A Facile Route to Fabricate Superhydrophobic Cu2O Surface for Efficient Oil–Water Separation
Abstract
:1. Introduction
2. Materials and Methods
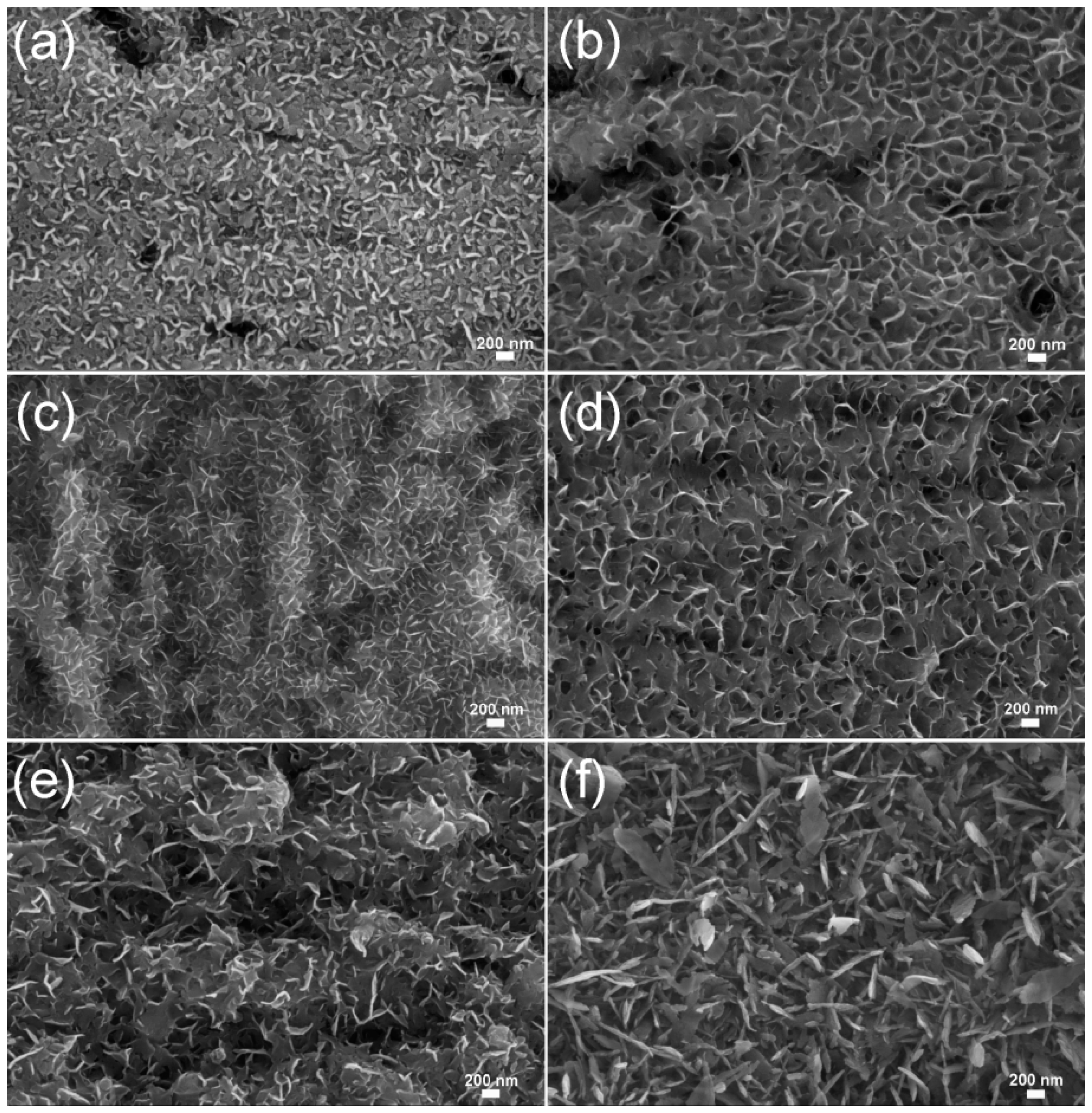
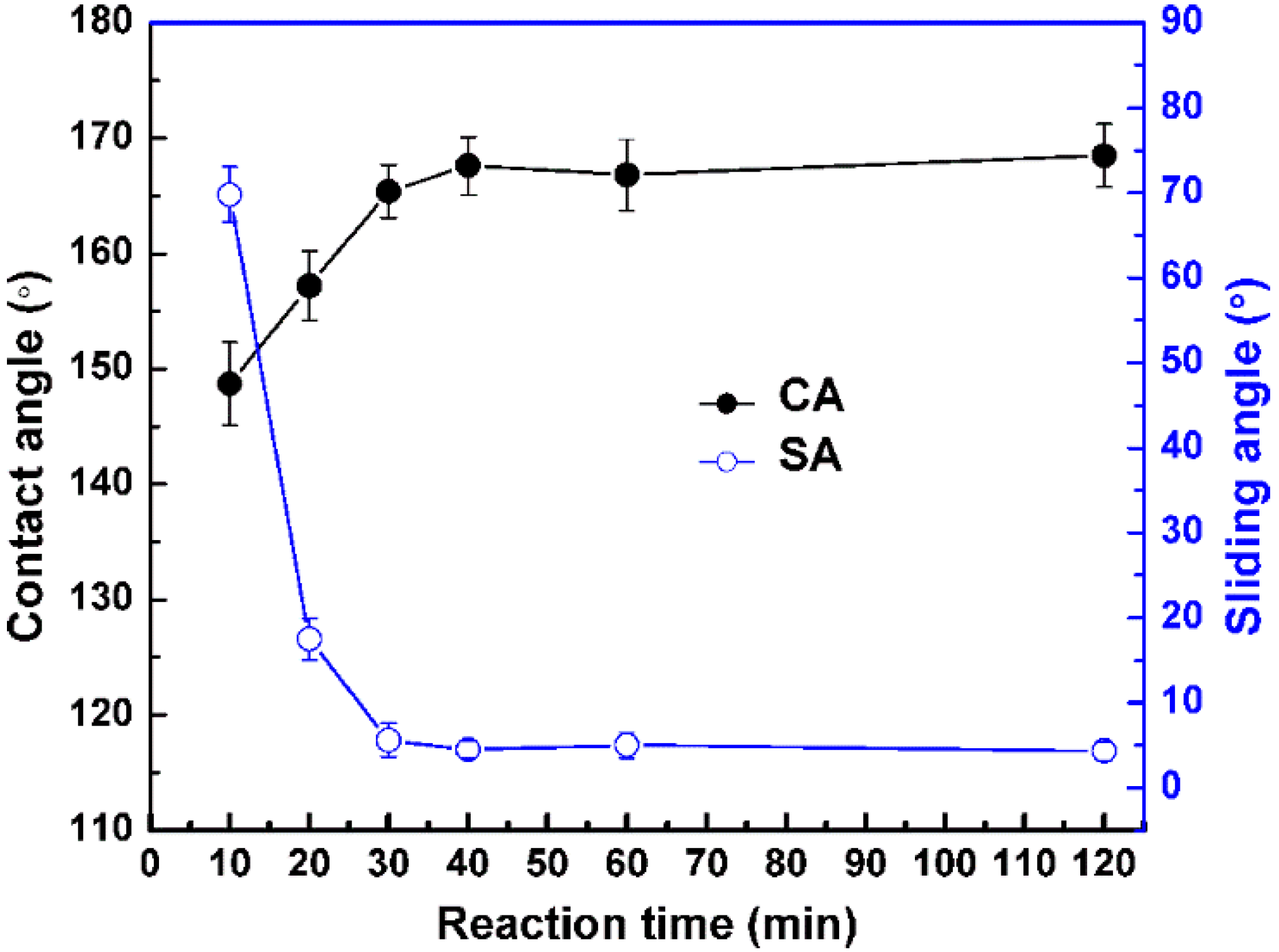
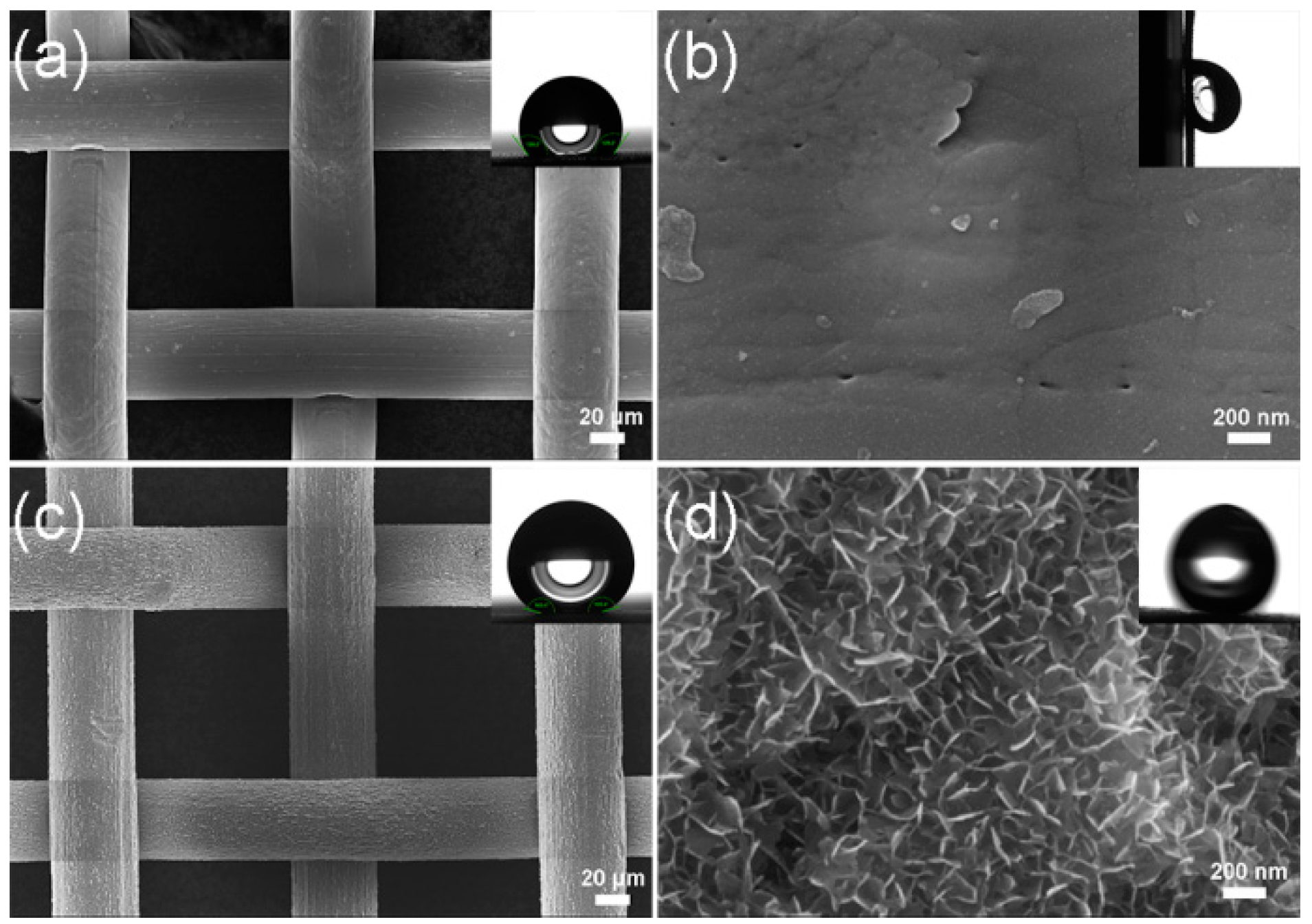
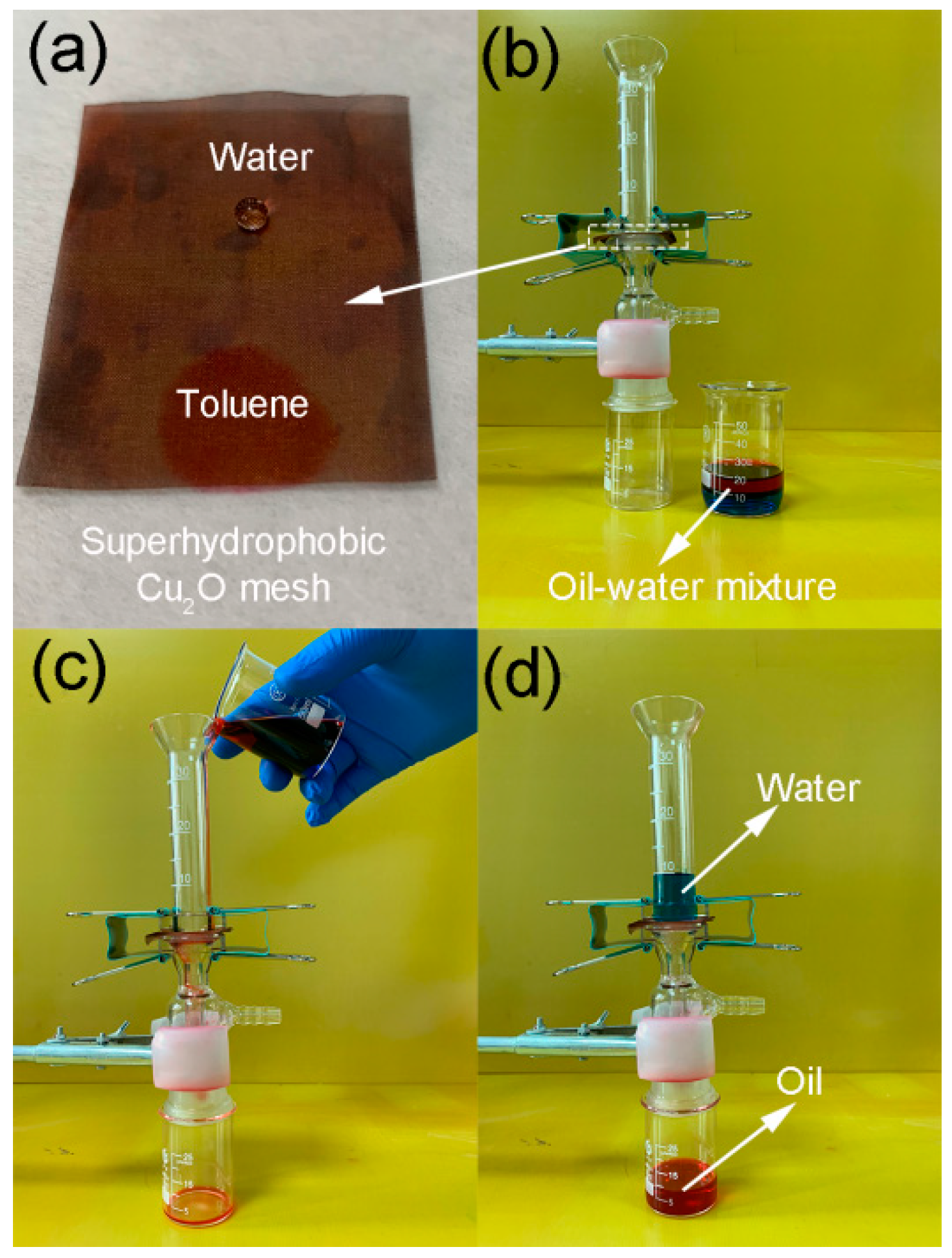
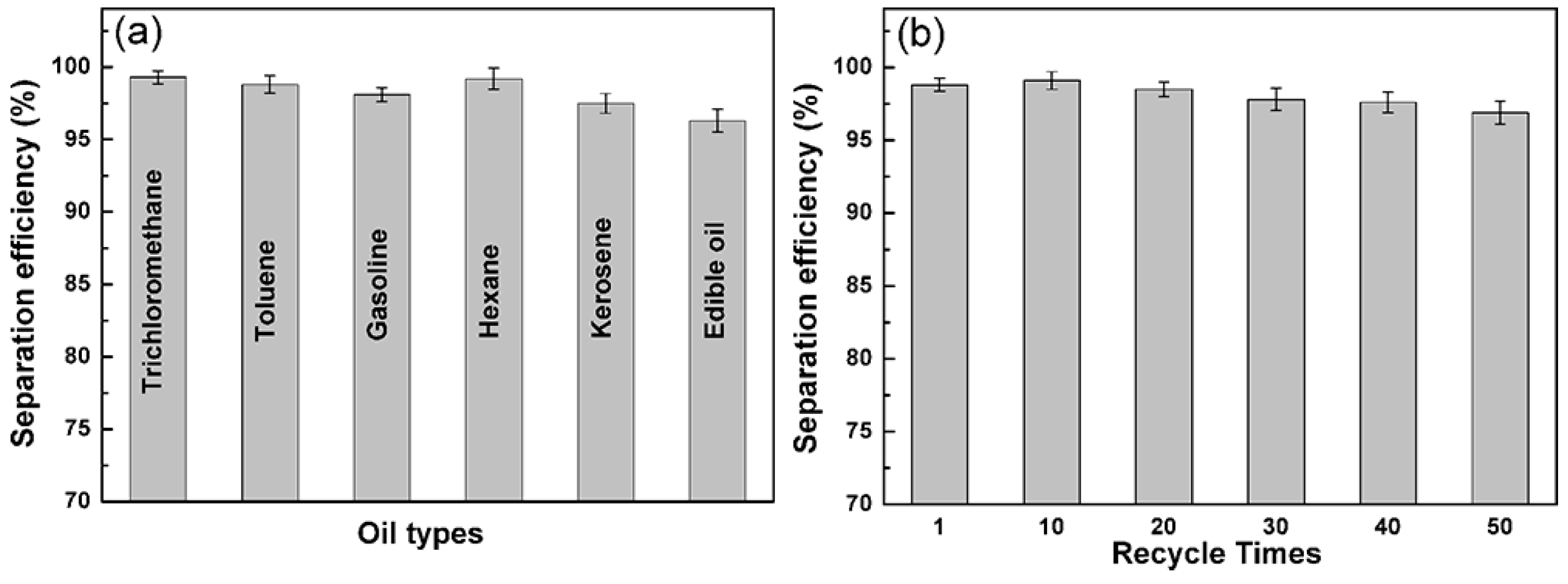
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, A.F.; Ahmad, J.; Basma, Y.; Ramzi, T. Assessment of alternative management techniques of tank bottom petroleum sludge in Oman. J. Hazard. Mater. 2007, 141, 557–564. [Google Scholar]
- Guterman, L. Exxon valdez turns 20. Science 2009, 323, 1558–1559. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; He, G.H. Separation of water and oil from water-in-oil emulsion by freeze/thaw method. Sep. Purif. Technol. 2003, 31, 83–89. [Google Scholar] [CrossRef]
- Mullin, J.V.; Champ, M.A. Introduction/overview to in situ burning of oil spills. Spill Sci. Technol. Bull. 2003, 8, 323–330. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Avranas, A. Treatment of oil-in-water emulsions by coagulation and dissolved-air flotation. Colloid. Surface A 2000, 172, 153–161. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: a review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Nyankson, E.; Olasehinde, O.; John, V.T.; Gupta, R.B. Surfactant-loaded halloysite clay nanotube dispersants for crude oil spill remediation. Ind. Eng. Chem. Res. 2015, 54, 9328–9341. