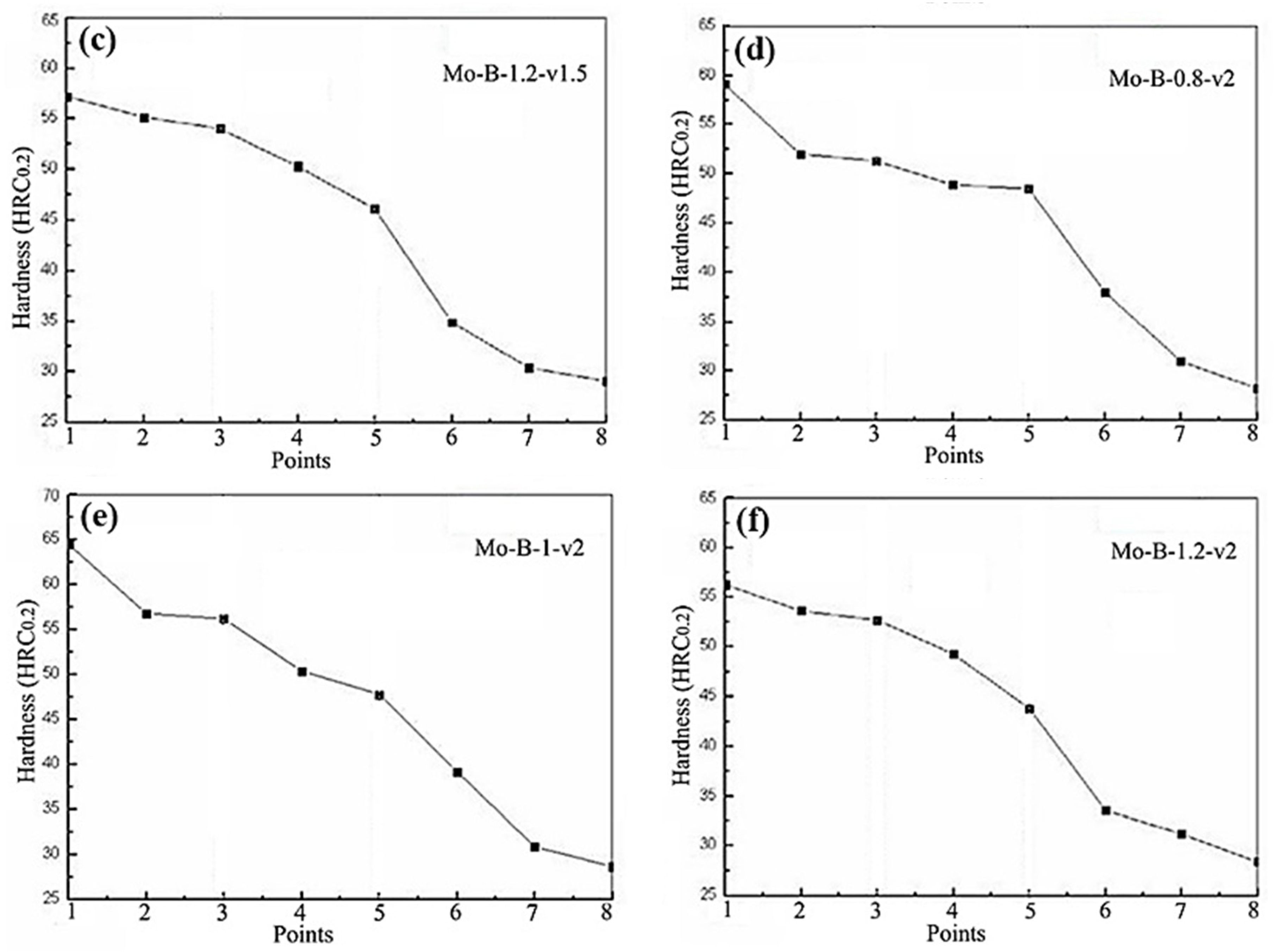
3.2. Hardness of the Mo2NiB2 Cermet Coatings
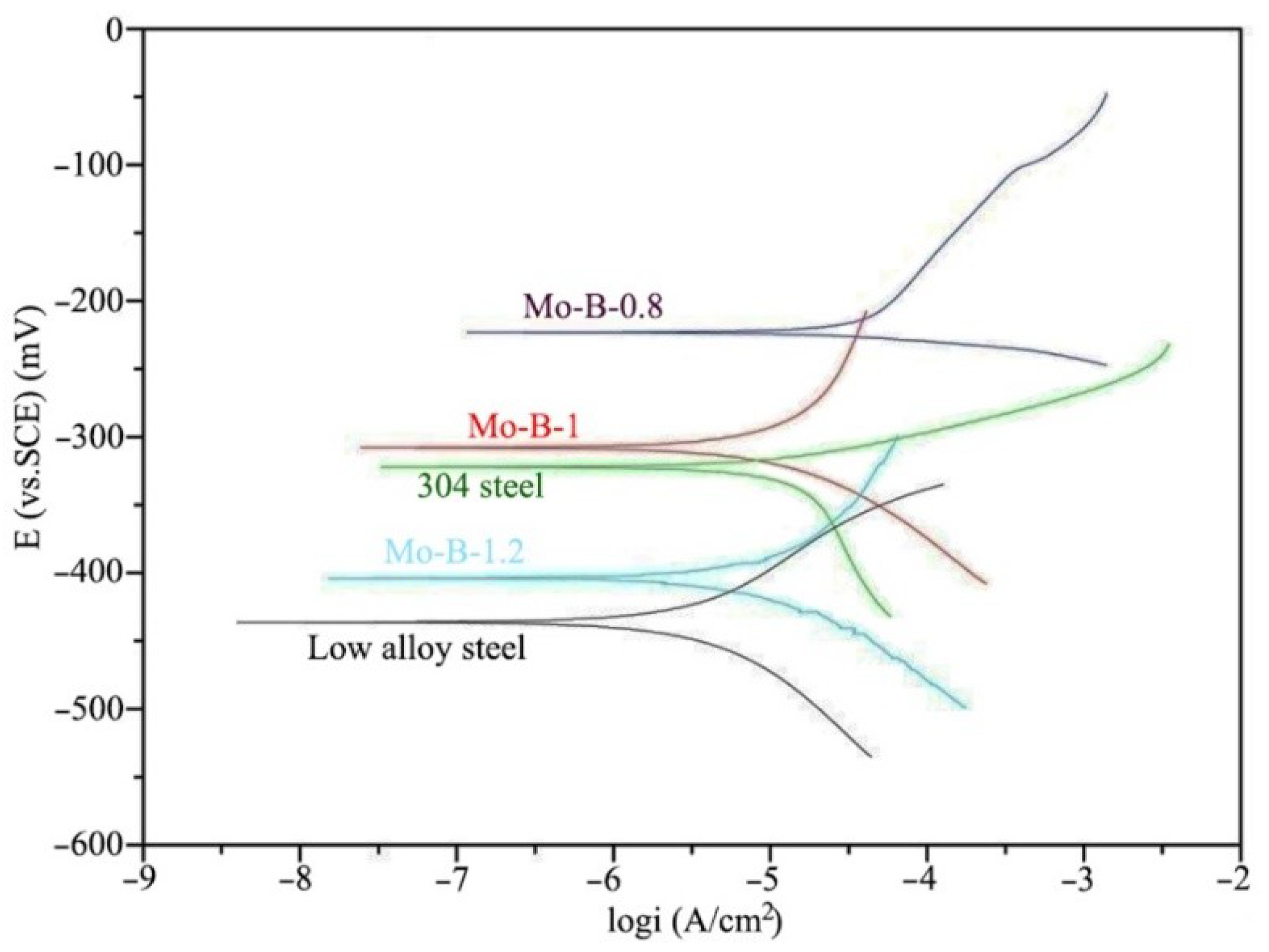
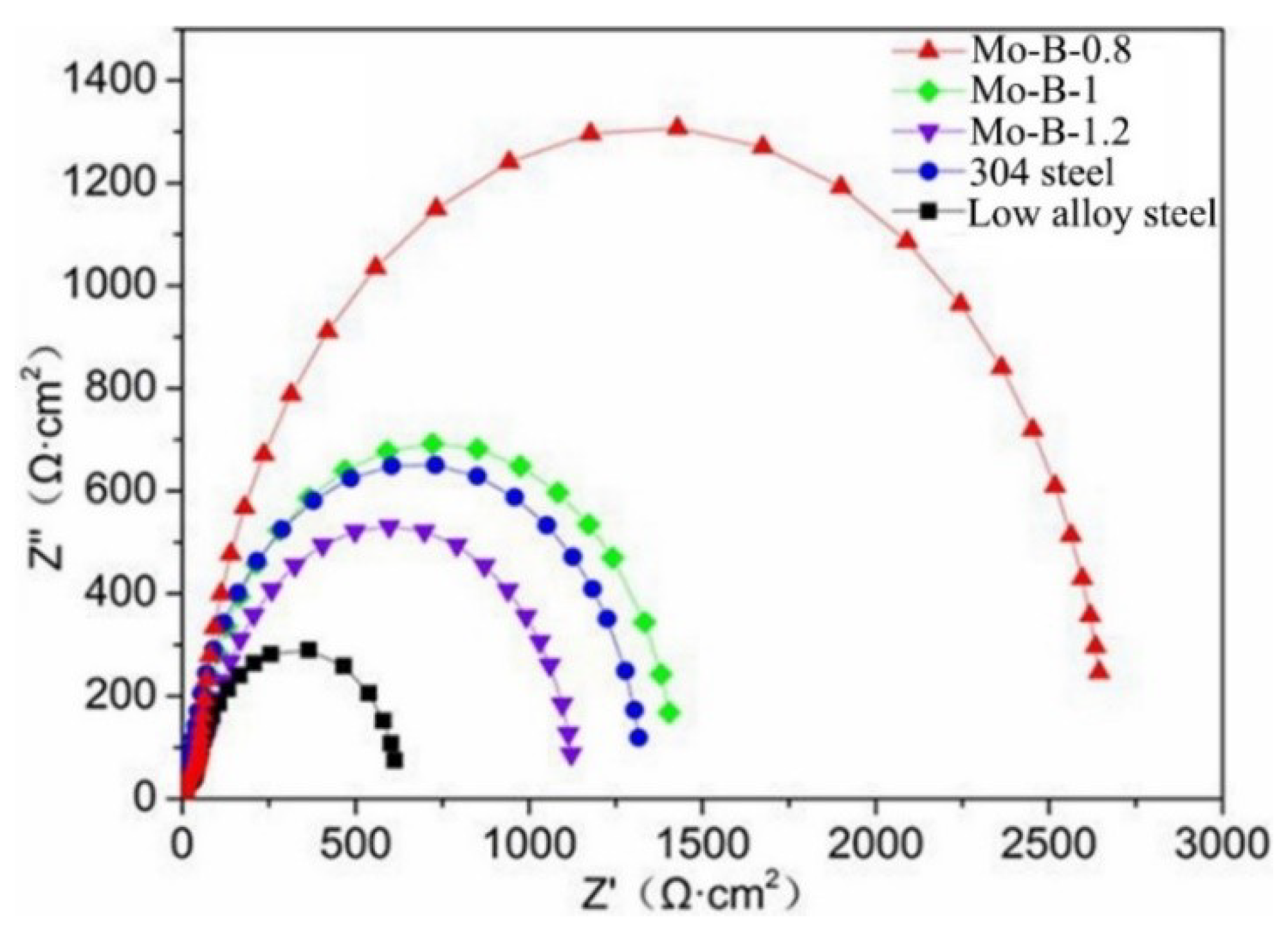
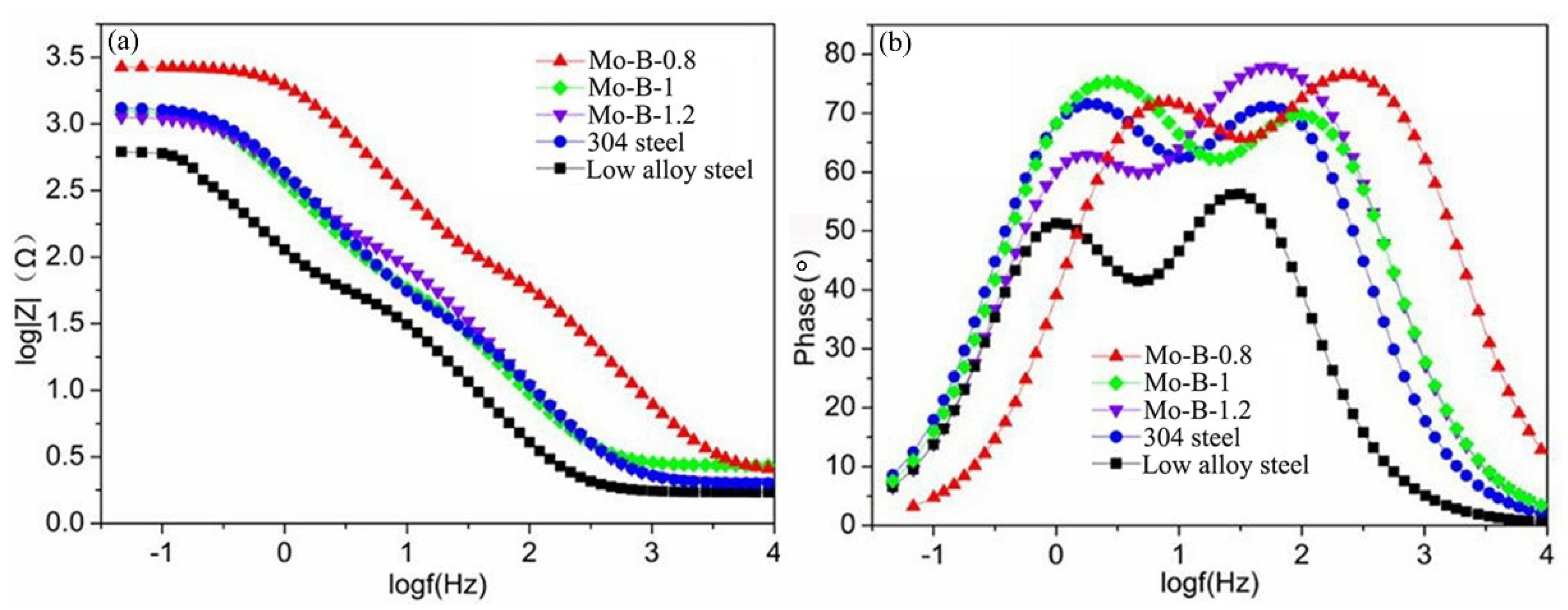
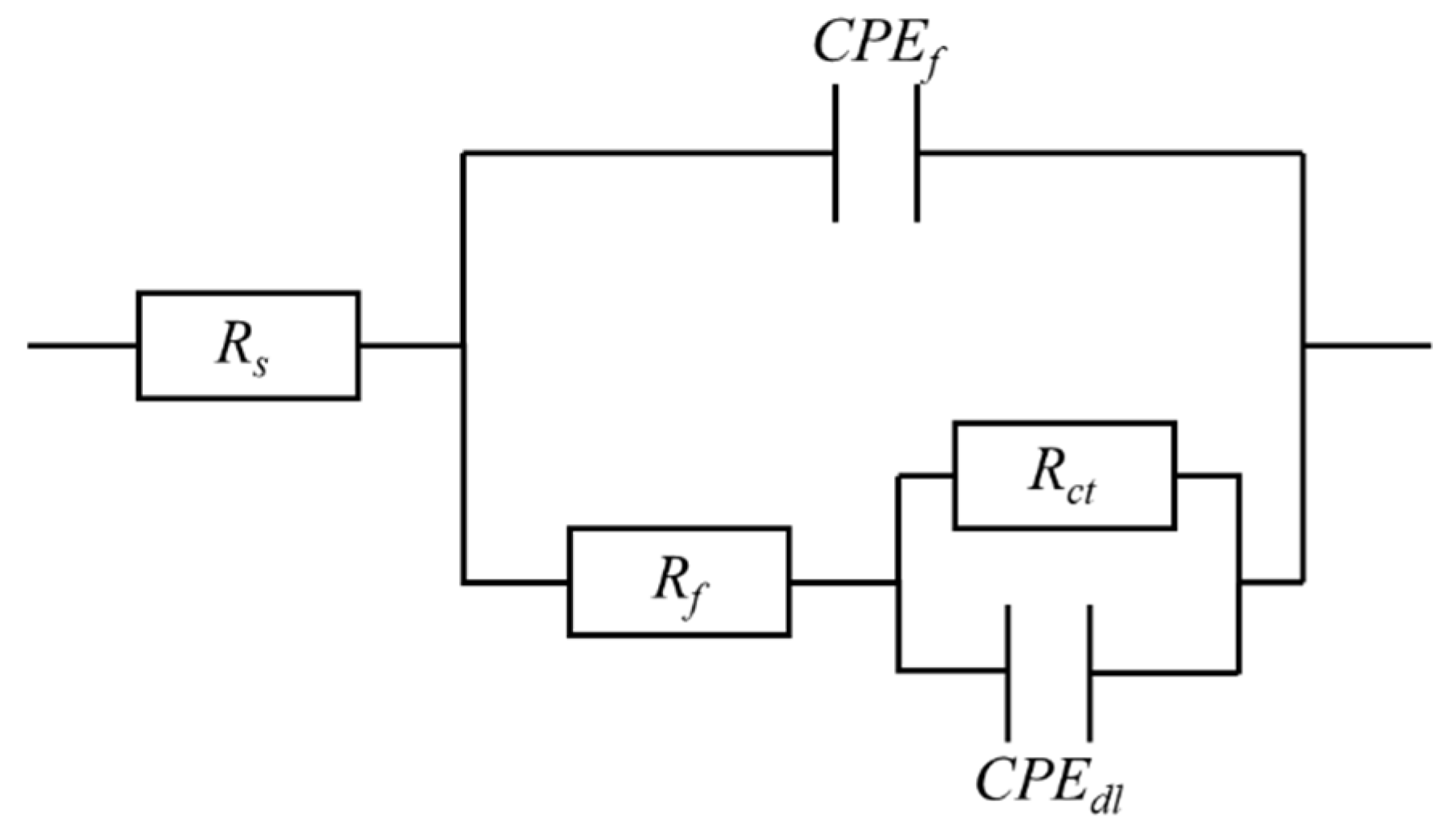
3.3. Corrosion Behaviors of the Mo2NiB2 Cermet Coatings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takagi, K.I. Development and application of high strength ternary boride base cermets. J. Solid State Chem. 2006, 179, 2809–2818. [Google Scholar] [CrossRef]
- Wang, H.Q.; Sun, J.S.; Li, C.N.; Geng, S.N.; Sun, H.G.; Wang, G.L. Microstructure and mechanical properties of molybdenum-ron-boron-chromium cladding using argon arc welding. Mater. Sci. Technol. 2016, 32, 1694–1701. [Google Scholar] [CrossRef]
- Zhang, T.; Yin, H.; Zhang, C.; Zhang, R.; Xue, J.; Zheng, Q.; Qu, X. First-principles study on the mechanical properties and electronic structure of V doped WCoB andW2CoB2 ternary borides. Materials 2019, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Togano, K.; Badica, P.; Nakamori, Y.; Orimo, S.; Takeya, H.; Hirata, K. Superconductivity in metal rich Li–Pd–B ternary boride. Phys. Rev. Lett. 2004, 93, 247004. [Google Scholar] [CrossRef]
