Abstract
The precursor for a lithium-ion sieve is prepared using an inorganic precipitation-peptization method with titanium sulfate as the titanium source and lithium acetate as the lithium source. The effects of Ni2+ (Nickel ions) doping on the stability of the sol, crystal morphology and interplanar spacing of Li2TiO3 are investigated. The results indicate that, after Ni2+ doping with varying fractions, the stability of the precursor sol first increases then decreases, and the maximum stabilization time of the precursor sol doped with 1% Ni2+ is 87 h. When doped with 1% Ni2+, the sol performance is most stable, the porous Li2TiO3 is obtained, and the specific surface area of Li2TiO3 increases by up to 1.349 m2/g from 0.911 m2/g. Accompanying the increase in calcination temperature, the inhibition of Ni2+ doping on the growth and crystallization of grains decreases. When the temperature is lower than 750 °C, Ni atoms replace the Ti atoms that are substituted for Li atoms in the original pure Li layer, forming lattice defects, resulting in the disappearance of (002) and (−131) diffraction peaks for Li2TiO3, the reduced ordering of crystal structure, a decrease in the interplanar spacing of the (002) plane, lattice expansion and an increase in the particle size to 100–200 nm. When the temperature exceeds 750 °C, with the increase of calcination temperature, the influence of Ni doping on the growth and crystallinity of grains decreases, and the (002) crystal surface starts to grow again.
1. Introduction
As a new energy metal, lithium has the advantages of cleanliness and high efficiency compared to traditional energy metals. Lithium is widely used, therefore, in lithium batteries [1], energy research [2] and other related industries; moreover, the demand for lithium in the market is increasing., Most lithium resources in China are distributed in ores, salt lake brine and seawater [3]. Since lithium reserves in salt lake brine and seawater are so rich, the extraction of lithium from such sources is the focus of further development. Presently, the main methods for extracting lithium from salt lake brine are the precipitation method, extraction method [4,5,6,7], ion exchange adsorption method [8,9], calcination method [10,11], and salting method [12]. Among these methods, the simple ion exchange adsorption method is suitable for most salt lake brines. The key to this method lies in the synthesis of an ion-exchange agent for selective absorption of lithium ions [13]. As a result, lithium-ion sieves have attracted extensive attention from chemical researchers around the world. Currently, the research on lithium-ion sieves mainly includes manganese and titanium lithium-ion sieves. Xu et al. [14] synthesized Li4Mn5O12 (Spinel Manganese Series lithium ion sieve precursor) using an ethylenediamine tetraacetic acid (EDTA)-citric acid complexation method, followed by pickling to obtain a manganese-based lithium-ion sieve with a maximum absorption capacity of 43.1 mg/g. The eluent, however, is liable to cause the dissolution of manganese and erosion of the manganese skeleton [15], which results in a low recycling efficiency and unsuitability for industrial lithium extraction. To address this problem, some researchers [16] propose that the dissolution rate can be controlled via doping, which can reduce the absorption capacity to a certain extent.
Compared with manganese-based lithium-ion sieves, titanium-based lithium-ion sieves have the advantages of simple preparation, large absorption capacity [17], and a low dissolution rate. Titanium-based lithium-ion sieves mainly are prepared by pickling the Li2TiO3 precursor with eluent. The main preparation methods for Li2TiO3 include solid-phase reaction [18], the hydrothermal method [19], the sol–gel process [20], precipitation–peptization [21]. Due to the difficulties of solid–liquid separation and poor fluidity, however, the efficiency of powdered lithium-ion sieves prepared by the solid-phase reaction and hydrothermal method is so slow that it is not feasible for column-loading operation. To solve the above issue, in our previous work [22], an H2TiO3-lithium absorbent, prepared using the sol–gel process, was loaded onto ceramic foams. The pickling and adsorption performances were investigated with an ion-exchange column. It was demonstrated that H2TiO3-lithium absorbent supported on ceramic foams had a good absorption performance [23]., The cost of the raw material, tetrabutyl titanate, is too high, however. During another work of our group [24], lithium absorbent also was synthesized, with lithium acetate as a lithium source and titanium sulfate as a titanium source, using the Xu precipitation–peptization method. The sol prepared by the traditional precipitation–peptization method has limited stability [24,25], however, which results in poor reusability of the sol. To improve the stability of the sol, doping metals such as Ni, Al, and La can be used. It has been reported [26] that a precursor manganese lithium-ion sieve, doped with aluminum, shows remarkable improvement in the stability and dissolution rate. Transition-metal lanthanum doping [27] also has a significant effect on improving the stability of samples, reducing the dissolution rate and improving the recycling ability.
As a transition metal with moderate cost, good formability and strong corrosion resistance, nickel also can enhance [28] the stability and reduce the dissolution rate of samples after doping. Following doping treatment with nickel, the precursors of a series of manganese samples showed X-ray diffraction patterns that are quite different from those of the standard cards, indicating that most of the nickel atoms enter the lattice of the lithium manganese spinel [29]. As a result, both the dissolution rate of manganese and its saturated absorption capacity were reduced [30]. Based on a series of previous studies on Ni-doped Mn-Li ion sieve precursors, innovated in this work, a Ni-doped Ti-Li ion sieve precursor is prepared to explore the influence of Ni-doping on colloidal stability, surface structure, the crystal size and crystal plane growth, and other aspects of an Li2TiO3 crystal. This work aims to prolong the stable existence time of sol, improve the reusability of sol, and obtain a kind of Ti-Li ion sieve precursor with a porous structure.
2. Experimental
Using the traditional high temperature solid-state method, the contact between a lithium source and solid metal oxide is not enough and the mixing is uneven, resulting in poor crystallinity and defects in the crystal structure. Regarding the sol–gel process, the reactants are mixed uniformly, however, the reaction time is long, the operation requirements are high, and the cost of the organic titanium source is high. The preparation of a Li-ion sieve by an inorganic precipitation–peptization method can make up for the shortcomings of the previous method. During this method, Ti4+ is transformed into precipitate by a suitable precipitant, the excess SO42− is removed by centrifugal washing, followed by adding a proper amount of complexing agent into the fresh precipitate to make the precipitate disperse automatically, forming sol. Compared to the former methods, this method is more operable and can reduce the cost greatly. Thus, in this work, the precursor of a lithium-ion sieve was prepared by doping transition metal nickel combined with an inorganic precipitation–peptization method. The specific operations are as follows.
2.1. Preparation of Ni-Doped Li2TiO3 Sol
Employing a water bath at 60 °C, 10.82 g Ti(SO4)2 and 6.61 g CH3COOLi were dissolved, respectively, using 200 mL of distilled water to form the transparent solution. NiCl2∙6H2O solution (Ni/Ti = 1%, 2%, 3%) was added if necessary. An ammonia solution was added to the Ti(SO4)2 solution at a speed of one drop per ten seconds under magnetic stirring until the pH value was 8.5. The obtained precipitate was centrifuged and washed with distilled water four times, then placed in a beaker, followed by the addition of 200 mL distilled water. The mixture was stirred while the lithium source was introduced at a uniform speed. Finally, 30% H2O2 (25 mL) was added dropwise into the mixing solution at a speed that was maintained at approximately 3 seconds/drop. Subsequently, the transparent yellow sol was obtained by stirring at room temperature for 1 h.
2.2. Preparation of Li2TiO3 Powders
Following aging at room temperature, the colloids were dried in an oven at 80 °C to obtain the dry gel. Then, the dry gel was ground evenly and calcined at different temperatures in a muffle furnace. The calcination temperature was set to 650–900 °C with a holding period of 2 h.
2.3. Characterization
The viscosity of the colloidal sol was measured by a digital viscometer (NDJ-5S, Shanghai Jingtian Electronic Instrument Co., Ltd., Shanghai, China). Thermal properties of the samples were estimated by Thermogravimetric-Differential Scanning Calorimetry (TG-DSC TA-Q600, TA Company, New Castle, DE, USA), the specimen was heated at a ramp rate of 10 °C·min−1 to 1000 °C in dry air conditions. The microscopic structures of the samples were characterized by scanning electronic microscopy (SEM, VEGA3, TESCAN, Brno, Czech Republic) and transmission electron microscopy (TEM, JEM 2010, Japan Electron Optics Laboratory Co., Ltd., Tokyo, Japan). The crystalline structure of the samples was investigated with an X-ray diffractometer (XRD, DX-2700, Dandong Haoyuan Instrument Co., Ltd., Liaoning, China) using Cu Kα radiation at a scanning rate of 0.05°·s−1 and a working voltage/current of 40 kV/40 mA. The pore size and specific surface area were measured on a surface area analyzer (BET, Autosorb iQ2, Quantachrome, Boynton Beach, FL, USA). The chemical composition and elemental chemical status were obtained by X-ray photoelectron spectroscopy (XPS, 250 Xi, Thermo Fisher Scientific, Waltham, MA, USA). The functional groups of the samples were revealed by Escalab Fourier-transform infrared spectroscopy (FT-IR, WQF-510A, Beijing Beifen–Ruili Analytical Instrument Co., Ltd., Beijing, China).
