Condensation Test and Simulation of Superhydrophobic Coatings
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of the Superhydrophobic Surface
3. Result and Discussion
3.1. Wettability and Dielectric Properties
3.2. Charged Condensation
3.3. Distribution Statistics of Surface Water Droplets
3.4. Analysis of Simulation Results
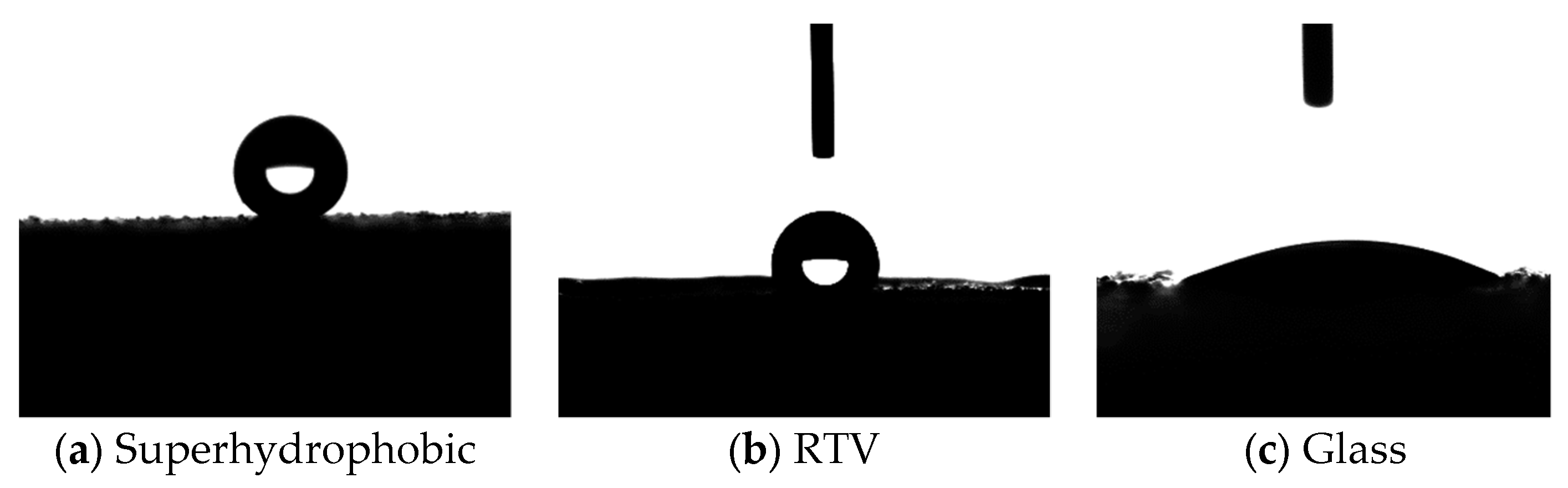
3.4.1. Static Contact Angle
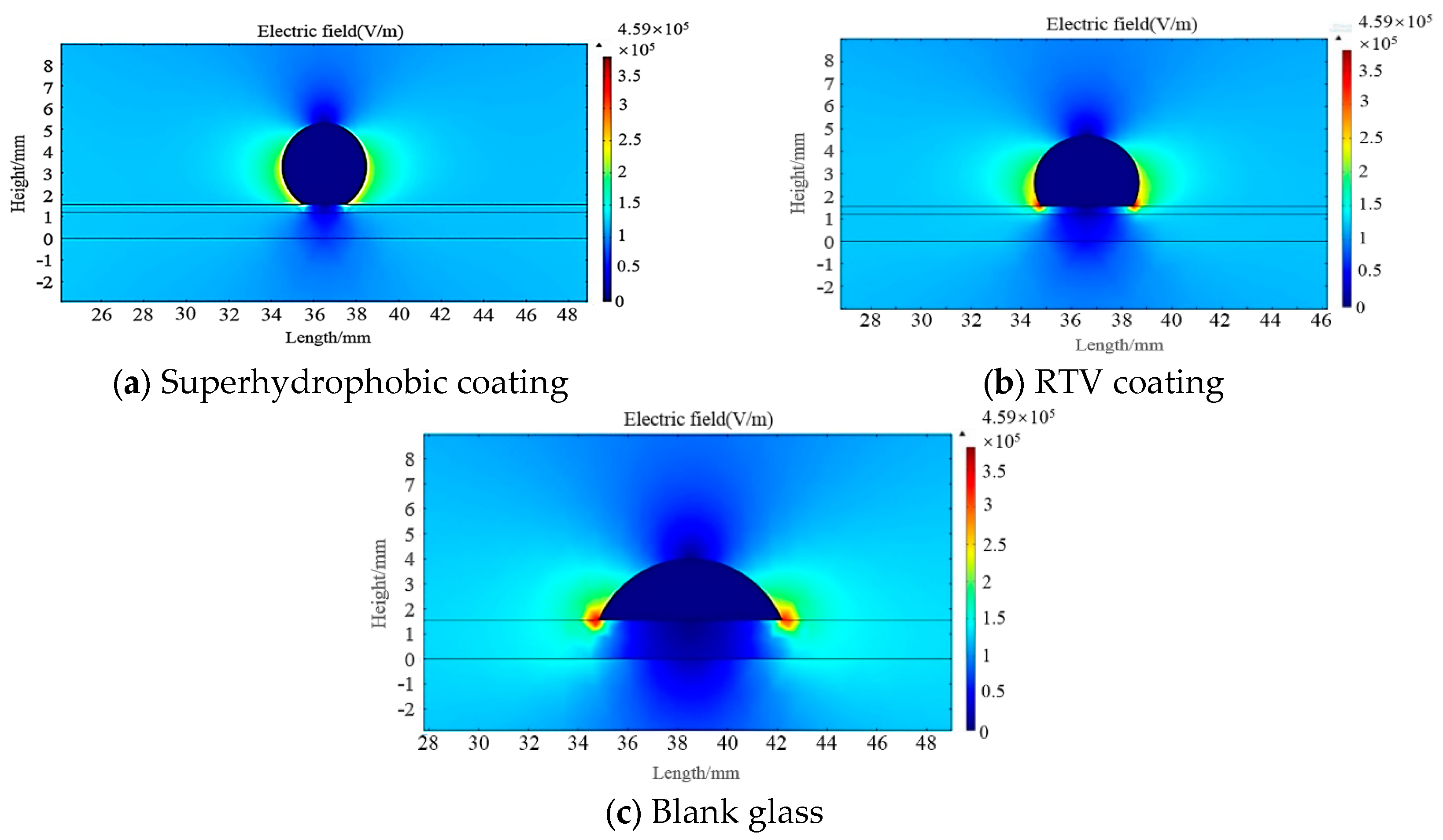
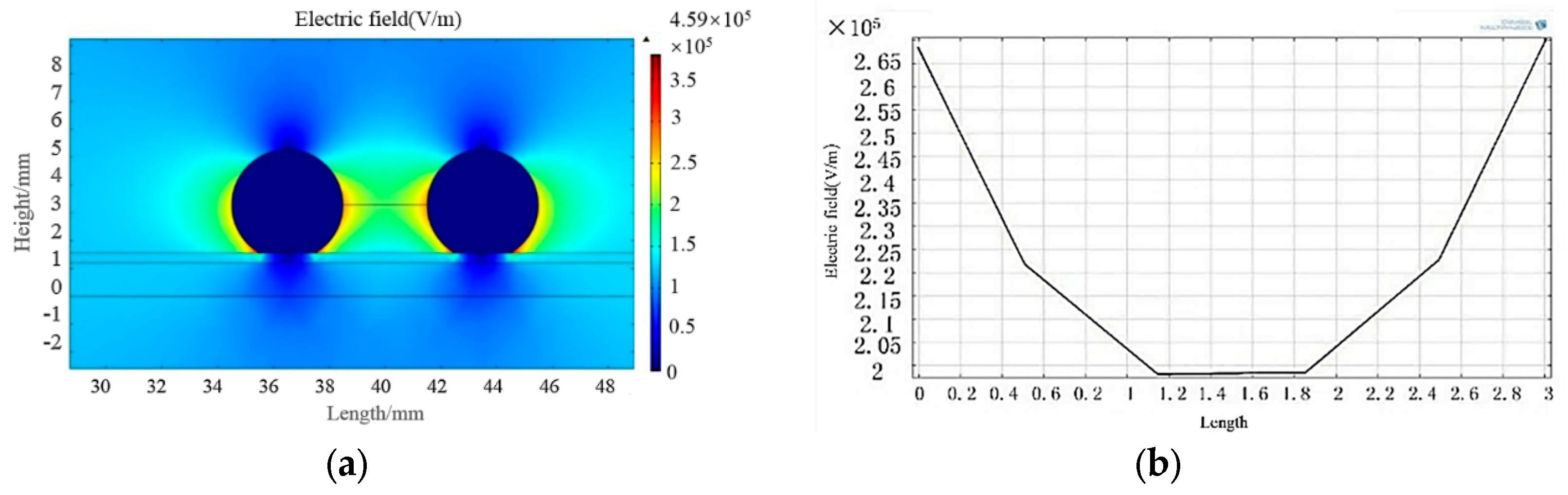
3.4.2. COMSOL Simulation
4. Conclusions
- (1)
- The micro-nano composite rough structure was constructed using PMMA particles and nano-SiO2 particles, and the superhydrophobic coating was prepared by spraying with PVDF for a low surface energy treatment.
- (2)
- The average static contact angle of the superhydrophobic coating was 155°, and the relative dielectric constant and conductivity were 2.8 and 3.3 × 10−13 S/m, respectively, at a power frequency. The prepared coating had excellent superhydrophobic and dielectric properties.
- (3)
- Based on the condensation test platform, we analyzed the distribution characteristics of water droplets on the superhydrophobic coating. The water droplets on the superhydrophobic coating were sparse, and the particle size was relatively small, which was beneficial for improving the dielectric properties of the coating. There were fewer water droplets on the surface of the superhydrophobic coating, the particle size was smaller, and the spacing was larger, which was beneficial for improving the dielectric properties of the coating.
- (4)
