Reduced Graphene Oxide–Epoxy Grafted Poly(Styrene-Co-Acrylate) Composites for Corrosion Protection of Mild Steel
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of EPSA
2.3. Preparation of rGO/EPSA Composites
2.4. Preparation of the Coating
2.5. Characterization
3. Results and Discussion
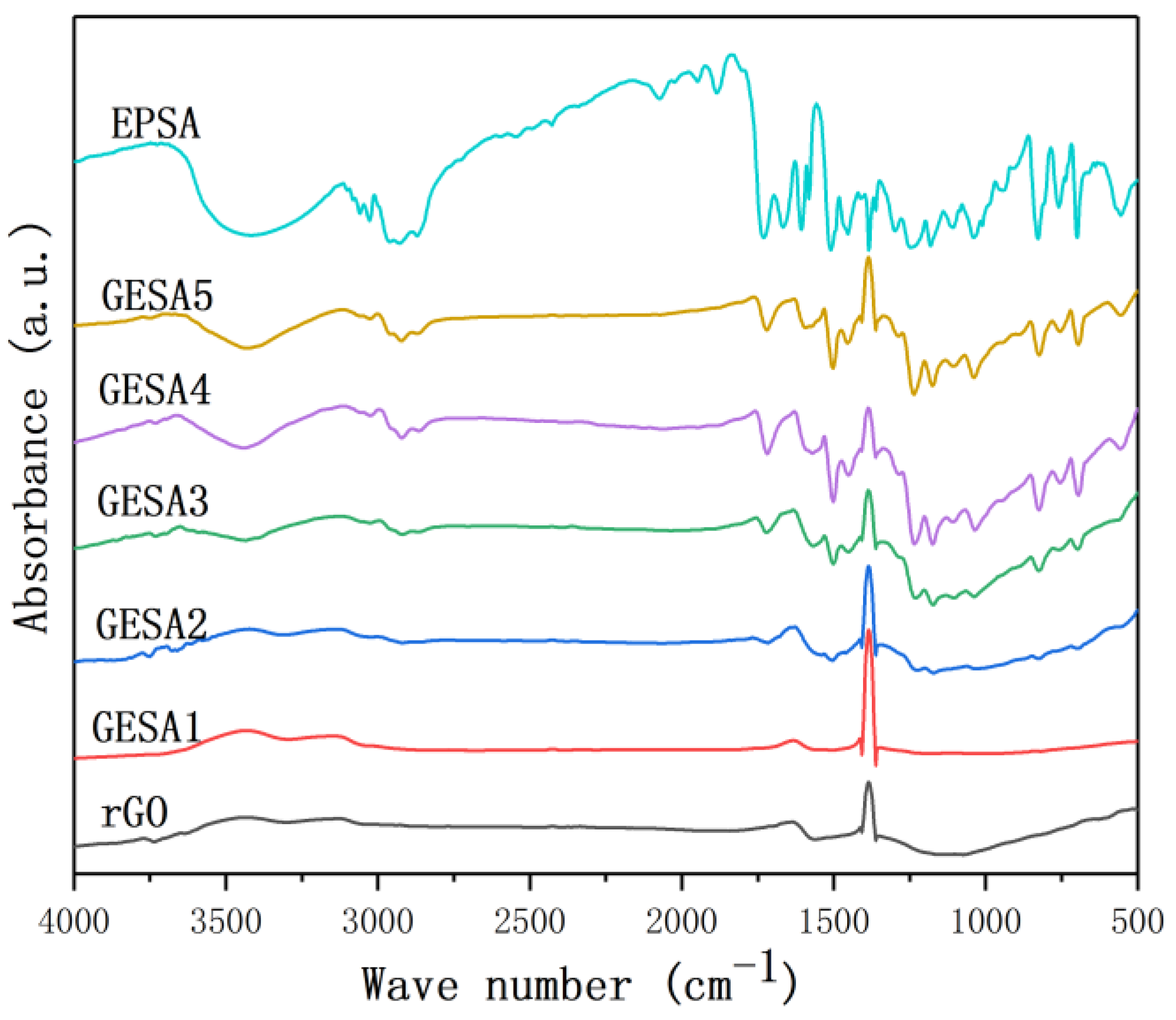
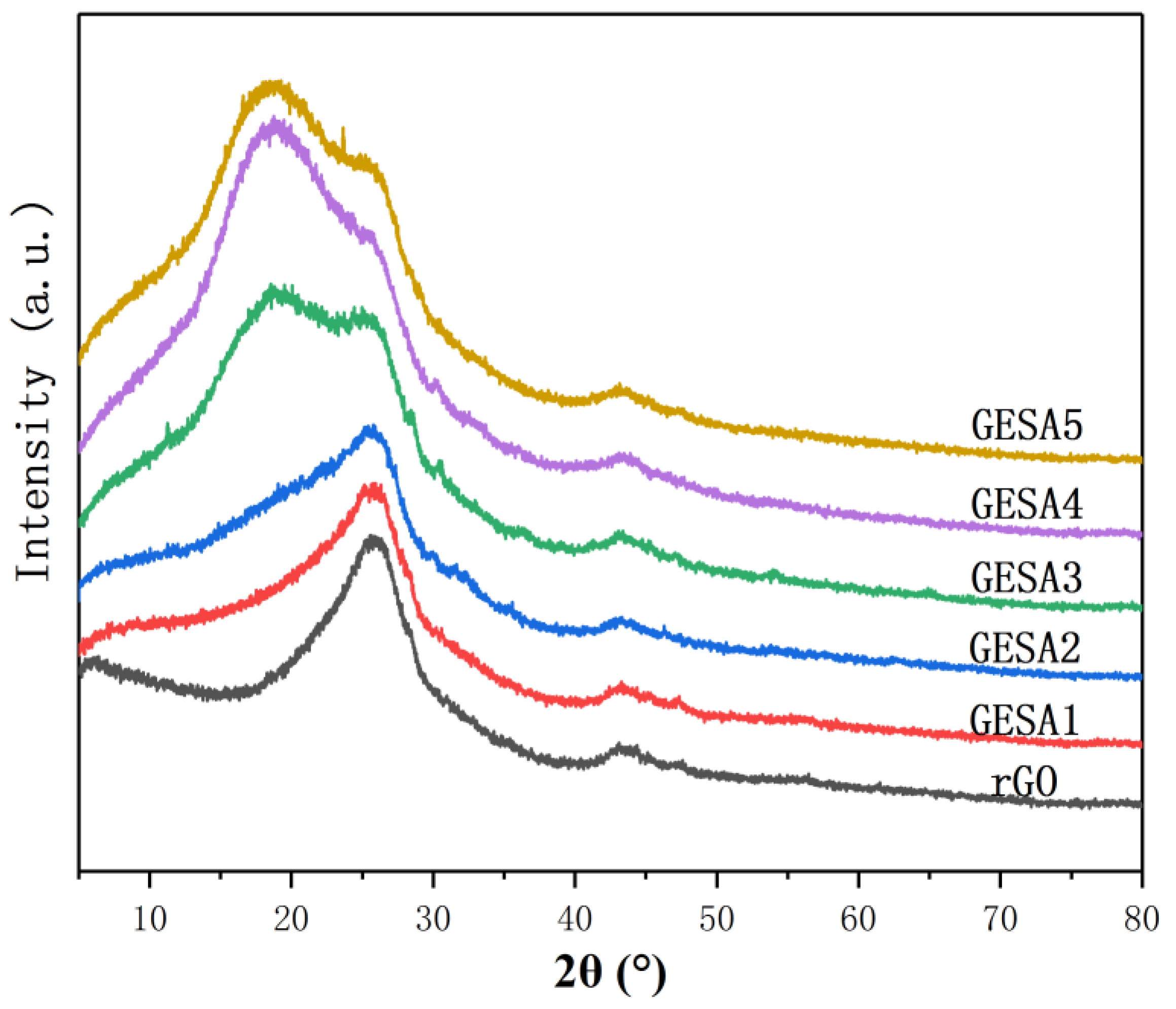
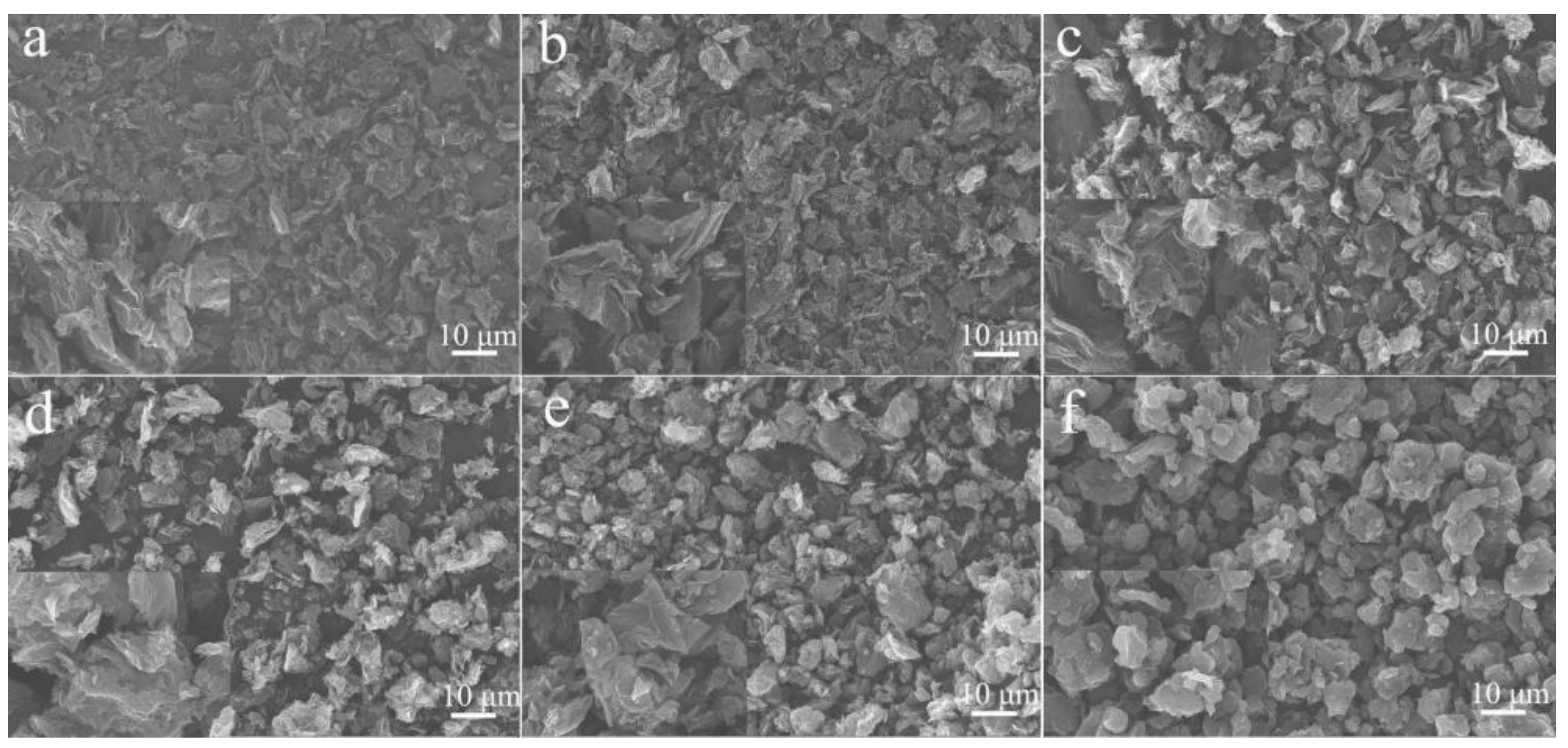
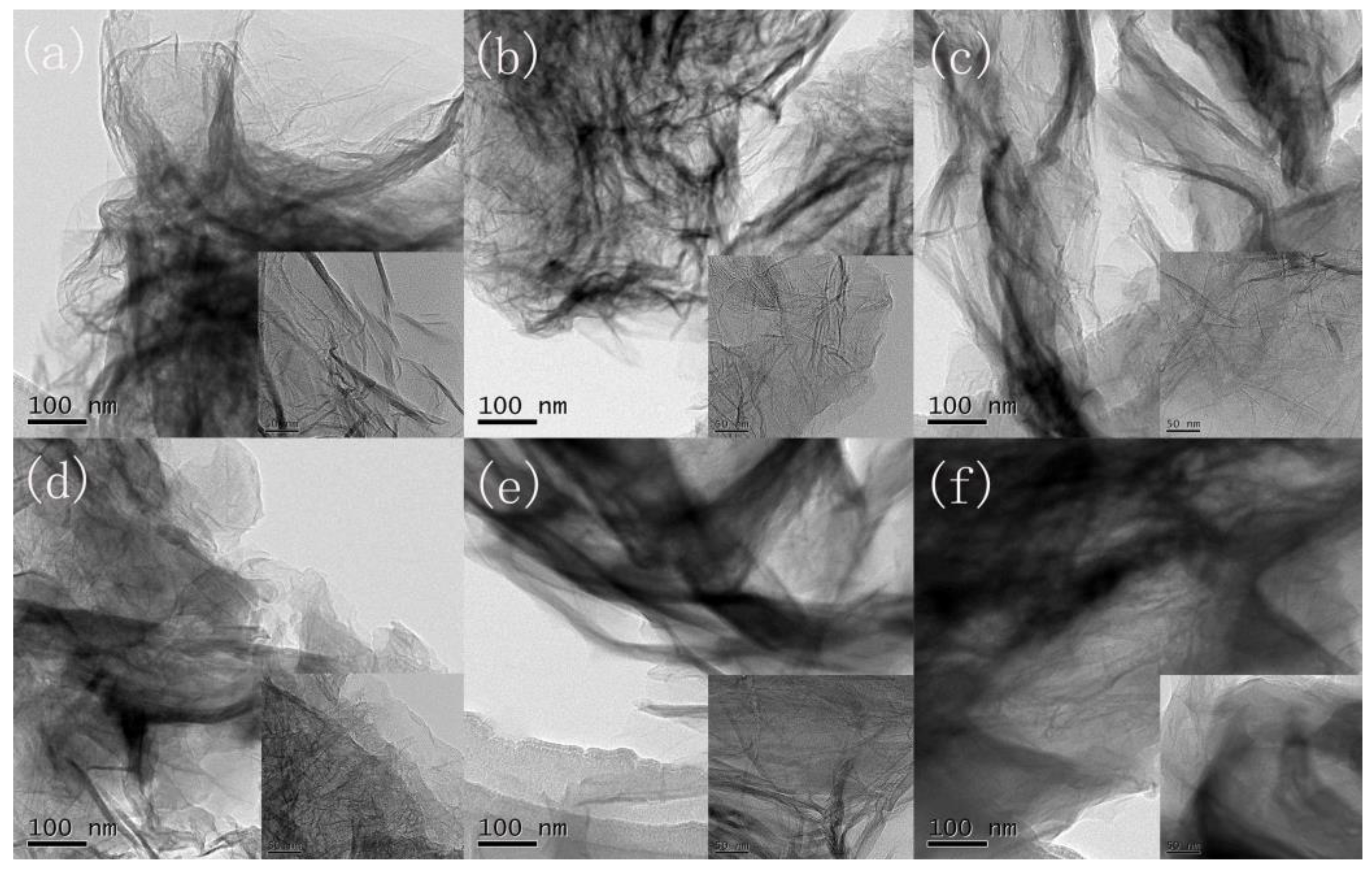
3.1. Characterization of GESA
3.2. Corrosion Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, L.; Pei, X.Y.; Mo, D.C.; Heng, Y.; Lyu, S.S.; Fu, Y.X. Facile fabrication of NiO flakes and reduced graphene oxide (NiO/rGO) composite as anode material for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2019, 30, 5874–5880. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Zabihi, Z.; Araghi, H.; Rodriguez, P.E.D.S.; Boujakhrout, A.; Villalonga, R. Vapor sensing and interface properties of reduced graphene oxide-poly (methyl methacrylate) nanocomposite. J. Mater. Sci. Mater. Electron. 2019, 30, 2908–2919. [Google Scholar] [CrossRef]
- Hu, X.R.; Ding, Z.T.; Fei, L.X.; Xiang, Y.; Lin, Y. Wearable piezoelectric nanogenerators based on reduced graphene oxide and in situ polarization-enhanced PVDF-TrFE films. J. Mater. Sci. 2019, 54, 6401–6409. [Google Scholar] [CrossRef]