3. Results and Discussion
3.1. Viscosity Test
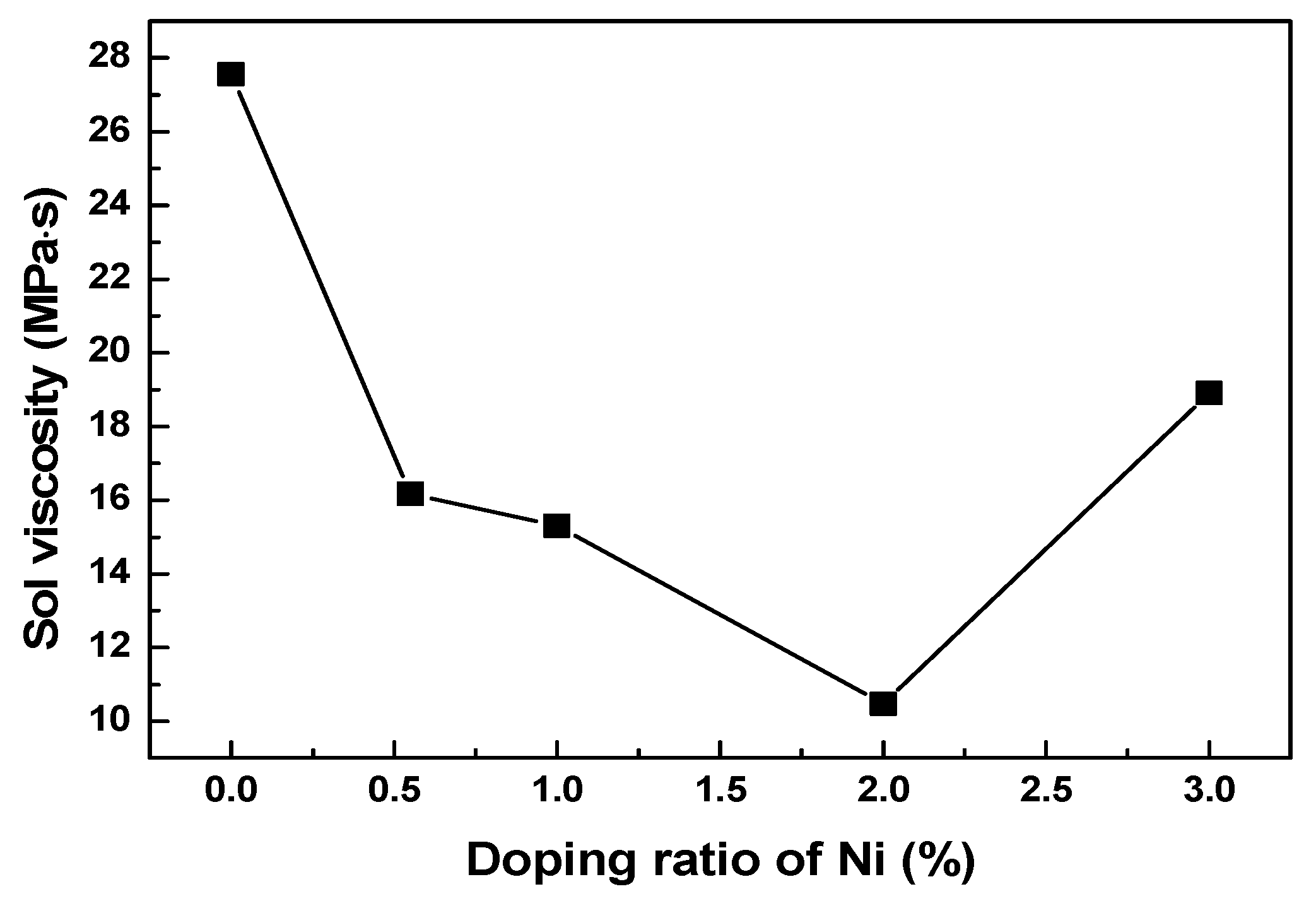
The stability of sol is related directly to its viscosity. To investigate the influence of Ni doping on the stability of sol, the viscosity value of sol was measured. Figure 1 shows the viscosity values for a sol doped with different ratios of nickel at 87 h. During this test, a lower viscosity indicates a better stability of the sol. The results shown in Figure 1 suggest the sol viscosity first decreases and then increases with an increasing nickel doping amount. This is because the characteristic adsorption of Ni2+ on the surface of the sol particle reaches a saturated state with an increasing doping amount. Excessive Ni2+ causes the spatial distribution of the sol particles to become denser. Additionally, the probability of interaction between the particles increases, while the intermolecular potential decreases. Thus, the flocculation of the sol system occurs [31], which results in the increased viscosity value. Previously, in our other work, it was demonstrated that sol could be loaded effectively onto the matrix when the maximum viscosity of the sol did not exceed 15 MPa·s. According to the results given in Figure 1, only the 2% nickel-doped sol showed a viscosity value lower than 15 MPa·s when the aging time was 87 h, and the viscosity value for the 1% nickel-doped sol was close to 15 MPa·s.
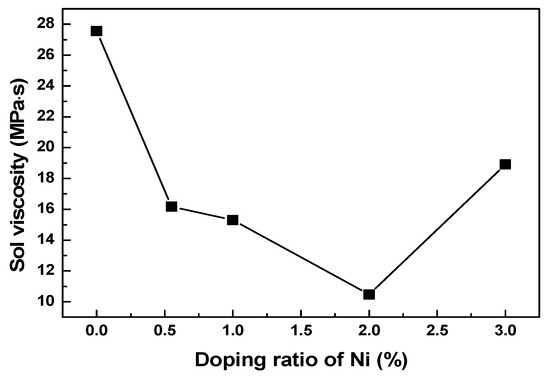
Figure 1.
The sol viscosity of sol doped with different mass ratios of Ni at 87 h.
The changes in colloidal viscosity with time, after doping with varying mass ratios of nickel, are listed in Table 1. It is evident that the viscosity of the sol with nickel was much lower compared to the undoped case, which is consistent with the above results. Considering 75–87 h, the sol viscosity of the nickel-doped precursors increased dramatically. The sol viscosity of nickel-doped precursors with 1% nickel doping was close to 15 MPa·s at 87 h, while that of precursors doped with 2% nickel was lower than 15 MPa·s. The sol viscosity change was less stable than that of the 1% nickel-doped sol during the first 75 h. Additionally, the low viscosity of the sol leads to weak adhesion on the substrate [21], which is not beneficial for loading. Therefore, the precursor sol doped with 1% nickel is more suitable for the loading of the substrate.

Table 1.
Variation of sol viscosity of Ni with varying mass percentage over time.
3.2. Performance of the Precursor Sol
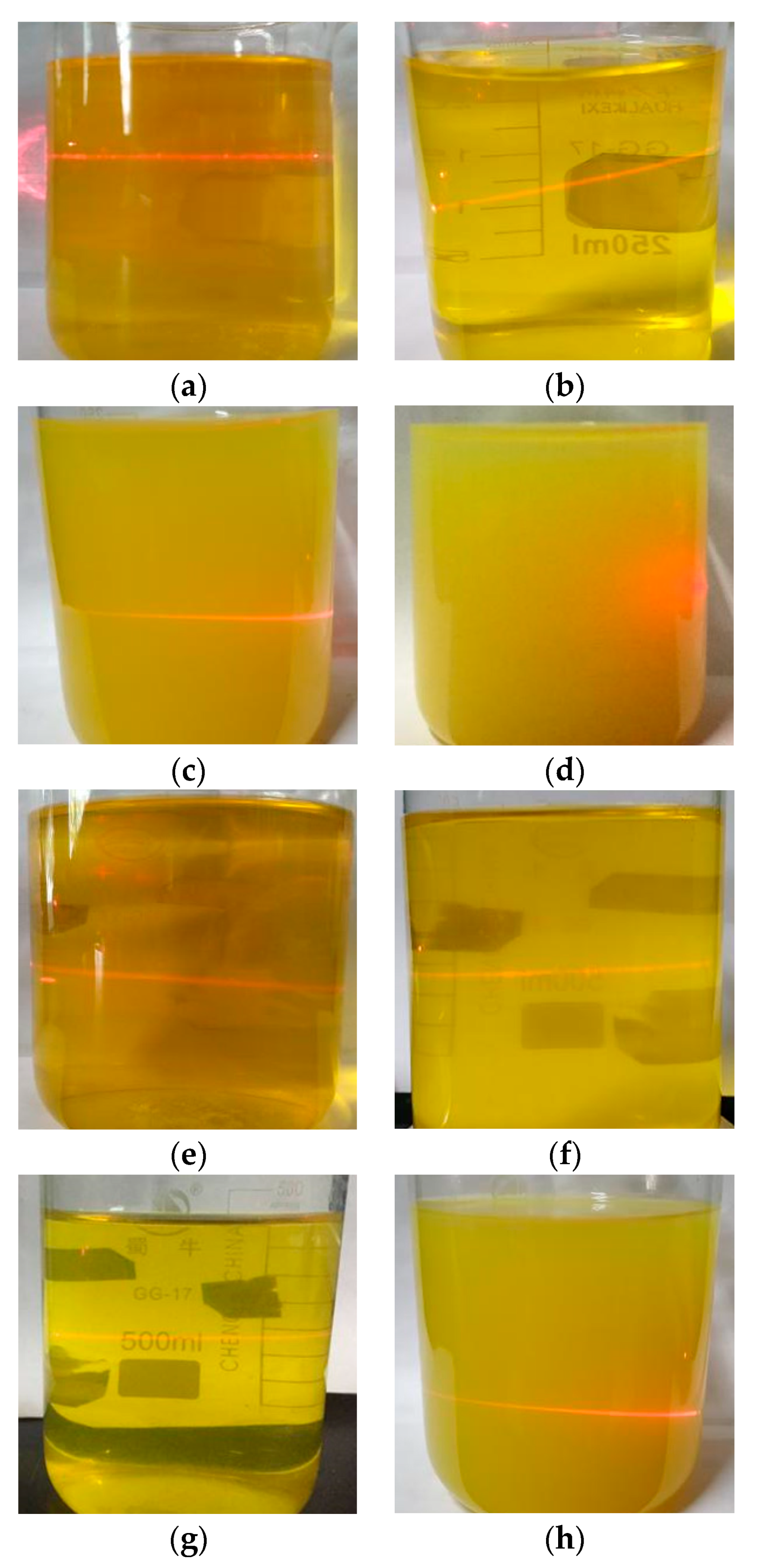
One of the obvious characteristics of colloids is the Tyndall effect. The stability of sol can be qualitatively reflected by the Tyndall effect produced by laser irradiation. The performance of the precursor sol as a function of aging time is shown in Figure 2. The new sols were transparent, orange-yellow and showed an obvious Tyndall effect. As the aging time was extended to 39 h, the undoped sol remained yellow, but became opaque, while the 1% Ni-doped sol remained yellow and transparent. Both samples still showed the Tyndall effect. When the aging time was further extended to 63 h, the undoped sol showed a tendency for flocculation and precipitation and the Tyndall effect was no longer observed, while the 1% Ni-doped sol became yellow and opaque, but still showed the Tyndall effect. This indicates that nickel doping is beneficial to improve the stability of the colloidal structure of the sol. We hypothesize that the main reason for this effect might be as follows: The small amount of NiCl2∙6H2O doped into the sol is dissolved in water and forms a conductive electrolyte solution. Ni2+ can interact with the charged particles in the sol and form a characteristic adsorption on the surface to form a sol particle with a certain size [32], which leads to a change in the electric double layer structure in the sol system. Thus, the repulsion between the colloidal particles is increased and the surface potential is enhanced, leading to the dispersion of the colloidal particles and an enhanced stability of the sol.
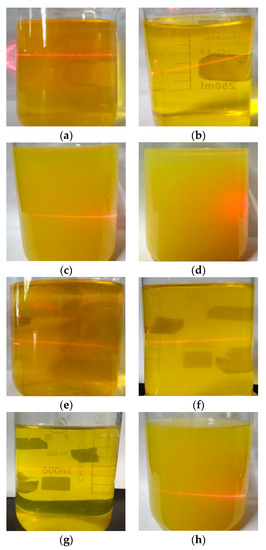
Figure 2.
