Modified Ethylsilicates as Efficient Innovative Consolidants for Sedimentary Rock
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Consolidants
- DYN40 was dissolved in isopropanol, in the presence of n-octylamine.
- Either PDMS, A200, R805, or VP was added.
- The sols were subjected to agitation in an ultrasonic bath for 30 min.
2.3. Characterization of Sols and Xerogels
2.4. Application on Stone and Evaluation of the Performance
2.4.1. Stone–Product Interaction
2.4.2. Effectiveness of the Products on the Stone
2.4.3. Negative Effects Induced by the Applied Products
3. Results and Discussion
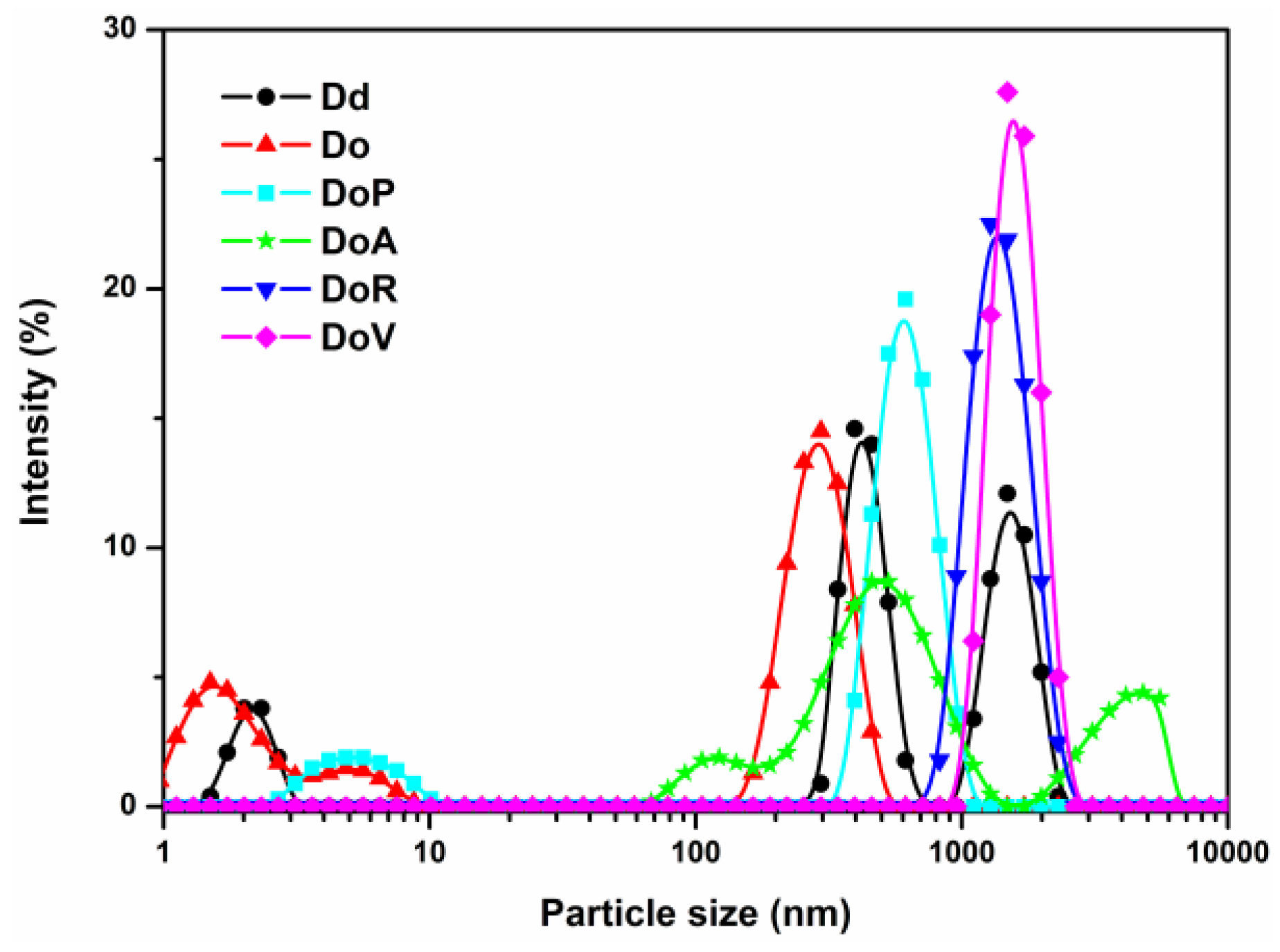
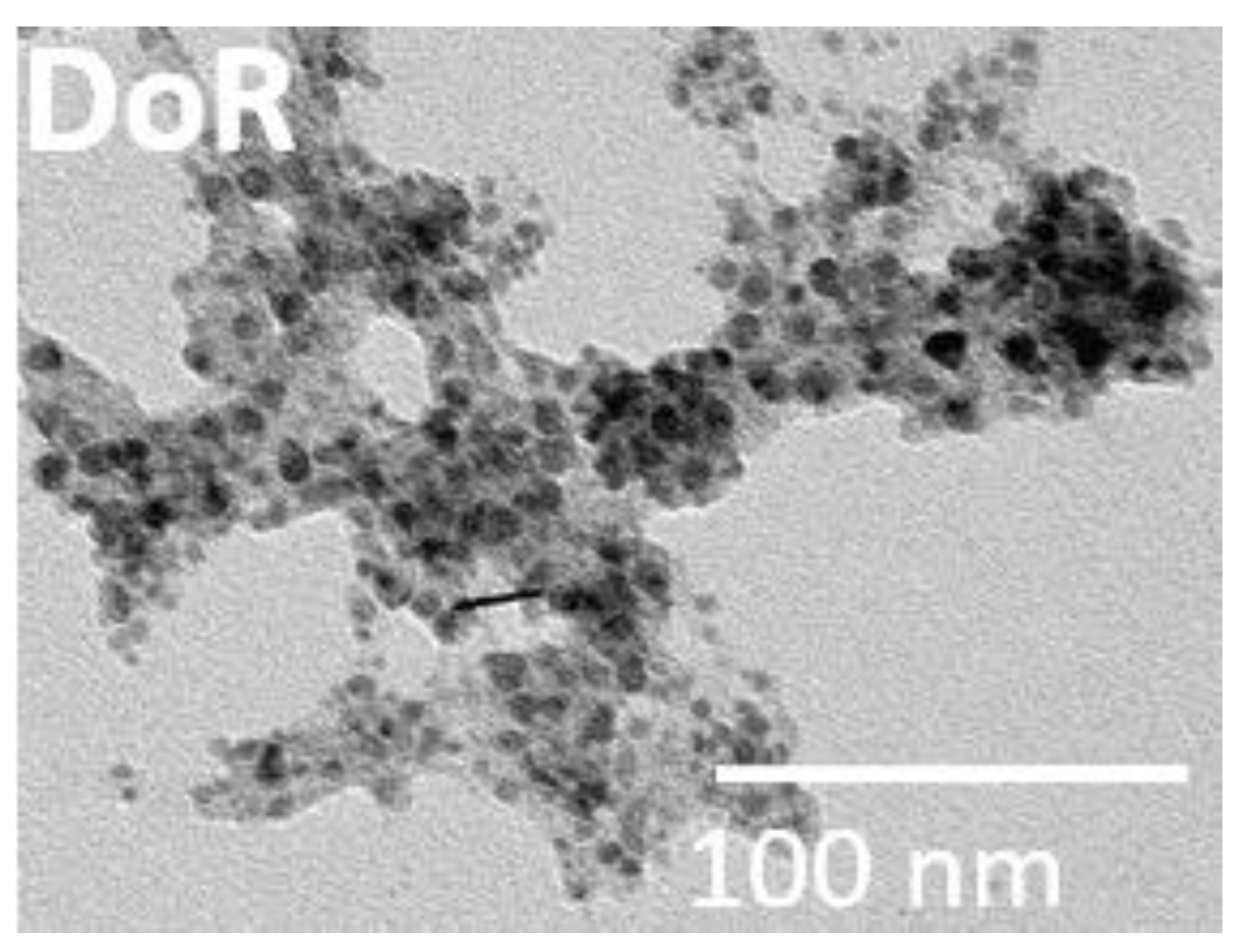
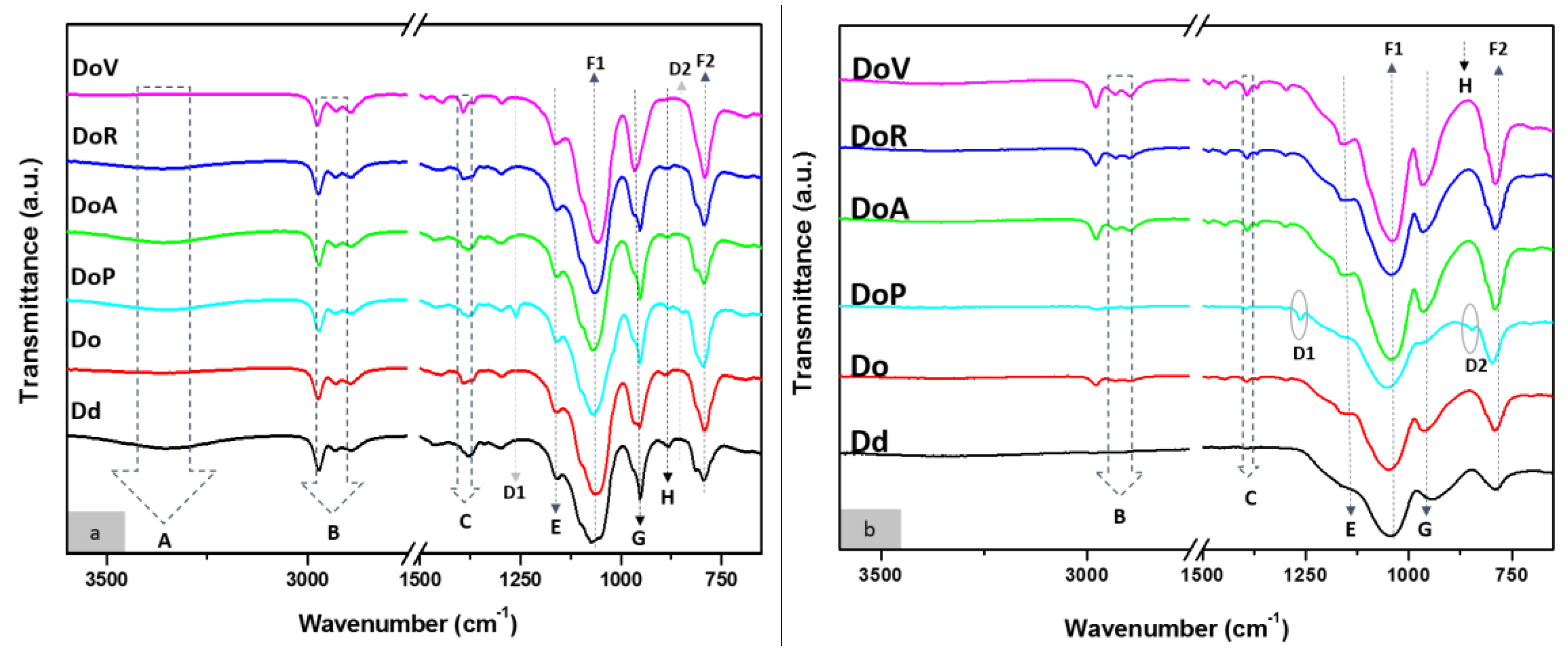
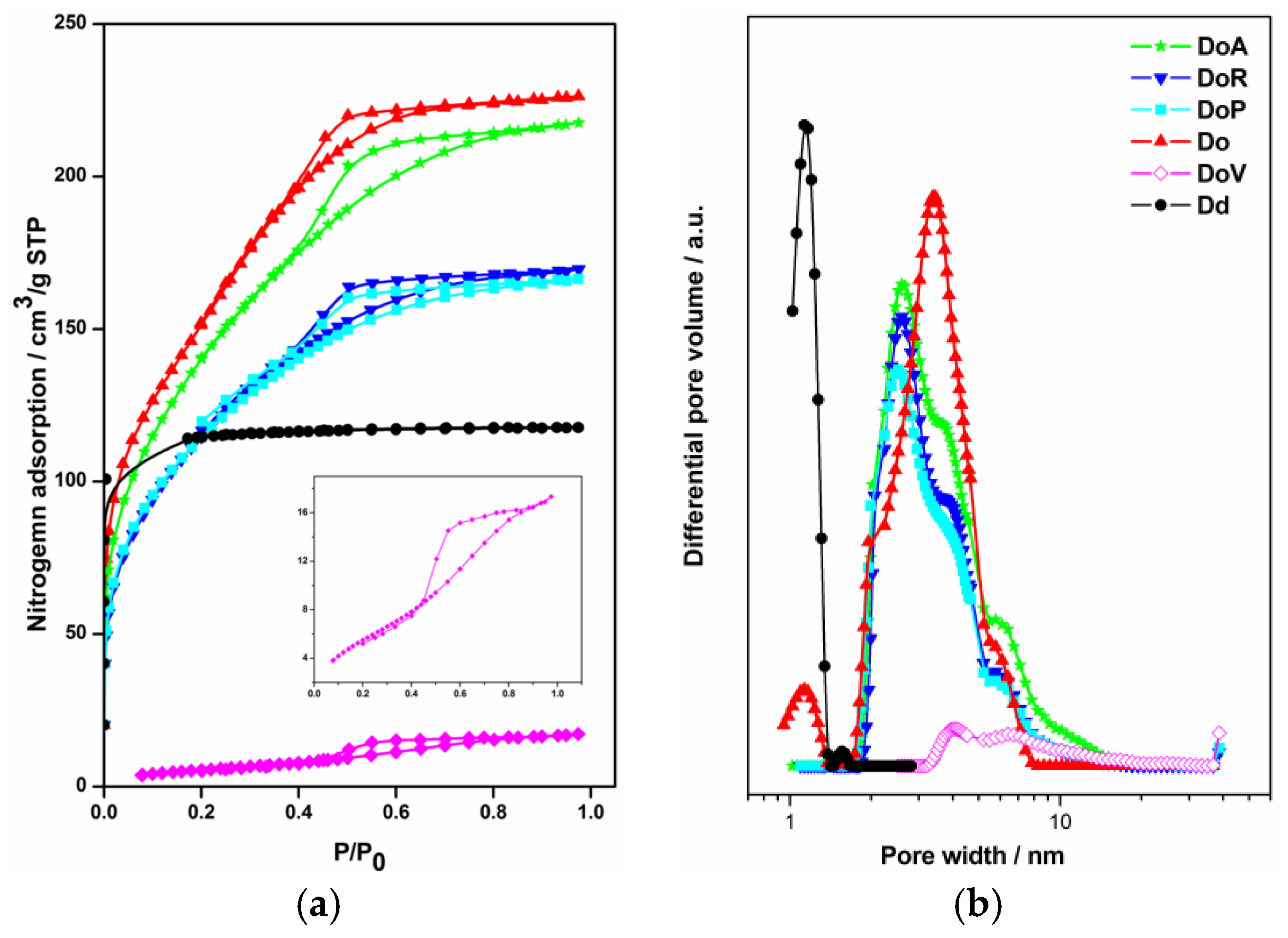
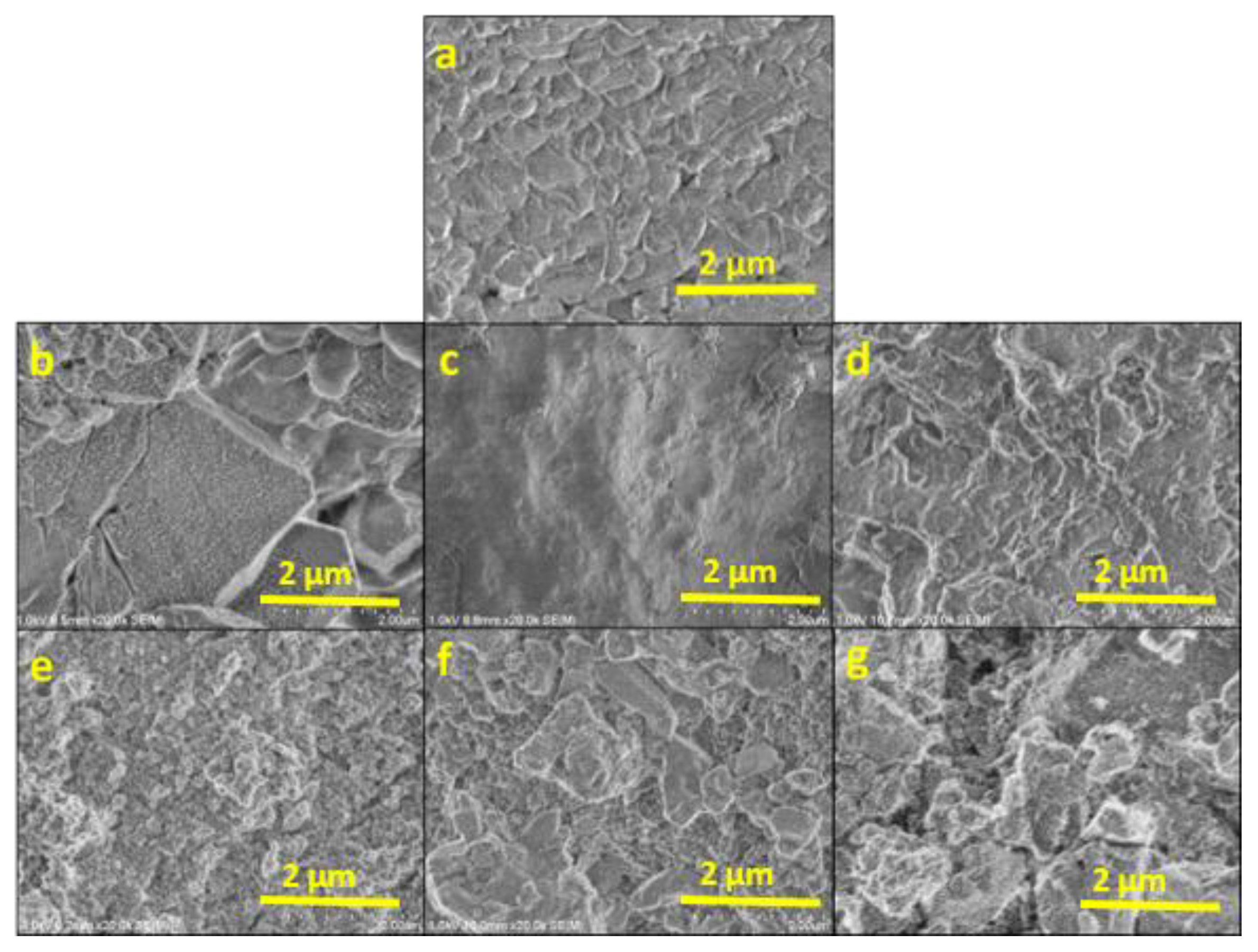
3.1. Characterization of the Consolidants
3.2. Application on Stone and Evaluation of the Performance
3.2.1. Stone–Product Interaction
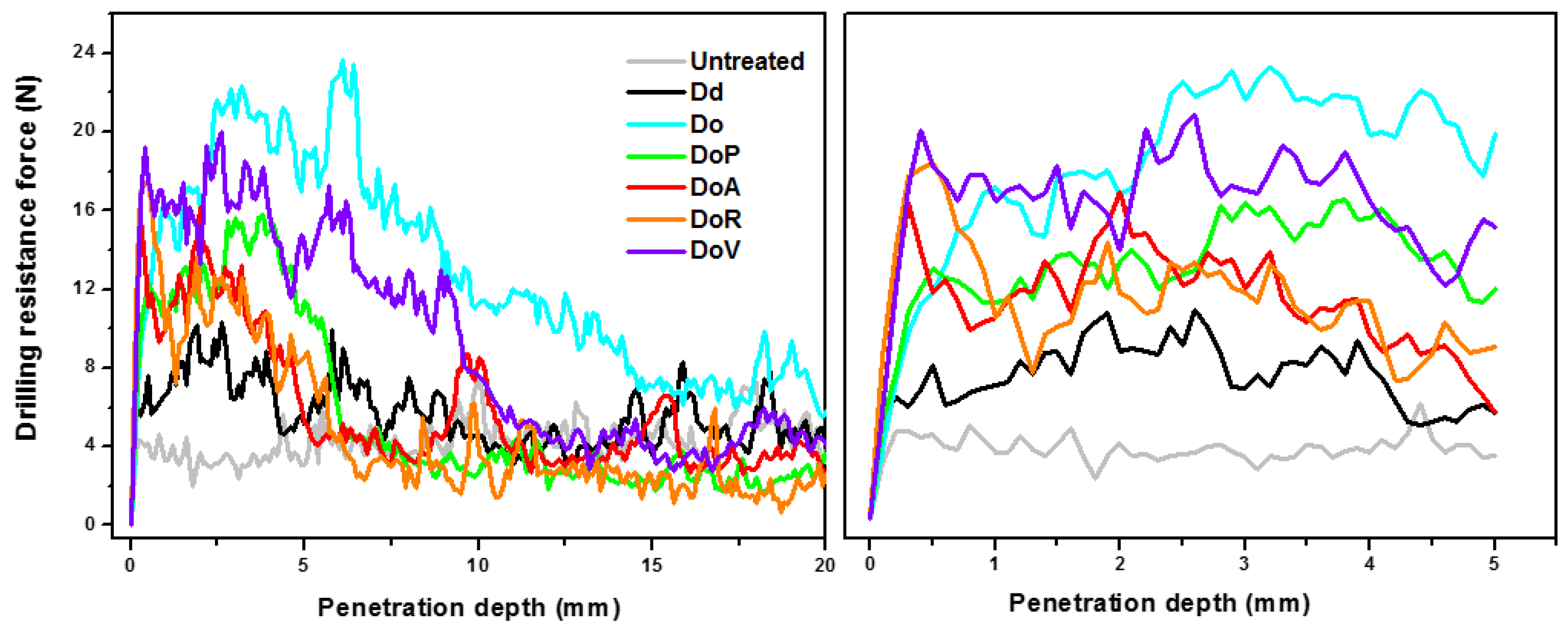
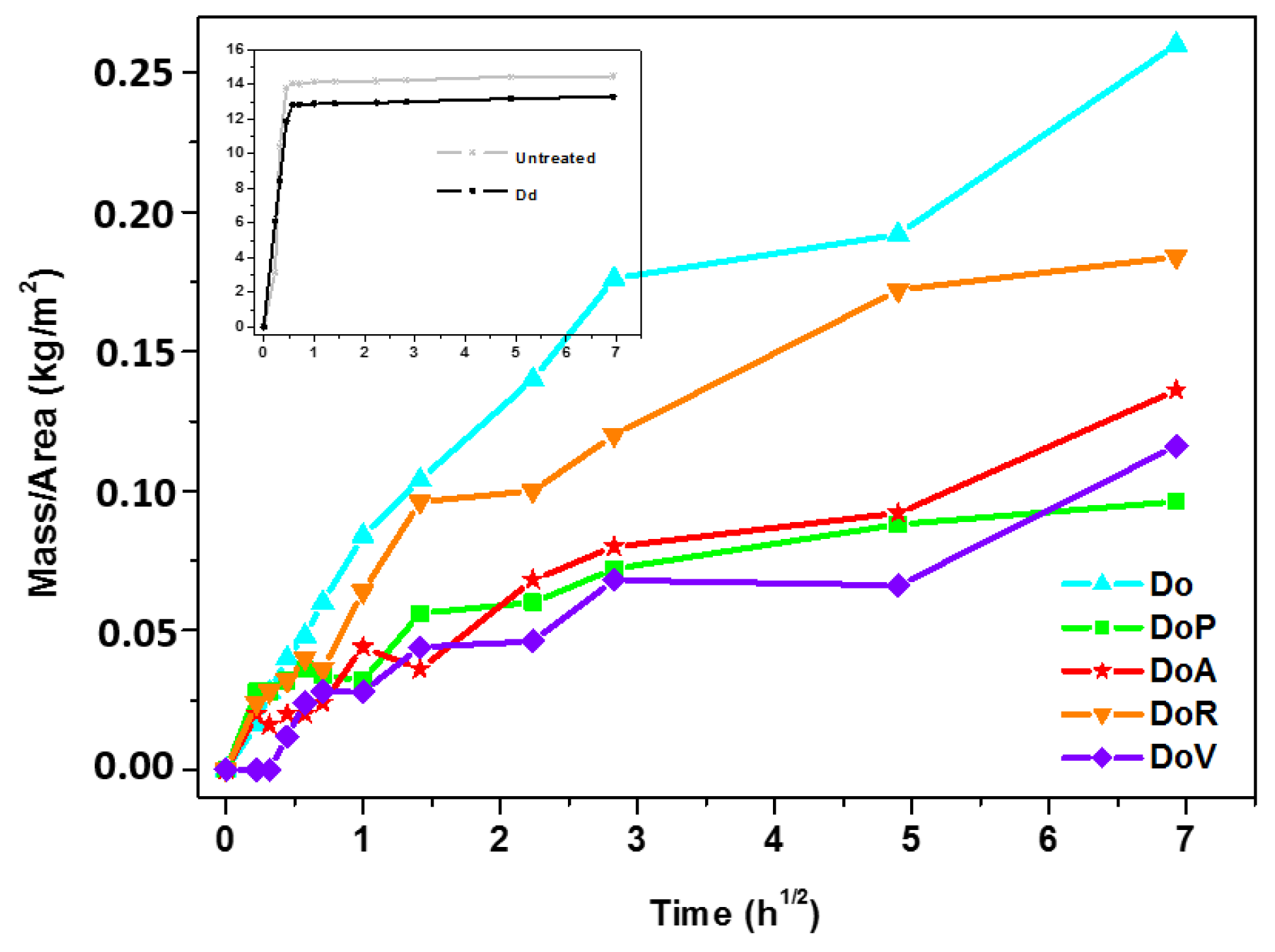
3.2.2. Effectiveness of the Products on Stone
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wheeler, G.; Goins, E.S. Alkoxysilanes and the Consolidation of Stone; The Getty Conservation Institute: Los Angeles, CA, USA, 2005; Volume 46. [Google Scholar]
