Rapid Electrodeposition of Fe–Ni Alloy Foils from Chloride Baths Containing Trivalent Iron Ions
Abstract
:1. Introduction
2. Experimental
2.1. Electrodeposition of Fe–Ni Alloy Foils
2.2. Characterization
2.3. Tensile Tests
3. Results and Discussion
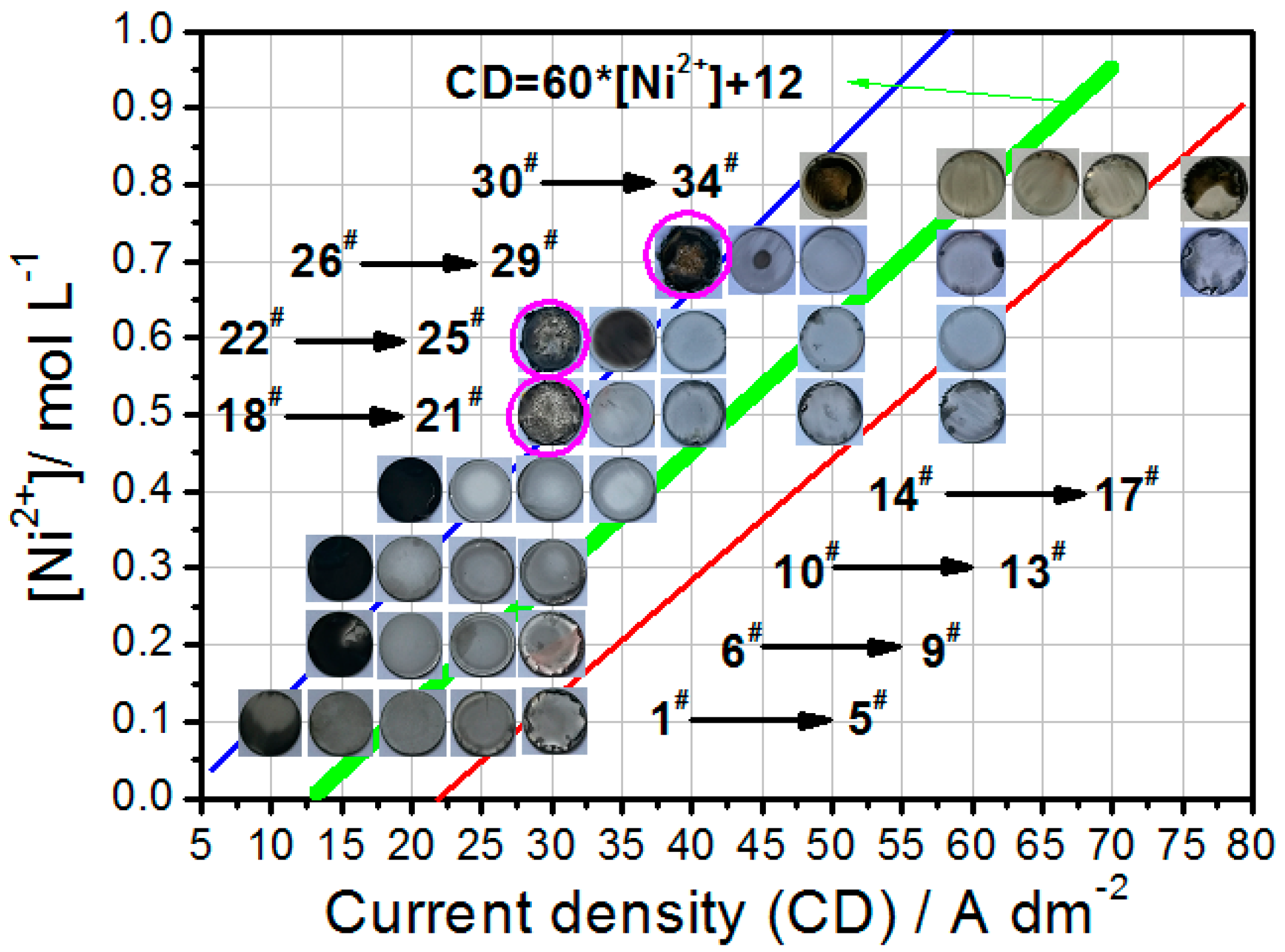
3.1. Electrodeposition of Fe–Ni Alloy Foils
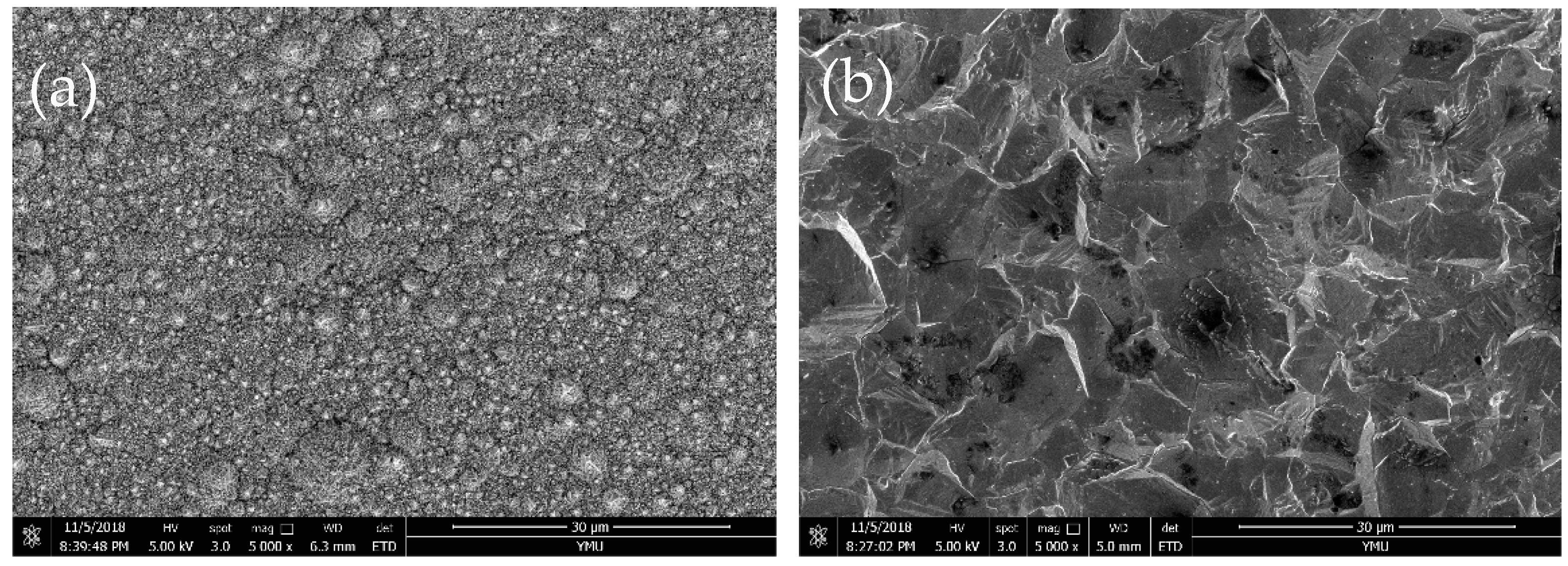
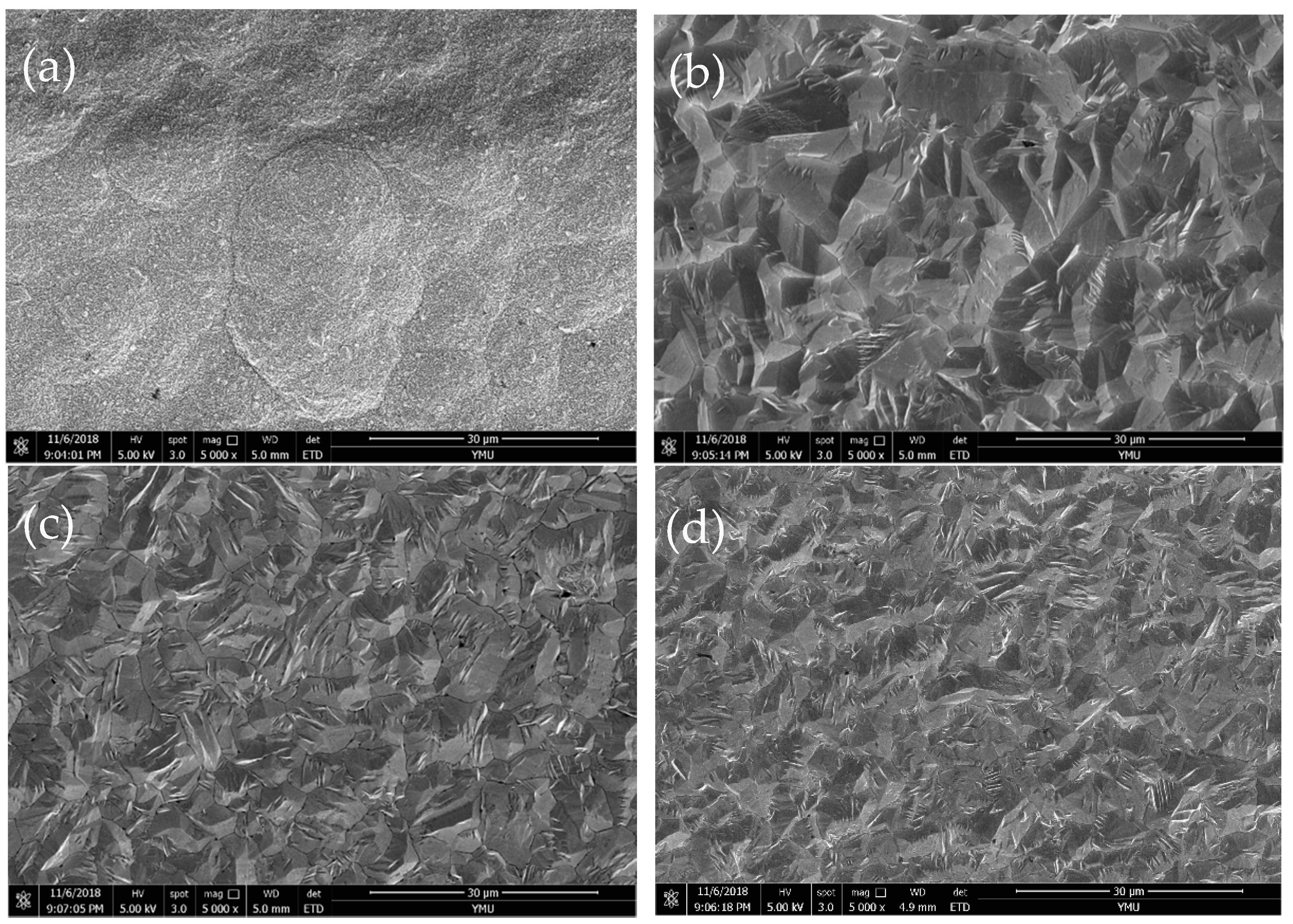
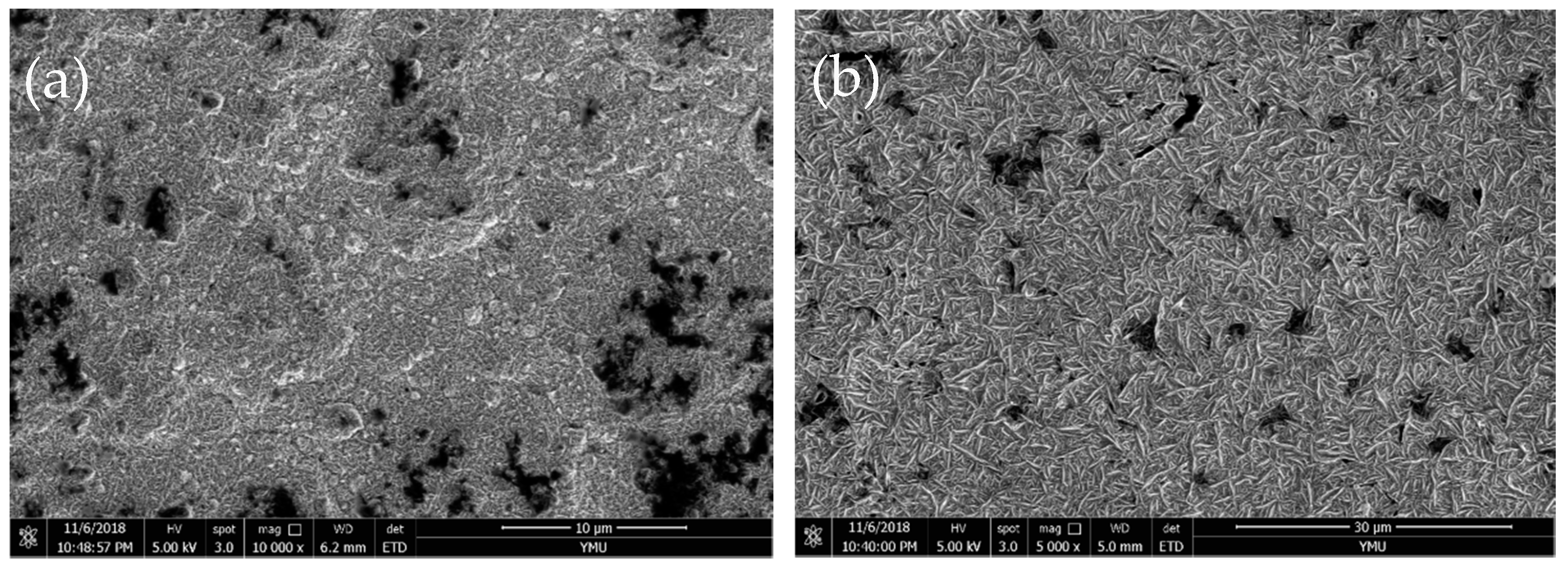
3.2. Chemical Composition and Surface Morphology of Fe–Ni Alloy Foils
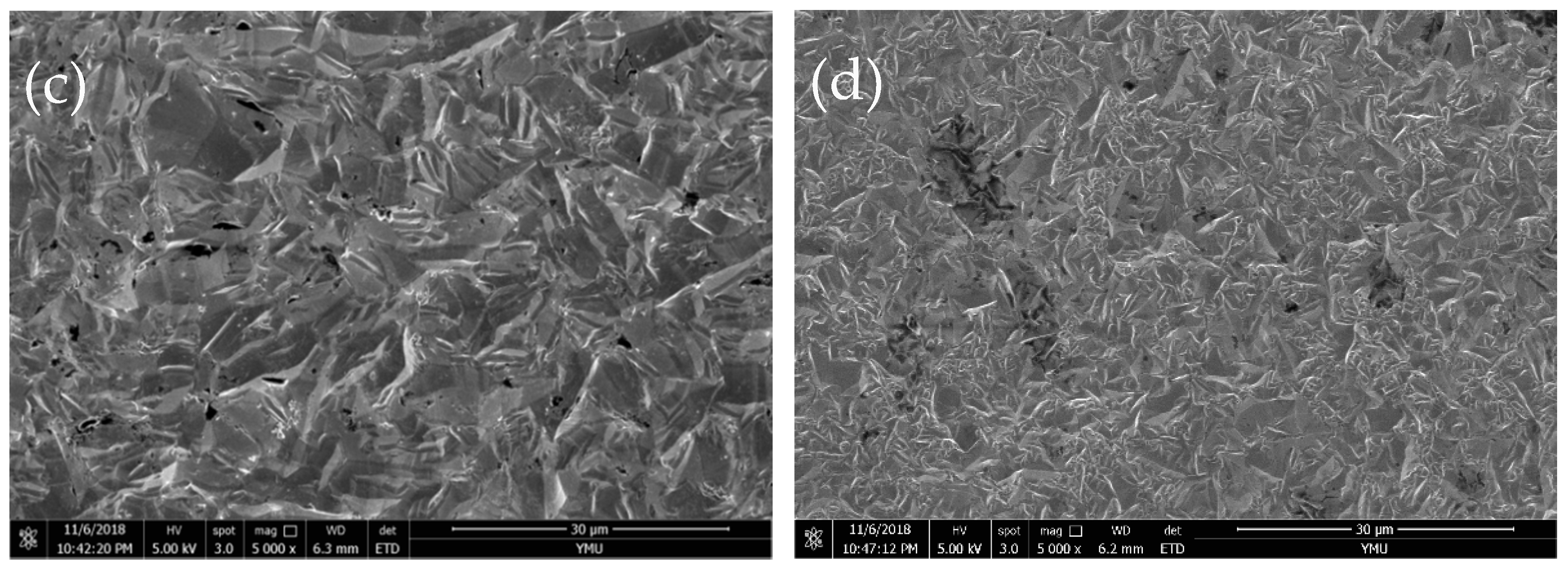
3.3. Crystal Structure of Fe–Ni Alloy Foils
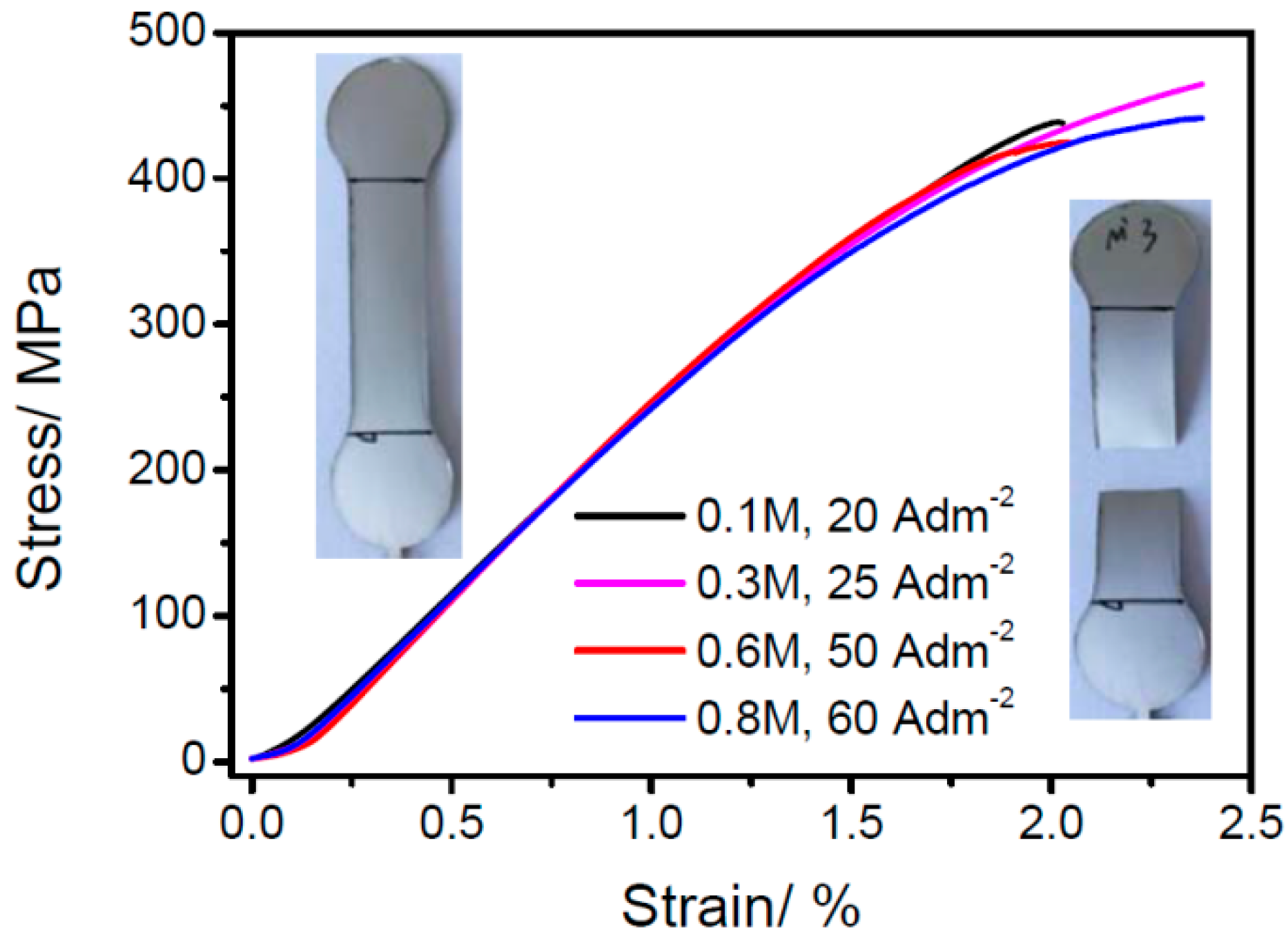
3.4. The Tensile Property of Electrodeposited Fe–Ni Alloy Foils