The state of the sol doped with Ni aged for varying times. (a) 0% Ni, 1 h; (b) 0% Ni, 18 h; (c) 0% Ni, 39 h; (d) 0% Ni, 63 h; (e) 1% Ni, 1 h; (f) 1% Ni, 18 h; (g) 1% Ni, 39 h; (h) 1% Ni, 63 h.
3.3. TG–DSC Analysis
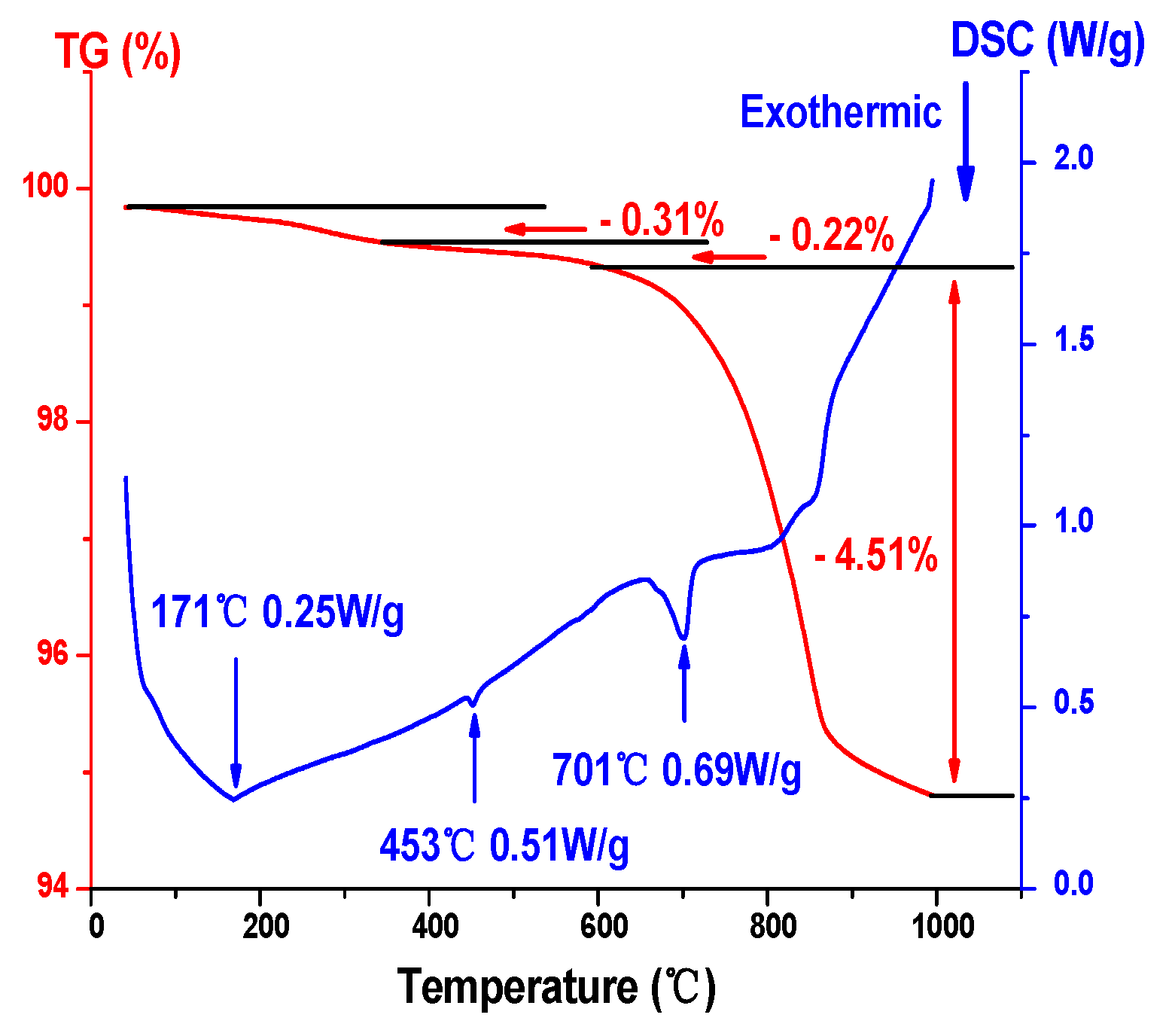
Following drying, the gel was obtained. The gel needed to be converted into a Ni-Li2TiO3 crystal by calcination and the determination of the calcination temperature depended on TG–DSC analysis. To study the thermal stability of Ni-doped xerogel precursors and the sample composition by changing temperature at the same time, TG–DSC analysis was carried out. Figure 3 shows the TG–DSC curves for the 1% Ni-doped precursor. Viewing Figure 3, the total weight loss of xerogel was 5.04% during the whole heat treatment. Three obvious exothermic peaks are observed on the DSC Curve, and the TG Curve shows a downward trend. Among them, the first mass loss (0.31%) occurs at 171 °C, which corresponds to the removal of evaporated free water in the precursor and desorption of acetic acid. The second mass loss occurs at 453 °C, the weight loss percentage of the sample is approximately 0.22%, which may be related to the partial decomposition of ammonium sulfate. When the calcination temperature is 701 °C, the TG curve shows that the weight loss percentage for the sample is 4.51% and the step change at approximately 701 °C may be due to the change in specific heat capacity after the increase in crystallization of the lithium titanate, which leads to a baseline drift [21].
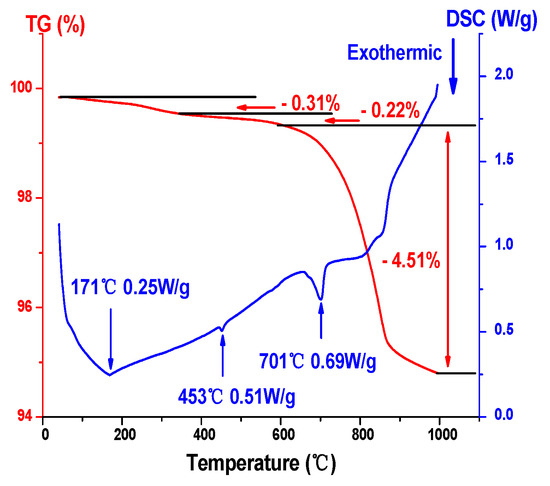
Figure 3.
TG–DSC curves for a 1% Ni-doped sample.
3.4. SEM Analysis
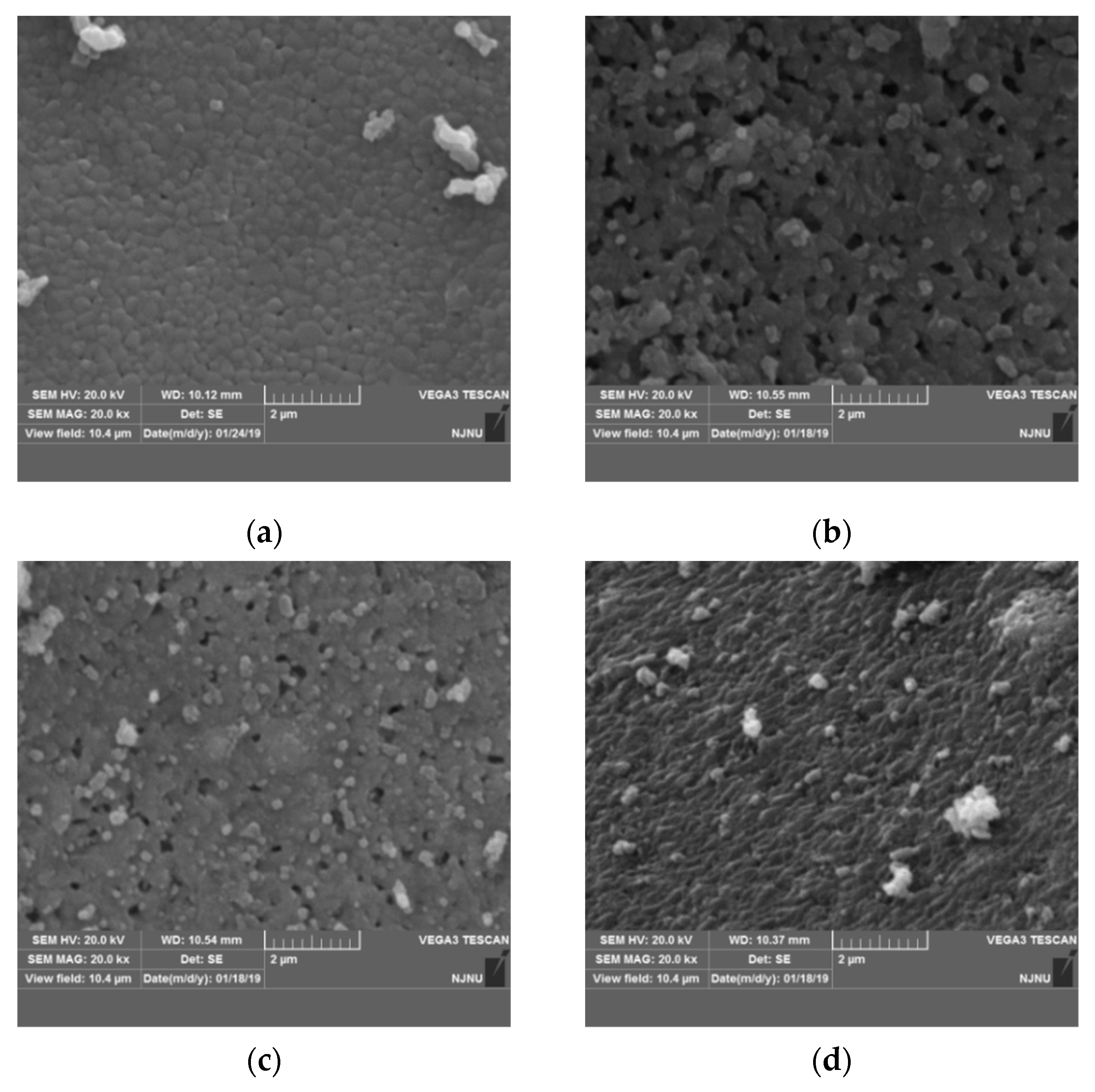
To study the effect of nickel doping on the surface morphology, particle size of Li2TiO3 crystal, and determine the optimum amount of Ni-doping in the precursor, samples were characterized by SEM. Figure 4 shows the SEM images of the precursor of the lithium-ion sieve with different nickel doping amounts. Some particles in the undoped sample are ellipsoid while the others are irregular with a smooth and dense surface and a compact and orderly arrangement, which is not conducive to the subsequent eluting transition process. Accompanying the increasing nickel doping amount, the roughness of the particle surface also increases. Compared with the pristine sample, the particle size of the sample with 1% Ni doping in Figure 4b increases to approximately 100–200 nm. The morphology of the sample is no longer ellipsoidal but granular. The results are consistent with the results of lattice distortion and the expansion effect in XRD analysis. Moreover, the lattice distribution of Li2TiO3 is changed by the nickel addition, thus affecting the formation and growth of the crystal nucleus and the formation of obvious particle accumulation pores, which is consistent with the following BET analysis.