- Kayhan, M.; Hildebrandt, E.; Frotscher, M.; Senyshyn, A.; Hofmann, K.; Alff, L.; Albert, B. Neutron diffraction and observation of superconductivity for tungsten borides, WB andW2B4. Solid State Sci. 2012, 14, 1656–1659. [Google Scholar] [CrossRef]
- Prakash, S.; Karacor, M.; Banerjee, S. Surface modification in microsystems and nanosystems. Surf. Sci. Rep. 2009, 64, 233–254. [Google Scholar] [CrossRef]
- Moraes, V.; Riedl, H.; Fuger, C.; Polcik, P.; Bolvardi, H.; Holec, D.; Mayrhofer, P. Ab initio inspired design of ternary boride thin films. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Kubliy, V.Z.; Bondar, A.A.; Utkin, S.V.; Petyukh, V.M.; Lysenko, S.I.; Velikanova, T.Y. Phase equilibria in the nickel corner of the Mo–Ni–B system at temperatures close to melting. Powder Metall. Met. Ceram. 2014, 47, 211. [Google Scholar] [CrossRef]
- Sanin, V.N.; Ikornikov, D.M.; Andreev, D.E.; Yukhvid, V.I.; Derin, B.; Yücel, O. Protective Mo2NiB2–Ni coatings by centrifugal metallothermic SHS. Int. J Self-Propag. High-Temp. Synth. 2015, 24, 161. [Google Scholar] [CrossRef]
