Synthesis of High Molecular Weight Vinylphenyl-Con Taining MQ Silicone Resin via Hydrosilylation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
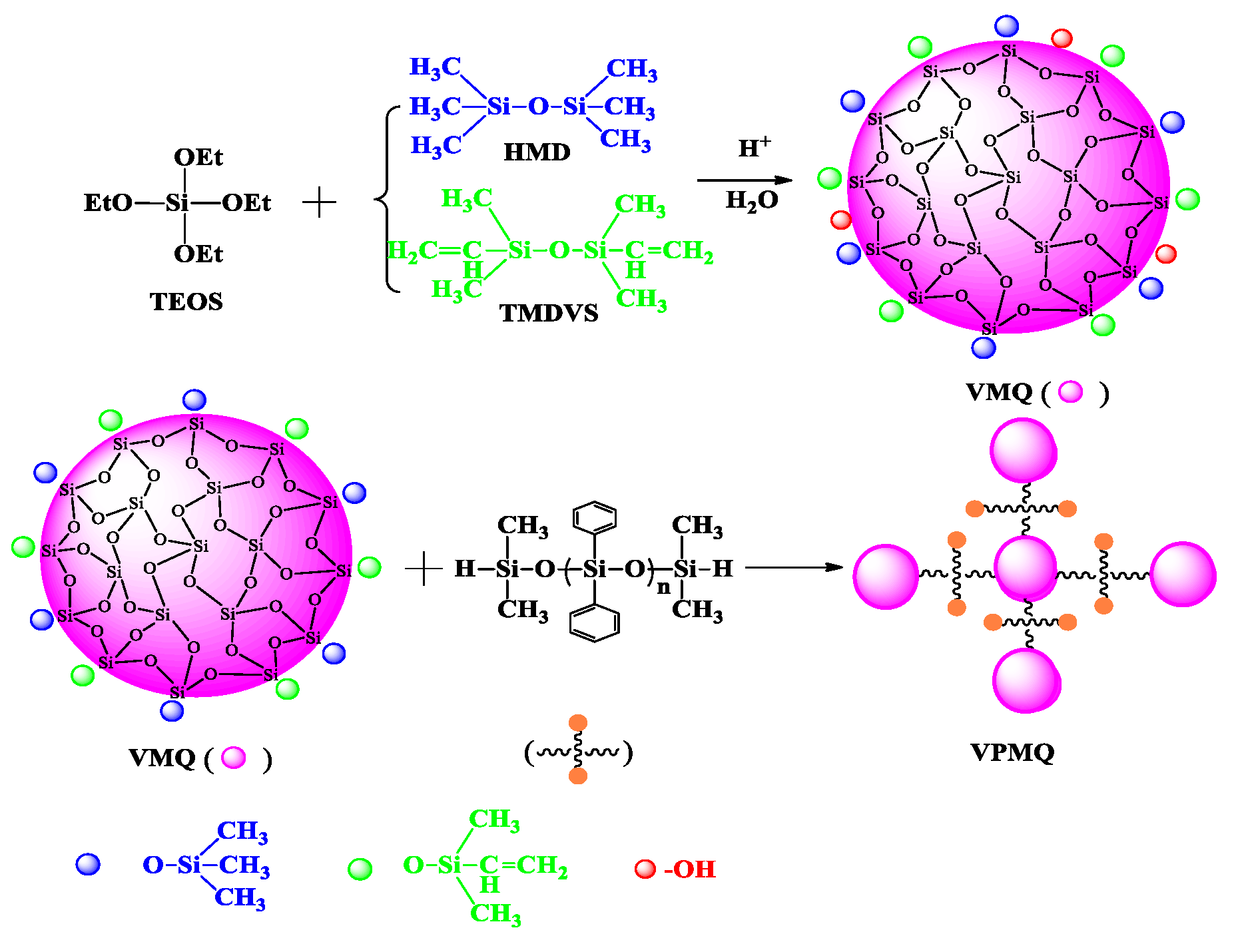
2.2. Synthesis of the VMQ
2.3. Synthesis of the VPMQ
2.4. Characterizations and Measurements
3. Results and Discussion
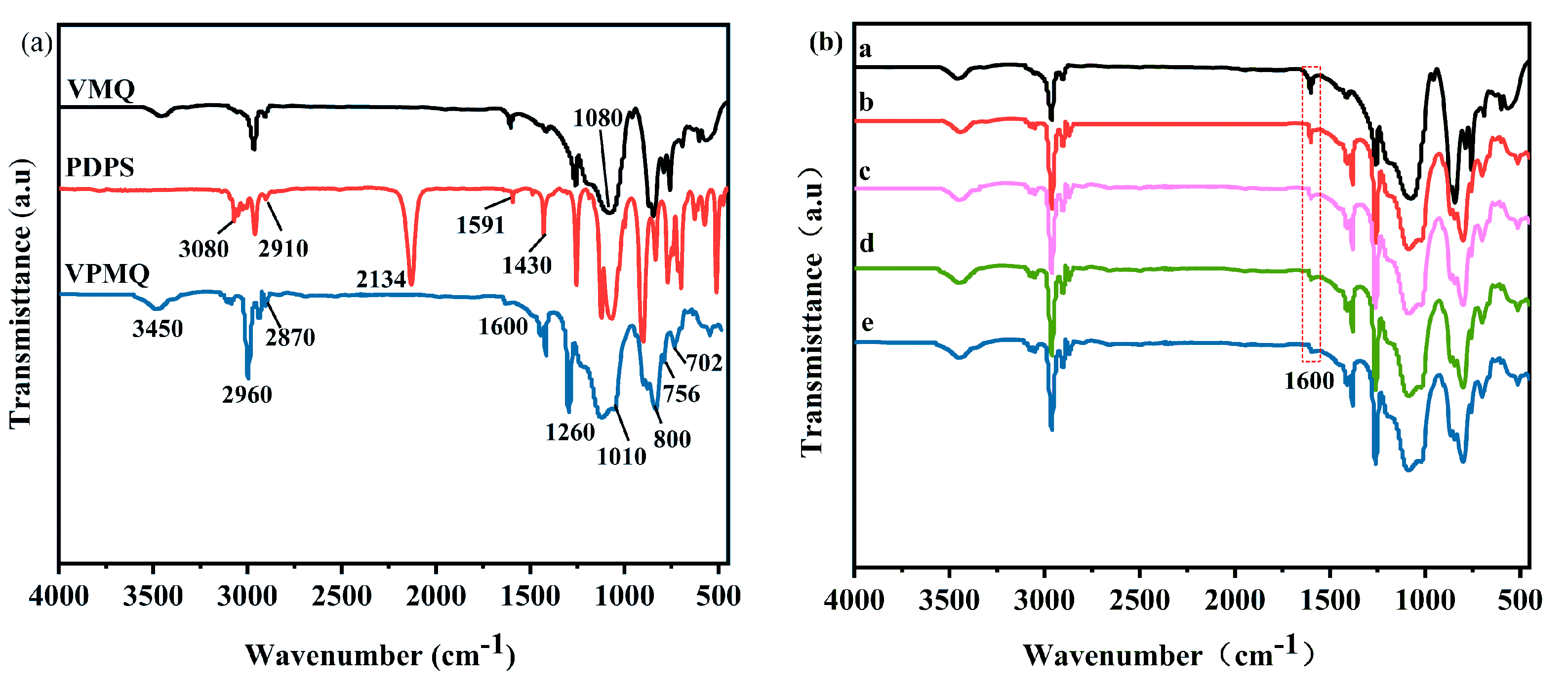
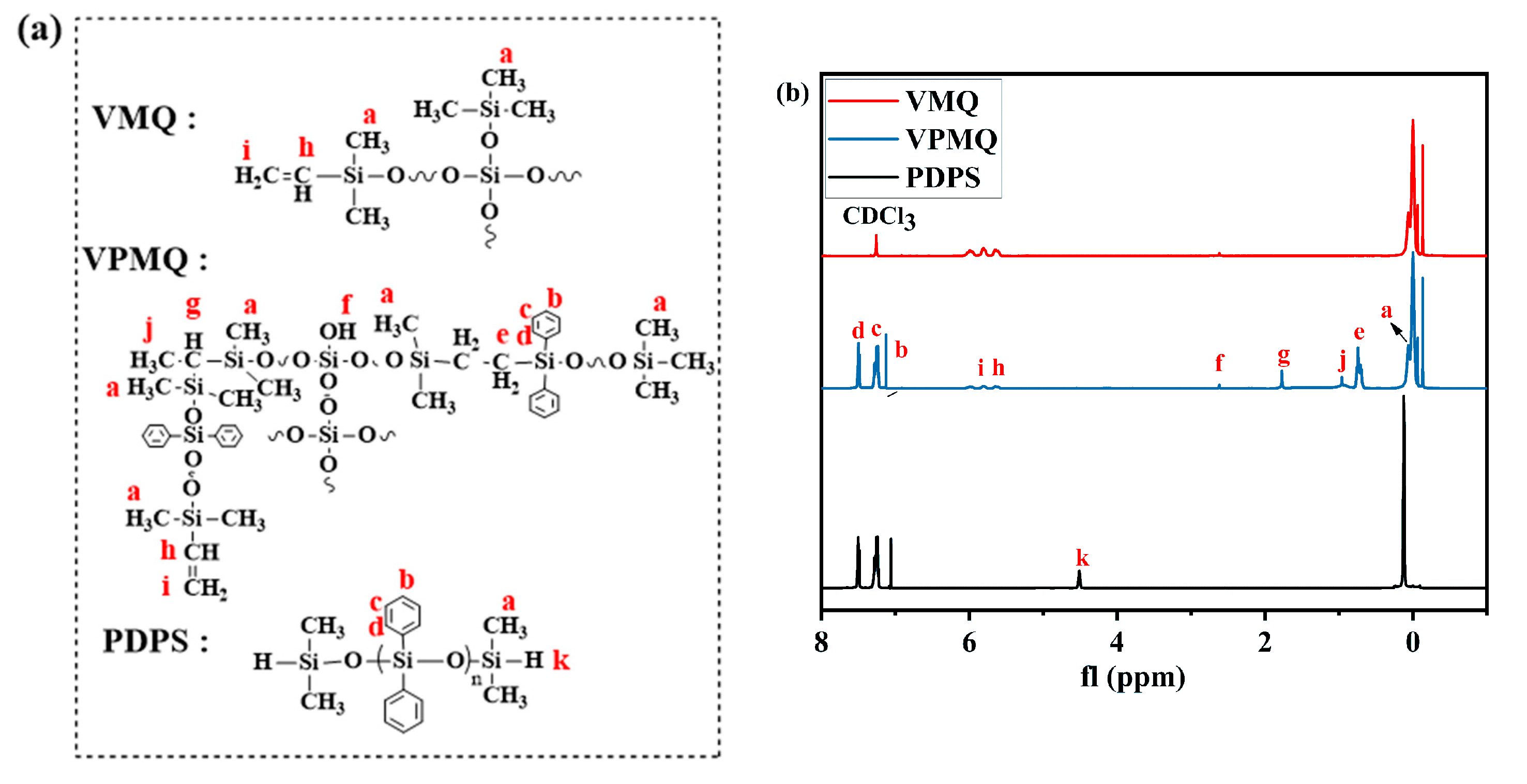
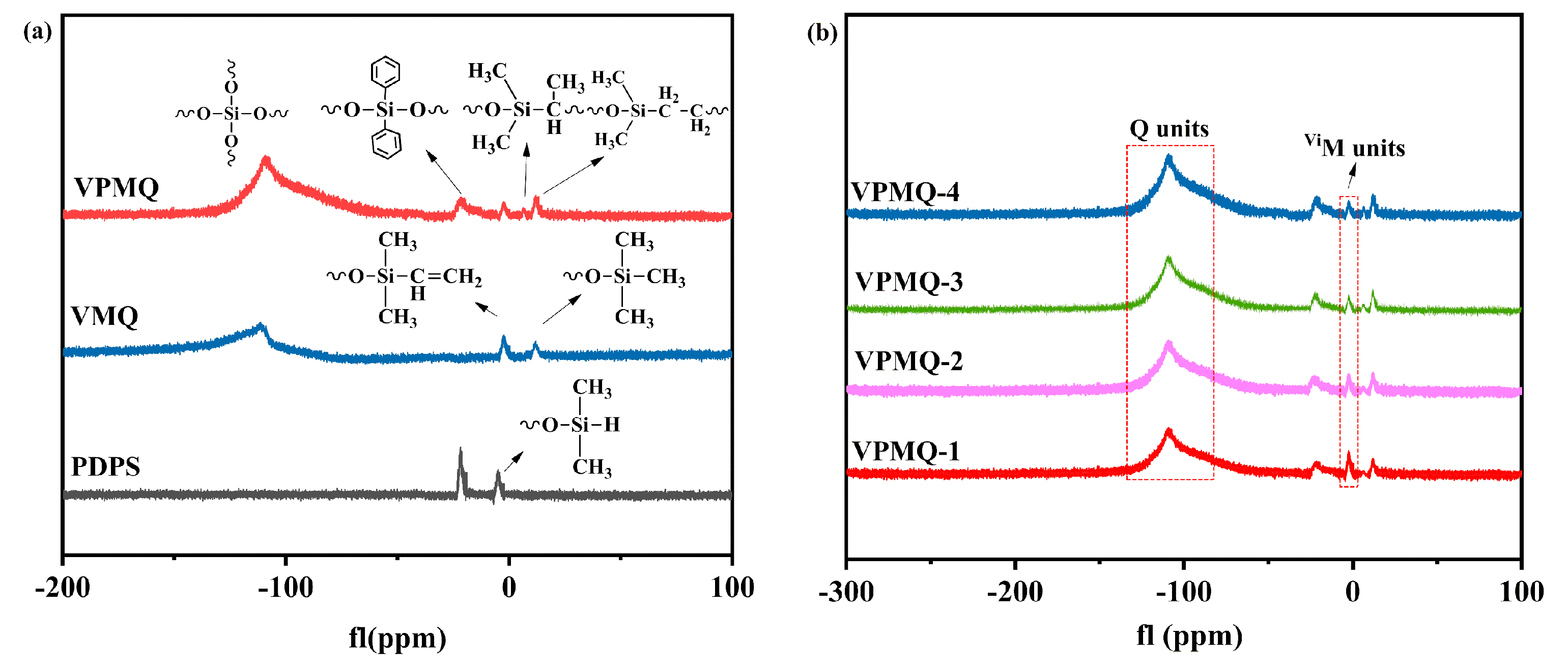
3.1. Structural Analysis
3.2. Refractive Index Analysis
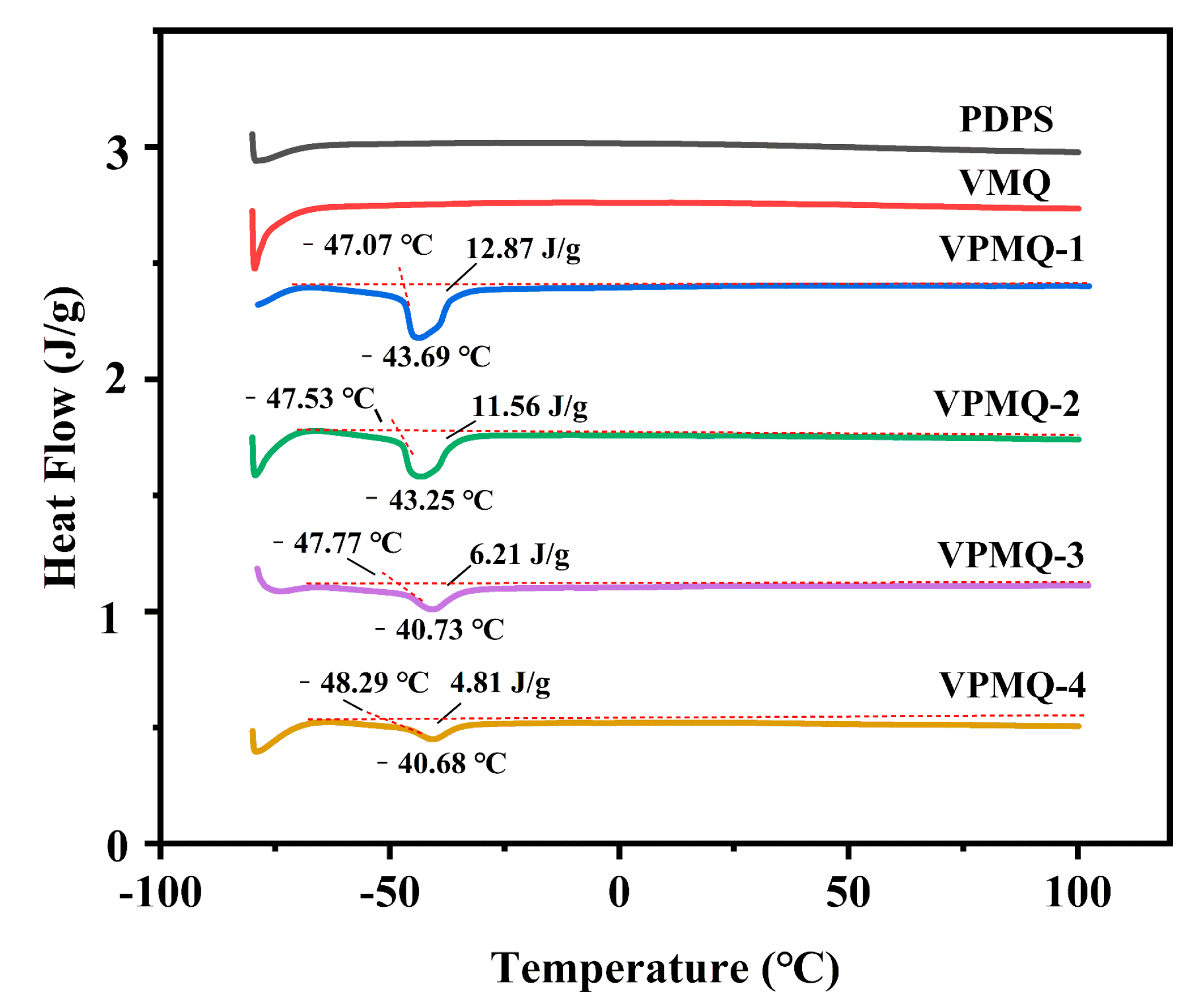
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Z.; Wu, J.; Liu, X.; Ji, H.; He, R.; Pimhataivoot, P.; Chen, X. Versatile cascade esterification route to MQ Resins. ACS Omega 2018, 3, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Ge, X.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Liang, W.; Ge, J.; Chen, X. Synthesis and characterization of room temperature vulcanized silicone rubber using methoxyl-capped MQ silicone resin as self-reinforced cross-linker. Polymers 2019, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, B.; Sun, Y.; Liu, B.; Wang, G. Synthesis of Vinylphenyl Oligomeric silsesquioxane based on MQ Silicone resin. Asian J. Chem. 2013, 25, 2541–2546. [Google Scholar] [CrossRef]