- According to the results of the simulation, the electric field intensity at the interface of the gas, liquid, and solid phases on the surface of superhydrophobic coatings was relatively low, which reduced the risk of partial discharge compared to the RTV coating.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Jiang, X.; Sun, C. Present situation and prospect of research on flashover characteristics of polluted insulators. Power Syst. Technol. 2006, 30, 35–40. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, X.; Sun, C.; Hu, J.; Shu, L.; Mao, F. Influence of air pressure on DC pollution flashover performance of insulator string. Trans. China Electrotech. Soc. 2010, 25, 38–43. [Google Scholar]
- Gençoğlu, M.T.; Cebeci, M. The pollution flashover on high voltage insulators. Electr. Power Syst. Res. 2008, 78, 1914–1921. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Jiang, X.; Hu, J.; Gao, D.W. Study on AC flashover performance for different types of porcelain and glass insulators with non-uniform pollution. IEEE Trans. Power Deliv. 2013, 28, 1691–1698. [Google Scholar] [CrossRef]
- Jiang, X.; Du, Y.; Lin, F.; Hu, J.; Zhang, Z.; Lu, J. Effect of permanent in-situ forming anti-fouling composite coating on insulator ice coating and AC ice flash voltage. Power Syst. Technol. 2008, 32, 71–75. [Google Scholar]
- Zeng, Z.; Gu, Z.; Huo, R.; Ye, Y.; Zhang, Z. Super-hydrophobic and Self-cleaning PVDF film fabricated by chemical bath deposition. J. Donghua Univ. (Engl. Ed.) 2010, 27, 451–457. [Google Scholar]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Hashimoto, K.; Watanabe, T.; Takai, K.; Yamauchi, G.; Fujishima, A. Transparent superhydrophobic thin films with self-cleaning properties. Langmuir 2000, 16, 7044–7047. [Google Scholar] [CrossRef]