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Alahyarazadeh, M.H.; Sabour Rouhaghdam, A. Electrodeposition of Ni-Fe alloys, composites and nano coatings—A review. J. Alloys Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.B. A review of fundamental aspects, characterization and applications of electrodeposited nanocrystalline iron group metals, Ni-Fe alloy and oxide ceramics reinforced nanocomposite coatings. J. Alloys Compd. 2018, 751, 194–214. [Google Scholar] [CrossRef]
- Harty, S.F.; Mcgeough, J.A.; Tulloch, R.M. A review of the electroforming of iron and iron-nickel alloy. Surf. Technol. 1981, 12, 39–55. [Google Scholar] [CrossRef]
- Gadad, S.; Haris, T.M. Oxygen incorporation during the electrodeposition of Ni, Fe and Ni-Fe alloys. J. Electrochem. Soc. 1998, 145, 3699–3703. [Google Scholar] [CrossRef]
- Kieling, V.C. Parameters influencing the electrodeposition of Ni-Fe alloys. Surf. Coat. Technol. 1997, 96, 135–139. [Google Scholar] [CrossRef]
- Tabakovic, I.; Gong, J.; Riemer, S.; Kautzky, M. Influence of surface roughness and current efficiency on composition gradients of thin NiFe films obtained by electrodeposition. J. Electrochem. Soc. 2015, 162, D102–D108. [Google Scholar] [CrossRef]
- Nagayama, T.; Yamamoto, T.; Nakamura, T. Thermal expansions and mechnical properties of elecctrodeposited Fe-Ni alloys in the invar compositionrange. Electrochim. Acta 2016, 205, 178–187. [Google Scholar] [CrossRef]
- Su, C.-W.; He, F.-J.; Ju, H.; Zhang, Y.-B.; Wang, E.-L. Electrodeposition of Ni, Fe and Ni-Fe alloy on the 316 stainless steel surface from fluorborate bath. Electrochim. Acta 2009, 54, 6257–6263. [Google Scholar] [CrossRef]