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.Y.; Mai, Z.H.; Ma, Y.M.; Liu, B.Q.; Jiang, L.; Zhu, D.B. A super-hydrophobic and super-oleophilie coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef]
- Gao, Y.F.; Cheng, M.J.; Wang, B.L.; Feng, Z.G.; Shi, F. Diving-surfacing cycle within a stimulus-responsive smart device towards developing functionally cooperating systems. Adv. Mater. 2010, 22, 5125–5128. [Google Scholar] [CrossRef]
- Pan, Q.M.; Wang, M. Miniature boats with striking loading capacity fabricated from superhydrophobic copper meshes. ACS Appl. Mater. Inter. 2009, 1, 420–423. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Geng, L.; Huan, Y.D. Design and fabrication of hydrophobic copper mesh with striking loading capacity and pressure resistance. J. Phys. Chem. C 2010, 114, 9370–9378. [Google Scholar] [CrossRef]
- Tian, D.L.; Zhang, X.F.; Zhai, J.; Jiang, L. Photocontrollable water permeation on the micro/nanoscale hierarchical structured ZnO mesh films. Langmuir 2011, 27, 4265–4270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Q.M.; Liu, F.T. Facile removal and collection of oils from water surfaces through superhydrophobic and superoleophilic sponges. J. Phys. Chem. C 2011, 115, 17464–17470. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhang, Z.Z.; Xu, X.H.; Men, X.H.; Zhu, X.T. Facile fabrication of superhydrophobic sponge with selective sbsorption and collection of oil from water. Ind. Eng. Chem. Res. 2013, 52, 9411–9416. [Google Scholar] [CrossRef]