- Kuo, C.F.J.; Chen, J.B.; Shih, C.Y.; Huang, C.Y. Silicone resin synthesized by tetraethoxysilane and chlorotrimethylsilane through hydrolysis–condensation reaction. J. Appl. Polym. Sci. 2014, 131, 40317. [Google Scholar] [CrossRef]
- Kuo, A.C.M. Silicone Release Coatings for the Pressure Sensitive Industry—Overview and Trends; DC Corporation: Barry, UK, 2003; pp. 1–4. [Google Scholar]
- Chen, W.; Zeng, X.; Lai, X.; Li, H.; Pan, Z. Effect and mechanism of ureido-modified MQ silicone resin and platinum on tracking and erosion resistance of silicone rubber. Polym. Test. 2018, 70, 162–169. [Google Scholar] [CrossRef]
- Lee, B.K.; Ryu, J.H.; Baek, I.B.; Kim, Y.; Jang, W.I.; Kim, S.H.; Yoon, Y.S.; Kim, S.H.; Hong, S.G.; Byun, S.; et al. Silicone-based adhesives with highly tunable adhesion force for Skin-contact applications. Adv. Healthc. Mater. 2017, 6, 1700621. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Wu, T.; Lai, X.; Liu, F.; Zeng, X. Efficiently enhancing the tracking and erosion resistance of silicone rubber by the synergism of fluorine-containing polyphenyl silsesquioxane and ureido-containing MQ silicone resin. Appl. Surf. Sci. 2018, 459, 483–491. [Google Scholar] [CrossRef]