- Xu, F.; Zeng, W.; Li, D. Recent advance in alkoxysilane-based consolidants for stone. Prog. Org. Coat. 2019, 127, 45–54. [Google Scholar] [CrossRef]
- Doehne, E.F.; Price, C.A. Stone Conservation: An Overview of Current Research, 2nd ed.; The Getty Conservation institute: Los Angeles, CA, USA, 2010. [Google Scholar]
- Scherer, G.W. Effect of drying on properties of silica gel. J. Non-Cryst. Solids 1997, 215, 155–168. [Google Scholar] [CrossRef]
- Scherer, G.W. Influence of viscoelasticity and permeability on the stress response of silica gel. Langmuir 1996, 12, 1109–1116. [Google Scholar] [CrossRef]
- Mosquera, M.J.; De Los Santos, D.M.; Montes, A.; Valdez-Castro, L. New nanomaterials for consolidating stone. Langmuir 2008, 24, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, M.J.; de los Santos, D.M.; Valdez-Castro, L.; Esquivias, L. New route for producing crack-free xerogels: Obtaining uniform pore size. J. Non-Cryst. Solids 2008, 354, 645–650. [Google Scholar] [CrossRef]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol–gel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Xu, F.; Li, D.; Zhang, Q.; Zhang, H.; Xu, J. Effects of addition of colloidal silica particles on TEOS-based stone protection using n-octylamine as a catalyst. Prog. Org. Coat. 2012, 75, 429–434. [Google Scholar] [CrossRef]