- Takagi, K. Effect of Mn on the mechanical properties and microstructure of reaction sintered Mo2NiB2 boride-based cermets. Met. Mater. Int. 2003, 9, 467. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, G.; Kan, Y.; Wang, P. Reactive synthesis and mechanical properties of Mo2NiB2 based hard alloy. Int. J. Refract. Met. Hard Mater. 2010, 28, 291–296. [Google Scholar] [CrossRef]
- Takagi, K.I.; Koike, W.; Momozawa, A.; Fujima, T. Effects of Cr on the properties of Mo2NiB2 ternary boride. Solid State Sci. 2012, 12, 1643–1647. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Zhong, N.; Wang, G. Microstructure and properties laser-clad Mo2NiB2 cermet coating on steel substrate. Steel Res. Int. 2014, 85, 1–9. [Google Scholar]
- Hu, Z.; Li, W.; Zhao, Y. Microstructure and properties of M3B2-type boride-based cermet coatings prepared by laser cladding synthesis. Coatings 2019, 9, 476. [Google Scholar] [CrossRef]
- Keränen, J.; Stenberg, T.; Mäntylä, T.; Lepistö, T. Micro structural characterization of detonation gun-sprayed boride-based cermet coatings. Surf. Coat Technol. 1996, 82, 29–37. [Google Scholar] [CrossRef]