- Peng, D.; Mu, Q.H.; Zhang, S.; Li, S.; Wang, F. Synthesis and Properties of Phenyl Silicone Resin Reinforced Addition Type Liquid Phenyl Silicone Rubber. Mater. Sci. Forum. 2018, 926, 39–44. [Google Scholar] [CrossRef]
- Jia, P.; Liu, H.; Liu, Q.; Cai, X. Thermal degradation mechanism and flame retardancy of MQ silicone/epoxy resin composites. Polym. Degrad. Stabil. 2016, 134, 144–150. [Google Scholar] [CrossRef]
- Roberts, C.; Cosgrove, T.; Schmidt, R.G.; Gordon, G.V. Diffusion of poly (dimethyl-siloxane) mixtures with silicate nanopartic les. Macromolecules 2001, 34, 538–543. [Google Scholar] [CrossRef]
- Kaneko, Y.; Coughlin, E.B.; Gunji, T.; Itoh, M.; Matsukawa, K.; Naka, K. Silse- squioxanes: Recent advancement and novel applications. Int. J. Polym. Sci. 2012, 2012, 453821. [Google Scholar]
- Robeyns, C.; Picard, L.; Ganachaud, F. Synthesis, characterization and modification of silicone resins: An “Augmented Review”. Prog. Org. Coat. 2018, 125, 287–315. [Google Scholar] [CrossRef]
- Elena, T.; Nataliya, V.; Aziz, M. Synthesis and Properties of MQ Copolymers: Current State of Knowledge. Molecules 2017, 22, 1768. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Yu, Y. Synthesis of MQ silicone resins through hydrol ytic condensation of ethyl polysilicate and hexamethyldisiloxane. J. Appl. Polym. Sci. 1998, 70, 1753–1757. [Google Scholar] [CrossRef]