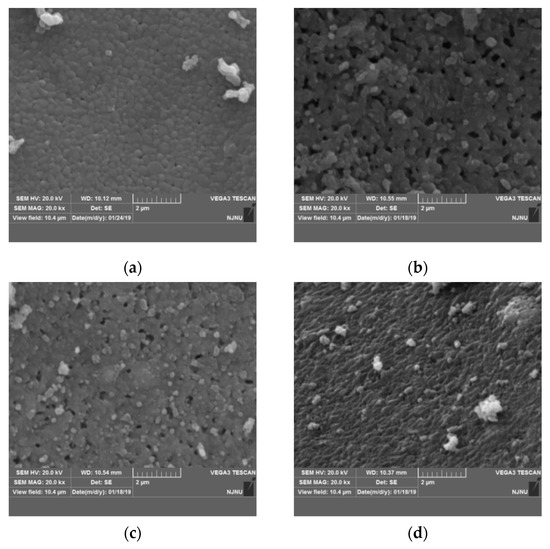
Figure 4.
SEM images of Li2TiO3 doped with different amounts of nickel at 750 °C. (a) 0% Ni; (b) 1% Ni; (c) 2% Ni; (d) 3% Ni.
Due to the increased extent of the mesoporous structure and the neat arrangement of mesopores, the surface presents a porous structure. Therefore, the sample doped with 1% nickel is conducive to elution in the pickling process and can achieve elution equilibrium in a shorter time [23]. Additionally, a porous structure is helpful to improve the absorption capacity of the adsorbent. The particle size of the sample with 2% nickel also is increased and contains some particle accumulation pores. The particle size and pore size are not uniform. Seen in Figure 4d, the sample completely loses the pore structure due to the excessive amount of nickel doping, which is not conducive to the subsequent elution transition process for Li2TiO3. The above phenomenon indicates that the nickel doping amount has a direct effect on the morphology of the crystal. An appropriate amount of Ni doping (1% in this work) can form a surface with an obvious porous structure, which is desired for the elution transition process. The physical adsorption capacity of the titanium lithium ion sieve also is improved.
3.5. BET Analysis
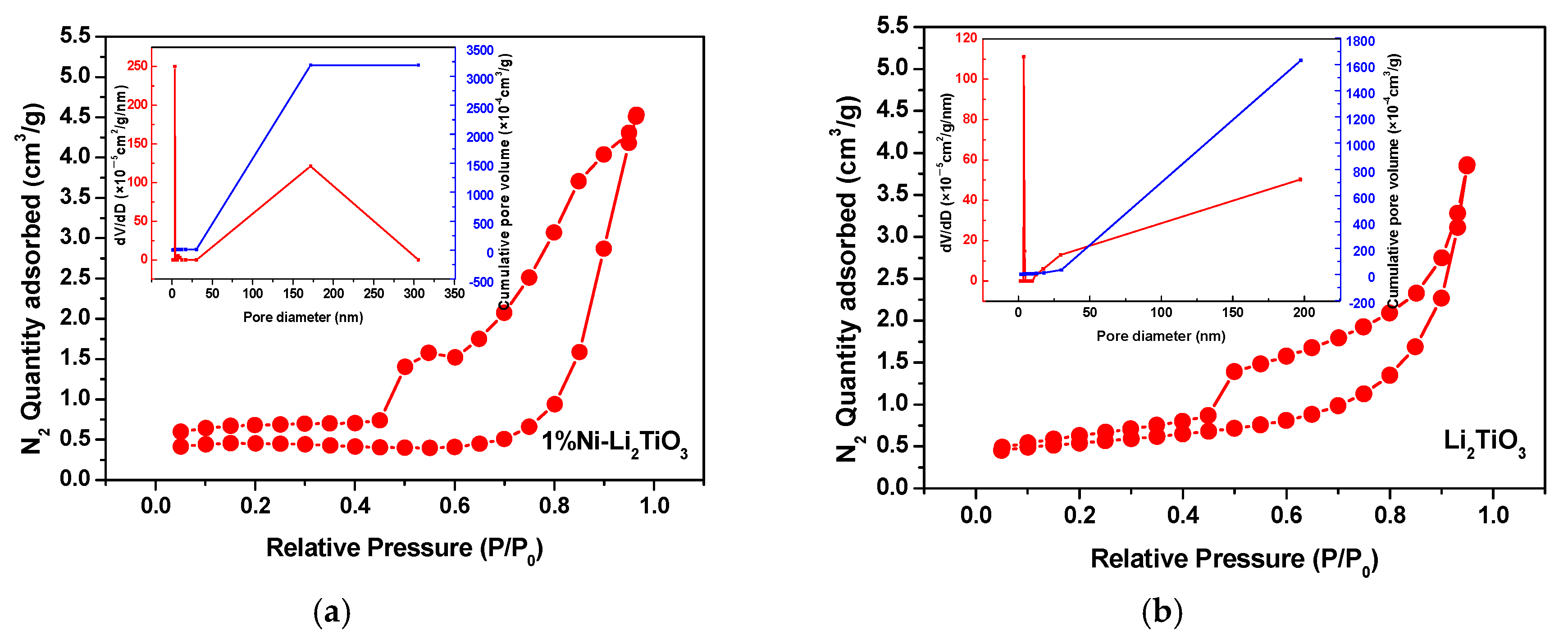
The above SEM test shows that both the pure sample and the Ni-doped sample have a porous structure. To further understand the specific situation of the pore structure of the samples, BET characterization is used. Figure 5 shows the N2 adsorption–desorption isothermal curves for Li2TiO3 doped with and without 1% nickel doping. Displayed in Figure 5, the absorption capacity increases steeply with increasing pressure when the relative pressure P/P0 < 0.4. The absorption capacity increases sharply with increasing pressure when the relative pressure P/P0 > 0.9. Both the samples show the characteristic type-Ⅳ adsorption–desorption isotherms, which is related to the fact that the sample contains a certain amount of mesoporous structure [33]. Additionally, slit holes or particle stacking holes also may exist [34]. The relative pressure required for the appearance of a hysteresis ring for a 1% nickel-doped sample is smaller than that for the pure sample, with its larger area, larger number of mesoporous structures, and greater N2 absorption capacity. This confirms that the absorption capacity of the sample has a certain relationship with its specific surface area and pore volume [35].
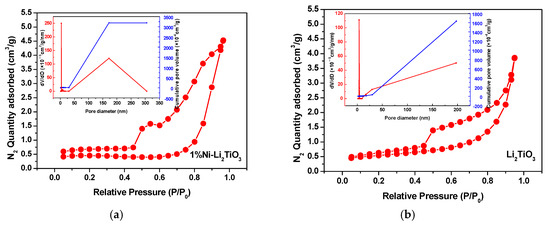
Figure 5.
Nitrogen adsorption–desorption isotherm and pore size distribution for Li2TiO3. (a) Li2TiO3; (b) 1% Ni-Li2TiO3.
The parameters for the pore structures of the samples are listed in Table 2. The pore diameter of the pure sample and 1% Ni-doped sample is approximately 3.8 nm, while the pore volume of the sample doped with 1% Ni is increased significantly by approximately twice that for the pure sample, which is because nickel enters the lattice of the lithium titanate, resulting in structural changes in the lithium titanate and the narrower orifice. Looking at the aperture distribution of the sample given in Figure 5, it also can be known that the maximum cumulative pore volume of the nickel doping samples is twice as that of the pure sample. Moreover, the presence of the mesoporous orifice can increase the specific surface area. Thus, the specific surface area and pore volume of Li2TiO3 are improved greatly [36] after Ni doping.

Table 2.
Pore structures of samples.