- Jin, M.; Feng, X.; Xi, J.; Zhai, J.; Cho, K.W.; Feng, L.; Jiang, L. Super-hydrophobic PDMS surface with ultra-low adhesive force. Macromol. Rapid Commun. 2005, 26, 1805–1809. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Miljkovic, N.; Preston, D.J.; Enright, R.; Wang, E.N. Electric-field-enhanced condensation on superhydrophobic nanostructured surfaces. ACS Nano 2013, 7, 11043–11054. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C.; Koch, K. Self-cleaning efficiency of artificial superhydrophobic surfaces. Langmuir 2009, 25, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, K.M.; Watson, J.A.; Qu, X.; Liu, F.J.; Watson, G.S.; Chen, C.H. Self-cleaning of superhydrophobic surfaces by self-propelled jumping condensate. Proc. Natl. Acad. Sci. USA 2013, 110, 7992–7997. [Google Scholar] [CrossRef] [PubMed]
- Boreyko, J.B.; Chen, C.H. Self-propelled dropwise condensate on superhydrophobic surfaces. Phys. Rev. Lett. 2009, 103, 184501. [Google Scholar] [CrossRef] [PubMed]
- Jun Lee, S.; Lee, S.; Hyoung Kang, K. Droplet jumping by electrowetting and its application to the three-dimensional digital microfluidics. Appl. Phys. Lett. 2012, 100, 81604. [Google Scholar] [CrossRef]
- Li, S.; Page, K.; Sathasivam, S.; Heale, F.; He, G.J.; Lu, Y.; Lai, Y.K.; Chen, G.Q.; Carmalt, C.J.; Parkin, I.P. Efficiently texturing hierarchical superhydrophobic fluoride-free translucent films by AACVD with excellent durability and self-cleaning ability. J. Mater. Chem. A 2018, 6, 17633–17641. [Google Scholar] [CrossRef]