- Cheng, H.H.; Ye, M.H.; Zhao, F.; Hu, C.G.; Zhao, Y.; Liang, Y.; Chen, N.; Chen, S.L.; Jiang, L.; Qu, L.T. A general and extremely simple remote approach toward graphene bulks with in situ multifunctionalization. Adv. Mater. 2016, 28, 3305–3312. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Kim, K.S.; Jeon, I.Y.; Ahn, S.N.; Kwon, Y.D.; Baek, J.B. Edge-functionalized graphene-like platelets as a co-curing agent and a nanoscale additive to epoxy resin. J. Mater. Chem. 2011, 21, 7337–7342. [Google Scholar] [CrossRef]
- Ghauri, F.A.; Raza, M.A.; Baig, M.S.; Ibrahim, S. Corrosion study of the graphene oxide and reduced graphene oxide-based epoxy coatings. Mater. Res. Express 2017, 4, 125601. [Google Scholar] [CrossRef]
- Huang, P.; Chen, T.; Zhang, G.M.; Zeng, B.Q.; Li, Z.M. An Electrically conducting polymer/graphene composite with a very low percolation threshold. Mater. Lett. 2010, 64, 2226–2229. [Google Scholar]
- Michael, M.; Slepchenkov, O.E. Mechanical and electroconductive properties of mono- and bilayer graphene–carbon nanotube films. Coatings 2019, 9, 74. [Google Scholar]