- Wang, C.F.; Lin, S.J. Robust superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from water. ACS Appl. Mater. Inter. 2013, 5, 8861–8864. [Google Scholar] [CrossRef]
- Li, S.H.; Huang, J.Y.; Chen, Z.; Chen, G.Q.; Lai, Y.K. A review on special wettability textiles: theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 2017, 5, 31–55. [Google Scholar] [CrossRef]
- Xu, Z.G.; Zhao, Y.; Wang, H.X.; Zhou, H.; Qin, C.X.; Wang, X.G.; Lin, T. Fluorine-free superhydrophobic coatings with pH-induced wettability transition for controllable oil-water separation. ACS Appl. Mater. Inter. 2016, 8, 5661–5667. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Zang, D.L.; Lu, Y.; Gao, Z.X.; Shi, J.Y.; Wang, C.Y. Preparation and characterization of cotton fabric with potential use in UV resistance and oil reclaim. Carbohyd. Polym. 2016, 137, 264–270. [Google Scholar] [CrossRef]
- Ou, J.F.; Wan, B.B.; Wang, F.J.; Xue, M.S.; Wu, H.M.; Li, W. Superhydrophobic fibers from cigarette filters for oil spill cleanup. RSC Adv. 2016, 6, 44469–44474. [Google Scholar] [CrossRef]
- Cheng, M.J.; Gao, Y.F.; Guo, X.P.; Shi, Z.Y.; Chen, J.F.; Shi, F. A functionally integrated device for effective and facile oil spill cleanup. Langmuir 2011, 27, 7371–7375. [Google Scholar] [CrossRef]