- Remzova, M.; Sasek, P.; Frankeova, D.; Slizkova, Z.; Rathousky, J. Effect of modified ethylsilicate consolidants on the mechanical properties of sandstone. Constr. Build. Mater. 2016, 112, 674–681. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Pinto, A.P.F. Laboratory and onsite study of barium hydroxide as a consolidant for high porosity limestones. J. Cult. Herit. 2016, 19, 467–476. [Google Scholar] [CrossRef]
- Muhyodin, G.; Zhu, X.; Abro, M.; Yasin, G.; Maitlo, I.; Akaram, M.Y.; Nie, J. Preparation and characteristics of self-floating silica. Prog. Org. Coat. 2018, 117, 1–6. [Google Scholar] [CrossRef]
- Pia, G.; Corcione, C.E.; Striani, R.; Casnedi, L.; Sanna, U. Thermal conductivity of porous stones treated with UV light-cured hybrid organic–inorganic methacrylic-based coating. Experimental and fractal modeling procedure. Prog. Org. Coat. 2016, 94, 105–115. [Google Scholar] [CrossRef]
- Elhaddad, F.; Carrascosa, L.A.; Mosquera, M.J. Long-term effectiveness, under a mountain environment, of a novel conservation nanomaterial applied on limestone from a roman archaeological site. Materials 