- Lotya, M.; King, P.J.; Khan, U.; Sukanta, D.; Coleman, J.N. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Luo, G.L.; Fan, Z.J.; Zheng, C.; Yan, J.; Yao, C.Z.; Li, W.F.; Zhang, C. Preparation of graphene nanosheet/polymer composites using in situ reduction–extractive dispersion. Carbon 2009, 47, 2296–2299. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Pro. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Yu, D.S.; Yang, Y.; Durstock, M.; Baek, J.B.; Dai, L.M. Soluble P3HT-grafted graphene for efficient bilayer-heterojunction photovoltaic devices. ACS Nano 2010, 4, 5633–5640. [Google Scholar] [CrossRef]
- Wang, N.; Diao, X.; Zhang, J. Corrosion resistance of waterborne epoxy coatings by incorporation of dopamine treated mesoporous-TiO2 particles. Coatings 2018, 8, 209. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.; Zhang, J. Effect of graphene oxide/ZSM-5 hybrid on corrosion resistance of waterborne epoxy coating. Coatings 2018, 8, 179. [Google Scholar] [CrossRef]
- Sainsbury, T.; Gnaniah, S.; Spencer, S.J.; Mignuzzi, S.; Belsey, N.A.; Paton, K.R.; Satti, A. Extreme mechanical reinforcement in graphene oxide based thin-film nanocomposites via covalently tailored nanofiller matrix compatibilization. Carbon 2017, 114, 367–376. [Google Scholar] [CrossRef]