- Jin, X.; Shi, B.R.; Zheng, L.C.; Pei, X.H.; Zhang, X.Y.; Sun, Z.Q.; Du, Y.; Kim, J.H.; Wang, X.L.; Dou, S.X.; et al. Bio-inspired multifunctional metallic foams through the fusion of different biological solutions. Adv. Funct. Mater. 2014, 24, 2721–2726. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Si, Y.; Tang, X.M.; Zhu, Z.G.; Ding, B.; Liu, L.F.; Zheng, G.; Luo, W.J.; Yu, J.Y. Gravity driven separation of emulsified oil-water mixtures utilizing in situ polymerized superhydrophobic and superoleophilic nanofibrous membranes. J. Mater. Chem. A 2013, 1, 14071–14074. [Google Scholar] [CrossRef]
- Fang, W.; Liu, L.; Li, T.; Dang, Z.; Qiao, C.; Xu, J.; Wang, Y. Electrospun N-substituted polyurethane membranes with self-healing ability for self-cleaning and oil/water separation. Chemistry 2016, 22, 878–883. [Google Scholar] [CrossRef]
- Gui, X.C.; Wei, J.Q.; Wang, K.L.; Cao, A.Y.; Zhu, H.W.; Jia, Y.; Shu, Q.K.; Wu, D.H. Carbon nanotube sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef]
- Wei, Y.B.; Qi, H.; Gong, X.; Zhao, S.F. Specially wettable membranes for oil-water separation. Adv. Mater. Inter. 2018, 1800576. [Google Scholar] [CrossRef]
- Yang, Y.; Tong, Z.; Ngai, T.; Wang, C.Y. Nitrogen-rich and fire-resistant carbon aerogels for the removal of oil contaminants from water. ACS Appl. Mater. Inter. 2014, 6, 6351–6360. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.J.; Xie, Y.F.; Cong, Q.; Zhao, B. Large-area unmodified superhydrophobic copper substrate can be prepared by an electroless replacement deposition. J. Colloid Interface Sci. 2009, 329, 208–211. [Google Scholar] [CrossRef]