2018, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Drdácký, M.; Lesák, J.; Niedoba, K.; Valach, J. Peeling tests for assessing the cohesion and consolidation characteristics of mortar and render surfaces. Mater. Struct. 2015, 48, 1947–1963. [Google Scholar] [CrossRef]
- Facio, D.S.; Mosquera, M.J. Simple strategy for producing superhydrophobic nanocomposite coatings in situ on a building substrate. ACS Appl. Mater. Interfaces 2013, 5, 7517–7526. [Google Scholar] [CrossRef]
- Carrascosa, L.A.; Facio, D.S.; Mosquera, M.J. Producing superhydrophobic roof tiles. Nanotechnology 2016, 27, 095604. [Google Scholar] [CrossRef] [PubMed]
- UNE-EN 1925 Natural Stone Test Methods. Determination of Water Absorption Coefficient by Capillarity; AENOR: Madrid, Spain, 1999.
- Mosquera, M.J.; Benítez, D.; Perry, S.H. Pore structure in mortars applied on restoration. Cem. Concr. Res. 2002, 32, 1883–1888. [Google Scholar] [CrossRef]
- ASTM E96-90 Standard Test Methods for Water Vapor Transmission of Material; ASTM: West Conshohocken, PA, USA, 1990; pp. 685–695.
- Berns, R.S. Billmeyer and Saltzman’s Principles of Color Technology, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Illescas, J.F.; Mosquera, M.J. Producing surfactant-synthesized