- Hua, D.; Rai, R.K.; Zhang, Y.; Chung, T.S. Aldehyde functionalized graphene oxide frameworks as robust membrane materials for pervaporative alcohol dehydration. Chem. Eng. Sci. 2017, 161, 341–349. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lee, T.C.; Hsiao, Y.S.; Ling, W.K.; Whang, W.T.; Chen, C.H. Facile synthesis of diamino-modified graphene/polyaniline semi-interpenetrating networks with practical high thermoelectric performance. ACS Appl. Mater. Interfaces 2018, 10, 4946–4952. [Google Scholar] [CrossRef]
- Yeh, J.M.; Chang, K.C.; Hsu, C.H.; Lu, H.I.; Ji, W.F.; Chang, C.H.; Li, W.Y.; Chuang, T.L.; Yeh, J.M.; Liu, W.R.; et al. Advanced anticorrosive coatings prepared from electroactive polyimide/graphene nanocomposites with synergistic effects of redox catalytic capability and gas barrier properties. Expr. Polym. Lett. 2014, 8, 243–255. [Google Scholar]
- Chang, C.H.; Huang, T.C.; Peng, C.W.; Yeh, T.C.; Lu, H.I.; Hung, W.I.; Weng, C.J.; Yang, T.I.; Yeh, J.M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lin, Y.Y.; Lin, C.H.; Chan, C.C.; Huang, Y.C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2013, 5, 535–550. [Google Scholar] [CrossRef]