- Xu, X.; Wu, C.; Zhang, B.; Dong, H. Preparation, structure characterization, and thermal performance of phenyl-modified MQ silicone resins. J. Appl. Polym. Sci. 2013, 128, 4189–4200. [Google Scholar] [CrossRef]
- Flagg, D.H.; McCarthy, T.J. Rediscovering silicones: MQ copolymers. Macromolecules 2016, 49, 8581–8592. [Google Scholar] [CrossRef]
- Pan, K.; Zeng, X.; Li, H.; Liao, F.; Zhang, H.L.; Yin, C.Y. Synthesis of phenyl silicone resin with epoxy and acrylate group and its adhesion enhancement for addition-cure silicone encapsulant with high refractive index. J. Adhes. Sci. Technol. 2016, 30, 2699–2709. [Google Scholar] [CrossRef]
- Xiang, H.; Ge, J.; Cheng, S. Synthesis and characterization of titania/MQ silicone resin hybrid nanocomposite via sol–gel process. J. Sol-Gel Sci. Technol. 2011, 59, 635. [Google Scholar] [CrossRef]
- Xie, C.; Zeng, X.; Fang, W.; Lai, X.; Li, H. Effect of alkyl-disubstituted ureido silanes with different alkyl chain structures on tracking resistance property of addition-cure liquid silicone rubber. Polym. Degrad. Stabil. 2017, 142, 263–272. [Google Scholar] [CrossRef]
- Xie, H.; Hu, W.; Wu, Z.; Hu, G.; Zhu, G.; Liu, Q.; Jia, Z. Study on synthesizing MQ silicone with solid acid as catalyst and its properties. China Adhes. 2016, 25, 308–316. (In Chinese) [Google Scholar]