nanomaterials in situ on a building substrate, without volatile organic compounds. ACS Appl. Mater. Interfaces 2012, 4, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, J.; Li, D.; Xiang, N.; Zhang, Q.; Shao, L. Solvent effects on structural properties of SiO2 gel using n-octylamine as a catalyst. J. Sol-Gel Sci. Technol. 2014, 71, 204–210. [Google Scholar] [CrossRef]
- Illescas, J.F.; Mosquera, M.J. Surfactant-synthesized PDMS/silica nanomaterials improve robustness and stain resistance of carbonate stone. J. Phys. Chem. C 2011, 115, 14624–14634. [Google Scholar] [CrossRef]
- Facio, D.S.; Carrascosa, L.A.; Mosquera, M.J. Producing lasting amphiphobic building surfaces with self-cleaning properties. Nanotechnology 2017, 28, 265601. [Google Scholar] [CrossRef]
- Matos, M.C.; Ilharco, L.M.; Almeida, R.M. The evolution of TEOS to silica gel and glass by vibrational spectroscopy. J. Non-Cryst. Solids 1992, 147–148, 232–237. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S. Adsorption by Powders and Porous Solids, 2nd ed.; Academic Press: Oxford, UK, 2014. [Google Scholar]
- Fidalgo, A.; Ilharco, L.M. Correlation between physical properties and structure of silica xerogels. J. Non-Cryst. Solids 2004, 347, 128–137. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Novotná, P.; Zita, J.; Krýsa, J.; Kalousek, V.; Rathouský, J. Two-component transparent TiO2/SiO2 and TiO2/PDMS films as efficient photocatalysts for environmental cleaning. Appl. Catal. B 2008, 79, 179–185. [Google Scholar] [CrossRef]
- Novotná, P.; Matoušek, J. Preparation and characterization of photocatalytical TiO2–SiO2–PDMS layers on glass. Thin Solid Films 2006, 502, 143–146. [Google Scholar] [CrossRef]
- Wang, S.D.; Jiang, Y.S. The durability of superhydrophobic films. Appl. Surf. Sci. 2015, 357, 1647–1657. [Google Scholar] [CrossRef]
| Sample | Additive | Additive Proportion | Catalyst | Catalyst Proportion |
|---|---|---|---|---|
| Dd | – | – | DOTL | 1 vol.% |
| Do | – | – | n-octylamine | 0.18 vol.% |
| DoP | PDMS | 5 vol.% | n-octylamine | 0.18 vol.% |
| DoA | A200 | 3 wt.% | n-octylamine | 0.18 vol.% |
| DoR | R805 | 3 wt.% | n-octylamine | 0.18 vol.% |
| DoV | VP | 3 wt.% | n-octylamine | 0.18 vol.% |
| Consolidant | Viscosity (mPa·s) | Gel Time (h) | Appearance | Stability (month) |
|---|---|---|---|---|
| Dd | 2.75 | 60 | Cracked | >18 |
| Do | 2.99 | 48 | Monolithic | >18 |
| DoP | 3.59 | 48 | Monolithic | =12 |
| DoA | 4.64 | 48 | Monolithic | >18 |