- Zhou, X.P.; Hu, X.B.; Xu, Y.S. The microstructure and properties of coating from Mo2FeB2 cermet on surface of H13 steel by reactive flame spraying. Adv. Mater. Res. 2010, 97, 1321–1327. [Google Scholar] [CrossRef]
- Zhuang, M.; Wei, W.; Zou, J.F.; Dong, S.Z.; Zhang, L.Y.; Li, Z.C. Preparation and properties of flame-sprayed Mo–FeB–Fe cermet coatings. Trans. Nonferrous Met. Soc. Chin. 2011, 21, 1314–1321. [Google Scholar]
- Vencl, A.; Mrdak, M.; Banjac, M. Correlation of microstructures and tribological properties of ferrous coatings deposited by atmospheric plasma spraying on Al–Si cast alloy substrate. Metall. Mater. Trans. A 2009, 40, 398–405. [Google Scholar] [CrossRef]
- Manna, I.; Majumdar, J.D.; Chandra, B.R.; Nayak, S.; Dahotre, N.B. Laser surface cladding of Fe–B–C, Fe–B–Si and Fe–BC–Si–Al–C on plain carbon steel. Surf. Coat. Technol. 2006, 201, 434–440. [Google Scholar] [CrossRef]
- Sexton, L.; Lavin, S.; Byrne, G.; Kennedy, A. Laser cladding of aerospace materials. J. Mater. Process. Technol. 2002, 122, 63–68. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Qin, Z.; Wang, C.; Xu, Z.; Jiang, C.; Ji, V. The roles of Ti particles in improving the corrosion resistance of electrochemically assembled Ni–Ti composite coatings. Corrosion 2017, 73, 1107–1118. [Google Scholar] [CrossRef]