- Wada, T.; Itoh, K. Heat-Curable Elastomeric Silicone Compositions. U.S. Patent 3,652,475, 28 March 1972. [Google Scholar]
- Li, B.; Zhang, Z.; Ma, D.; Cai, Q.; Feng, S.; Zhang, J. Preparation and kinetic analysis of room-temperature vulcanized methylethylsilicone rubbers. J. Appl. Polym. Sci. 2015, 132, 42656. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, A.S.; Lee, H.S.; Jeon, H.Y.; Baek, K.Y.; Choi, D.H.; Hwang, S.S. Synthesis and chara- cterization of UV-curable ladder-like polysilsesquioxane. J. Polym. Sci. A Polym. Chem. 2011, 49, 5012–5018. [Google Scholar] [CrossRef]
- Chang, S.; Matsumoto, T.; Matsumoto, H.; Unno, M. Synthesis and characterization of heptacyclic ladder- siloxanes and ladder polysilsesquioxane. Appl. Organomet. Chem. 2010, 24, 241–246. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Z.; Huang, H.; Zeng, X.; Chen, Z. Preparation and properties of vinylphenyl-silicone resins and their application in LED packaging. RSC Adv. 2016, 6, 71924–71933. [Google Scholar] [CrossRef]
- Witteman, L.; Evers, T.; Shu, Z. Hydrosilylation in Aryliminopyrrolide Substituted Silanes. Chem. Eur. J. 2016, 22, 6087–6099. [Google Scholar] [CrossRef]
- Antosik, A.K.; Czech, Z. Pressure-Sensitive Adhesives (PSA) Based on Silicone. Adv. Mater. Interfaces 2016, 7, 249–274. [Google Scholar]