- Chang, F.M.; Cheng, S.L.; Hong, S.J.; Sheng, Y.J.; Tsao, H.K. Superhydrophilicity to superhydrophobicity transition of CuO nanowire films. Appl. Phys. Lett. 2010, 96, 114101. [Google Scholar] [CrossRef]
- Wang, F.J.; Lei, S.; Xue, M.S.; Ou, J.F.; Li, W. In Situ separation and collection of oil from water surface via a novel superoleophilic and superhydrophobic oil containment boom. Langmuir 2014, 30, 1281–1289. [Google Scholar] [CrossRef]
- Ding, Y.B.; Li, Y.; Yang, L.L.; Li, Z.Y.; Xin, W.H.; Liu, X.; Pan, L.; Zhao, J.P. The fabrication of controlled coral-like Cu2O films and their hydrophobic property. Appl. Surf. Sci. 2013, 266, 395–399. [Google Scholar] [CrossRef]
- Singh, V.; Sheng, Y.J.; Tsao, H.K. Facile fabrication of superhydrophobic copper mesh for oil/water separation and theoretical principle for separation design. J. Taiwan Inst. Chem. E 2018, 87, 1–8. [Google Scholar] [CrossRef]
- Monte, M.; Munuera, G.; Costa, D.; Conesa, J.C.; Martinez, A.A. Near-ambient XPS characterization of interfacial copper species in ceria-supported copper catalysts. Phys. Chem. Chem. Phys. 2015, 17, 29995–30004. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luo, X.; Zhu, Y.; Wang, X. Immobilization of Cu(II) in KIT-6 supported Co3O4 and catalytic performance for epoxidation of styrene. App. Surf. Sci. 2015, 359, 609–620. [Google Scholar] [CrossRef]