- Baghery, P.; Farzam, M.; Mousavi, A.B.; Hosseini, M. Ni–TiO2 nanocomposite coating with high resistance to corrosion and wear. Surf. Coat. Technol. 2010, 204, 3804–3810. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Z.F.; Shen, Y.P.; Li, K.M.; Cao, Z.; Jian, Y.X.; Ren, Z.J. High-temperature compressive properties and tribological behaviour of Mo2NiB2–Ni cermets. Ceram. Int. 2019, 45, 18413–18421. [Google Scholar] [CrossRef]
- Gong, J.; Wilkinson, A.J. A microcantilever investigation of size effect, solid-solution strengthening and second-phase strengthening for<a> prism slip in alpha-Ti. Acta Mater. 2011, 59, 5970–5981. [Google Scholar] [CrossRef]
- Furukawa, M.; Horita, Z.; Nemoto, M.; Veliev, R.Z.; Langdon, T.G. Microhardness measurements and the Hall-Petch relationship in an Al–Mg alloy with submicrometer grain size. Acta Mater. 1996, 44, 4619–4629. [Google Scholar] [CrossRef]
- Cai, F.; Jiang, C.; Zhang, Z.; Muttini, E.; Fu, P.; Zhao, Y.; Ji, V. Fabrication and characterization of Ni–Zr composite coatings using electrodepositing technique. J. Alloys Compd. 2015, 635, 73–81. [Google Scholar] [CrossRef]