- Ji, J.; Ge, X.; Liang, W.; Liang, R.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Chen, X.; Ge, J. A Simple Preparation Route for Bio-Phenol MQ Silicone Resin via the Hydrosilylation Method and its Autonomic Antibacterial Property. Polymers 2019, 11, 1389. [Google Scholar] [CrossRef]
- Wei, Q.; Zan, X.; Qiu, X.; Öktem, G.; Sahre, K.; Kiriy, A.; Voit, B. High Refract ive Index Hyperbranched Polymers Prepared by Two Naphthalene-Bearing Monomers via Thiol-Yne Reaction. Macromol. Chem. Phys. 2016, 217, 1977–1984. [Google Scholar] [CrossRef]
- Yang, S.; Kim, J.; Jin, J.; Kwak, S.; Bae, B. Cycloaliphatic epoxy oligosiloxane-derived hybrid materials for a high-refractive index LED encapsulant. J. Appl. Polym. Sci. 2011, 122, 2478–2485. [Google Scholar] [CrossRef]
- Atkins, G.R.; Krolikowska, R.M.; Samoc, A. Optical properties of an ormosil system comprising methyl and phenyl substituted silica. J. Non-Cryst. Solids 2000, 265, 210–220. [Google Scholar] [CrossRef]
- Takahashi, T.; Kaschta, J.; Münstedt, H. Melt rheology and structure of silicone resins. Rheol. Acta 2001, 40, 490–498. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. Kinetic aspects of the thermal degradation of poly (dimethyl siloxane) and poly (dimethyl diphenyl siloxane). Polym. Degrad Stabil. 2002, 76, 17–24. [Google Scholar] [CrossRef]