- Su, C.-W.; Wang, E.-L.; Zhang, Y.-B.; He, F.-J. Ni1−xFex (0.1 < x < 0.75) alloy foils prepared from a fluorborate bath using electrochemical deposition. J. Alloys Compd. 2009, 474, 190–194. [Google Scholar]
- Grimmett, D.L.; Schwartz, M.; Nobe, K. Pulsed electrodeposition of iron-nickel alloys. J. Electrochem. Soc. 1990, 137, 3414–3418. [Google Scholar] [CrossRef]
- Trudeau, M.L. Nanocrystalline Fe and Fe-riched Fe-Ni through electrodeposition. Nanostruct. Mater. 1999, 12, 55–60. [Google Scholar] [CrossRef]
- Dubin, V.M.; Lisunova, M.O.; Walton, B.L. Invar electrodeposition for controlled expansion interconnects. J. Electrochem. Soc. 2017, 164, D321–D326. [Google Scholar] [CrossRef]
- Brankovic, S.R.; Bae, S.-E.; Litvinov, D. The effect of Fe3+ on magnetic moment of electrodepositied CoFe alloys-experimental study and analytical model. Electrochim. Acta 2008, 53, 5934–5940. [Google Scholar] [CrossRef]
- Su, C.-W.; Yang, W.; Guo, J.-M.; Zhang, Y.-J. Modulated morphology of Fe foils electrodeposited at a high current density by adding cations. Vacuum 2012, 86, 2095–2101. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys; Academic Press, Inc.: New York, NY, USA, 1963. [Google Scholar]
- Zhou, Q.; He, F. Insight into the mechanism of the Fe-Ni alloys co-deposition from ploy-nuclear complexes. J. Electrochem. Soc. 2018, 165, D681–D686. [Google Scholar] [CrossRef]


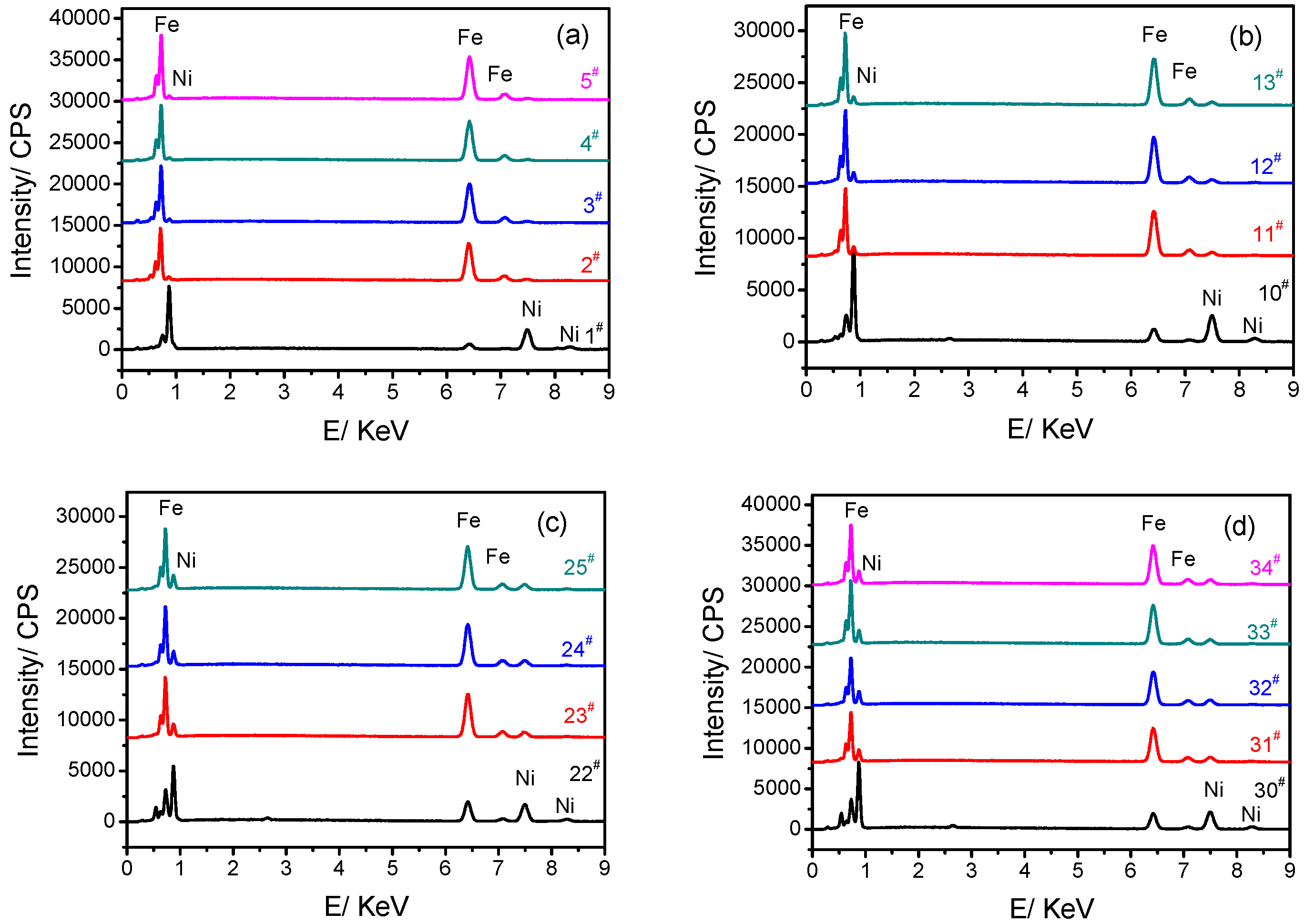




| Sample | Element | Weight % | Atomic % |
|---|---|---|---|
| 1# | O K | 1.060 | 3.780 |
| Ni K | 88.16 | 85.26 | |
| Fe K | 10.78 | 10.97 | |
| 2# | O K | 1.740 | 5.850 |