3.6. XRD Analysis
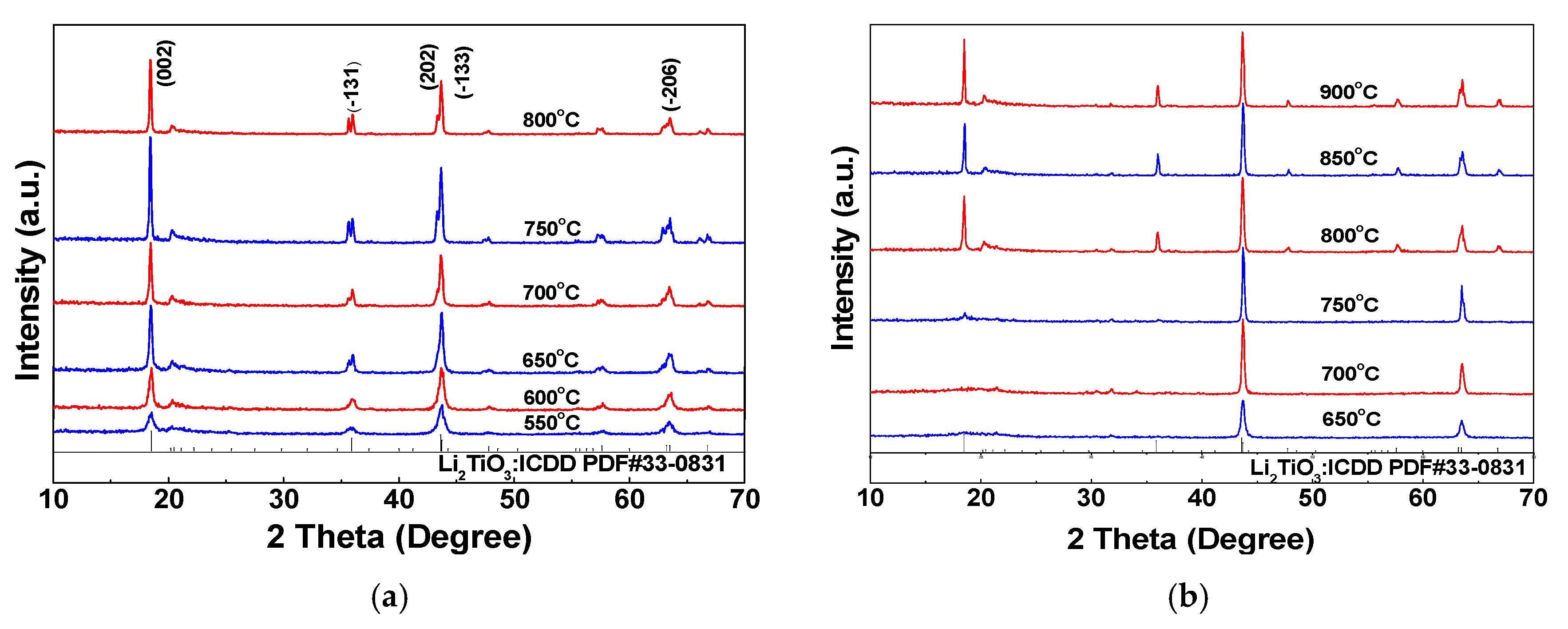
To determine the crystal phases of the samples and investigate the influence of the calcination temperature and nickel doping amount on the crystal size and crystal plane growth of Li2TiO3, samples are analyzed by XRD. According to the XRD patterns for undoped samples at different calcination temperatures (Figure 6), the XRD peaks for the undoped samples at different calcination temperatures are coincident with the standard card, and no heteropeaks appear, indicating that the Li2TiO3 product formed has a relatively high purity. Peak division is observed for the (−131) crystal plane at 650–800 °C. This is because the X-ray used in the test is the characteristic Ka emission from the metal target, and the Ka ray actually is composed of Ka1 and Ka2 rays with very similar wavelengths. Since a special monochromator is not used in the test, the collected XRD pattern is actually a superimposed spectrum of two kinds of diffraction patterns and, thus, the data appear to show two split peaks. A splitting in the other peaks is not obvious due to peak broadening. It also can be seen from Figure 6a that the diffraction peak intensity for lithium titanate obtained at a low calcination temperature (550–600 °C) is low, which indicates that the obtained crystal has a low crystallinity and small grain size. As the calcination temperature increases, however, the intensity of the characteristic diffraction peak increases correspondingly and the peak becomes sharper, illustrating the growing grain size and the more complete crystal structure. The diffraction peak intensity of the crystal plane (−133) is generally high at 650–800 °C, indicating that the lithium and titanium ions in the interstitial plane (Li2 layer) are arranged in order. As the calcination temperature increases, the ratio I(002)/I(−133) for the Li2TiO3 powders is increased gradually, indicating that the crystal supercellular structure is developed gradually and the particle uniformity of the powder is better. However, this is not conducive to the subsequent elution and absorption reactions of the samples due to the large grain size and the correspondingly reduced specific surface area.
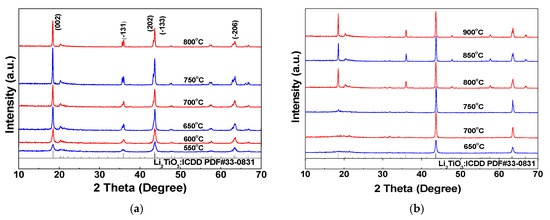
Figure 6.
XRD patterns for samples at different calcination temperatures. (a) Li2TiO3; (b) Li2TiO3 doped with 1% Ni.
According to the XRD patterns for the sample at different calcination temperatures with a Ni doping amount of 1% (Figure 6b) and the standard card, it can be seen that, when the calcination temperature is lower than 750 °C, the characteristic peaks due to the (002) and (−131) crystal faces of the obtained Li2TiO3 powders do not appear. This may be due to the different ionic radii of Ni2+ and Ti4+ (rNi²⁺ = 0.069 nm, rTi⁴⁺ = 0.0605 nm). When a small amount of Ni2+ is doped, Ni2+ replaces a part of Ti4+ in the lattice to form a substitutional point defect, causing lattice distortion. Moreover, it also leads to lattice expansion and reduces the order of the original crystal structure. Thus, the intensity for the corresponding crystal plane diffraction peak of the product is weakened or even completely suppressed [37]. When the calcination temperature is 750 °C, the diffraction peaks due to the (002) and (−131) planes appear in Li2TiO3 with a Ni doping amount of 1%, but the diffraction peak intensity is quite weak, indicating that the crystal grain development is incomplete. However, compared with the undoped samples, the grain size of the samples shows a growing trend, which is consistent with the SEM analysis results. The intensity of the characteristic diffraction peak increases correspondingly as the calcination temperature increases; furthermore, the peak becomes sharp, which indicates increased grain size and grain growth integrity. The grain sizes of undoped and doped 1% Ni samples at different calcination temperatures are calculated according to the Scherrer formula (Equation (1)) and listed in Table 3 and Table 4, which shows that the grain size of the sample increases with temperature. This is because the atomic thermal activation energy and the diffusion coefficient of the atoms both increase with increasing temperature, which makes it easier for the atoms to migrate from the grain boundary to another crystal cell and form the state of atomic aggregation, thus making the overall energy of the crystal more stable [38]. When the calcination temperature is too high, however, the pore size and pore volume of the mesoporous crystal are reduced significantly, the skeletal density of the crystal increases accordingly, and the surface of the sample becomes dense, which is not conducive to ion intercalation and is drawn-out [39]. Shown in Table 4, when the calcination temperature is 750 °C, the crystal plane growth is incomplete. Following the addition of a small amount of NiCl2∙6H2O dispersant, however, when calcined at 750 °C, the growth speed of the nucleus is greater than that of crystal nucleation [40], which results in the largest grain size of the crystal at this temperature. Accompanying the increase of the calcination temperature to 800 °C, however, the grain size of the crystal decreases. This could be because, with the increase of the calcination temperature, the cell parameters a, b and c increase, resulting in the decrease of grain size (crystal grain miniaturization) and the increase of lattice distortion [41]. Alongside the further increase of the calcination temperature to 850 and 900 ℃, the crystal grain size increases to a certain extent and then tends to be stable, which may be due to the distribution of fine Ni oxide particles in the crystal. The grain boundary is pinned by the oxide particles, and the grain growth is restrained. Thus, the grain size tends to be stable after growing to a certain extent. Viewing Table 3 and Table 4, it can be determined that the grain size of the sample after doping with Ni is larger than that of the undoped sample at the same calcination temperature. This probably is due to the fact that the Ni doping not only increases the specific surface area and pore volume of the mesoporous sample but also reduces the unit cell shrinkage [42]. According to previous report [43], the unit cell shrinkage increases as the grain size decreases. Thus, the unit cell shrinkage decreases with increasing grain size.

Table 3.
Cell parameters for pure samples at different calcination temperatures.

Table 4.
Lattice parameters for 1% Ni-doped samples at different calcination temperatures.
D = Kλ/(βcosθ)
3.7. TEM Analysis
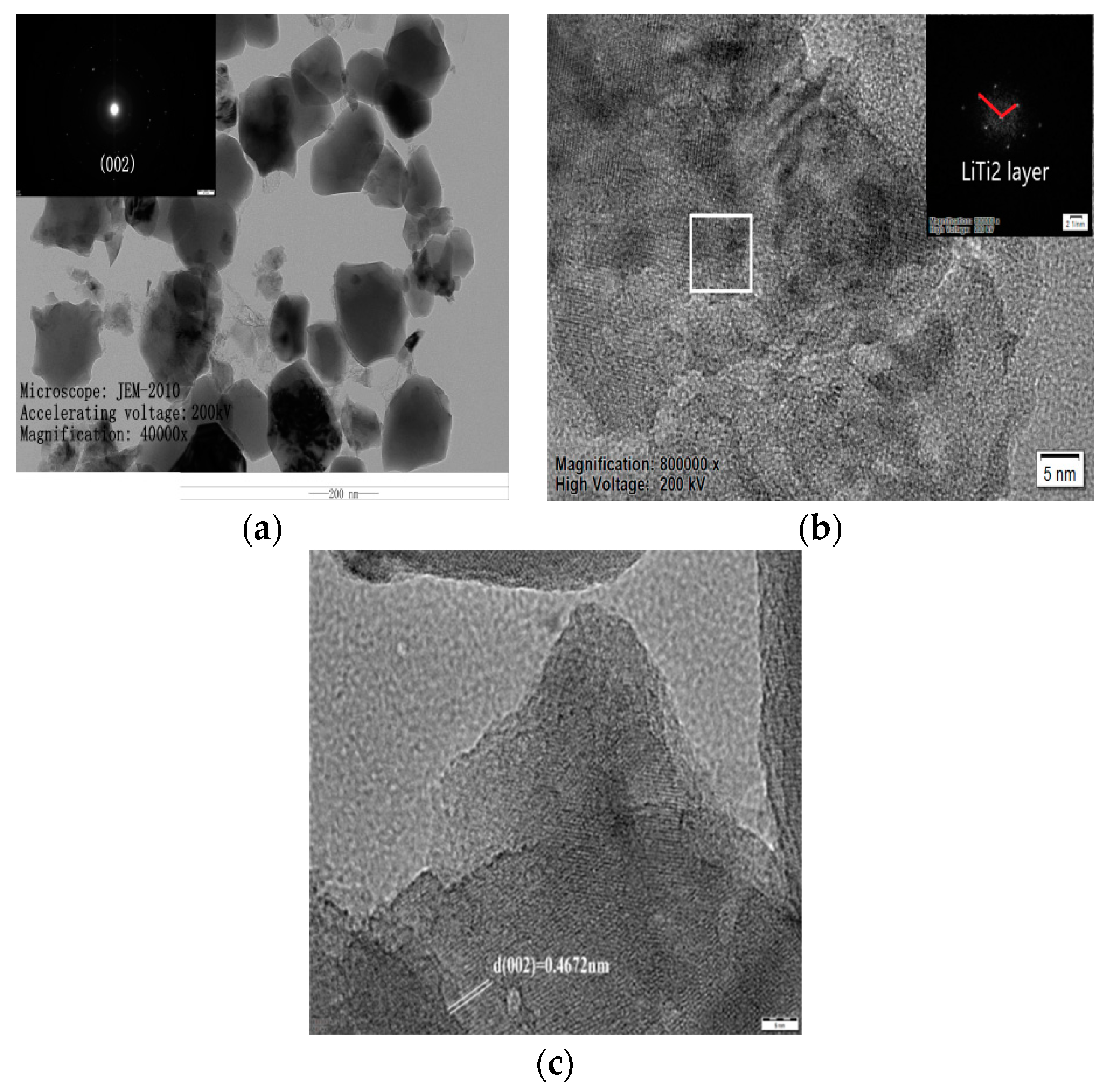
To further determine the influence of nickel doping on atom arrangement and interplanar spacing, TEM characterization is carried out. TEM images and an electron diffraction map for the Li2TiO3 after doping 1% Ni are shown in Figure 7. It is evident that the doped sample has a block structure with a particle size of 100–200 nm (Figure 7a, High Voltage: 200 KV, Magnification: 40,000x, Ruler: 200 nm). According to the electron diffraction map, the Ni-doped sample has a polycrystalline structure in which the electron diffraction ring corresponds to the (002) crystal surface, indicating that the atoms in the pure Li layer of the doped sample are arranged in order after calcination at 750 °C [19]. While the bright spot strength is quite low, which indicates that the grain development is not complete, the atoms in the pure Li layer are arranged with low order. This is consistent with the weak peak strength of the (002) crystal surface at 750 °C in the XRD pattern. Looking at Figure 7b (Magnification: 800,000×, High Voltage: 200 KV, Ruler: 5 nm), the layered structure of the constituency corresponds to the LiTi2 layer along the C-axis direction and there are no periodic conversion spots in the vertical direction of the LiTi2 layer in the selected SADE graph, which is consistent with the work by Yu et al. [19]. Periodic bright spots in the direction of 110° appear, however, which is 8° larger than that reported by Yu et al. [19]. It is suggested that Ni atoms replace the Ti atoms that are substituted for the Li atom in the original pure Li layer. Following calcination at 750 °C, the Li atom forms a lattice defect, resulting in a low arrangement order of atoms in the pure Li layer. Figure 7c (Ruler: 5 nm) demonstrates that the (002) interplanar spacing is 0.4672 nm, which is smaller than the theoretical value of (002) (0.4800 nm). This is due to the lattice distortion caused by the Ni2+ replacing Ti4+ in the lattice due to the larger ion radius, causing a change in the cell parameters and, eventually, leading to a decreased interplanar spacing.