- Pham, Q.T.; Chern, C.S. Thermal stability of organofunctional polysiloxanes. Thermochim. Acta 2013, 565, 114–123. [Google Scholar] [CrossRef]

| Entry | Si–H/ Si–CH=CH2 | VMQ (g) | PDPS (g) | H2PtCI6 (g) | Toluene (g) | Physical State | Dispersion in Toluene |
|---|---|---|---|---|---|---|---|
| VPMQ-1 | 0.2/1 | 30.00 | 1.60 | 0.06 | 6.0 | viscous liquid | clear |
| VPMQ-2 | 0.4/1 | 30.00 | 3.20 | 0.06 | 6.0 | viscous liquid | clear |
| VPMQ-3 | 0.6/1 | 30.00 | 4.80 | 0.06 | 6.0 | viscous liquid | clear |
| VPMQ-4 | 0.8/1 | 30.00 | 6.00 | 0.07 | 6.0 | semisolid | clear |
| VPMQ-5 | 1.0/1 | 30.00 | 7.20 | 0.07 | 6.0 | semisolid | gelatinous |
| Entry | Mw (dal·mol−1) | Mn (dal·mol−1) | Mw/Mn | |
|---|---|---|---|---|
| PDPS | 800 | 512 | 1.56 | 1.5440 |
| VMQ | 4801 | 2562 | 1.87 | 1.4840 |
| VPMQ-1 | 31,099 | 17,357 | 1.79 | 1.4850 |
| VPMQ-2 | 77,321 | 28,902 | 2.68 | 1.4889 |
| VPMQ-3 | 197,199 | 137,585 | 1.43 | 1.4890 |
| VPMQ-4 | 391,817 | 285,494 | 1.37 | 1.4994 |
| Entry | Tmax (°C) | T5 (°C) | T10 (°C) | Residual Yield (%) |
|---|---|---|---|---|
| PDPS | − | − | − | 0 |
| VMQ | 509.17 | 281.33 | 358.33 | 17.91 |
| VPMQ-1 | 496.17 | 341.67 | 416.13 | 21.29 |