- Luo, X.H.; Zhong, J.W.; Zhou, Q.L.; Du, S.; Yuan, S.; Liu, Y.L. Cationic Reduced Graphene Oxide as Self-aligned Nanofiller in the Epoxy Nanocomposited Coating with Excellent Anticorrosive Performance and Its High Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 18400–18415. [Google Scholar] [CrossRef] [PubMed]
- Kotutha, I.; Swatsitang, E.; Meewassana, W.; Maensiri, S. One-pot hydrothermal synthesis, characterization, and electrochemical properties of rGO/MnFe2O4 nanocomposites. Jpn. J. Appl. Phys. 2015, 54, 12273–12279. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.G.; Ding, Y.; Han, Y.C. Decreasing the aggregation of PCBM in P3HT/PCBM blend films by cooling the solution. Colloids Surf. A Physicochem. Eng. Aspects 2013, 421, 135–141. [Google Scholar] [CrossRef]
- Mohammadi, A.; Barikani, M.; Doctorsafaei, A.H.; Isfahani, A.P.; Shams, E.; Ghalei, B. Aqueous dispersion of polyurethane nanocomposites based on calixarenes modified graphene oxide nanosheets: Preparation, characterization, and anti-corrosion properties. Chem. Eng. J. 2018, 349, 466–480. [Google Scholar] [CrossRef]