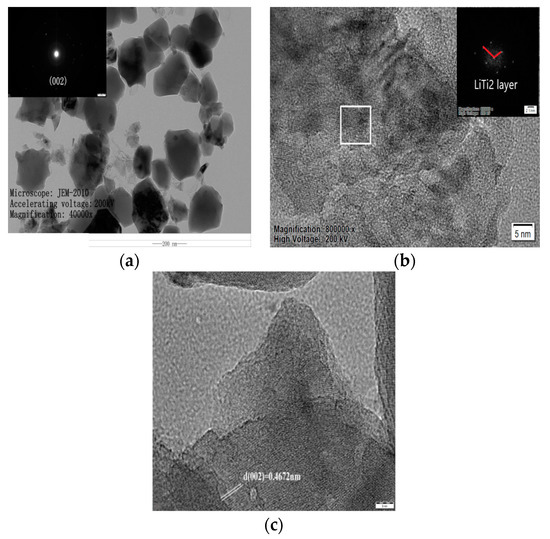
Figure 7.
TEM images of samples after adding 1% Ni. (a) Sample Magnified 40,000×; (b) Selected area electron diffraction pattern; (c) (002) Crystal plane spacing.
3.8. FT–IR Analysis
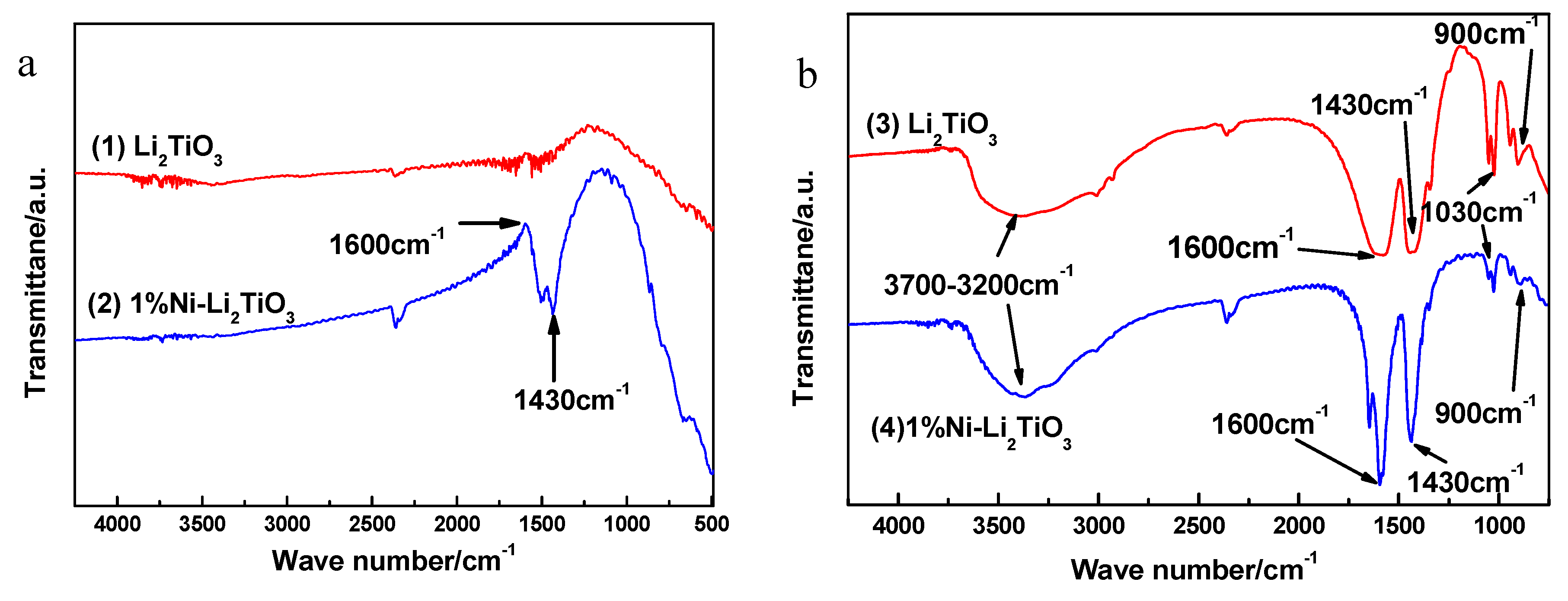
To determine the influence of nickel doping on the functional group structure of the samples, FT–IR analysis is used. Figure 8a shows the infrared spectra for the sample calcined at 750 °C. Curve (1) is pure Li2TiO3 and Curve (2) corresponds to the sample doped with 1% Ni. Seen in Curve (1), the characteristic peaks at 3700–3200, 1600, 1430, 1030 and 900 cm−1 disappear because H2O, SO42−, NH4+ and oxygen groups are removed with an increasing calcination temperature. Compared to Curve (1), the characteristic peaks at 3700–3200, 1030 and 900 cm−1 in Curve (2) also disappear, which is due to the increase in calcination temperature. The negative peaks at 1600 cm−1 in Curve (2) are caused by the decrease in water content in the environment. The characteristic peak at 1430 cm−1 is because six hydrated nickel chloride is coated on the surface of the sample, resulting in a portion of NH4+ not being removed completely. The wide peaks at 3700–3200 cm−1 in Curves (3) and (4) are the stretching vibration peaks of O–H in their association with water. The absorption peaks near 1600 cm−1 are caused by the bending vibration of water molecules and the absorption peaks near 1430 cm−1 are caused by the telescopic vibration of the N–H bond in NH4+ ions. Additionally, the small absorption peaks at approximately 1030 cm−1 are caused by SO42−, indicating that SO42− and NH4+ are not removed completely during the preparation of the sample. The peak at 900 cm−1 belongs to the characteristic absorption peak of –O–O– (peroxide group), which indicates that a peroxide bond exists in the sol. The unstable hydrogen peroxide basically is decomposed completely in the process of drying, and the influence of the peroxide group in the hydrogen peroxide can be ruled out, suggesting that the sol system is composed of peroxide titanium complexes. Curve (4), however, shows a sharper characteristic peak at 1600 cm−1 and 1430 cm−1 compared to Curve (3), which indicates that the structure of the sample surface is affected by the nickel chloride hexahydrate cladding layer.
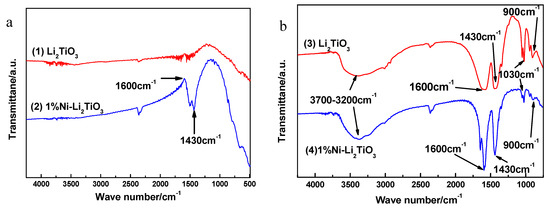
Figure 8.
Infrared spectra for Li2TiO3 prepared under different conditions. (a) After calcination; (b) Before calcination.