| DoR | 4.41 | 48 | Monolithic | >18 |
| DoV | 3.23 | 48 | Phase separation | >18 |
| Consolidant | Micropore Volume (cm3·g−1) | Mesopore Volume (cm3·g−1) | BET Surface Area (m2·g−1) | Pore Width (nm) | Total Porosity (%) ** |
|---|---|---|---|---|---|
| Dd | 0.15 | 0.00 | 1.6 * | 0.8 | 25 |
| Do | 0.00 | 0.35 | 555.0 | 3.2 | 43 |
| DoP | 0.00 | 0.26 | 414.0 | 2.4 | 37 |
| DoA | 0.00 | 0.34 | 514.0 | 2.4 | 45 |
| DoR | 0.00 | 0.26 | 420.0 | 2.4 | 37 |
| DoV | 0.00 | 0.04 | 32.0 | 3.5 | 8 |
| Sample | Peeling Test* (mg) | Vickers Hardness Test (kP/mm2) | ΔE * | Static Angle (°) | Vap. Diffusivity (×10−6) (m2·s−1) | Porosity (%) | Static Angle after WAC Experiment (°) |
|---|---|---|---|---|---|---|---|
| Untreated | 0.9 ± 0.4 | 15.41 ± 1.76 | – | nd | 3.13 | 37.2 | nd |
| Dd | 0 | 17.5 ± 3.71 | 3.75 ± 1.05 | 0 | 2.71 | 37.1 | nd |
| Do | 0.1 ± 0.1 | 18.91 ± 1.91 | 4.68 ± 1.2 | 140 ± 1 | 2.78 | 40.8 | nd |
| DoP | 0 | 19.43 ± 2.53 | 7.59 ± 0.34 | 149 ± 6 | 2.46 | 42.4 | 135 ± 5 |
| DoA | 0.6 ± 0.1 | 19.10 ± 2.93 | 4.74 ± 0.14 | 144 ± 3 | 3.05 | 47.6 | 125 ± 9 |
| DoR | 0.1 ± 0.1 | 18.44 ± 5.61 | 5.79 ± 0.42 | 129 ± 5 | 3.01 | 40.5 | 113 ± 13 |
| DoV | 0 | 17.06 ± 2.74 | 4.39 ± 0.58 | 120 ± 5 | 3.11 | 43.4 | 108 ± 8 |