| Ni K | 5.330 | 4.870 | |
| Fe K | 92.92 | 89.28 | |
| 3# | O K | 1.450 | 4.890 |
| Ni K | 4.870 | 4.480 | |
| Fe K | 93.68 | 90.62 | |
| 4# | O K | 0.930 | 3.180 |
| Ni K | 4.100 | 3.830 | |
| Fe K | 94.74 | 92.89 | |
| 5# | O K | 0.940 | 3.220 |
| Ni K | 4.100 | 3.820 | |
| Fe K | 94.96 | 92.97 | |
| 10# | O K | 1.490 | 5.220 |
| Ni K | 81.11 | 77.34 | |
| Fe K | 17.40 | 17.44 | |
| 11# | O K | 1.330 | 4.530 |
| Ni K | 11.20 | 10.39 | |
| Fe K | 87.17 | 84.97 | |
| 12# | O K | 1.050 | 3.600 |
| Ni K | 11.18 | 10.44 | |
| Fe K | 87.54 | 85.88 | |
| 13# | O K | 1.050 | 3.580 |
| Ni K | 9.790 | 9.130 | |
| Fe K | 88.97 | 87.22 | |
| 22# | O K | 4.010 | 13.09 |
| Ni K | 61.21 | 54.42 | |
| Fe K | 34.77 | 32.49 | |
| 23# | O K | 1.030 | 3.540 |
| Ni K | 16.96 | 15.88 | |
| Fe K | 81.75 | 80.48 | |