3.9. XPS Analysis
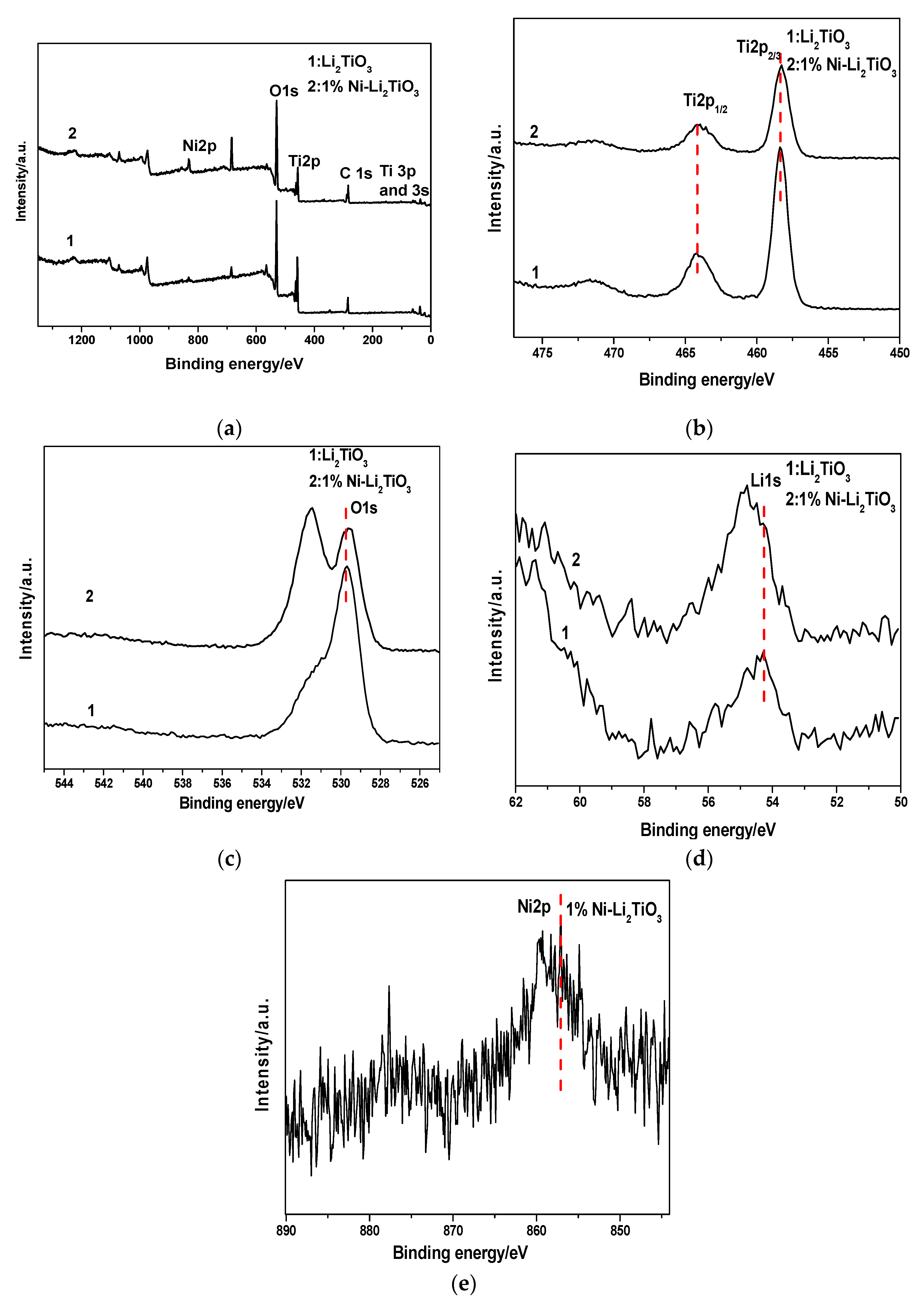
XPS characterization is used to determine the surface element composition and the relative atomic concentrations, the corresponding chemical bond, and valence state of the pure sample and Ni-doped sample at 750 °C. Thereby, the effect of Ni doping on the chemical environment of the original elements in the sample can be investigated. The X-ray source is a monochromatic Al Kaa (hvn = 1486.6 eV) with a power of 150 W. The vacuum degree of the chamber is better than 1 × 10−7 Pa, the fixed transmission energy of the energy analyzer is 30 eV, and the size of the beam spot is 500 μm. The charge correction of the sample spectrum is carried out using the carbon pollutant C1s with a binding energy of 284.60–284.80 eV. The results are shown in Figure 9. The XPS full spectrum of the undoped sample (Curve 1) and the Ni-doped sample (Curve 2) are given in Figure 9a. It can be known that the undoped sample mainly is composed of Li, O, and Ti. Following Ni doping, the sample is mainly composed of Li, O, Ti and Ni, wherein element C is introduced by the pretreatment process before the sample test. The two peaks with binding energies of 464.08 eV and 458.28 eV in Figure 9b correspond to Ti 2p1/2 and Ti 2p3/2, respectively. As seen in Figure 9b, the peak signal at 458.28 eV is stronger, indicating that Ti4+ loading is increased in the sample. Regarding the Ni-doped sample, almost no significant shift in the two peaks is observed compared to the undoped sample. It preliminarily can be concluded that the incorporation of Ni does not change the chemical environment of Ti in Li2TiO3 calcined at 750 °C and that the outer electrons of the titanium atom do not undergo significant transfer. This indicates that the outer electron density and chemical state of the titanium atom are not changed [44]. The intensities of the Ti 2p1/2 and Ti 2p3/2 peaks for the undoped samples are 7935.44 s and 22302.7 s, respectively, and they are reduced to 5020.69 s and 12708.8 s, respectively, which show that the content of Ti decreases after Ni-doping. The Ni2p spectrum, and the peak at 857.08 eV corresponding to the Ni–O bond, are given in Figure 9e.
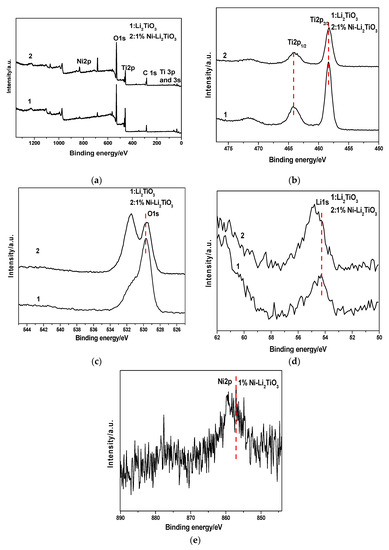
Figure 9.
XPS for samples before and after Ni doping. (a) Survey; (b) Ti 2p peaks; (c) O 1s peaks; (d) Li 1s peaks; (e) Ni 2p peaks.
The figure shows that, after the incorporation of nickel, a part of the Ni combines with O to form a Ni-O bond, which replaces the Ti–O bond in Li2TiO3. Thus, the Ti content decreases after Ni doping, and both the intensities of the Ti2p1/2 and Ti2p3/2 peaks are reduced. Figure 9c represents the O1s component, in which the peak signal of the undoped sample at 529.28 eV represents the Ti–O binding energy. Subsequent to 1% Ni-doping, the corresponding binding energy of the peak is 529.58 eV, which shifts toward higher energy by 0.30 eV.
This result shows the impacts of the Ni doping on the oxygen in titanium oxide—the outer electron density of it decreases, its shielding effect decreases, and the inner electron binding energy increases. Overall, the chemical state of oxygen changes accordingly [45]. According to the Curve (2) in Figure 9c, the O1s peak in the figure contains two low-energy peaks [45]. The peak at Eb = 529.58 eV belongs to lattice oxygen in Ti–O, while that at 531.48 eV is due to oxygen adsorption including surface hydroxyls and adsorbed water, most of which is due to surface hydroxyls [46]. Figure 9d shows the XPS spectra of the Li1s orbital component. The spectra show that, after doping with 1% Ni, the Li1s peak has an obvious chemical shift, the binding energy increases from 54.28 to 54.78 eV, moving 0.50 eV toward a higher binding energy. This shows that, after Ni-doping, the cell volume increases, and the diffusion of lithium ions are promoted because the radius of the nickel ion is larger than that of the titanium ion. Therefore, the outer electron density of lithium in lithium titanate decreases and the chemical state of lithium changes, which also is consistent with the previous TEM analysis.
Table 5 quantitatively shows the relative atomic concentrations of C 1s, O 1s, Li 1s, Ti 2p and Ni 2p in Li2TiO3 and 1% Ni–Li2TiO3 calcined at 750 °C. Viewing Table 5, the relative ratio of the atomic concentration of Li to Ti in Li2TiO3 is about 2:1, which is in accordance with the theoretical stoichiometric ratio, however, that in the sample 1% Ni-Li2TiO3 is greater than 2:1, indicating that the Ti content is reduced. Ni atoms account for 1.21 at %, however, which indicates that, after doping, Ni enters the crystal structure of Li2TiO3 and replaces part of Ti, which is consistent with the results of XRD and TEM analysis.

Table 5.
The relative atomic concentrations of C, O, Li, Ti and Ni before and after Ni doping.
The relative atomic concentrations of C 1s and O 1s changes before and after Ni doping. The reason for the change of C is there is a certain C content range due to the residual orbital composition in the pretreatment process before the test of the sample. The decrease of the relative atomic concentration of O is because the positive charge in the crystal is reduced after the replacement of Ti by Ni. To balance the positive charge, oxygen vacancy is produced and, thus, the relative atomic concentration of O is decreased after Ni doping.
4. Conclusions
Accompanying an increasing amount of Ni doping, the stability of the viscosity of the precursor sol first increased then decreased, and the maximum stabilization time of the precursor sol doped with 1% Ni was 87 h. Following doping with 1% Ni, the number of mesopores increased and the specific surface area increased by up to 1.349 m2/g. Along with the increase of calcination temperature, the inhibition of Ni doping on the growth and crystallization of grains decreased. When the calcination temperature was lower than 750 °C, Ni doping caused lattice defects, inhibited crystal plane growth, and reduced the ordering of crystal structure. When the calcination temperature was 750 °C, the grain size was the largest. Increasing the calcination temperature further, the effects of Ni doping on the growth and crystallization degree of crystal gradually were reduced.
Author Contributions
Performed the experiments, data collection and analysis, L.-Y.Z.; wrote the original draft, Y.S.; reviewed and edited the paper, L.-L.Z.; language editing, P.Z.; drawed the figures with software, W.-Y.X.; study design, Y.-H.Y.