- Cao, L.; Li, Z.H.; Su, K.M.; Cheng, B.W. Hydrophilic graphene preparation from gallic acid modified graphene oxide in magnesium self-propagating high temperature synthesis process. Sci. Rep. 2016, 6, 35184. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Wei, G.C.; Yan, M.M.; Dong, R.H.; Wang, D.; Zhou, X.Z.; Chen, J.F.; Hao, J.C. Covalent modification of reduced graphene oxide by means of diazonium chemistry and use as a drug-delivery system. Chem. A Eur. J. 2012, 18, 14708–14716. [Google Scholar] [CrossRef]
- Tu, W.G.; Zhou, Y.; Zou, Z.G. Versatile graphene-promoting photocatalytic performance of semiconductors: Basic principles, synthesis, solar energy conversion, and environmental applications. Adv. Funct. Mater. 2013, 23, 4996–5008. [Google Scholar] [CrossRef]
- Sherif, E.; Park, S.M. Effects of 1, 4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim. Acta 2006, 51, 1313–1321. [Google Scholar] [CrossRef]
- Sherif, E.; Park, S.M. Effects of 1, 5-naphthalenediol on aluminum corrosion as a corrosion inhibitor in 0.50 M NaCl. J. Electrochem. Soc. 2005, 152, B205–B211. [Google Scholar] [CrossRef]
- Hikku, G.S.; Jeyasubramanian, K.; Venugopal, A.; Ghosh, R. Corrosion resistance behaviour of graphene/polyvinyl alcohol nanocomposite coating for aluminium-2219 alloy. J. Alloys Comp. 2017, 716, 259–269. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Almajid, A.A. Electrochemical corrosion behavior of API X-70 5L grade steel in 4.0 wt.% sodium chloride solutions after different immersion periods of time. Int. J. Electrochem. Sci. 2015, 10, 34–45. [Google Scholar]

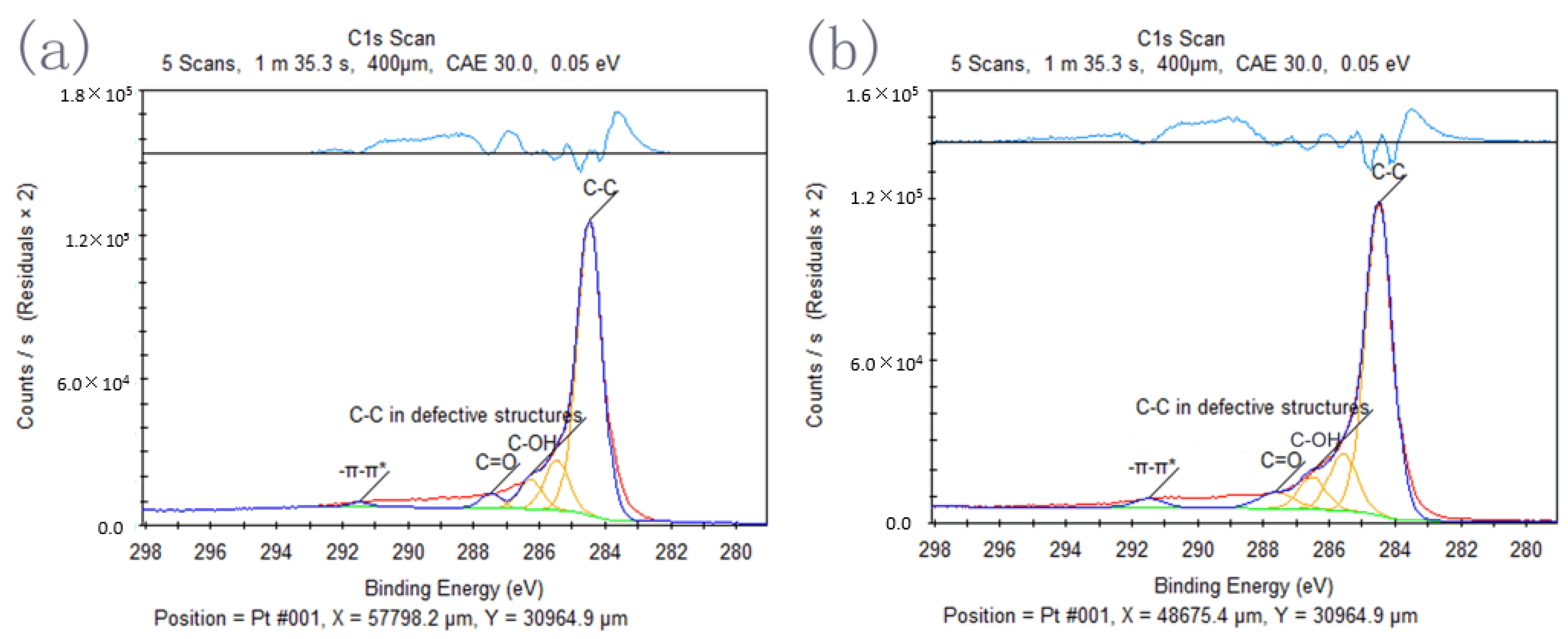

| Name | C–C | C–C in Defective Structures | C–OH | C=O | –π–π* | |
|---|---|---|---|---|---|---|
| Peak (eV) | rGO | 284.48 | 285.48 | 286.28 | 287.50 | 291.50 |
| GESA3 | 284.50 | 285.58 | 286.53 | 287.63 | 291.50 | |
| Atomic | rGO | 76.47 | 12.61 | 7.09 | 2.90 | 0.93 |
| GESA3 | 72.12 | 13.63 | 7.22 | 4.95 | 2.08 | |
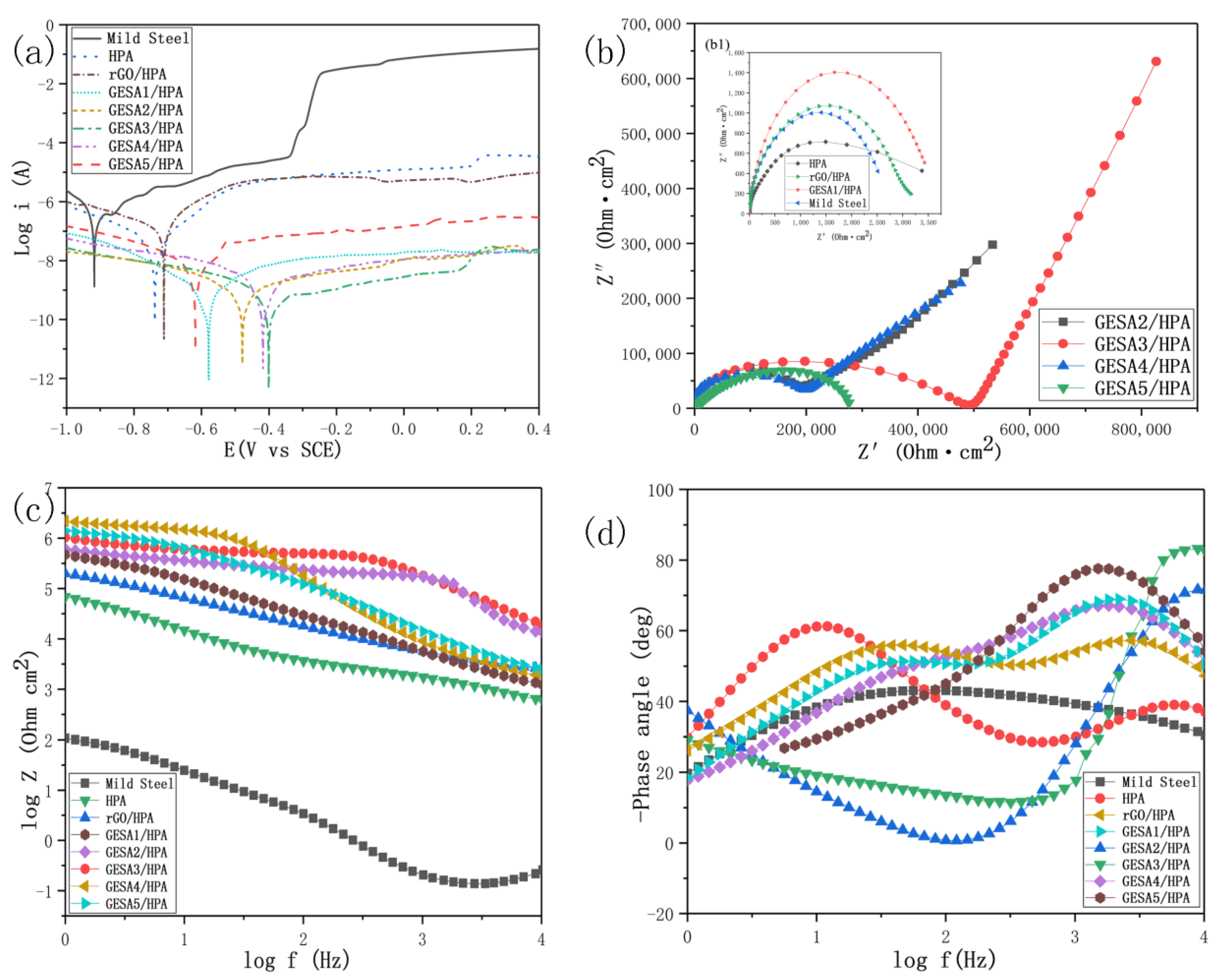
| Specimen | Corrosion Potential, Ecorr (V) | Corrosion Current Density, icorr (A) | Corrosion Rate, vcorr (mm/a) |
|---|---|---|---|
| Bare mild steel | −0.9178 | 2.980 × 10−5 | 3.509 × 10−1 |
| HPA coated mild steel | −0.7115 | 2.911 × 10−8 | 3.427 × 10−3 |
| rGO/HPA coated mild steel | −0.7382 | 9.216 × 10−9 | 1.085 × 10−3 |
| GESA1/HPA coated mild steel | −0.5187 | 4.629 × 10−10 | 5.450 × 10−6 |
| GESA2/HPA coated mild steel | −0.4792 | 2.147 × 10−10 | 2.528 × 10−6 |
| GESA3/HPA coated mild steel | −0.4006 | 1.184 × 10−10 | 1.394 × 10−6 |