| Sample | Uptake (%) | Dry-Matter (%) | Penetration Depth (mm) |
|---|---|---|---|
| Dd | 1.0 ± 0.2 | 0.6 ± 0.1 | – |
| Do | 2.2 ± 0.3 | 1.3 ± 0.2 | 12.5 ± 1.3 |
| DoP | 1.2 ± 0.1 | 0.8 ± 0.1 | 9.1 ± 0.9 |
| DoA | 1.1 ± 0.1 | 0.8 ± 0.1 | 5.4 ± 1.3 |
| DoR | 1.2 ± 0.3 | 0.8 ± 0.2 | 4.1 ± 0.7 |
| DoV | 1.4 ± 0.1 | 0.9 ± 0.1 | 8.4 ± 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remzova, M.; Carrascosa, L.A.M.; Mosquera, M.J.; Rathousky, J. Modified Ethylsilicates as Efficient Innovative Consolidants for Sedimentary Rock. Coatings 2019, 9, 6. https://doi.org/10.3390/coatings9010006
Remzova M, Carrascosa LAM, Mosquera MJ, Rathousky J. Modified Ethylsilicates as Efficient Innovative Consolidants for Sedimentary Rock. Coatings. 2019; 9(1):6. https://doi.org/10.3390/coatings9010006
Chicago/Turabian StyleRemzova, Monika, Luis A. M. Carrascosa, María J. Mosquera, and Jiri Rathousky. 2019. "Modified Ethylsilicates as Efficient Innovative Consolidants for Sedimentary Rock" Coatings 9, no. 1: 6. https://doi.org/10.3390/coatings9010006
APA StyleRemzova, M., Carrascosa, L. A. M., Mosquera, M. J., & Rathousky, J. (2019). Modified Ethylsilicates as Efficient Innovative Consolidants for Sedimentary Rock. Coatings, 9(1), 6. https://doi.org/10.3390/coatings9010006