Funding
This work was financially supported by Sichuan Science and Technology Program (Grant No. 2019YJ0383) and The Undergraduate Innovative Project of Neijiang Normal University (X2018075 and X2018025).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo, Y.Z.; Xu, X.; Zhang, Y.X.; Pi, Y.Q.; Zhao, Y.L.; Tian, X.C.; An, Q.Y.; Wei, Q.L.; Mai, L.Q. Hierarchical carbon decorated Li3V2(PO4)3 as a bicontinuous cathode with high-rate capability and broad temperature adaptability. Adv. Energy Mater. 2014, 4, 1400107. [Google Scholar] [CrossRef]
- Huang, H.; Faulkner, T.; Barker, J.; Saidi, M.Y. Lithium metal phosphates, power and automotive applications. J. Power Sources 2009, 189, 748. [Google Scholar] [CrossRef]
- Luo, Q.P.; Guo, P.C.; Li, C.Z.; Chen, L. Distribution of lithium resources and research status on lithium extraction technology. Hydrometall. China 2012, 2, 67–70. [Google Scholar]
- Zhao, X.; Wu, H.H.; Duan, M.S.; Hao, X.C.; Yang, Q.W.; Zhang, Q.; Huang, X.P. Liquid-liquid extraction of lithium from aqueous solution using novel ionic liquid extractants via COSMO-RS and experiments. Fluid Phase Equilib. 2018, 459, 129. [Google Scholar] [CrossRef]
- Zhang, L.C.; Li, L.J.; Shi, D.; Li, J.F.; Peng, X.W.; Nie, F. Selective extraction of lithium from alkaline brine using HBTA-TOPO synergistic extraction system. Sep. Purif. Technol. 2017, 188, 167. [Google Scholar] [CrossRef]
- Shi, C.L.; Jing, Y.; Xiao, J.; Wang, X.Q.; Jia, Y.Z. Liquid-liquid extraction of lithium using novel phosphonium ionic liquid as an extractant. Hydrometallurgy 2017, 169, 314. [Google Scholar] [CrossRef]
- Xiang, W.; Liang, S.K.; Zhou, Z.Y.; Qin, W.; Fei, W.Y. Extraction of lithium from salt lake brine containing borate anion and high concentration of magnesium. Hydrometallurgy 2016, 166, 9. [Google Scholar] [CrossRef]
- Wang, S.L.; Zheng, S.L.; Wang, Z.M.; Cui, W.W.; Zhang, H.L.; Yang, L.R.; Zhang, Y.; Li, P. Superior lithium adsorption and required magnetic separation behavior of iron-doped lithium ion-sieves. Chem. Eng. J. 2018, 332, 160. [Google Scholar] [CrossRef]
- Paranthaman, M.P.; Li, L.; Luo, J.Q.; Hoke, T.; Ucar, H.; Moyer, B.A.; Harrison, S. Recovery of lithium from geothermal brine with lithium–aluminum layered double hydroxide chloride sorbents. Environ. Sci. Technol. 2017, 51, 13481. [Google Scholar] [CrossRef]
- Song, J.F.; Nghiem, L.D.; Li, X.M.; He, T. Lithium extraction from chinese salt-lake brines: opportunities, challenges, and future outlook. Environ. Sci. Wat. Res. Technol. 2017, 3, 593. [Google Scholar] [CrossRef]
- He, L.H.; Xu, W.H.; Song, Y.F.; Liu, X.H.; Zhao, Z.W. Selective removal of magnesium from a lithium-concentrated anolyte by magnesium ammonium phosphate precipitation. Sep. Purif. Technol. 2017, 187, 214. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Li, S.P.; Sun, S.Y.; Yin, X.S.; Yu, J.G. LiMn2O4 spinel direct synthesis and lithium ion selective adsorption. Chem. Eng. Sci. 2010, 65, 169. [Google Scholar] [CrossRef]
- Xu, H.; Chen, C.G.; Song, Y.H. Synthesis and properties of lithium ion-sieve precursor Li4Mn5O12. J. Nonferr. Metal. 2013, 28, 720. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Yuan, J.S.; Li, X.G. Extraction of solanesol by dynamic saponification method. Chem. Eng. 2007, 35, 9. [Google Scholar]
- Hagh, N.M.; Amatucci, G.G. Effect of cation and anion doping on microstructure and electrochemical properties of the LiMn1.5Ni0.5O4−δ spinel. J. Power Sources 2014, 256, 457. [Google Scholar] [CrossRef]
- Dong, D.Q.; Zhang, F.B.; Zhang, G.L.; Liu, Y.F. Synthesis of Li4Ti5O12 and its exchange kinetics with Li+. Acta Phys. Chim. Sin. 2007, 23, 950. [Google Scholar]
- Shi, X.C.; Zhang, Z.B.; Zhou, D.F.; Zhang, L.F.; Chen, B.Z.; Yu, L.L. Synthesis of Li+ adsorbent (H2TiO3) and its adsorption properties. T. Nonferr. Metal. Soc. 2013, 23, 253. [Google Scholar] [CrossRef]
- Yu, C.L.; Yanagisawa, K.; Kamiya, S.; Kozawa, T.; Ueda, T. Monoclinic Li2TiO3 nano-particles via hydrothermal reaction: Processing and structure. Ceram. Int. 2014, 40, 1901. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhou, D.L.; Yao, Q.Q.; Zhou, J.B. Preparation of H2TiO3-lithium adsorbent by the sol-gel process and its adsorption performance. App. Surf. Sci. 2016, 368, 82. [Google Scholar] [CrossRef]
- Yao, Q.Q.; Zhou, J.; Zhang, L.; Yang, S.; Zhou, D. Study on H2TiO3-1ithium exchanger synthesized by precipitation-peptization method. Iron Steel Vanadium Titan. 2017, 38, 38. [Google Scholar]
- Zhang, L.Y.; Zhou, D.L.; He, G.; Yao, Q.Q.; Wang, F.H.; Zhou, J.B. Synthesis of H2TiO3-lithium adsorbent loaded on ceramic foams. Mater. Lett. 2015, 145, 351. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Liu, Y.W.; Zhou, D.L.; Yao, Q.Q. Dynamic adsorption and elution performances of H2TiO3-lithium adsorbent loaded on ceramic foams. J. Sichuan Univ. (Nat. Sci. Edi.) 2017, 54, 1275. [Google Scholar]
- Zhang, L.Y.; Liu, Y.W.; Huang, L.; Li, N. A novel study on preparation of H2TiO3-lithium adsorbent with titanyl sulfate as titanium source by inorganic precipitation-peptization method. RSC Adv. 2018, 8, 1385. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, P.; Xiang, W.; Ran, F.Y.; Cao, W.B. A novel inorganic precipitation-peptization method for VO2 sol and VO2 nanoparticles preparation: Synthesis, characterization and mechanism. J. Colloid Interf. Sci. 2016, 462, 42. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Ma, L.B.; Zhang, Q. Research progress of cationic doping of lithium manganese oxide ion-sieve. J. Salt Sci. Chem. Ind. 2018, 47, 8. [Google Scholar]
- Mohan, P.; Ranjith, B.; Kalaignan, G.P. Structure and electrochemical performances of co-substituted LiSmxLa0.2-xMn1.80O4 cathode materials for rechargeable lithium-ion batteries. J. Solid State Electr. 2014, 18, 2183. [Google Scholar] [CrossRef]
- Kamata, H.; Tian, Z.Q.; Izumi, Y. Dispersed and high loading Ni catalyst stabilized in porous SiO2 matrix for substituted natural gas production. Catal. Today. 2018, 299, 193. [Google Scholar] [CrossRef]
- Todorov, Y.M.; Hideshima, Y.; Noguchi, H.; Yoshio, M. Determination of theoretical capacity of metal ion-doped LiMn2O4 as the positive electrode in Li-ion batteries. J. Power Sources 1999, 77, 198. [Google Scholar] [CrossRef]
- Feng, L.Y.; Jiang, X.X.; Wang, S.D.; Fan, Y.Q.; Zhang, L.H.; Jiang, W.; Yang, X.W. The adsorption performance of doped lithium ion sieve. Nonferr. Metal (Ext. Met.) 2008, 6, 31. [Google Scholar]
- Humphreys, B.A.; Wanless, E.J.; Webber, G.B. Effect of ionic strength and salt identity on poly(N-isopropylacrylamide) brush modified colloidal silica particles. J. Colloid Interf. Sci. 2018, 516, 153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, H.J.; Guo, Y.M.; Li, B.; Hou, H.Y. Stability of Ni/SiO2 sol and phase-chemical structure of its gel material. Chem. Eng. 2019, 47, 47. [Google Scholar]
- Dai, Y.M.; Mao, I.H.; Chen, C.C. Evaluating the optimum operating parameters of biodiesel production process from soybean oil using the Li2TiO3 catalyst. J. Taiwan Inst. Chem. Eng. 2017, 70, 260. [Google Scholar] [CrossRef]
- Liu, L.C.; Ji, Z.Y.; Zou, W.X. In situ loading transition metal oxide clusters on TiO2 nanosheets as co-catalysts for exceptional high photoactivity. ACS. Catal. 2013, 3, 2052. [Google Scholar] [CrossRef]
- Xiang, Z.H.; Mercado, R.; Huck, J.M.; Wang, H.; Guo, Z.H.; Wang, W.C.; Cao, D.P.; Haranczyk, M.; Smit, B. Systematic tuning and multifunctionalization of covalent organic polymers for enhanced carbon capture. J. Am. Chem. Soc. 2015, 137, 13301. [Google Scholar] [CrossRef]
- Xu, Q.; Zou, L.X.; Xiong, X.Q. Structure and property of nano crystalline WO3·NiO·0.33H2O photo-catalyst. Chem. Ind. Eng. Prog. 2010, 29, 260. [Google Scholar]
- Long, F.; Zhang, J.; Zhang, M.Y.; He, J.Y.; Wu, X.L.; Zou, Z.G. Ni-doped FeS2: Solvothermal synthesis and the visible-light photocatalytic properties. Chinese J. Inorg. Chem. 2015, 31, 1119. [Google Scholar]
- Sadanov, E.V.; Dudka, O.V.; Ksenofontov, V.A.; Mikhailovskij, I.M.; Maziova, T.I.; Manakin, V.L.; Starchenko, I.V. Binding energy of self-interstitial atoms to grain boundaries: An experimental approach. Mater. Lett. 2016, 183, 139. [Google Scholar] [CrossRef]
- Zhou, H.M.; Yuan, J.S.; Zhang, L.; Fu, Y.P.; Hou, J. Hydrothermal synthesis of doped chromium spinel-type lithium ion-sieve and its lithium absorption properties. J. Funct. Mater. 2011, 42, 621. [Google Scholar]
- Tang, J.; Zhang, D.; Bi, X.G.; Niu, W.; Dong, Y.N.; Zhou, W.P.; Zhang, R.Q.; Sun, X.D. Preparation and characterization of micron-level TiO2 powders for rutile single crystal growth using verneuil method. J. Funct. Mater. 2018, 49, 9184. [Google Scholar]
- Han, F.; Jia, L.; Qiao, X.L.; Jin, Y.; Fan, B.G. Effect of crystal structure on the desulfurization reactivity of magnesium slag. Chem. Ind. Eng. Prog. 2019, 38, 3319. [Google Scholar]
- Duan, Z.H.; Luo, L.T.; Shao, G.X.; Zhang, X.H. Effects of template and Ni dope on mesoporous titania material. J. Mol. Catal. 2005, 19, 298. [Google Scholar]
- Song, Z.H.; Guo, H.Q.; Zhang, C.H.; Wang, G.L.; Wu, C.H.; Wu, Q.S.; Wu, Z.J. Characterization of nano-tungsten powder and its lattice distortion analysis. Mater. Rev. 2009, 23, 106. [Google Scholar]
- Xu, G.M.; Shi, Z.; Deng, J. Characterization of adsorption of antimony and phosphate by using IOCS with XRD, FTIR and XPS. Acta Sci. J. Environ. Sci.-China 2007, 27, 402. [Google Scholar]
- Qiu, Y.; Wang, L.M.; Shen, Y.; Xu, L.H.; Chen, C. Preparation of Ni/ZnO and its application on modified polyester fabric. J. Funct. Mater. 2018, 49, 8189. [Google Scholar]
- Chen, G.; Song, C.; Chen, C.; Cao, S.; Zeng, F.; Pan, F. Resistive switching and magnetic modulation in cobalt-doped ZnO. Adv. Mater. 2012, 24, 3515. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).