| Specimen No. | Mo:B Ratios | Scanning Speed (mm/s) | Laser Power (kW) | Focus Distance (mm) | Lapping Distance (mm) | Spot Size (mm) |
|---|---|---|---|---|---|---|
| 1 | 0.8 | 1.5 | 2.5 | 25 | 1 | 6 × 1 |
| 2 | 0.8 | 2 | ||||
| 3 | 1 | 1.5 | ||||
| 4 | 1 | 2 | ||||
| 5 | 1.2 | 1.5 | ||||
| 6 | 1.2 | 2 |
| Locations | Mo | Ni | B | Fe | Cr |
|---|---|---|---|---|---|
| 1 | 5.7 | 16.3 | 0 | 74.5 | 3.5 |
| 2 | 55.9 | 24.2 | 4.8 | 10.3 | 4.8 |
| Specimens | Ecorr (mV) | βa (mV·dec−1) | βc (mV·dec−1) | Icorr (A·cm−2) |
|---|---|---|---|---|
| Mo:B = 0.8 | −223.2 | 99.007 | −9.456 | 1.58 × 10−5 |
| Mo:B = 1 | −307.9 | 245.286 | −87.655 | 1.64 × 10−5 |
| Mo:B = 1.2 | −403.8 | 165.839 | −80.153 | 2.67 × 10−5 |
| 304 steel | −322.2 | 48.296 | −202.883 | 1.76 × 10−5 |
| Low alloy steel | −436.3 | 62.323 | −104.151 | 2.41 × 10−4 |
| Specimens | Rs (Ω·cm2) | CPEf (F·cm−2) | Rf (Ω·cm2) | CPEdl (F·cm−2) | Rct (Ω·cm2) |
|---|---|---|---|---|---|
| Mo:B = 0.8 | 2.897 | 2.70 × 10−5 | 202.8 | 3.47 × 10−5 | 2153 |
| Mo:B = 1 | 2.004 | 1.47 × 10−4 | 59.82 | 2.16 × 10−4 | 1310 |
| Mo:B = 1.2 | 2.042 | 1.49 × 10−4 | 178.5 | 2.18 × 10−5 | 1248 |