| GESA4/HPA coated mild steel | −0.4182 | 1.782 × 10−10 | 2.098 × 10−6 |
| GESA5/HPA coated mild steel | −0.6179 | 2.568 × 10−9 | 3.023 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Hu, Y.; Zhang, Y.; Lu, J.; Chen, X.; Niu, J.; Li, N.; Chen, D. Reduced Graphene Oxide–Epoxy Grafted Poly(Styrene-Co-Acrylate) Composites for Corrosion Protection of Mild Steel. Coatings 2019, 9, 666. https://doi.org/10.3390/coatings9100666
Fan X, Hu Y, Zhang Y, Lu J, Chen X, Niu J, Li N, Chen D. Reduced Graphene Oxide–Epoxy Grafted Poly(Styrene-Co-Acrylate) Composites for Corrosion Protection of Mild Steel. Coatings. 2019; 9(10):666. https://doi.org/10.3390/coatings9100666
Chicago/Turabian StyleFan, Xinchuan, Yue Hu, Yijun Zhang, Jiachen Lu, Xiaofeng Chen, Jun Niu, Ningyan Li, and Dianyu Chen. 2019. "Reduced Graphene Oxide–Epoxy Grafted Poly(Styrene-Co-Acrylate) Composites for Corrosion Protection of Mild Steel" Coatings 9, no. 10: 666. https://doi.org/10.3390/coatings9100666
APA StyleFan, X., Hu, Y., Zhang, Y., Lu, J., Chen, X., Niu, J., Li, N., & Chen, D. (2019). Reduced Graphene Oxide–Epoxy Grafted Poly(Styrene-Co-Acrylate) Composites for Corrosion Protection of Mild Steel. Coatings, 9(10), 666. https://doi.org/10.3390/coatings9100666