- Embden, J.V.; Tachibana, Y. Synthesis and characterisation of famatinite copper antimony sulfide nanocrystals. J. Mater. Chem. 2012, 22, 11466–11469. [Google Scholar] [CrossRef]
- Lv, Y.Q.; Shi, B.Z.; Qi, Y.; Su, X.F.; Liu, L.; Tian, L.H.; Ding, J. Synthesis and characterization of the maize-leaf like Cu2O nanosheets array film via an anodic oxidation method. J. Alloy. Compd. 2019, 773, 706–712. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Xu, J.M.; Zhong, H.; Chu, X.Z.; Song, J. Repeatable synthesis of Cu2O nanorods by a simple and novel reduction route. Mater. Lett. 2011, 65, 1871–1874. [Google Scholar] [CrossRef]
- Zhou, C.L.; Cheng, J.; Hou, K.; Zhu, Z.T.; Zheng, Y.F. Preparation of CuWO4@Cu2O film on copper mesh by anodization for oil/water separation and aqueous pollutant degradation. Chem. Eng. J. 2017, 307, 803–811. [Google Scholar] [CrossRef]
- Ai, Z.H.; Zhang, L.Z.; Lee, S.C.; Ho, W.K. Interfacial hydrothermal synthesis of Cu@Cu2O core-shell microspheres with enhanced visible-light-driven photocatalytic activity. J. Phys. Chem. C 2009, 113, 20896–20902. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of polycrystalline copper thin films at ambient conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I. Modification of the wettability of polymer surfaces using nanoparticles. Prog. Org. Coat. 2014, 77, 331–338. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Chen, F.; Xu, W.; Carmalt, C.J.; Parkin, I.P. Creating superhydrophobic mild steel surfaces for water proofing and oil-water separation. J. Mater. Chem. A 2014, 2, 11628–11634. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Li, H.; Li, W.; Zha, F.; Lei, Z. Underwater superoleophobic palygorskite coated meshes for efficient oil/water separation. J. Mater. Chem. A 2015, 3, 14696–14702. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, S.; Fang, X.; Wang, F.; Xue, M.; Ou, J.; Li, C.; Li, W. A Facile Route to Fabricate Superhydrophobic Cu2O Surface for Efficient Oil–Water Separation. Coatings 2019, 9, 659. https://doi.org/10.3390/coatings9100659
Lei S, Fang X, Wang F, Xue M, Ou J, Li C, Li W. A Facile Route to Fabricate Superhydrophobic Cu2O Surface for Efficient Oil–Water Separation. Coatings. 2019; 9(10):659. https://doi.org/10.3390/coatings9100659
Chicago/Turabian StyleLei, Sheng, Xinzuo Fang, Fajun Wang, Mingshan Xue, Junfei Ou, Changquan Li, and Wen Li. 2019. "A Facile Route to Fabricate Superhydrophobic Cu2O Surface for Efficient Oil–Water Separation" Coatings 9, no. 10: 659. https://doi.org/10.3390/coatings9100659
APA StyleLei, S., Fang, X., Wang, F., Xue, M., Ou, J., Li, C., & Li, W. (2019). A Facile Route to Fabricate Superhydrophobic Cu2O Surface for Efficient Oil–Water Separation. Coatings, 9(10), 659. https://doi.org/10.3390/coatings9100659