| 304 steel | 2.748 | 1.83 × 10−4 | 113.0 | 2.85 × 10−5 | 1266 |
| Low alloy steel | 3.175 | 2.70 × 10−5 | 22.98 | 3.47 × 10−5 | 948.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Wang, S.; Zhao, Y.; Li, W.; Jiao, X. Investigation on Microstructure, Hardness, and Corrosion Resistance of Mo–Ni–B Coatings Prepared by Laser Cladding Technique. Coatings 2019, 9, 856. https://doi.org/10.3390/coatings9120856
Ni X, Wang S, Zhao Y, Li W, Jiao X. Investigation on Microstructure, Hardness, and Corrosion Resistance of Mo–Ni–B Coatings Prepared by Laser Cladding Technique. Coatings. 2019; 9(12):856. https://doi.org/10.3390/coatings9120856
Chicago/Turabian StyleNi, Xiaojie, Shengze Wang, Yuantao Zhao, Wenge Li, and Xiong Jiao. 2019. "Investigation on Microstructure, Hardness, and Corrosion Resistance of Mo–Ni–B Coatings Prepared by Laser Cladding Technique" Coatings 9, no. 12: 856. https://doi.org/10.3390/coatings9120856
APA StyleNi, X., Wang, S., Zhao, Y., Li, W., & Jiao, X. (2019). Investigation on Microstructure, Hardness, and Corrosion Resistance of Mo–Ni–B Coatings Prepared by Laser Cladding Technique. Coatings, 9(12), 856. https://doi.org/10.3390/coatings9120856