| 24# | O K | 1.100 | 3.770 |
| Ni K | 18.20 | 16.83 | |
| Fe K | 80.69 | 79.32 | |
| 25# | O K | 1.020 | 3.490 |
| Ni K | 17.64 | 16.53 | |
| Fe K | 81.14 | 79.90 | |
| 30# | O K | 4.760 | 15.32 |
| Ni K | 66.73 | 58.50 | |
| Fe K | 28.34 | 26.12 | |
| 31# | O K | 1.190 | 4.080 |
| Ni K | 19.42 | 18.07 | |
| Fe K | 79.39 | 77.79 | |
| 32# | O K | 0.990 | 3.420 |
| Ni K | 20.80 | 19.53 | |
| Fe K | 78.21 | 76.97 | |
| 33# | O K | 1.090 | 3.730 |
| Ni K | 16.65 | 15.47 | |
| Fe K | 82.16 | 80.71 | |
| 34# | O K | 1.060 | 3.640 |
| Ni K | 16.94 | 15.75 | |
| Fe K | 81.99 | 80.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Zhao, L.; Tian, L.; Wen, B.; Xiang, M.; Bai, W.; Guo, J. Rapid Electrodeposition of Fe–Ni Alloy Foils from Chloride Baths Containing Trivalent Iron Ions. Coatings 2019, 9, 56. https://doi.org/10.3390/coatings9010056
Su C, Zhao L, Tian L, Wen B, Xiang M, Bai W, Guo J. Rapid Electrodeposition of Fe–Ni Alloy Foils from Chloride Baths Containing Trivalent Iron Ions. Coatings. 2019; 9(1):56. https://doi.org/10.3390/coatings9010056
Chicago/Turabian StyleSu, Changwei, Linfu Zhao, Liang Tian, Bixia Wen, Mingwu Xiang, Wei Bai, and Junming Guo. 2019. "Rapid Electrodeposition of Fe–Ni Alloy Foils from Chloride Baths Containing Trivalent Iron Ions" Coatings 9, no. 1: 56. https://doi.org/10.3390/coatings9010056
APA StyleSu, C., Zhao, L., Tian, L., Wen, B., Xiang, M., Bai, W., & Guo, J. (2019). Rapid Electrodeposition of Fe–Ni Alloy Foils from Chloride Baths Containing Trivalent Iron Ions. Coatings, 9(1), 56. https://doi.org/10.3390/coatings9010056