| VPMQ-2 | 503.17 | 346.17 | 421.70 | 25.06 |
| VPMQ-3 | 496.17 | 363.33 | 424.74 | 29.71 |
| VPMQ-4 | 492.18 | 364.18 | 428.33 | 33.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Ge, X.; Liang, W.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Chen, X.; Ge, J. Synthesis of High Molecular Weight Vinylphenyl-Con Taining MQ Silicone Resin via Hydrosilylation Reaction. Coatings 2019, 9, 605. https://doi.org/10.3390/coatings9100605
Ji J, Ge X, Liang W, Pang X, Liu R, Wen S, Sun J, Chen X, Ge J. Synthesis of High Molecular Weight Vinylphenyl-Con Taining MQ Silicone Resin via Hydrosilylation Reaction. Coatings. 2019; 9(10):605. https://doi.org/10.3390/coatings9100605
Chicago/Turabian StyleJi, Jianye, Xin Ge, Weijie Liang, Xiaoyan Pang, Ruoling Liu, Shuyi Wen, Jiaqi Sun, Xunjun Chen, and Jianfang Ge. 2019. "Synthesis of High Molecular Weight Vinylphenyl-Con Taining MQ Silicone Resin via Hydrosilylation Reaction" Coatings 9, no. 10: 605. https://doi.org/10.3390/coatings9100605
APA StyleJi, J., Ge, X., Liang, W., Pang, X., Liu, R., Wen, S., Sun, J., Chen, X., & Ge, J. (2019). Synthesis of High Molecular Weight Vinylphenyl-Con Taining MQ Silicone Resin via Hydrosilylation Reaction. Coatings, 9(10), 605. https://doi.org/10.3390/coatings9100605