- Schmitt, M.; Heib, F. A more appropriate procedure to measure and analyse contact angles/drop shape behaviours. Adv. Contact Angle Wettabil. Adhes. 2018, 3, 1–57. [Google Scholar]
- Extrand, C.W. Model for contact angles and hysteresis on rough and ultraphobic surfaces. Langmuir 2002, 18, 7991–7999. [Google Scholar] [CrossRef]
- Patankar, N.A. On the modeling of hydrophobic contact angles on rough surfaces. Langmuir 2003, 19, 1249–1253. [Google Scholar] [CrossRef]
- Marmur, A. Soft contact: Measurement and interpretation of contact angles. Soft Matter 2006, 2, 12–17. [Google Scholar] [CrossRef]




| Coatings | 100~300 μm | 300~500 μm | 500~1000 μm | 1000~2000 μm | >2000 μm |
|---|---|---|---|---|---|
| Superhydrophobic coating | 249 | 12 | 3 | 0 | 0 |
| RTV coating | 348 | 52 | 35 | 20 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Yang, Y.; Guo, X.; Xia, H. Condensation Test and Simulation of Superhydrophobic Coatings. Coatings 2019, 9, 743. https://doi.org/10.3390/coatings9110743
He Y, Yang Y, Guo X, Xia H. Condensation Test and Simulation of Superhydrophobic Coatings. Coatings. 2019; 9(11):743. https://doi.org/10.3390/coatings9110743
Chicago/Turabian StyleHe, Yunhua, Yuling Yang, Xinliang Guo, and Huanhuan Xia. 2019. "Condensation Test and Simulation of Superhydrophobic Coatings" Coatings 9, no. 11: 743. https://doi.org/10.3390/coatings9110743
APA StyleHe, Y., Yang, Y., Guo, X., & Xia, H. (2019). Condensation Test and Simulation of Superhydrophobic Coatings. Coatings, 9(11), 743. https://doi.org/10.3390/coatings9110743




